Abstract
A non-steroidal anti-inflammatory drug (NSAID) has many adverse effects including cardiovascular (CV) risk. Diclofenac among the nonselective NSAIDs has the highest CV risk such as congestive heart failure, which resulted commonly from the impaired cardiac pumping due to a disrupted excitation-contraction (E-C) coupling. We investigated the effects of diclofenac on the L-type calcium channels which are essential to the E-C coupling at the level of single ventricular myocytes isolated from neonatal rat heart, using the whole-cell voltage-clamp technique. Only diclofenac of three NSAIDs, including naproxen and ibuprofen, significantly reduced inward whole cell currents. At concentrations higher than 3 µM, diclofenac inhibited reversibly the Na+ current and did irreversibly the L-type Ca2+ channels-mediated inward current (IC50=12.89±0.43 µM) in a dose-dependent manner. However, nifedipine, a well-known L-type channel blocker, effectively inhibited the L-type Ca2+ currents but not the Na+ current. Our finding may explain that diclofenac causes the CV risk by the inhibition of L-type Ca2+ channel, leading to the impairment of E-C coupling in cardiac myocytes.
Keywords: Diclofenac, L-type Ca2+ current, Rat cardiac myocytes, NSAID
INTRODUCTION
Diclofenac, a nonselective non-steroidal anti-inflammatory drug (nonselective NSAID), has been widely used as an anti-inflammatory, analgesic, and antipyretic drug. Medication with diclofenac has many adverse effects on gastrointestinal, renal, hepatic, and the cardiovascular (CV) system (Bort et al., 1998; Kearney et al., 2006). Clinical observations have shown that long-term treatment with diclofenac correlates with the onset or aggravation of the congestive heart failure (CHF), which can cause serious CV thromboembolic events, such as myocardial infarction and stroke (Hudson et al., 2007; Waksman et al., 2007). A recent systemic study claimed that diclofenac has the highest CV risk score of the nonselective NSAIDs (McGettigan and Henry, 2006).
Heart failure (HF) is an impairment of cardiac pumping, rendering in insufficient to meet the body's demand. This is frequently associated with electrical instability and reduced contractile force in the ventricles (Bodi et al., 2005; Dalla Libera et al., 2008; Hombach, 2008). Changes in the Na+ current can slow myocardial conduction and cause conduction defects and reentrant arrhythmia (Pinto and Boyden, 1999; Tan et al., 2001). Reduced systolic Ca2+ with prolonged Ca2+ transient can result in a decreased generation capacity and a reduction in the decay rate of the contraction force in the failing heart (Pieske, 1999). This has been demonstrated by the decreasing Na+ and Ca2+ current densities in experimentally induced CHF in dog's heart and in ventricular myocytes from patients with terminal heart failure (Lindner et al., 1998; Maltsev et al., 2002; Cha et al., 2004).
In normal cardiac muscle, Ca2+ influx through the L-type Ca2+ channels (LCC) is a key to initiate the excitation-contraction (E-C) coupling via Ca2+-induced Ca2+ release (CICR) from the sarcoplasmic reticulum (SR). The impairment of LCC function is a potential mechanism for altered CICR and E-C coupling disorders (McGettigan and Henry, 2006). Therefore, altered LCC activity can be a serious factor in heart failure. Little is known about interference of NSAIDs with function of heart. Some NSAIDs were found to impair normal activity of cardiac pacemaker cells by inhibiting LCC (Morales et al., 1992; Morales et al., 1993). Considering its ability to modulate several ion channels, diclofenac also may modulate functioning of excitable membranes. Diclofenac can inhibit voltage-dependent Na+ channels in cardiac myoblasts and neurons (Lee et al., 2003; Yang and Kuo, 2005; Fei et al., 2006). It also activates neuronal K+ channels, such as the transient outward K+ currents and the ATP-sensitive potassium (KATP) channel (Tonussi and Ferreira, 1994; Asomoza-Espinosa et al., 2001; Alves and Duarte, 2002; Ortiz et al., 2002; Liu et al., 2005). However, to date, there has been no evidence of a suppressive effect of diclofenac on LCC, which is critical in working myocytes.
