Abstract
The role of apurinic/apyrimidinic endonuclease1/redox factor-1 (Ref-1) on the lead (Pb)-induced cellular response was investigated in the cultured endothelial cells. Pb caused progressive cellular death in endothelial cells, which occurred in a concentration- and time-dependent manner. However, Ref-1 overexpression with AdRef-1 significantly inhibited Pb-induced cell death in the endothelial cells. Also the overexpression of Ref-1 significantly suppressed Pb-induced superoxide and hydrogen peroxide elevation in the endothelial cells. Pb exposure induced the downregulation of catalase, it was inhibited by the Ref-1 overexpression in the endothelial cells. Taken together, our data suggests that the overexpression of Ref-1 inhibited Pb-induced cell death via the upregulation of catalase in the cultured endothelial cells.
Keywords: Apurinic/apyrimidinic endonuclease1/redox factor-1 (Ref-1), Lead, Cell death, Endothelial cells, Catalase
INTRODUCTION
Lead (Pb) is a persistent and common environmental metal ion. Lead increases free radical production and reduces the availability of antioxidants reserve, resulting in oxidative cellular and tissue injuries (Ercal et al., 2001; Patrick, 2006). Lead-induced oxidative stress has been previously identified as the primary contributing agent in the pathogenesis of lead poisoning (Patrick, 2006). The lead-induced disruption of free radicals and antioxidant balance might result in tissue injury. In particular, oxidative stress has also been implicated in lead-induced cardiovascular system injuries such as hypertension. Chronic exposure to low levels of lead has been shown to induce sustained arterial hypertension in human and experimental animals (Sharp et al., 1988; Gonick et al., 1997; Vaziri, 2002).
In addition to oxidative stress, cell death is an important phenomenon in lead-induced toxicity. A number of studies have revealed that lead exposure is capable of inducing cell death in a variety of cell types (Fox et al., 1998; Cheng et al., 2002; Wang et al., 2009). Even though the mechanisms relevant to lead-induced cell death have yet to be fully elucidated, some have implied a relationship between oxidative stress and cell death in lead-induced cell death. Oxidative stress resulting in transcriptional regulation has been proposed as an important mechanism in the expression of specific genes associated with endothelial cell death and survival (Nakamura et al., 1997).
Apurinic apyrimidinic endonuclease 1/redox factor-1 (Ref-1) is a ubiquitous multifunctional protein, which is involved in the base excision repair pathways of oxidatively damaged DNA (Demple et al., 1991). Also, Ref-1 functions as a redox co-factor of a variety of transcriptional factors, including AP1, nuclear factor (NF)-κB, and early growth response protein-1 in different cell systems (Tell et al., 2005). Additionally, Ref-1 is a critical factor in cellular protection against cell death resulting from oxidative stresses, including endothelial cell activation and inflammation. Ref-1 has also been shown to suppress intracellular oxidative stress and apoptosis (Angkeow et al., 2002; Ozaki et al., 2002; Yoo et al., 2004). It was recently reported that vascular endothelial cell activation and vascular inflammation were inhibited by Ref-1 overexpression (Kim et al., 2006; Song et al., 2008; Lee et al., 2009).
Although the DNA repair and transcriptional regulation of Ref-1 have been investigated extensively, the functions of Ref-1 in the regulation of oxidative stress and cell death resulting from exposure to lead remain largely unknown. In this study, we report a protective role of Ref-1 in the lead-induced cell death of endothelial cells. The Ref-1-induced negative regulation in lead-induced cell death is mediated through the modulation of hydrogen peroxide production and catalase expression.
METHODS
Cell culture and reagent
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and were grown and maintained in endothelial growth medium. Cells were used between passages 3 and 6. Anti-Ref-1 and Anti-β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Catalase was obtained from Sigma (St. Louis, MO, USA). HRP-labeled anti-rabbit and anti-mouse antibodies were obtained from Amersham (Buckinghamshire, UK). Lead (Pb) acetate (C4H6O4Pb, MW 379.33) was purchased from Riedel-deHaeon (Germany). All other chemicals were obtained from Sigma (St. Louis, MO. USA).
