Abstract
Osteoclasts, derived from multipotent myeloid progenitor cells, play homeostatic roles in skeletal modeling and remodeling, but may also destroy bone in pathological conditions such as osteoporosis and rheumatoid arthritis. Osteoclast development depends critically on a differentiation factor, the receptor activator of NF-κB ligand (RANKL). In this study, we found that the hexane soluble fraction of the common fig Ficus carica (HF6-FC) is a potent inhibitor of osteoclastogenesis in RANKL-stimulated RAW264.7 cells and in bone marrow-derived macrophages (BMMs). HF6-FC exerts its inhibitory effects by suppression of p38 and NF-κB but activation of ERK. In addition, HF6-FC significantly decreased the expression of NFATc1 and c-Fos, the master regulator of osteoclast differentiation. The data indicate that components of HF6-FC may have therapeutic effects on bone-destructive processes such as osteoporosis, rheumatoid arthritis, and periodontal bone resorption.
Keywords: Ficus carica, Osteoclast differentiation, RAW264.7 cells, Bone marrow-derived macrophages, Bone lytic diseases
INTRODUCTION
Ficus carica L. (Moraceae), the common Fig, is widely distributed in South Korea, and the leaves of this plant have been used as folk medicine for hemorrhoids, neuralgia, warts, diarrhea and carbuncles in Korea and China (Lee, 1996). The leaves of this plant contain terpenoid, saponin and flavonoid compounds (el-Kholy and Shaban, 1966; Ahmed et al., 1988), which may be related to their alleged antiviral effects on herpes simplex virus (HSV), and hypoglycemic activity in type-I diabetic patients (Serraclara et al., 1998; Canal et al., 2000). But no study has yet reported the regulatory effects of F. carica leaf on osteogenic differentiation.
A balance between osteoclast-regulated bone resorption and osteoblast-induced bone formation determines bone mass in adults. Perturbations in this balance contribute to diseases such as osteoporosis, osteomalacia, and osteopetrosis (Alliston et al., 2002; Oh et al., 2007). The multinucleated osteoclasts are generated from hematopoietic monocyte/macrophage precursor cells under the control of two cytokines, macrophage colony-stimulating factor (MCSF) and receptor activator of NF-κB ligand (RANKL). Osteoblasts produce these two cytokines in membrane-bound or secreted form, when activated by interleukin-1, prostaglandin E2, and vitamin D3 (Miyaura et al., 2003; Wei et al., 2005).
RANKL induces precursor cells to differentiate into osteoclasts (Theill et al., 2002), whereas M-CSF provides an osteoclast survival signal (Yoshida et al., 1990). RANKL is expressed in osteoblasts and induces the signaling essential for precursor cells to differentiate into osteoclasts (Theill et al., 2002), whereas M-CSF, secreted by osteoblasts, provides the survival signal to these cells (Yoshida et al., 1990). Binding of RANKL to its receptor RANK activates TNF receptor-associated factor 6 (TRAF6), which is linked to the nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPKs) (Kobayashi et al., 2001; Lee et al., 2002). Active extracellular signal-regulated kinase (ERK) can directly phosphorylate c-Fos and active c-Jun-N-terminal kinase (JNK) phosphorylates c-Jun. Thus AP-1 transcription factor, a heterodimer composed of a Fos family (c-Fos, FosB, Fra-1, and Fra-2) and a Jun family (c-Jun, JunB, and JunD) protein, can be a target of ERK and JNK in response to RANKL in osteoclast precursor cells. In addition, RANKL induces the key transcription factor for osteoclastogenesis, nuclear factor of activated T cells c1 (NFATc1) (Zhou et al., 2002; Yamashita et al., 2007).
In this study, we assessed the effects of the hexane soluble fraction of F. carica leaf (HF6-FC) on RANKL-induced osteoclastogenesis in murine monocytes/macrophage RAW 264.7 cells and bone marrow-derived macrophages (BMMs). HF6-FC significantly suppressed osteoclast differentiation in both situations, and inhibited RANKL-induced activation of NF-κB and c-Fos. Our results suggest that HF6-FC may be useful as a therapeutic agent for bone lytic diseases such as osteoporosis, osteomalacia, and osteopetrosis.