In our preliminary study to test the adverse effects of three nonselective NSAIDs, diclofenac, naproxen, and ibuprofen, we found that only diclofenac inhibited the inward currents in single myocytes, whereas the others did not. In this study, we focused on the effects of diclofenac on ion channels, in particular, its modulation of LCC. We found that diclofenac inhibits LCC and the Na+ current in neonatal rat cardiomyocytes. Our findings may provide some clues to the diverse adverse effects of diclofenac on the heart, such as diclofenac-associated high risk for heart failure.
METHODS
Cells
This study was performed in accordance with the Gyeongsang National University Institutional Guidelines for the Care and Use of Laboratory Animals. Neonatal rat ventricular cardiomyocytes were isolated from rat pups on postnatal day 1 and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and supplemented with 100 units/ml penicillin, and 100 mg/ml streptomycin at 37℃ in a humidity-controlled incubator with 5% CO2 (Fu et al., 2005). The experiments began the next day after plating.
Electrophysiology
The standard extracellular (bath) solution for whole-cell current measurements contained, (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 5.5 glucose, 5 BaCl2, 10 HEPES, and was adjusted to pH 7.35 with HCl. The standard pipette solution contained, (in mM): 100 K-glutamate, 5 NaCl, 5 KCl, 1 MgCl2, 23/10 KOH/EGTA, 10 HEPES, 4 ATP potassium salt and adjusted at pH 7.20. For the measurement of L-type Ca2+ currents the Na-free bath solution was used contained, (in mM): 140 TEA-Cl, 5 KCl, 1 MgCl2, 5.5 glucose, 5 BaCl2, 10 HEPES, and was adjusted at pH 7.35 with HCl. The pipette solution was 50 CsOH, 80 CsCl, 40 aspartate, 5 HEPES, 10 EGTA, 4 MgATP (pH 7.2). Diclofenac, naproxen, and ibuprofen were purchased from Sigma (St. Louis, MO, USA).
Whole-cell currents were recorded with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, USA). The current-voltage (I-V) relationship was measured by applying step pulses from a holding potential (HP) of -100 or -50 mV. In particular, an HP of -50 mV was applied to isolate LCC Ca2+ currents. Step pulses were up to +60 mV in 10 mV increments. The duration of the step pulses was 200 ms. The recorded currents were filtered at 5 kHz and sampled at 5 kHz. Currents were analyzed with Clampfit software (Axon Instruments, USA). Statistical analysis was performed with Origin 7.5 software. Data are given as mean values±SE. Cell membrane capacitance were 13.14±0.97 pF (n=18). All experiments were performed at room temperature.
RESULTS
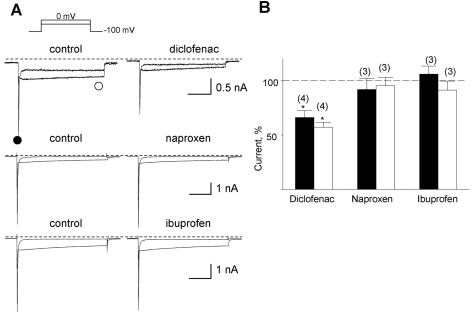
In our preliminary study, we examined the effects of three NSAIDs, diclofenac, naproxen, and ibuprofen, on ion currents in single cardiac myocytes. These drugs are known as nonspecific cyclooxygenase (COX) I and II inhibitors. Only diclofenac significantly inhibited the inward currents elicited by step depolarization, whereas the other drugs did not (Fig. 1), suggesting that current inhibition by diclofenac is COX-independent.
Fig. 1.
Inhibition of the Na+- and the Ca2+-sensitive inward current by three NSAIDs. (A) Representative currents before and after application of the drugs denoted above the corresponding trace. The drugs were applied at a concentration of 10 µM each. The amplitudes of the initial transient component and the slowly decayed components were measured at positions marked by closed (●) and open circles (○), respectively. (B) Summary of the normalized data for the effect of drugs on two components. Relative inhibition (%) for the Na+-sensitive initial transient (black bar) and the nifedipine-sensitive slowly decayed components (open bar) are shown with number of observations. Data were normalized to currents measured before application of each drug.