Determination of cell viability
Cell viability was evaluated via a modified MTT assay (Mosmann, 1983). In brief, the cells (1×105 cells/well) were seeded in 12-well plates and treated with Pb. After lead treatment, 100 µl of a MTT solution (0.5 mg/ml in phosphate buffered saline) was added to each well and incubated for an additional 2 h at 37℃. 100 µl of dimethyl sulfoxide (DMSO) was subsequently added to each well to solubilize any deposited formazan. After transfer to 96-well plates, the optical density (OD) of each well was measured at 540 nm with a microplate reader (Sunrise, TECAN, Austria).
Western blot analysis
48 hours after transfection with adenoviruses, protein expressions (40 µg) were evaluated by Western blotting as previously described (Jeon et al., 2004). HUVECs were harvested with 100 µl of lysis buffer containing 20 mM Tris-Cl, pH 7.5, 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM Na3VO3, 1 mM β-glycerophosphate, 4 mM Na pyrophosphate, 5 mM NaF, and 1% Triton X-100, and protease inhibitor cocktail. The lysate was centrifuged at 12,000 rpm for 20 min and the supernatant was collected. Protein was separated by 10% SDS-PAGE and electrotransfered onto nitrocellulose membranes. After blocking with 5% skim milk for 1 hour at room temperature, blots were incubated for overnight at 4℃ with specific primary antibody (1 : 1,000), and subsequent detection with horseradish peroxidase-conjugated secondary antibody was performed. Blots were developed for visualization using an enhanced chemiluminescence detection kit (Pierce Biotechnology, Rockford, IL).
Adenoviral transfections
Adenoviruses encoding for β-galactosidase (Adβgal) and full-length Ref-1 (AdRef-1) were generated via homologous recombination in human embryonic kidney 293 cells, as described in a previous study (Jeon et al., 2004). In some experiments, HUVECs were infected with a 20~200 multiplicity of infection (MOI) of adenovirus for 24 hours. A total of 200 MOI of adenovirus was balanced with Adβgal.
Detection of superoxide and hydrogen peroxide production
Intracellular superoxide and hydrogen peroxide production were detected using the superoxide-sensitive fluorophore dihydroethidine (DHE) and the peroxide-sensitive fluorophore 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA) as described previously (Angkeow et al., 2002; Kim et al., 2006). In order to detect DHE or DCF-DA fluorescence, the cells were cultivated in chamber slides (2×105 cells/well) (Nalge Nunc International). The cells were rinsed three times and incubated for 30 min with 5 µM DHE and for 30 min with 10 µM DCF-DA at 37℃ in Krebs-HEPES buffer and Hanks buffered salts solution, respectively. The absolute fluorescence of 20~25 random cells was quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Values are expressed as the means±SEM. Statistical evaluation was conducted via one-way ANOVA, followed by a Turkey post hoc test, and p<0.05 was considered statistically significant.
RESULTS
Effect of lead on the cellular viability
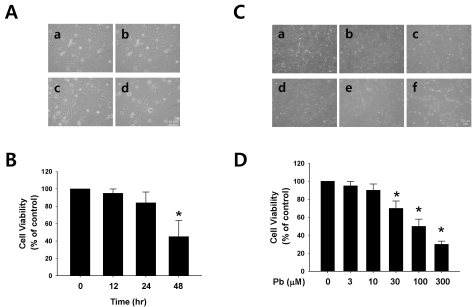
Endothelial cell damage is one of the most important phenomena in the pathogenesis of cardiovascular disorder. First, the effects of lead (Pb) on the cell viability were evaluated in the endothelial cells. Lead caused progressive cellular death in endothelial cells, which occurred in a concentrationand time-dependent manner during lead exposure (Fig. 1). Regarding the exposure time selected for this experiment, the cells were treated with 30 µM of Pb acetate for 12, 24, and 48 hr. As shown in Fig. 1A, Pb significantly affected the cell viability after 24 hr of incubation, resulting in a cell viability of 69% in the endothelial cells. The cells were treated with a range of lead acetate concentrations (0~300 µM) for 24 h. Pb in a range of 30~300 µM resulted in a significant and concentration-dependent reduction in the viability of the endothelial cells.