METHODS
Reagents
The leaves of F. carica were collected in July 2008 in Youngam, Chonnam, Korea and air-dried. A voucher specimen was deposited in the herbarium of the College of Pharmacy, Woosuk University (WSU-08-025). Murine monocyte/marcrophage RAW 264.7 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture media and supplements, including minimum essential medium-alpha (α-MEM), Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, trypsin-EDTA and TRIzol were obtained from GIBCO (Invitrogen Inc, NY, USA). RANKL was purchased from PeproTech (Rocky Hill, NJ, USA); macrophage-colony stimulating factor (M-CSF), from R&D Systems (Minneapolis, MN, USA); antibodies against Akt, ERK, p38, JNK, IκBα, phospho-ERK, phospho-p38, phospho-JNK and phospo-IκBα, from Cell Signaling Technology (Beverly, MA, USA); and anti-β-actin antibody, from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were purchased from Sigma and/or the same distributors used in previous studies (Soh et al., 2000; Soh et al., 2003; Choi et al., 2008), unless otherwise indicated.
Extraction, solvent fractionation and identification of fig leaf components
The shade-dried plant material (600 g) was extracted three times with methanol at room temperature and filtered. The extracts were combined and evaporated in vacuo at 40℃. The resulting methanolic extract (60 g) was partitioned successively with n-hexane, methylene chloride, ethylacetate and n-butanol. The n-hexane-soluble extract, which had the most anti-osteoclastogenic activity, was fractionated on a silica gel column (n-hexane-ethyl acetate, 10:1-1:1) to give eight fractions (H1-H8). Of these eight, fraction H6, the most active one (HF6-FC), was analyzed by GS-MS to identify its constituents.
Cell culture and Isolation of bone marrow-derived macrophages
The murine monocyte/macrophage cell line RAW264.7 was cultured with DMEM containing 10% heat-inactivated FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml). All cells were grown in a humidified atmosphere containing 5% CO2 at 37℃. To induce osteoclast differentiation, RAW264.7 cells were suspended in α-MEM containing 10% FBS, 2 mM L-glutamate, 100 U/ml penicillin, and 100 µg/ml streptomycin; seeded at 3×103 cells/well in 96-well culture plates; and cultured with 50 ng/ml soluble RANKL for 6 days. The medium and HF6-FC were changed every two or three days. Six-week-old ICR (Institute of Cancer Research) mice were purchased from Damool Science (Daejeon, Korea). Cells obtained from tibia and femur bone marrow were cultured in α-MEM with 10% fetal bovine serum (FBS) containing 30 ng/ml macrophage colony-stimulating factor (M-CSF). After culturing for three days, the nonadherent cells were removed by washing and adherent cells were used as bone marrow-derived macrophages (BMMs). BMM were cultured for three days in medium containing M-CSF (30 ng/ml) and RANKL (200 ng/ml).
MTT assay
Cells (5×103 per well) were seeded in a 96-well plate with medium supplemented with 10% FBS and incubated for 24 h. Cells were treated with various concentrations of HF6-FC for 24 h, then washed three times with phosphate-buffered saline (PBS) and treated with medium containing 100 µg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 2 h at 37℃. Cells were then washed with PBS and solubilized in 200 µl of DMSO. The intracellular purple formazan concentrations were determined from the absorbance at 540 nm.
Tartrate-resistant acid phosphatase (TRAP) staining
TRAP staining was performed as described (Han et al., 2007). Briefly, cells were washed with PBS and fixed with 3.7% formaldehyde for 10 min. After washing with PBS, cells were made permeable with 0.1% (v/v) Triton X-100 for 1 min and washed with distilled water. Cells were incubated at 37℃ in a humid and light-protected incubator for 40 min in the reaction mixture of the Leukocyte Acid Phosphatase Assay kit (Sigma, Cat No. 387), as directed by the manufacturer. Cells were washed three times with distilled water and TRAP-positive multinucleated cells containing three or more nuclei were counted under a light microscope.