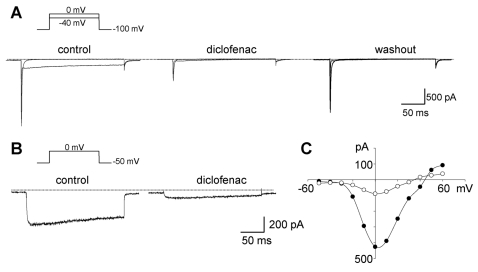
The whole-cell currents elicited by depolarization steps from -100 mV of HP to +60 mV were characterized by the rapid transients (-340.7±53.4 pA/pF, n=5) with the peak current at -40 mV, followed by a slowly decayed component (-31.7±4.3 pA/pF, n=5; left in Fig. 2A) with the peak at 0 mV (Fig. 2C). Diclofenac (100 µM) irreversibly inhibited the second component. However, the rapid transient current was restored upon the removal of diclofenac (right in Fig. 2A).
Fig. 2.
Representative traces of whole-cell currents elicited by step depolarizations in single cardiac myocytes. (A) Inhibition of the inward current induced by diclofenac. Changes in whole-cell currents evoked at -40 and 0 mV from a holding potential of -100 mV in bath solution containing 140 mM Na+ before (left) and after adding diclofenac (middle), and following washout (right), respectively. Dotted lines in A and B indicate the zero current level. (B) Currents induced by depolarization as indicated above the traces, in Na+-free bath solution before and after the addition of diclofenac. (C) Current-voltage relationship measured from the peak current of the traces in panel B. Diclofenac of 100 µM was applied. Outward components were not detected due to the presence of Ba2+, instead of Ca2+ in the bath.
To investigate the ionic nature of both components, we used a Na+-free solution for the bath. The rapid component was abolished but the slowly decayed component was still observed in Na+-free solution, indicating that the initial inward current was carried by Na+ through voltage-dependent Na+ channels (data not shown). To examine whether the second component is permeable to Ba2+ through the LCC, step depolarization from HP of -50 mV to 0 mV was applied. The slowly decayed component was still observed under these conditions (Fig. 2B, 2C). This component was completely blocked with 1 µM nifedipine, a specific LCC blocker, strongly suggesting that the slowly decayed component is the LCC-mediated Ba2+ current (IBa). As shown in Fig. 2B and 1C, 100 µM diclofenac drastically inhibited IBa.
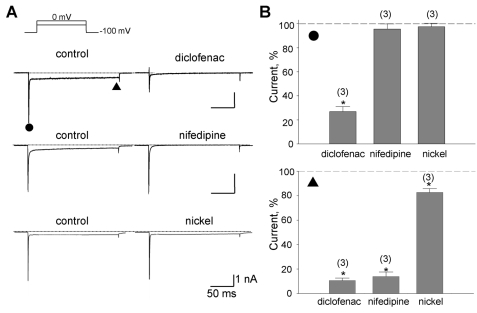
The transient low-voltage activated (T-type) Ca2+ current can be transiently activated by depolarizations from HP of -100 mV (Perez-Reyes, 1998). The initial transient inward component is possibly mingled with the T-type Ca2+ current. Thus, we examined whether T-type Ca2+ current might be activated by step depolarization from -100 mV and sensitive to diclofenac. This was done by comparing the effects of diclofenac with those of nifedipine and Ni2+, which is known to be more specific blocker of T-type Ca2+ channel (Lee et al., 1999; Perez-Reyes et al., 1999; Doering and Zamponi, 2003).
In this experiment using Ba2+ instead of Ca2+, step depolarization from -100 mV to 0 mV elicited both initial rapidly transient and sustained inward currents (IBa). As shown in Fig. 3, diclofenac significantly reduced the rapidly transient component by 74.6±4.8% (n=3) and the IBa component by 89.5±2.7% (n=3). Nifedipine (1 µM) and Ni2+ (300 µM) reduced IBa component by 85.2±4.5% (n=3) and by 17.6±3.3% (n=3), respectively. However neither had any effect on the initial transient component and blocking potency of Ni2+ for IBa was negligible (lower bar chart in Fig. 3B), suggesting that the T-type Ca2+ channels were not detected especially in the initial transient inward currents. These results confirmed again that diclofenac inhibits the current through LCC as well as the Na+ current.