Fig. 1.
Effect of lead (Pb) on endothelial cell viability. (A) Typical endothelial cell morphology after treatment with Pb acetate (30 µM) for 12~48 hr (a, control; b, 12 hr; c, 24 hr; d, 48 hr). (B) Summarized data are plotted in Fig. 1B. (C) Typical endothelial cell morphology after treatment with Pb acetate for 24 hr. (a, control; b, 3 µM of Pb; c, 10 µM of Pb; d, 30 µM of Pb; e, 100 µM of Pb; f, 300 µM of Pb). (D) Summarized data was plotted in Fig. 1D. Total acetate concentration (300 µM) was balanced with Na acetate in Fig. 1C. Endothelial cell viability was measured via a MTT assay as described in the Materials and Methods section. The data are expressed as the means±SEM for 3 separate experiments. *p<0.05 compared with control.
Effect of Ref-1 on the Pb-induced cell toxicity
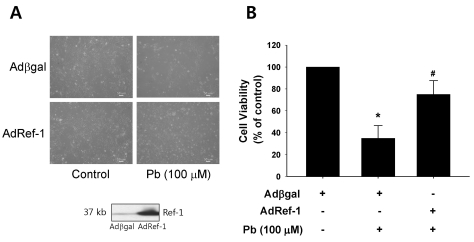
Ref-1 protein is an intracellular surveillance protein that functions in cellular protection against cell death as the result of oxidative stresses, including endothelial cell activation and inflammation. In order to ascertain whether Ref-1 regulates Pb-induced cell death, the AdRef-1-treated endothelial cells were treated for 24 h with Pb (100 µM). The Western blot analysis data in Fig. 2A revealed Ref-1 overexpression in the endothelial cells transfected with AdRef-1 (200 MOI). Ref-1 overexpression significantly inhibited Pb-induced cell death, as compared with that observed in the Adβgal-treated endothelial cells, thereby suggesting that Ref-1 performs a protective function against Pb-induced endothelial death. The quantitative data is plotted in Fig. 2B.
Fig. 2.
Ref-1 overexpression inhibited lead (Pb) induced endothelial cell death. (A) Endothelial cell morphology in the Pb (100 µM)-treated endothelial cells which were transfected with Adβgal or AdRef-1. Adenoviral transfection of AdRef-1 successfully overexpressed Ref-1 in the endothelial cells as compared with Adβgal. (B) Ref-1 overexpression induced by AdRef-1 inhibited Pb (100 µM)-induced endothelial cell death (in the bottom of Fig. 2A). (B) Summarized data was plotted in Fig. 2B. Endothelial cell viability was measured with an MTT assay as described in the Materials and Methods section. The data are expressed as the means±SEM for 4 separate experiments. *p<0.05 compared with control cells. #p<0.05 compared with Adβgal+Pb.
Effect of Ref-1 on Pb-induced reactive oxygen species (ROS) production
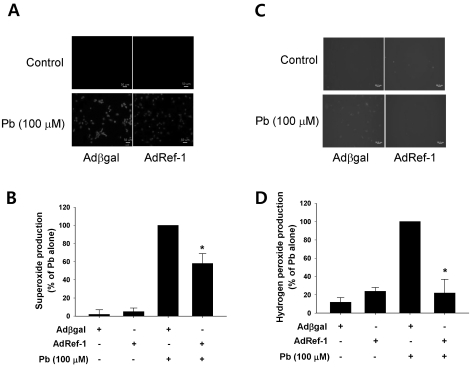
Lead-induced oxidative stress has been previously identified as the primary contributory agent in the pathogenesis of lead poisoning (Hsu and Guo, 2002). 24 h of exposure to 30 µM of Pb resulted in significant elevations of both superoxide and hydrogen peroxide in endothelial cells (Fig. 3). However, the overexpression of Ref-1 with AdRef-1 significantly suppressed Pb-induced superoxide and hydrogen peroxide elevation in the endothelial cells. Interestingly, Ref-1 overexpression markedly abrogated Pb-induced hydrogen peroxide production in the endothelial cells. The quantitative data are plotted in Fig. 3C and 3D.
Fig. 3.
