Abstract
Intracranial headaches, including migraines, are mediated by nociceptive activation of the trigeminal nucleus caudalis (TNC), but the precise mechanisms are poorly understood. We previously demonstrated that selective blockage of spinal sigma-1 receptors (Sig-1R) produces a prominent antinociceptive effect in several types of pain models. This study evaluates whether the Sig-1R antagonist (BD1047) has an antinociceptive effect on capsaicin (a potent C-fiber activator) induced headache models in rats. Intracisternal infusion of capsaicin evoked pain behavior (face grooming), which was significantly attenuated by BD1047 pretreatment. BD1047 consistently reduced capsaicin-induced Fos-like immunoreactivity (Fos-LI), a neuronal activator, in the TNC in a dose-dependent manner. Moreover, capsaicin-induced phosphorylation of N-methyl-D-aspartate receptor subunit 1 was reversed by BD1047 pretreatment in the TNC. These results indicate that the Sig-1R antagonist has an inhibitory effect on nociceptive activation of the TNC in the capsaicin-induced headache animal model.
Keywords: Fos, Headache, N-methyl-D-aspartate receptor, Sigma-1 receptor, Trigeminal nucleus caudalis
INTRODUCTION
Migraine is a very common neurobiological headache disorder. Despite the prevalence of neurovascular headaches, their pathophysiology is unclear. However, the involvement of the trigeminovascular system is generally accepted. Unmyelinated C-fibers of the trigeminal nerve transmit painful stimuli from the meninges to the trigeminal nucleus caudalis (TNC) within the brain stem via the trigeminal ganglion neurons. Therefore, migraine attacks may be associated with nociceptive activation of the TNC (Goadsby and Edvinsson, 1993).
Research techniques in animal models provide insight into the pathophysiology of pain processing in migraines (Oshinsky et al., 2006). TNC nociceptive activation is evoked by a direct electrical, mechanical, or chemical stimulus (e.g., capsaicin as a potent C-fiber activator) into the dura. Nociceptive dural stimulations increase Fos like immunoreactive (Fos-LI) neurons, makers of neuronal activation, within the TNC (Park et al., 1997; Mitsikostas and Sanchez del Rio, 2001). Dural stimulation increases neuronal sensitivity of the TNC through phosphorylation of NMDA receptor subunit 1 (pNR1) without changing NR1 expression (Maneepak et al., 2009). There is also a strong correlation between the amount of pNR1 expression and Fos-LI cells in the TNC following dural stimulation (Maneepak et al., 2009). Thus, these neurological changes could evaluate pharmacological agents associated with headache disorders.
Sigma-1 receptors (Sig-1R) may serve as amplifiers for intracellular signal transduction through modulation of calcium channels or N-methyl-D-aspartate receptor (NMDA) receptor modulation in the central nervous system (Guitart et al., 2004). Knockout studies indicate that Sig-1R plays an important role in pain processing (Cendan et al., 2005). In addition, we recently demonstrated that selective blockage of spinal Sig-1R can reduce pain behaviors and spinal elevation of pNR1 in both formalin-induced inflammatory pain and a rodent neuropathic pain model (Kim et al., 2008; Roh et al., 2008). Moreover, we observed that the activation of spinal Sig-1R enhances NMDA-induced pain behaviors via pNR1 upregulation (Kim et al., 2008). From these findings, we postulated that Sig-1R regulates various types of pain symptoms through the modulatory role of NMDA receptor function in the spinal cord. This study aims to investigate whether Sig-1R affects the TNC neuronal activity (i.e. Fos and pNR1 expression) as a key mechanism underlying the generation of headaches.
METHODS
Animals
Male Sprague-Dawley rats (Dae Han Biolink Co., Eumsung, South Korea) were housed in colony cages with free access to food and water and maintained in temperature and light controlled rooms (23±2℃, 12/12 h light/dark cycle with lights on at 08:00). All of the methods used in the present study were approved by the Institute of Animal Care and Use Committee at Chonbuk National University and conform to NIH guidelines (NIH publication No. 86-23, revised in 1985).