RT-PCR analysis
Total RNA was isolated from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instruction. PCR was performed with mouse-specific primers, shown here with the corresponding gene sequences: c-Fos, 5'-atgggctctcctgtcaacac-3' (forward) and 5'-tggagtttattttggcagcc-3' (reverse); NFATc1, 5'-gggtcagtgtgaccgaagat-3' (forward) and 5'-tccacccacttctgacttcc-3' (reverse); GAPDH, 5'-accacagtccatgccatcac-3' (forward) and 5'-tacagcaacagggtggtgga-3' (reverse). Thermal cycling parameters were 95℃ for 5 min, followed by 25~35 cycles for 30 sec at 95℃, 30 sec at 55~60℃, and 30 sec at 72℃, and 10 min at 72℃ for the final elongation. The number of cycles for each gene was determined to be in the range of linear amplification through an optimization experiment. PCR products were separated on 1.5% agarose gels, visualized by ethidium bromide staining, and analyzed densitometrically using a Phosphoimager and Quantity One software (Version 4.3.1) (Bio-Rad, Hercules, CA). The optical densities for each gene were normalized to the corresponding values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot analysis
Cells were harvested, washed three times with ice-cold phosphate buffered saline containing 1 mM sodium vanadate, and lysed in a buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 1 mM phenylmethylsulfonylfluoride (PMSF), and 1×protease inhibitor cocktail. Total cell lystes were incubated for 20 min and centrifuged at 16,000× g for 15 min at 4℃, and supernatants were used as cell extracts. Cell extracts (30~40 µg) were separated on an 8~10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membranes were blocked for 1 h at room temperature with 5% nonfat skim milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TTBS), and then probed with specific antibodies in 5% nonfat skim milk in TTBS, for 16 h at 4℃. Horseradish peroxidase-conjugated anti-rabbit or mouse antibodies (Santa Cruz Biotechnology) were used as secondary antibodies (1:5,000~1:10,000 dilution in 5% nonfat skim milk in TTBS) for 1 h incubation at room temperature. The antigen-antibody complexes were detected with an ECL Plus kit (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
All data are expressed as the mean±SD of three or more replicates, unless otherwise indicated. Group data were compared using one-way analysis of variance (ANOVA) followed by a Student's t-test, and p values less than 0.05 were considered significant.
RESULTS
Characterization of the hexane soluble fraction of F. carica
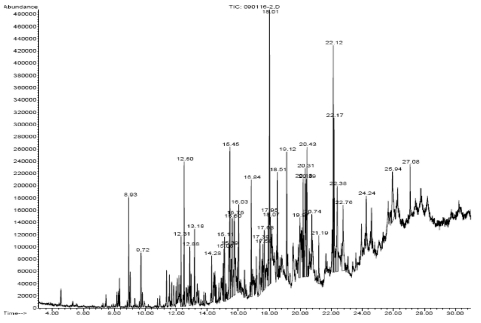
The n-hexane soluble fraction of F. carica showed a significant anti-osteoclastogenic activity in the murine monocyte/macrophage cell line RAW 264.7. Of the eight silica gel fractions (H1-H8), H6 (HF6-FC) exhibited the strongest anti-osteoclastogenic effect (data not shown). Analysis by GS-MS (Fig. 1) identified the following components of HF6-FC: octadecane, pentadecane, hexadecane, heptadecane, octadecane, 2H-1-benzopyran-2-one, nonadecane, hexadecanoic acid methyl ester, octadecanoic acid methyl ester, tridecane, tetradecane, eicosane, 9,12,-octadecadienoic acid methyl ester and 8-octadecenoic acid. HF6-FC was used for all cell experiments. DMSO was used as control vehicle to solubilize HF6-FC.
Fig. 1.
GC/MS spectrum of HF6-FC.