Ref-1 overexpression inhibited lead (Pb)-induced superoxide and hydrogen peroxide production. Pb (100 µM) was administered for 24 hr in the endothelial cells transfected with Adβgal or AdRef-1. Intracellular superoxide (A) and hydrogen peroxide production (C) were evaluated using the superoxide-sensitive fluorophore dihydroethidine (DHE) and the peroxide-sensitive fluorophore 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA). (B, D) The summarized data was plotted in Figs. 3B and 3D. The data are expressed as the means±SEM for 3 separate experiments. *p<0.05 compared with Adβgal+Pb.
Effect of Ref-1 on Pb-induced catalase suppression
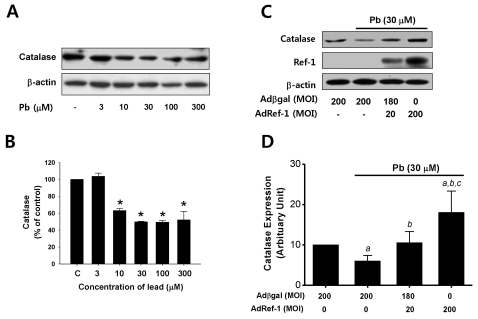
As Pb has been previously demonstrated to induce ROS production, we attempted to determine the effects of Pb on catalase expression, which is known to be involved in the conversion of hydrogen peroxide into water. After the endothelial cells were treated for 24 h with Pb in a range of 3~300 µM, catalase expression was measured by Western blot analysis. As shown in Fig. 4A, Pb exposure, particularly exposure to 10 µM of Pb or more, induced the downregulation of catalase in a dose-dependent manner. This data indicated that the Pb-induced downregulation of catalase is the principal cause of ROS production in endothelial cells.
Fig. 4.
Ref-1 inhibited lead-induced catalase suppression in the endothelial cells. (A) Lead (Pb) treatment resulted in the downregulation of catalase expression. Endothelial cells were exposed to lead (3~300 µM) for 24 hr. Western blot analysis for catalase expression was conducted. β-actin was used as a loading control. (B) Summarized data was plotted in Fig. 4B. ap<0.05 compared with control. The data were expressed as the means±SEM for 3 separate experiments. (C) Effect of Ref-1 on the Pb-induced suppression of catalase expression in the endothelial cells. Endothelial cells were exposed by lead (30 µM) for 24 hr in the endothelial cells transfected with 20 or 200 MOI of AdRef-1. A total of 200 MOI of adenoviral transfection was balanced with Adβgal. (D) The summarized data was plotted in Fig. 4D. The data were expressed as the means±SEM for 3 separate experiments. ap<0.05 compared with Adβgal 200 MOI, bp<0.05 compared with AdβGal+Pb, and cp<0.05 compared with AdRef-1 20 MOI+Pb.
Finally, we turned our focus to the mechanism by which Ref-1 suppressed Pb-induced hydrogen peroxide production in endothelial cells. We evaluated the effects of Ref-1 overexpression on Pb-induced (30 µM) catalase downregulation in AdRef-1-treated endothelial cells. As shown in Fig. 4B, the Pb-induced suppression of catalase expression was inhibited by the Ref-1 overexpression induced by 20 MOI of AdRef-1 in the endothelial cells. Interestingly, catalase expression in the endothelial cells transfected with high doses of AdRef-1 (200 MOI) was greater than the basal level of catalase in the Adβgal-treated endothelial cells.
DISCUSSION
Vascular endothelial cells line the luminal surfaces of all blood vessels. Vascular endothelial cells, then, would naturally be exposed to any lead (Pb) circulating in the bloodstream. When Pb reaches highly toxic levels in the circulating blood, the endothelial cells may be injured or damaged. Oxidative stress performs a central function in the pathogenesis of vascular disorders, including atherosclerosis and hypertension. Therefore, increased ROS generation in Pbexposed endothelial cells may prove to be an important risk factor in the development of atherosclerosis or hypertension in lead-exposed animal and human subjects.