Cisterna magna cannula implantation and drug infusion
Rats (200~220 g) were anesthetized with an intraperitoneal injection mixture of ketamine (90 mg/kg) and xylazine (9 mg/kg). A 2 mm wide craniotomy was performed above the junction of the superior sagittal and transverse sinuses, and an intracisternal (IC) cannula (7 cm length PE-10 tube connected with a 6.5 mm length 30 gage stainless steel needle) was fixed to the bone around the opening in the skull using small screws and dental cement. The cannula's needle end opened onto the dura over the transverse sinus along the midline. The end of the PE-10 tube was led to the back and closed. Correct placement of the cannula was confirmed by cerebrospinal fluid (CSF) withdrawal. After surgery, rats were allowed 3 days to recover.
Capsaicin stock solution [3.05 mg capsaicin (Sigma, USA) per 1 ml of vehicle buffer (saline-ethanol-Tween 80; 8:1:1; v/v)] was diluted with artificial CSF (aCSF; 132 mM NaCl, 3 mM KCl, 0.6 mM MgCl2, 1.5 mM CaCl2, 49 mM NaHCO3, 6.6 mM urea, 7.4 mM D (+) glucose, 5 mM HEPES, pH=7.40) to a final concentration of 2.5 nmol. Selective Sig-1R antagonist [BD1047 (10, 30 and 100 nmol) from Tocris] was dissolved in aCSF and intracisternally pretreated 10 minutes prior to capsaicin infusion. All drugs or vehicle (aCSF) with 50 µl volume were infused into the dura via IC cannula by micro-infusion pump (Model 310 Plus, KD Scientific Inc, USA) with a speed rate of 50 µl per min.
Behavioral assay
Immediately after final drug infusion, the rat was placed in a transparent individual home cage and its behavior was recorded with a video camera. Face grooming is considered to reflect a specific nocifensive response in several experimental facial nociceptive conditions induced by noxious chemical stimuli (Kemper et al., 1998, Yao and Sessle, 2008). In preliminary observations, the capsaicin (2.5 nmol/rat) induced grooming/scratching behavior was evident for 10 minutes after infusion. The first 2 min after the infusion was considered a conditioning period and behavior was not analyzed. The remaining 8 min were analyzed by two experienced investigators who were blinded to the experimental conditions.
Immunohistochemistry
Two hours after final infusion, rats were deeply anesthetized with 5% isoflurane and perfused transcardially with calcium-free Tyrode's solution followed by a fixative containing 4% paraformaldehyde. The brain was removed after perfusion, post-fixed at 4℃ for 4 hours in the same fixative and finally cryoprotected in 30% sucrose in phosphate buffered saline (PBS, pH 7.4) for 48 hours.
Frozen serial frontal sections (40 µm) were cut from -0.5 to +0.5 mm of the obex region using a cryostat (Microm, Walldorf, Germany). After eliminating endogenous peroxidase activity with 0.3% hydrogen peroxide in PBS and preblocking with 3% normal serum (goat serum for rabbit IgG, rabbit serum for mouse IgG) and 0.3% triton X-100 in PBS, the free-floating sections were incubated in rabbit anti-c-fos antibody (Calbiochem, 1:10,000), rabbit anti-NR1 antibody (1:I1,000, Upstate Biotechnology) or rabbit anti-pNR1 antibody (1:I1,000, Upstate Biotechnology; this antibody is specific for NR1 phosphorylated on Ser897) at 4℃ overnight. The sections were subsequently incubated with goat anti-rabbit IgG (Vector, 1:200) at room temperature for 2 hours, processed using the avidin-biotin-peroxidase procedure and visualized using a 3-3 diamino-benzidine reaction intensified with 0.2% nickel chloride.
For quantitative analysis of Fos and pNR1, individual sections were digitized with 4,096 gray levels using a cooled CCD camera (CoolSnap ES, Roper, Japan) connected to a computer-assisted image analysis system (Metamorph, Universal Imaging Co., West Chester, PA) as previously described (Kwon et al., 2001; Kim et al., 2008).
Data and statistical analysis
Data values were expressed as the mean±SEM. All data were analyzed using the commercially available software GraphPad Prizm 5.0 (Graphpad Software, San Diego, CA, USA). Statistical analysis was carried out using One-way analysis of variance (ANOVA) for repeated measures followed by Post hoc Newman-Keuls Multiple Comparison test.