Fig. 3.
Inhibition of the Na+ and the Ba2+ components by diclofenac. (A) Representative currents inhibited by drugs denoted above the right trace. With the application of diclofenac (100 µM), nifedipine (1 µM), or nickel (300 µM), reduced currents were shown on the right. The amplitudes of the initial transient component and the slowly decayed components were measured at the positions marked by the closed circle (●) and triangle (▲), respectively. (B) Summary of the normalized data for the effect of drugs on the two components. Relative inhibitions (%) of the Na+-sensitive initial transient and the nifedipine-sensitive components are shown in upper and lower panel with number of observations, respectively. Data were normalized to currents measured before application of each drug. Scale bars are equal to 1 nA and 50 ms.
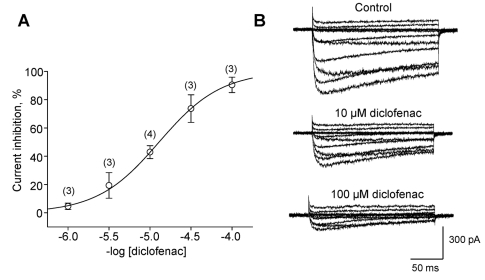
In Na+-free bath solution, diclofenac dose-dependently inhibited the LCC-mediated IBa with an IC50=12.89±0.43 µM (Fig. 4A). Diclofenac reduced the current amplitude without changing its kinetics in inactivation process (see current traces in Fig. 4B). This led to an implication that diclofenac did not at least play as an open channel blocker which remarkably accelerates inactivation process or decay phase (Nawrath et al., 1998). Although not shown here, diclofenac also depressed Ca2+ transients elicited by high K+ (25 mM)-induced depolarization. This depression was done with a similar fashion introduced by nifedipine.
Fig. 4.
Dose-dependent inhibition of the L-type current by diclofenac. (A) Dose-response relationship of the inhibitory effect of diclofenac on peak L-type (IBa) currents in cardiomyocytes, with the numbers of cells. The molar concentration of diclofenac is given. (B) Representative traces of L-type currents reduced by diclofenac. Step depolarizations were applied from HP of -50 mV to +60 mV in 10 mV increments.
DISCUSSION
Recommendation of diclofenac for patients with CHF or other cardiac problems has been under debate, remaining unknown about the molecular mechanism of its adverse effects. The present study focused side effects of diclofenac on ion channels since heart problems such as CHF could be initiated or triggered commonly by ionic disturbances. Here we provided the first finding that diclofenac dose-dependently suppressed LCC which is crucial to excitation-contraction coupling. In addition, diclofenac reversibly inhibitied the voltage-activated Na+ currents, which is consistent with other studies (Lee et al., 2003; Yang and Kuo, 2005; Fei et al., 2006).
In heart cells, L-type Ca2+ channels are major source to increase intracellular Ca2+ level ([Ca2+]i) via CICR. Restriction of Ca2+ entry by blocking LCC should reduce [Ca2+]i and weaken the cardiac muscle contraction. This could be confirmed with the result that diclofenac (30 µM), as well as nifedipine (10 µM), significantly suppressed Ca2+ transients elicited by high (25 mM) K+-induced depolarization, although not shown as data in the present study. Since diclofenac inhibited the LCC activity, it could disturb the muscle contraction for the efficient pumping as did by other LCC blockers such as verapamil, Cd2+, nifedipine, and Ni2+ (Ferrier and Howlett, 1995; Hobai et al., 1997; Howlett et al., 1998; Ferrier et al., 2000; Zhu and Ferrier, 2000). The voltage-dependent Na+ channel is essential to generate the cardiac action potentials (APs) and its propagation throughout the whole heart. Due to its inhibitory action on Na+ currents as shown in Fig. 2A and 3A, diclofenac might fail to, or generate APs inadequate to conduct the electrical excitation, which can induce arrhythmia. These combined effects observed at the cellular level can at least partly explain why diclofenac reveals severe cardiac risks such as the congestive heart failure.