Effect of HF6-FC on osteoclastogenesis in murine monocyte/macrophage RAW 264.7 cells
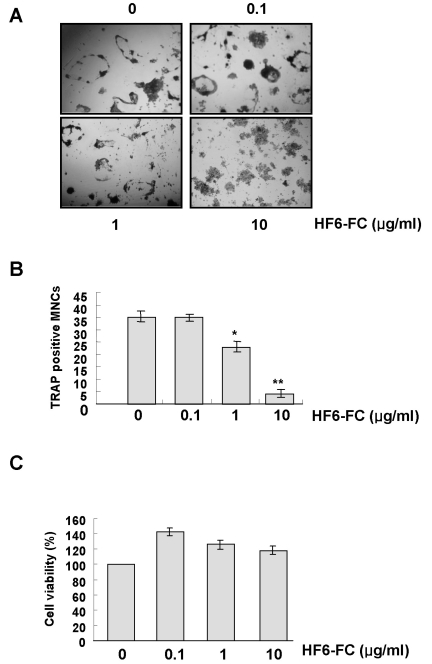
Using the murine monocyte/macrophage cell line RAW 264.7 as a model for osteoclastogenesis, we found that RANKL (50 ng/ml) induced TRAP-positive multinucleated osteoclasts. The HF6-FC, however, inhibited osteoclast differentiation in a concentration-dependent manner (Fig. 2A), reducing the numbers of TRAP-positive multinucleated cells by 34.3±4.2% and 88.6±1.5% at 1 and 10 µg/ml, respectively (Fig. 2B). To test the effects of HF6-FC on cell growth, cells were treated with various concentrations of HF6-FC for 24 h and growth and viability were tested in an MTT assay. In this study, the HF6-FC did not did not adversely affect the cell growth rate of RAW 264.7 cells (Fig. 2C). Hence, the anti-osteoclastogenic action of HF6-FC does not apparently stem from cell toxicity.
Fig. 2.
Effect of HF6-FC on RANKL-induced osteoclastogenesis in RAW 264.7 cells. (A) RAW 264.7 cells were cultured in the presence of RANKL (50 ng/ml) for 6 days. HF6-FC was added to the culture medium at final concentrations of 0.1, 1, and 10 µg/ml, and cells were stained for TRAP activity on day 6. (B) TRAP-positive multinucleated osteoclasts were counted. Data represent the mean±SD of three independent experiments. *p<0.05 and **p<0.01, as compared to the control without HF6-FC, respectively. (C) RAW 264.7 cells were seeded into 96-well plates and incubated with HF6-FC for 24 h. Cell proliferation was evaluated with the MTT assay.
Effect of HF6-FC on osteoclastogenesis in bone marrow-derived macrophages
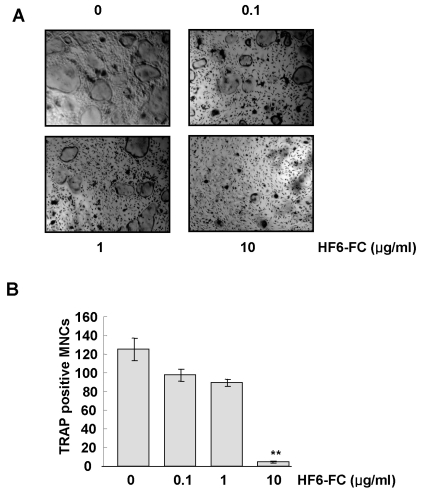
Bone marrow-derived macrophages (BMMs) were induced to differentiate into osteoclasts in the presence of M-CSF and RANKL. In this system, HF6-FC reduced the formation of TRAP-positive multinucleated cell (MNC) in a concentration-dependent manner (Fig. 3A), with almost complete inhibition of osteoclastogenesis at 10 µg/ml and reductions in TRAP-positive multinucleated cells by 28.7±3.8% and 96.4±0.7% at 1 µg/ml and 10 µg/ml, respectively (Fig. 3B). HF6-FC did not affect the growth of BMMs at the concentrations used in this study (data not shown).
Fig. 3.