RESULTS
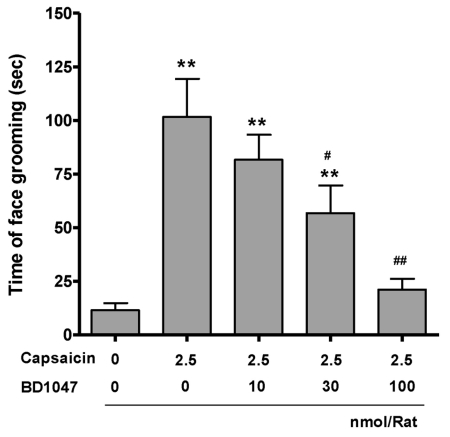
Dural stimulation via intracisternal capsaicin infusion (101.6±17.7) increased face grooming behavior more than vehicle infusion (11.5±3.3). Intracisternal BD1047 pre-infusion (10, 30, 100 nmol/rat) dose-dependently reduced capsaicin-induced pain behavior upto vehicle control level (Fig. 1).
Fig. 1.
The time spent on face grooming behavior within 10 minutes after capsaicin infusion (2.5 nmol/rat). BD1047 was introduced 10 min prior to capsaicin infusion. **p<0.01 compared to vehicle control group (first column). #p<0.05, ##p<0.01 compared to capsaicin group (second column). Each group contains 6 animals.
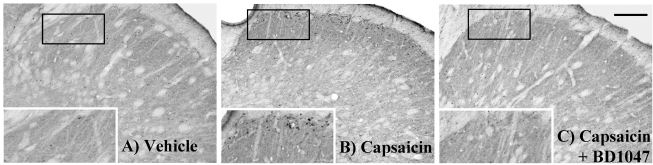
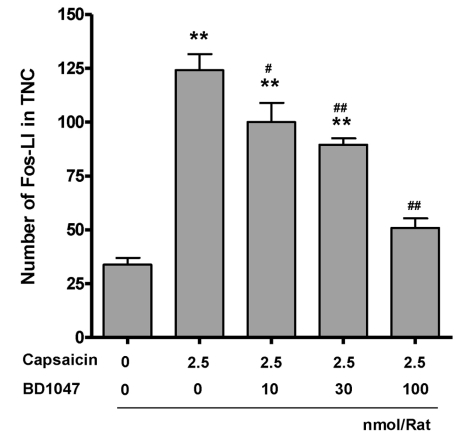
Control rats, receiving vehicle in the cisterna magna, showed low levels of Fos immunoreactivity (Fos-LI) in the TNC (33.8±3.1, Fig. 2A and Fig. 3). Intracisternal capsaicin infusion evoked prominent Fos-LI in layers I and II of the TNC (124.1±7.4, Fig. 2B and Fig. 3). Intracisternal BD1047 pre-infusion (10, 30, 100 nmol/rat) dose-dependently decreased capsaicin induced Fos-LI in the TNC (Fig. 2C and Fig. 3). The highest dose of BD1047 dramatically reversed capsaicin induced Fos-LI activity to vehicle control levels (50.8±4.5, Fig. 3).
Fig. 2.
Fos-like immunoreactivity (Fos-LI) in the trigeminal nucleus caudalis (TNC) at 2 hours after intracisternal drug infusion. (A) Vehicle (artificial CSF) control, (B) capsaicin (2.5 nmol), (C) capsaicin (Cap, 2.5 nmol)+BD1047 (BD, 100 nmol). Scale bar=200 µm.
Fig. 3.
Number of Fos-like immunoreactive (Fos-LI) neurons in the trigeminal nucleus caudalis (TNC) at 2 hours after intracisternal capsaicin or vehicle infusion. BD1047 was introdced 10 min prior to capsaicin infusion. **p<0.01 compared to vehicle control group (first column). #p<0.05, ##p<0.01 compared to capsaicin group (second column). Each group contains 6 animals.