Based on our knowledge, it is well known that lead exposure increases the occurrence of cell death via apoptosis, such as neural (Suresh et al., 2006; Chetty et al., 2007), renal (Stacchiotti et al., 2009) and testicular cells (Adhikari et al., 2001; Massanyi et al., 2007). The cellular toxic effect of lead may be mediated by apoptosis. The present study demonstrated that short-term lead exposure caused endothelial cell death in a range of 30~300 µM of Pb (6.2~62.2 ppm of Pb). The lead-exposed endothelial cells evidenced increases in superoxide and hydrogen peroxide generation as compared to the cultured human endothelial cells. Considering the crucial role of catalase in the detoxification of hydrogen peroxide, the downregulation of catalase induced by Pb exposure, as shown in Fig. 4A, undoubtedly contributes to oxidative stress in lead-exposed endothelial cells. This finding is consistent with a previous report, in which it was demonstrated that catalase levels declined significantly in vascular smooth muscle cells chronically exposed to lead (Ni et al., 2004). Catalase expression can be downregulated by lipopolysaccharide (Clerch et al., 1996), ionizing radiation (Ushakova et al., 1999; Nenoi et al., 2001), and tumor necrosis factor-alpha (Clerch and Massaro, 1992; Chovolou et al., 2003; Lupertz et al., 2008), which stimulated cell death or oxidative stress in the cells. Therefore, in this study, the downregulation of catalase was related to Pb-induced cell death in endothelial cells.
Catalase is very effective in high-level oxidative stress, and protects cells against hydrogen peroxide production within the cell. The enzyme is particularly important under limited glutathione content conditions, such as obtain in lead (Pb)-exposed animals (Hsu, 1981; Hunaiti et al., 1995), and plays a significant role in the development of tolerance to oxidative stress in the adaptive responses of cells (Wassmann et al., 2004).
Interestingly, our study showed that Ref-1 overexpression inhibited Pb-induced cell death, oxidative stress, and the downregulation of catalase in the endothelial cells. The reduction of Pb-induced cell death by Ref-1 overexpression may be attributable to the reduction in oxidative stress mediated by catalase upregulation in the Ref-1 overexpressed cells. Ref-1 exerts important effects against oxidative stress. The reduction of Ref-1 activity by antisense RNA has been previously reported to sensitize cells to oxidative DNA damage (Grosch et al., 1998; Silber et al., 2002). Conversely, overexpression of Ref-1 repair functions stimulates an increase in resistance to certain alkylating agents and oxidative stresses (Tomicic et al., 1997; Grosch et al., 1998; Kim et al., 2006; Lee et al., 2009). Ref-1 and p53 proteins are required for selenium protection from DNA damage in the human and mouse fibroblasts (Fischer et al., 2006). However, we remain unable to dismiss the possibility that the base excision repair function of Ref-1 against oxidatively damaged DNA may be involved in the protective role played by Ref-1 against Pb-induced cell death (Evans et al., 2000).
In the present study, the reduced oxidative stress by Ref-1 was assumed to be attributable to the upregulation of catalase. However, the cellular mechanism underlying the Ref-1-mediated upregulation of catalase has yet to be clearly elucidated. Ref-1 has been established to function as a transcriptional co-activator, similarly to AP-1, NF-κB, and Egr-1 (Tell et al., 2005), which are found in the promoter region of catalase (Nenoi et al., 2001); thus, the promoter activity of catalase in endothelial cells may be modulated by Ref-1. However, the inhibition of NADPH oxidase by Ref-1 is another pathway by which intracellular ROS levels might be reduced. It was reported previously that Ref-1 inhibited ROS production via the inhibition of the activity of rac1 GTPase, one subunit of NADPH oxidase (Ozaki et al., 2002; Lee et al., 2009). A recent study reported the upregulation of the gp91phox subunit of NADPH oxidase in the brain and renal cortex of lead-exposed animals (Vaziri et al., 2003).
This study had some limitations. First, our study was performed only with cultured endothelial cells. Therefore, further studies will be necessary to confirm this beneficial effect in vivo in the future. Another limitation of this study is that it was conducted on Ref-1 overexpressed cells prior to lead (Pb) exposure. Even though this finding implies a beneficial effect in the Pb-induced cell deaths and oxidative stress, any clinical applications regarding lead toxicity should be approached with great care.