Effect of HF6-FC on RANKL-induced osteoclastogenesis in bone marrow-derived macrophages (BMM). (A) BMMs were cultured in the presence of M-CSF (20 ng/ml) and RANKL (100 ng/ml) for 3 days. HF6-FC was added to the culture medium at final concentrations of 0.1, 1, and 10 µg/ml. (B) After three days, cells were fixed and stained for TRAP, and TRAP-positive multinuclear cells (MNC) were counted. Data represent the mean±SD of three independent experiments. **p<0.01 versus the control without HF6-FC.
Effect of HF6-FC on MAPKs in RANKL-stimulated RAW 264.7 cells
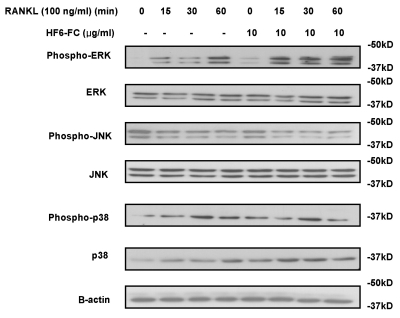
The mitogen-activated protein kinases (MAPKs), which include the ERK, JNK, and p38 kinase families, participate in RANKL-induced osteoclast differentiation. To investigate the role of MAPK signaling in the anti-osteoclastogenic action of HF6-FC, we tested the effect of the extract on ERK, JNK, and p38 activities in RANKL-induced RAW264.7 cells. We measured these activities by immunoblot analysis, using antibodies specifically directed against the phosphorylated forms of the enzymes, and for comparison, antibodies specific for the unphosphorylated forms. As shown in Fig. 4, RANKL induced both ERK and p38. HF6-FC further increased the ERK activity, but inhibited the RANKL-induced p38 kinase activation.
Fig. 4.
Effect of HF6-FC on MAPK activation by RANKL in RAW 264.7 cells. Cells were serum-starved for 16 h, pretreated with or without HF6-FC (10 µg/ml) for 30 min, and stimulated with RANKL (100 ng/ml) for times indicated. Whole cell lystes were used for western blotting with MAPK-specific antibodies. Blots were stripped and reprobed with other antibodies.
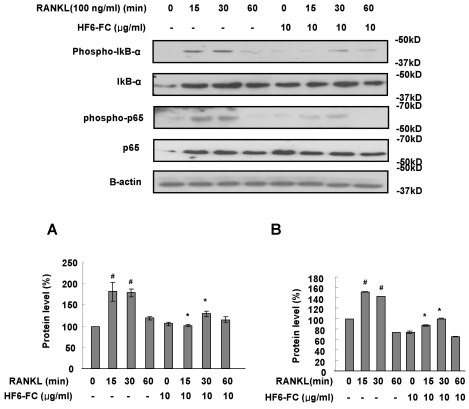
NF-κB expression in RANKL-stimulated RAW 264.7 cells
Activation of the transcription factor NF-κB is an essential step in osteoclast differentiation (Wong, 1998; Kotake, 1999). NF-κB activation occurs through release from inhibitory κB (IκB), which is phosphorylated, ubiquitinated, and degraded in the proteasome. In RANKL-treated RAW 264.7 cells, the level of phosphorylated IκB-αprotein increased after a 15 min exposure. The presence of HF6-FC, however, significantly reduced that increase (Fig. 5A). At 30 min, the RANKL treatment also increased the phosphorylation state (and hence the transactivation potential) of p65 (Vaira et al., 2008). Addition of HF6-FC significantly suppressed the level of phosphorylated p65 (Fig. 5B). These results suggest that HF6-FC suppresses the NF-κB induction by RANKL.
Fig. 5.
Effect of HF6-FC on NF-κB activation by RANKL in RAW 264.7 cells. Cells were serum-starved for 16 h, pretreated with or without HF6-FC (10 µg/ml) for 30 min and stimulated with RANKL (100 ng/ml) for indicated times. Whole cell lysates were immunoblotted with antibodies specific for phospho-IκB-α and phospo-p65. Blots were stripped and reprobed with control antibodies. The histograms represent the level of the phospho-IκB-α (A) and phospo-p65 (B). The asterisk (*) indicates a significant difference (p<0.05) compared with the control (#) at same time period.