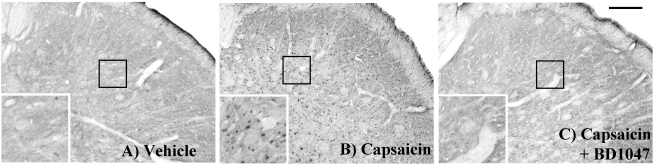
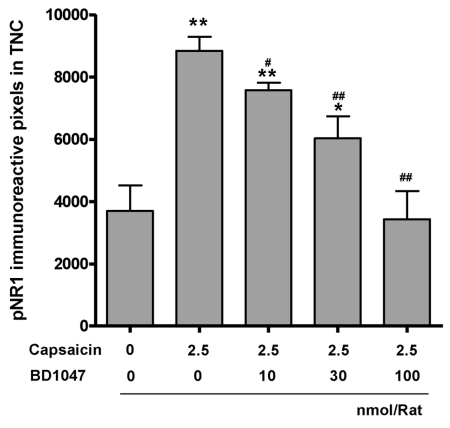
We also found that intracisternal capsaicin infusion (8,843.8±453.2) significantly increased pNR1 immunoreactivity in the TNC as compared with the vehicle infusion group (3,702.1±818.6), was and BD1047 dose-dependently reduced this increase (Fig. 4 and Fig. 5). Overall NR1 expression did not change after capsaicin or BD1047 treatment (data not shown).
Fig. 4.
The phosphorylation of NMDA-receptor subunit 1 (pNR1) positive immunoreactivity in the trigeminal nucleus caudalis (TNC) at 2 hours after intracisternal drug infusion. (A) Vehicle (artificial CSF) control, (B) capsaicin (2.5 nmol), (C) capsaicin (Cap, 2.5 nmol)+BD1047 (BD, 100 nmol). Scale bar=200 µm.
Fig. 5.
The phosphorylation of NMDA-receptor subunit 1 (pNR1) immunoreactivity in the trigeminal nucleus caudalis (TNC) at 2 hours after intracisternal capsaicin or vehicle infusion. BD1047 was introduced 10 min prior to capsaicin infusion. *p<0.05, **p<0.01 compared to vehicle control group (first column). #p<0.05, ##p<0.01 compared to capsaicin group (second column). Each group contains 6 animals.
DISCUSSION
We observed that intracisternal capsaicin infusion activates a nociceptive pain behavior (face grooming) and in turn increases TNC neuronal activity (i.e., elevation of Fos-LI and pNR1 expression). This serves as a key mechanism underlying the generation of headaches. Using this animal model, we obtained experimental evidence for the putative role of Sig-1R in the pathophysiology of neurovascular headaches. The ligand, BD1047, shows a high affinity for Sig-1R (Ki=0.93 nM) without other receptor interaction and thus is commonly used to evaluate the specific role of the Sig-1R (Kim et al., 2006; Kim et al., 2008).
In the present study, capsaicin induced TNC neuronal activity (i.e., elevation of Fos-LI expression) was dose-dependently blocked by BD1047, indicating that Sig-1R antagonists may be effective in the treatment of migraines. In general, the amount of Fos-LI in the TNC has a strong positive correlation with the intensity of nociceptive dural stimulation (Ter Horst et al., 2001). More importantly, we demonstrated that capsaicin-induced pain behavior also decreases with BD1047 pretreatment. Therefore, the specific activation of Sig-1R may be intimately associated with the pathophysiology of pain processing in migraines.
The NMDA receptor plays a facilitatory role in Fos-LI and nociceptive transmission in the TNC (Otahara et al., 2003; Maneepak et al., 2009), and Sig-1R appears to be functionally coupled to NMDA receptors (Bermack et al., 2002; Nuwayhid et al., 2003). A supportive finding shows the overactivation of Sig-1R directly enhances brain-derived neurotrophic factor-induced glutamate release (Yagasaki et al., 2006). Similarly, activation of Sig-1R promotes an NMDA-induced nociceptive response through the upregulation of pNR1 (Kim et al., 2008). In the present study, we observed that BD1047 dose-dependently reduced capsaicin-induced pNR1 in the TNC. Therefore, our data suggests that activation of Sig-1R facilitates the nociceptive response of NMDA receptors, which may be involved in the development of central sensitization and pain hypersensitivity in the TNC.
Agents typically used in migraine headache treatment include sumatriptan, topiramate, and gabapentin. However, a recent clinical study reveals a potent therapeutic effect of memantine on headache symptoms in women with typical chronic migraines (Spengos et al., 2008). Memantine is a well-known and well-tolerated, noncompetitive, low-affinity NMDA receptor antagonist that preferentially blocks excessive NMDA receptor activity (Lipton, 2007). Thus, weak blockage of over-activated NMDA receptors will not disrupt normal activity and has become a new therapeutic treatment strategy for migraines. Considering the good safety profile of existing Sig-1R antagonists such as haloperidol (Entrena et al., 2009), the indirect modulation of NMDA receptor by Sig-1R antagonists may be a promising strategy for the treatment of headache disorders.