In this study, we have examined the effect of diclofenac mainly on the LCC present in ventricular myocytes isolated from one-day old rat hearts. The quantitative analysis of the expression and distribution of Ca2+ channels demonstrated the expression of four types of Ca2+ channels in rat hearts, Cav1.2, Cav2.3, Cav3.1, and Cav3.2. The level of Cav3.1 and Cav3.2, the phenotypes of the T-type Ca2+ channel, is not changed significantly during development and become undetectable at five weeks postpartum. Cav2.3, an R-type Ca2+ channel, gradually declines after four weeks, when it reaches its peak expression. Of the four channel types, the phenotype of LCC, Cav1.2 is 10~100 times more abundant than other types and remains steadily its expression throughout development (Larsen et al., 2002). In accordance with others, the LCC density in neonatal rat cardiomyocytes corresponds to ca. 85% of that in adult rats (Katsube et al., 1998). Diclofenac can therefore induce cardiac problems to the adult from the neonates, due to its inhibitory effect on LCC.
During short-term therapeutic intake of diclofenac, its plasma concentration has been reported to reach 1.50~3.0 µg/ml (corresponding to 5~10 µM (Willis et al., 1979; Leucuta et al., 2004), which is close in the range of the concentrations effective on the LCC block in this study (refer to Fig. 4A). Because of its irreversible action, repeated intakes of diclofenac may progressively aggravate the LCC function. Diclofenac more than 10 µM also blocks the Na+ channels which are responsible to generate action potentials. Combined together, diclofenac may depress cardiac excitability and the contractility simultaneously. Therefore, its dual effects provide insight into how to bring about side effects on the heart and why it is more critical to patients with heart problems. To explain clearly, one should explore whether diclofenac suppresses Ca2+ transients induced by Ca2+ entry (i.e. CICR) and is sensitively offensive to the patients. The present study could not address the differences in the sensitivity to diclofenac between normal and cardiac cells from the heart with impaired function, since we could not find the appropriate rat model with experimentally induced heart failure.
In conclusion, this study showed that diclofenac reversibly inhibited the Na+ currents and irreversibly the L-type Ca2+ channel currents in cardiac muscle cells as our first finding. This finding provides a clue to explain at least partly why diclofenac play as a critical risk factor on heart as well as smooth muscle cells at the cellular/molecular level. The further study is required to assay the effects of long-term administration of therapeutic concentrations of diclofenac on the L-type Ca2+ channel and the E-C coupling in muscle cells.
ACKNOWLEDGEMENTS
This research was supported by a grant (08172KFDA507) from the Korea Food & Drug Administration in 2008. O.V.Y. and E.M.H were supported by the Korea Research Foundation Grant (KRF-2006-005-J04204). D.K. is supported by a scholarship from the BK21 Program and J.C.Y. is supported by the Brain Korea 21 Programs.
ABBREVIATIONS
- APs
action potentials
- CHF
congestive heart failure
- CICR
Ca2+-induced Ca2+ release
- CV
cardiovascular
- E-C coupling
excitation-contraction coupling
- LCC
L-type Ca2+ channel
- NSAIDs
non-steroidal anti-inflammatory drugs
References
- 1.Alves D, Duarte I. Involvement of ATP-sensitive K+ channels in the peripheral antinociceptive effect induced by dipyrone. Eur J Pharmacol. 2002;444:47–52. doi: 10.1016/s0014-2999(02)01412-7. [DOI] [PubMed] [Google Scholar]
- 2.Asomoza-Espinosa R, Alonso-Lopez R, Mixcoatl-Zecuatl T, Aguirre-Banuelos P, Torres-Lopez JE, Granados-Soto V. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol. 2001;418:195–200. doi: 10.1016/s0014-2999(01)00956-6. [DOI] [PubMed] [Google Scholar]