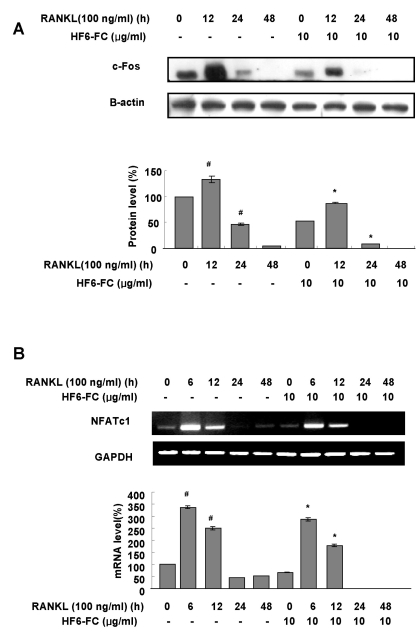
c-Fos and NFATc1 expression in RANKL-stimulated RAW 264.7 cells
In osteoclast precursor cells, RANKL increases the level of c-Fos, a key transcription factor in osteoclast differentiation (Wong et al., 1998; Chang et al., 2007). As shown in Fig. 6A, RANKL induced c-Fos protein in RAW 264.7 cells at 12 h. HF6-FC significantly restricted this increase in c-Fos. We also examined the regulation of nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (NFATc1) in RANKL-induced RAW 264.7 cells, and found that HF6-FC significantly reduced RANKL-induced increase of NFATc1 mRNA (Fig. 6B). These results suggest that HF6-FC suppresses c-Fos as well as NFATc1 induction by RANKL in the RAW 264.7 cells.
Fig. 6.
Effects of HF6-FC on c-Fos protein and NFATc1 mRNA in RANKL-stimulated RAW 264.7 cells. Cells were serum-starved for 16 h, pretreated with or without HF6-FC (10 µg/ml) for 30 min and stimulated with RANKL (100 ng/ml) for times indicated. (A) Whole cell lysates were analyzed by immunoblotting with c-Fos-and β-actin-specific antibodies. (B) NFATc1 mRNA level was determined by RT-PCR and compared with that of GAPDH. The asterisk (*) indicates a significant difference (p<0.05) compared with the control (#) at same time period.
DISCUSSION
Bone destruction and inflammation are closely linked in diseases such as periodontitis and rheumatoid arthritis (Jimi et al., 2004). Inflammatory cytokines and prostaglandins up-regulate RANKL in osteoblasts, synovial fibroblasts and activated T cells (Mino et al., 1998; Coon et al., 2007), and signaling interactions of RANKL and RANK stimulate osteoclast formation, resorption activity, and survival (Han et al., 2007). Regulation of osteoclast development and the signaling pathway of RANK have been well characterized (Teitelbaum and Ross, 2003).
The signaling mechanism of RANKL has been extensively studied. Cells of the osteoblast lineage express a membranebound form of RANKL, a member of the TNF cytokine family. RANK, like other members of the TNF receptor superfamily, strongly activates the NF-κB pathway. Binding of RANKL to its receptor RANK in BMMs recruits TRAF family proteins such as TRAF6, which interacts with NF-κB and JNK pathways (Wong et al., 1998; Takayanagi et al., 2002; Yamashita et al., 2007). In mammals, the NF-κB family has five members: RelA/p65, RelB, c-Rel, NF-κB1/p50, and NF-κB2/p52 (Vaira et al., 2008). In the canonical NF-κB pathway, ligation of RANK activates the inhibitor of the IκB kinase (IKK) complex, which phosphorylates NF-κB-associated IκBα, and leads to its ubiquitination and proteosomal degradation. These events release NF-κB dimers containing RelA and c-Rel into the cytosol, from which they enter the nucleus to enhance transcription of target genes (Luo et al., 2005). HF6-FC inhibited NF-κB transcriptional activity as well as phosphorylation of IκB and p65, which RANKL markedly induces.