Taken together, our data suggests that the overexpression of Ref-1 inhibited Pb-induced cell death in the cultured endothelial cells. This protective effect may be strongly correlated with reduced oxidative stress via the upregulation of the catalase antioxidant system.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science & Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01000-0) and Chungnam National University.
ABBREVIATIONS
- Pb
lead
- Ref-1
apurinic apyrimidinic endonuclease1/redox factor-1
- HUVEC
human umbilical vein endothelial cell
References
- 1.Adhikari N, Sinha N, Narayan R, Saxena DK. Lead-induced cell death in testes of young rats. J Appl Toxicol. 2001;21:275–277. doi: 10.1002/jat.754. [DOI] [PubMed] [Google Scholar]
- 2.Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 3.Cheng YJ, Yang BC, Hsieh WC, Huang BM, Liu MY. Enhancement of TNF-alpha expression does not trigger apoptosis upon exposure of glial cells to lead and lipopolysaccharide. Toxicology. 2002;178:183–191. doi: 10.1016/s0300-483x(02)00225-1. [DOI] [PubMed] [Google Scholar]
- 4.Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective effect of 17-beta-estradiol in human neurocellular models of lead exposure. Neurotoxicology. 2007;28:396–401. doi: 10.1016/j.neuro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Chovolou Y, Watjen W, Kampkotter A, Kahl R. Resistance to tumor necrosis factor-alpha (TNF-alpha)-induced apoptosis in rat hepatoma cells expressing TNF-alpha is linked to low antioxidant enzyme expression. J Biol Chem. 2003;278:29626–29632. doi: 10.1074/jbc.M208665200. [DOI] [PubMed] [Google Scholar]
- 6.Clerch LB, Massaro D. Oxidation-reduction-sensitive binding of lung protein to rat catalase mRNA. J Biol Chem. 1992;267:2853–2855. [PubMed] [Google Scholar]
- 7.Clerch LB, Wright A, Chung DJ, Massaro D. Early divergent lung antioxidant enzyme expression in response to lipopolysaccharide. Am J Physiol. 1996;271:L949–L954. doi: 10.1152/ajplung.1996.271.6.L949. [DOI] [PubMed] [Google Scholar]
- 8.Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991;88:11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 10.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 11.Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899–904. [PubMed] [Google Scholar]
- 12.Fox DA, He L, Poblenz AT, Medrano CJ, Blocker YS, Srivastava D. Lead-induced alterations in retinal cGMP phosphodiesterase trigger calcium overload, mitochondrial dysfunction and rod photoreceptor apoptosis. Toxicol Lett. 1998;102-103:359–361. doi: 10.1016/s0378-4274(98)00232-x. [DOI] [PubMed] [Google Scholar]
- 13.Gonick HC, Ding Y, Bondy SC, Ni Z, Vaziri ND. Lead-induced hypertension: interplay of nitric oxide and reactive oxygen species. Hypertension. 1997;30:1487–1492. doi: 10.1161/01.hyp.30.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.Grosch S, Fritz G, Kaina B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 1998;58:4410–4416. [PubMed] [Google Scholar]
- 15.Hsu JM. Lead toxicity as related to glutathione metabolism. J Nutr. 1981;111:26–33. doi: 10.1093/jn/111.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Hsu PC, Guo YL. Antioxidant nutrients and lead toxicity. Toxicology. 2002;180:33–44. doi: 10.1016/s0300-483x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 17.Hunaiti A, Soud M, Khalil A. Lead concentration and the level of glutathione, glutathione S-transferase, reductase and peroxidase in the blood of some occupational workers from Irbid City, Jordan. Sci Total Environ. 1995;170:95–100. doi: 10.1016/0048-9697(95)04606-2. [DOI] [PubMed] [Google Scholar]