- 3.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bort R, Ponsoda X, Jover R, Gomez-Lechon MJ, Castell JV. Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. J Pharmacol Exp Ther. 1998;288:65–72. [PubMed] [Google Scholar]
- 5.Brater DC. Renal effects of cyclooxygyenase-2-selective inhibitors. J Pain Symptom Manage. 2002;23:S15–S20. doi: 10.1016/s0885-3924(02)00370-6. [DOI] [PubMed] [Google Scholar]
- 6.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 7.Dalla Libera L, Vescovo G, Volterrani M. Physiological basis for contractile dysfunction in the heart failure. Curr Pharm Des. 2008;14:2572–2581. doi: 10.2174/138161208786071254. [DOI] [PubMed] [Google Scholar]
- 8.Doering CJ, Zamponi GW. Molecular pharmacology of high voltage-activated calcium channels. J Bioenerg and Biomem. 2003;35:491–505. doi: 10.1023/b:jobb.0000008022.50702.1a. [DOI] [PubMed] [Google Scholar]
- 9.Fei XW, Liu LY, Xu JG, Zhang ZH, Mei YA. The non-steroidal anti-inflammatory drug, diclofenac, inhibits Na+ current in rat myoblasts. Biochem Biophys Res Commun. 2006;346:1275–1283. doi: 10.1016/j.bbrc.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Ferrier GR, Howlett SE. Contractions in guinea-pig ventricular myocytes triggered by a calcium-release mechanism separate from Na+ and L-currents. J Physiol. 1995;484:107–122. doi: 10.1113/jphysiol.1995.sp020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrier GR, Redondo IM, Mason CA, Mapplebeck C, Howlett SE. Regulation of contraction and relaxation by membrane potential in cardiac ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1618–H1626. doi: 10.1152/ajpheart.2000.278.5.H1618. [DOI] [PubMed] [Google Scholar]
- 12.Fu J, Gao J, Pi R, Liu P. An optimized protocol for culture of cardiomyocytes from neonatal rat. Cytotechnol. 2005;49:109–116. [Google Scholar]
- 13.Graham DJ. COX-2 inhibitors, other NSAIDs, and cardiovascular risk: the seduction of common sense. JAMA. 2006;296:1653–1656. doi: 10.1001/jama.296.13.jed60058. [DOI] [PubMed] [Google Scholar]
- 14.Hobai IA, Howarth FC, Pabbathi VK, Dalton GR, Hancox JC, Zhu JQ, Howlett SE, Ferrier GR, Levi AJ. Voltage-activated Ca2+ release in rabbit, rat and guinea-pig cardiac myocytes, and modulation by internal cAMP. Pflugers Arch. 1997;435:164–173. doi: 10.1007/s004240050496. [DOI] [PubMed] [Google Scholar]
- 15.Hombach V. Electrocardiography of the failing heart. Cardiol Clin. 2006;24:413–426. doi: 10.1016/j.ccl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Howlett SE, Zhu JQ, Ferrier GR. Contribution of a voltage-sensitive calcium release mechanism to contraction in cardiac ventricular myocytes. Am J Physiol. 1998;274:H155–H170. doi: 10.1152/ajpheart.1998.274.1.H155. [DOI] [PubMed] [Google Scholar]
- 17.Hudson M, Rahme E, Richard H, Pilote L. Risk of congestive heart failure with nonsteroidal anti-inflammatory drugs and selective cyclooxygenase 2 inhibitors: a class effect? Arthritis and Rheumatism. 2007;57:516–523. doi: 10.1002/art.22614. [DOI] [PubMed] [Google Scholar]
- 18.Katsube Y, Yokoshiki H, Nguyen L, Yamamoto M, Sperelakis N. L-type Ca2+ currents in ventricular myocytes from neonatal and adult rats. Can J Physiol Pharmacol. 1998;76:873–881. doi: 10.1139/cjpp-76-9-873. [DOI] [PubMed] [Google Scholar]
- 19.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. Br Med J. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen JK, Mitchell JW, Best PM. Quantitative analysis of the expression and distribution of calcium channel α1 subunit mRNA in the atria and ventricles of the rat heart. J Mol Cell Cardiol. 2002;34:519–532. doi: 10.1006/jmcc.2001.1534. [DOI] [PubMed] [Google Scholar]
- 21.Lee HM, Kim HI, Shin YK, Lee CS, Park M, Song JH. Diclofenac inhibition of sodium currents in rat dorsal root ganglion neurons. Brain Research. 2003;992:120–127. doi: 10.1016/j.brainres.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block a1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leucuta A, Vlase L, Farcau D, Nanulescu M. No effect of short term ranitidine intake on diclofenac pharmakinetics. Rom J Gastroenterol. 2004;13:306–308. [PubMed] [Google Scholar]