In addition to the NF-κB pathways, RANKL activates ERK, JNK and p38 in osteoclasts and their precursor cells (Yoshida et al., 1990; Lee et al., 2002; Chang et al., 2007). Downstream targets of ERK and JNK include the AP-1 transcription factor. While ERK can induce and activate c-Fos, JNK increases AP-1 transcriptional activity through c-Jun phosphorylation. In differentiated osteoclasts, ERK activity correlates with cell survival, but not with resorption function (Miyazaki et al., 2000). While HF6-FC inhibited the RANKL-induced p38 kinase activation, it did not inhibit the ERK activation, but instead prolonged ERK activity, suggesting that HF6-FC influences osteoclast survival through ERK, and osteoclastogenesis via p38 kinase. Both RANKL and M-CSF activate Akt, a key regulator of osteoclast survival (Stern et al., 2007). RANKL activates Akt through the signaling sequence TRAF6-Src-PI 3-kinase (Wong et al., 1999).
The transcription factor NFATc1 also plays a critical role in RANKL-induced osteoclastogenesis (Takayanagi et al., 2002). Genes such as TRAP, cathepsin K, and MMP-9, which are specifically induced during terminal osteoclast differentiation, contain multiple NFAT and AP-1 binding sites (Reddy et al., 1995; Motyckova et al., 2001). Both AP-1 and NF-κB binding sites are present within the promoter region of the NFATc1 gene (Zhou et al., 2002). It is therefore possible that these two transcription factors initiate induction of the gene, which is then followed by NFATc1-mediated autoamplification of gene induction. Accordingly, we investigated the up-regulation of specific genes such as MMP9, TRAP, RANK, and cathepsin K in RANKL-induced RAW 264.7 cells. However, HF6-FC did not affect the expression of theses genes (data not shown).
In summary, HF6-FC inhibited the osteoclastic differentiation of RANKL-stimulated macrophages and BMMs. In RANKL-stimulated RAW 264.7 cells, HF6-FC inhibited the activation of c-Fos and p38 kinase, NFATc1 and NF-κB, possibly through the inhibition of IκB phosphorylation; however, HF6-FC increased the ERK activity. Although further study is needed to confirm its effectiveness in vivo, HF6-FC may potentially provide a novel therapy for disorders associated with bone loss.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (The Regional Research Universities Program/Center for Healthcare Technology Development) and by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. R01-2008-000-20556-0).
ABBREVIATIONS
- BMMs
bone marrow-derived macrophages
- ERK
extracellular signal-regulated kinase
- HF6-FC
hexane soluble fraction of Ficus carica
- JNK
c-Jun-N-terminal kinase
- NFATc1
nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1
- RANKL
receptor activator of NF-κB ligand
- TRAF6
TNF receptor-associated factor 6
References
- 1.Ahmed W, Khan AQ, Malik A. Two Triterpenes from the Leaves of Ficus carica. Planta Med. 1988;54:481. doi: 10.1055/s-2006-962522. [DOI] [PubMed] [Google Scholar]
- 2.Alliston T, Derynck R. Medicine: interfering with bone remodelling. Nature. 2002;416:686–687. doi: 10.1038/416686a. [DOI] [PubMed] [Google Scholar]
- 3.Canal JR, Torres MD, Romero A, Pérez C. A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptootocin-includede diabetes. Acta Physiol Hung. 2000;87:71–76. doi: 10.1556/APhysiol.87.2000.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Chang EJ, Kim HJ, Ha J, Kim HJ, Ryu J, Park KH, Kim UH, Lee ZH, Kim HM, Fisher DE, Kim HH. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 2007;120:166–176. doi: 10.1242/jcs.03310. [DOI] [PubMed] [Google Scholar]
- 5.Choi HJ, Song BJ, Gong YD, Gwak WJ, Soh Y. Rapid degradation of hypoxia-inducible factor-1alpha by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 2008;154:114–125. doi: 10.1038/bjp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coon D, Gulati A, Cowan C, He J. The role of cyclooxygenase-2 (COX-2) in inflammatory bone resorption. J Endod. 2007;33:432–436. doi: 10.1016/j.joen.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.El-Kholy IS, Shaban MA. Constituents of the leaves of Ficus carica, L. II. Isolation of a psi-taraxasteryl ester, rutin, and a new steroid sapogenin. J Chem Soc Perkin 1. 1966;13:1140–1142. doi: 10.1039/j39660001140. [DOI] [PubMed] [Google Scholar]