- 18.Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, Berkowitz DE, Irani K. Apurinic/apyrmidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- 19.Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006;69:520–526. doi: 10.1016/j.cardiores.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009;104:219–227. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 21.Lupertz R, Chovolou Y, Kampkotter A, Watjen W, Kahl R. Catalase overexpression impairs TNF-alpha induced NF-kappaB activation and sensitizes MCF-7 cells against TNF-alpha. J Cell Biochem. 2008;103:1497–1511. doi: 10.1002/jcb.21538. [DOI] [PubMed] [Google Scholar]
- 22.Massanyi P, Lukac N, Makarevich AV, Chrenek P, Forgacs Z, Zakrzewski M, Stawarz R, Toman R, Lazor P, Flesarova S. Lead-induced alterations in rat kidneys and testes in vivo. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42:671–676. doi: 10.1080/10934520701244474. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 25.Nenoi M, Ichimura S, Mita K, Yukawa O, Cartwright IL. Regulation of the catalase gene promoter by Sp1, CCAAT-recognizing factors, and a WT1/Egr-related factor in hydrogen peroxide-resistant HP100 cells. Cancer Res. 2001;61:5885–5894. [PubMed] [Google Scholar]
- 26.Ni Z, Hou S, Barton CH, Vaziri ND. Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int. 2004;66:2329–2336. doi: 10.1111/j.1523-1755.2004.66032.x. [DOI] [PubMed] [Google Scholar]
- 27.Ozaki M, Suzuki S, Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 28.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11:114–127. [PubMed] [Google Scholar]
- 29.Sharp DS, Osterloh J, Becker CE, Bernard B, Smith AH, Fisher JM, Syme SL, Holman BL, Johnston T. Blood pressure and blood lead concentration in bus drivers. Environ Health Perspect. 1988;78:131–137. doi: 10.1289/ehp.8878131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 2002;8:3008–3018. [PubMed] [Google Scholar]
- 31.Song YJ, Lee JY, Joo HK, Kim HS, Lee SK, Lee KH, Cho CH, Park JB, Jeon BH. Tat-APE1/ref-1 protein inhibits TNF-alpha-induced endothelial cell activation. Biochem Biophys Res Commun. 2008;368:68–73. doi: 10.1016/j.bbrc.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Stacchiotti A, Morandini F, Bettoni F, Schena I, Lavazza A, Grigolato PG, Apostoli P, Rezzani R, Aleo MF. Stress proteins and oxidative damage in a renal derived cell line exposed to inorganic mercury and lead. Toxicology. 2009;264:215–224. doi: 10.1016/j.tox.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Suresh C, Dennis AO, Heinz J, Vemuri MC, Chetty CS. Melatonin protection against lead-induced changes in human neuroblastoma cell cultures. Int J Toxicol. 2006;25:459–464. doi: 10.1080/10915810600959576. [DOI] [PubMed] [Google Scholar]
- 34.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 35.Tomicic M, Eschbach E, Kaina B. Expression of yeast but not human apurinic/apyrimidinic endonuclease renders Chinese hamster cells more resistant to DNA damaging agents. Mutat Res. 1997;383:155–165. doi: 10.1016/s0921-8777(96)00055-9. [DOI] [PubMed] [Google Scholar]
- 36.Ushakova T, Melkonyan H, Nikonova L, Afanasyev V, Gaziev AI, Mudrik N, Bradbury R, Gogvadze V. Modification of gene expression by dietary antioxidants in radiation-induced apoptosis of mice splenocytes. Free Radic Biol Med. 1999;26:887–891. doi: 10.1016/s0891-5849(98)00281-0. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri ND. Pathogenesis of lead-induced hypertension: role of oxidative stress. J Hypertens Suppl. 2002;20:S15–S20. [PubMed] [Google Scholar]
- 38.Vaziri ND, Lin CY, Farmand F, Sindhu RK. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int. 2003;63:186–194. doi: 10.1046/j.1523-1755.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wang H, Hu M, Cao J, Chen D, Liu Z. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 2009;83:417–427. doi: 10.1007/s00204-009-0425-z. [DOI] [PubMed] [Google Scholar]
- 40.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 41.Yoo YH, Lim YJ, Park SE, Kim JM, Park YC. Overexpression of redox factor-1 negatively regulates NO synthesis and apoptosis in LPS-stimulated RAW 264.7 macrophages. FEBS Lett. 2004;556:39–42. doi: 10.1016/s0014-5793(03)01361-9. [DOI] [PubMed] [Google Scholar]