- 24.Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- 25.Liu LY, Fei XW, Li ZM, Zhang ZH, Mei YA. Diclofenac, a nonsteroidal anti-inflammatory drug, activates the transient outward K+ current in rat cerebellar granule cells. Neuropharmacology. 2005;48:918–926. doi: 10.1016/j.neuropharm.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Maltsev VA, Sabbab HN, Undrovinas AI. Down-regulation of sodium current in chronic heart failure: effect of long-term therapy with carvediol. Cell Mol Life Sci. 2002;59:1561–1568. doi: 10.1007/s00018-002-8529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systemic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase-2. JAMA. 2006;296:1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 28.Morales MA, Inostroza L, Salazar T, Paeile C. Effects of clonixin on the electrical activity of cardiac pacemaker cells. Gen Pharmacol. 1992;23:515–521. doi: 10.1016/0306-3623(92)90121-y. [DOI] [PubMed] [Google Scholar]
- 29.Morales MA, Salazar T, Paeile C. Effects of flunixin and mefenamic acid on cardiac pacemaker cells. Structure-activity relationship and comparison with clonixin. Gen Pharmacol. 1993;24:775–780. doi: 10.1016/0306-3623(93)90245-s. [DOI] [PubMed] [Google Scholar]
- 30.Nawrath H, Klein G, Rupp J, Wegener JRW, Shainberg A. Open state block by fendiline of L-type Ca2+ channels in ventricular myocytes from rat heart. J Pharmacol Exp Ther. 1998;285:546–552. [PubMed] [Google Scholar]
- 31.Ortiz MI, Torres-Lopez JE, Castaneda-Hernandez G, Rosas R, Vidal-Cantu GC, Granados-Soto V. Pharmacological evidence for the activation of K+ channels by diclofenac. Eur J Pharmacol. 2002;438:85–91. doi: 10.1016/s0014-2999(02)01288-8. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Reyes E. Molecular characterization of a novel family of low voltage-activated, T-type, calcium channels. J Bioenerg Biomembr. 1998;30:313–318. doi: 10.1023/a:1021981420839. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Reyes E, Lee JH, Cribbs LL. Molecular characterization of two members of the T-type calcium channel family. Ann N Y Acad Sci. 1999;868:131–143. doi: 10.1111/j.1749-6632.1999.tb11283.x. [DOI] [PubMed] [Google Scholar]
- 34.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999;42:284–297. doi: 10.1016/s0008-6363(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 36.Tan HL, Bink-Boelkens MT, Bezzina CR, Viswanathan PC, Beaufort-Krol GC, van Tintelen PJ, van den Berg MP, Wilde AA, Balser JR. A sodium channel mutation causes isolated cardiac conduction disease. Nature. 2001;409:1043–1047. doi: 10.1038/35059090. [DOI] [PubMed] [Google Scholar]
- 37.Tonussi CR, Ferreira SH. Mechanism of diclofenac analgesia: direct blockade of inflammatory sensitization. Eur J Pharmacol. 1994;251:173–179. doi: 10.1016/0014-2999(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 38.Waksman JC, Brody A, Phillips SD. Nonselective nonsteroidal anti-inflammatory drugs and cardiovascular risk: are they safe? Ann Pharmacother. 2007;41:1163–1173. doi: 10.1345/aph.1H341. [DOI] [PubMed] [Google Scholar]
- 39.Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16:405–410. doi: 10.1007/BF00568201. [DOI] [PubMed] [Google Scholar]
- 40.Yang YC, Kuo CC. An inactivation stabilizer of the Na+ channel acts as an opportunistic pore blocker modulated by external Na+ J Gen Physiol. 2005;125:465–481. doi: 10.1085/jgp.200409156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu JQ, Ferrier GR. Regulation of a voltage-sensitive release mechanism by Ca2+-calmodulin dependent kinase in cardiac myocytes. Am J Physiol. 2000;279:H2104–H2115. doi: 10.1152/ajpheart.2000.279.5.H2104. [DOI] [PubMed] [Google Scholar]