- 8.Han KY, Yang D, Chang EJ, Lee Y, Huang H, Sung SH, Lee ZH, Kim YC, Kim HH. Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem Pharmacol. 2007;74:911–923. doi: 10.1016/j.bcp.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective in hibition of NF-kappa B block osteoclastogenesis and prevents inflammatory bone destructin in vivo. Nat Med. 2004;6:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30:71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee WT. Coloured standard illustrations of Korean plants. Seoul: Academy press; 1996. p. 624. [Google Scholar]
- 13.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mino T, Sugiyama E, Taki H, Kuroda A, Yamashita N, Maruyama M, Kobayashi M. Interleukin-1alpha and tumor necrosis factor alpha synergistically stimulate prostaglandin E2-dependent production of interleukin-11 in rheumatoid synovial fibroblasts. Arthritis Rheum. 1998;41:2004–2013. doi: 10.1002/1529-0131(199811)41:11<2004::AID-ART16>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, Ito A. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 2003;197:1303–1310. doi: 10.1084/jem.20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S. Reciprocal role of ERK and NF-kappaB pathwaysin survival and activation of osteoclasts. J Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motyckova G, Weilbaecher KN, Horstmann M, Rieman DJ, Fisher DZ, Fisher DE. Linking osteopetrosis and pycnodysostosis: regulation of cathepsin K expression by the microphthalmiatranscription factor family. Proc Natl Acad Sci U S A. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozar A, Haren N, Chasseraud M, Louvet L, Maziere C, Wattel A, Mentaverri R, Morliere P, Kamel S, Brazier M, Maziere JC, Massy ZA. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J Cell Physiol. 2008;215:47–54. doi: 10.1002/jcp.21283. [DOI] [PubMed] [Google Scholar]
- 19.Oh SY, Aryal DK, Kim YG, Kim HG. Effects of R. Glutinosa and E. Senticosus on Postmenopausal Osteoporosis. Korean J Physiol Pharmacol. 2007;11:121–127. [Google Scholar]
- 20.Reddy SV, Hundley JE, Windle JJ, Alcantara O, Linn R, Leach RJ, Boldt DH, Roodman GD. Characterization of the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res. 1995;10:601–606. doi: 10.1002/jbmr.5650100413. [DOI] [PubMed] [Google Scholar]
- 21.Rubnov S, Kashman Y, Rabinowitz R, Schlesinger M, Mechoulam R. Suppressors of cancer cell proliferation from Fig (Ficus carica)Resin: isolation and structure elucidation. J Nat Prod. 2001;64:993–996. doi: 10.1021/np000592z. [DOI] [PubMed] [Google Scholar]
- 22.Serraclara A, Hawkins F, Pérez C, Domínguez E, Campillo JE, Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39:19–22. doi: 10.1016/s0168-8227(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 23.Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol. 2000;58:534–541. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- 24.Soh Y, Shin MH, Lee JS, Jang JH, Kim OH, Kang H, Surh YJ. Oxidative DNA damage and glioma cell death induced by tetrahydropapaveroline. Mutat Res. 2003;544:129–142. doi: 10.1016/j.mrrev.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Stern PH. Antiresorptive agents and osteoclast apoptosis. J Cell Biochem. 2007;101:1087–1096. doi: 10.1002/jcb.21311. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 27.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells,bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 28.Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest. 2008;118:2088–2097. doi: 10.1172/JCI33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 31.Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaBactivation by the TRANCE receptor. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, Cron RQ, Wu B, Genin A, Wang Z, Liu S, Robson P, Baldwin HS. Regulation of the murine Nfatc1 gene by NFATc2. J Biol Chem. 2002;277:10704–10711. doi: 10.1074/jbc.M107068200. [DOI] [PubMed] [Google Scholar]