ACKNOWLEDGEMENTS
This work was supported by a Korea Research Foundation Grant funded by the Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2005-205-E00012).
ABBREVIATIONS
- CSF
cerebrospinal fluid
- Fos-LI
Fos-like immunoreactivity
- NMDA
N-methyl-D-aspartate receptor
- pNR1
phosphorylation of NMDA receptor subunit 1
- Sig-1R
sigma-1 receptors
- TNC
trigeminal nucleus caudalis
References
- 1.Bermack J, Lavoie N, Dryver E, Debonnel G. Effects of sigma ligands on NMDA receptor function in the bulbectomy model of depression: a behavioural study in the rat. Int J Neuropsychopharmacol. 2002;5:53–62. doi: 10.1017/S1461145701002760. [DOI] [PubMed] [Google Scholar]
- 2.Cendán CM, Pujalte JM, Portillo-Salido E, Baeyens JM. Formalin-induced pain is reduced in sigma (1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Entrena JM, Cobos EJ, Nieto FR, Cendán CM, Baeyens JM, Del Pozo E. Antagonism by haloperidol and its metabolites of mechanical hypersensitivity induced by intraplantar capsaicin in mice: role of sigma-1 receptors. Psychopharmacology (Berl) 2009;205:21–33. doi: 10.1007/s00213-009-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 5.Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 6.Kemper RH, Spoelstra MB, Meijler WJ, Ter Horst GJ. Lipopolysaccharide-induced hyperalgesia of intracranial capsaicin sensitive afferents in conscious rats. Pain. 1998;78:181–190. doi: 10.1016/S0304-3959(98)00125-0. [DOI] [PubMed] [Google Scholar]
- 7.Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006;148:490–498. doi: 10.1038/sj.bjp.0706764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon YB, Lee JD, Lee HJ, Han HJ, Mar WC, Kang SK, Beitz AJ, Lee JH. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90:271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- 10.Lipton SA. Pathologically-activated therapeutics for neuroprotection: Mechanism of NMDA receptor block by memantine and S- nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- 11.Maneepak M, Grand SL, Srikiatkhachorn A. Serotonin depletion increases nociception-evoked trigeminal NMDA receptor phosphorylation. Headache. 2009;49:375–382. doi: 10.1111/j.1526-4610.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitsikostas DD, Sanchez del Rio M. Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Brain Res Rev. 2001;35:20–35. doi: 10.1016/s0165-0173(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 13.Nuwayhid SJ, Werling LL. Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther. 2003;304:364–369. doi: 10.1124/jpet.102.043398. [DOI] [PubMed] [Google Scholar]
- 14.Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1):S39–S44. doi: 10.1111/j.1526-4610.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 15.Otahara N, Ikeda T, Sakoda S, Shiba R, Nishimori T. Involvement of NMDA receptors in Zif/268 expression in the trigeminal nucleus caudalis following formalin injection into the rat whisker pad. Brain Res Bull. 2003;62:63–70. doi: 10.1016/j.brainresbull.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Park BR, Doh NY, Kim MS, Chun SW, Lee MY, Lee SH. Correlation between electrical activity of type I neuron and c-Fos expression in the medial vestibular nuclei following unilateral labyrinthectomy in rats. Korean J Physiol Pharmacol. 1997;1:505–513. [Google Scholar]
- 17.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH. receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 1;109:879–889. doi: 10.1097/ALN.0b013e3181895a83. [DOI] [PubMed] [Google Scholar]
- 18.Spengos K, Theleritis C, Paparrigopoulos T. Memantine and NMDA antagonism for chronic migraine: a potentially novel therapeutic approach? Headache. 2008;48:284–286. doi: 10.1111/j.1526-4610.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 19.Ter Horst GJ, Meijler WJ, Korf J, Kemper RH. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia. 2001;21:963–975. doi: 10.1046/j.1468-2982.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 20.Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- 21.Yao D, Sessle BJ. Nitroglycerin facilitates calcitonin gene-related peptide-induced behavior. Neuroreport. 2008;19:1307–1311. doi: 10.1097/WNR.0b013e32830b0f9d. [DOI] [PubMed] [Google Scholar]