Abstract
Induced activation of the gamma-aminobutyric acidA (GABAA) receptor in the retina of goldfish caused the fish to rotate in the opposite direction to that of the spinning pattern during an optomotor response (OMR) measurement. Muscimol, a GABAA receptor agonist, modified OMR in a concentration-dependent manner. The GABAB receptor agonist baclofen and GABAC receptor agonist CACA did not affect OMR. The observed modifications in OMR included decreased anterograde rotation (0.01~0.03 µM), coexistence of retrograde rotation and decreased anterograde rotation (0.1~30 µM) and only retrograde rotation (100 µM~1 mM). In contrast, the GABAA receptor antagonist bicuculline blocked muscimolinduced retrograde rotation. Based on these results, we inferred that the coding inducing retrograde movement of the goldfish retina is essentially associated with the GABAA receptor-related visual pathway. Furthermore, from our novel approach using observations of goldfish behavior the induced discrete snapshot duration was approximately 573 ms when the fish were under the influence of muscimol.
Keywords: Discrete snapshot duration, Goldfish, Illusory motion reversal (IMR), Optomotor response (OMR), Retina
INTRODUCTION
The phenomenon in which rotating spokes look as if they are rotating in the opposite direction is called the wagon wheel illusion (WWI) or illusory motion reversal (IMR). This is observed not only in humans but also in other animals under specific conditions (Götz, 1964; Purves et al., 1996; Maaswinkel and Li, 2003; Kline et al., 2004; VanRullen et al, 2006; VanRullen, 2007). IMR occurs very often at low temporal frequency/high spatial frequency domain (Götz, 1964; Purves et al., 1996; Maaswinkel and Li, 2003). Although the occurrence of motion detection and IMR can be explained theoretically by the Reichardt and correlation detector model (Reichardt, 1987; Purves et al., 1996; Srinivasan et al., 1999; Zanker et al., 1999; Maaswinkel and Li, 2003; Kline et al., 2004), there are no reports of the induction of IMR via intraocular injections of drugs in the retina of the goldfish.
We were the first to discover the potency of intraocular injection of specific drugs in inducing IMR in goldfish under specific conditions. Since goldfish always move relative to the movements of their perceived surroundings, it is very appropriate to use the goldfish when observing the arousal of IMR. Motion detection in animals such as the goldfish is typically assessed with behavioral experiments such as the optomotor response (OMR) and optokinetic response (OKR), or with directional selectivity measurements (Barlow and Hill, 1963; Oyster et al., 1972; Schaerer and Neumeyer, 1996). There are reports that suggest that signals activating motion detection and directional selectivity are formed in the inner plexiform layer and amacrine cells, and have a central role in this process (Barlow and Levick, 1965; Werblin, 1969). Diverse types of amacrine cells, which are activated by bipolar cells, generate inhibitory or excitatory signals. These two types of signals are inputted into ganglion cells with time differences and, when combined, lead to directional selectivity. This directional selectivity is induced or suppressed by agonists that are related to the inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and glycine (Wyatt and Daw, 1976; Egelhaaf et al., 1990; Pan and Slaughter, 1991; Demb, 2007). Although research has focused mainly on the effects of neurotransmitters on directional selectivity, only a few investigations on motion detection have been undertaken.
Unlike many previous investigations, we presently examined the effects of neurotransmitters on motion detection by observing the changes of the recognition of motion detection by using OMR with the GABA agonist muscimol, which acts on GABAA receptors. In these experiments, we discovered that intraocular injection of drugs can trigger the goldfish to spin in the opposite direction to that of their perceived moving surroundings-in this case the spinning patterns-when under continuous stimuli. The maximum discrete snapshot duration, which forms in the retina by the activation of GABAA receptors, was about 573 ms with the rotation velocity of IMR. In addition, IMR induction required adaptation time of about 13 sec (the time required to induce IMR).
These results lead to the following conclusions: (1) recognition of the difference in light intensity of the moving object decreases when the object rotates, (2) the long adaptation time (the time required to induce the reversed rotation) creates the circuit for snapshot duration in the goldfish retina (perhaps with a GABAA related pathway), (3) the central signal intensifies every 573 ms, and (4) this evident signal induces the reversed recognition by producing a stroboscopic effect (influenced by the intraocular-injection of muscimol). Therefore, the GABAA related circuit may create snapshots by enforcing relatively stronger central signals periodically.
METHODS
Animals
Goldfish (carassius auratus) used in our experiment were approximately 7~9 cm in length and weighed 10~16 g. They were purchased from a local fish shop and were kept under an alternating 12 h light and dark cycle to maintain their circadian rhythm at room temperature in an aquarium before the experiment.
Experimental setup and light intensities
We used an experimental setup similar to the one used by Schaerer and Neumeyer (1996): a 12 cm-diameter fishbowl containing a goldfish was placed in the center of a cylinder with 6 alternating blue and white stripes (each strip was 3.8 cm in width and the wavelength of the deep blue was 441 nm). The patterned cylinder rotated mostly at 12 rpm but rotated at other various velocities if needed. The light source used herein was from on a XG-SV1A LCD projector (Sharp, Tokyo, Japan). The optical density of the pattern surface measured using a model IL 1400A radiometer (International light, Newburyport, MA) was adjusted to approximately 100 nW/cm2. To prevent the entrance of external light, the setup was covered in two layers of black curtains. The movement of the goldfish and pattern was recorded using a SK-2005X miniature camera (Huviron, Gyenggi-do, Korea) set over the fishbowl.
Measurement of optomotor response
Prior to the experiment, the goldfish was allowed to adapt for approximately 20 minutes in the fishbowl to become acclimated to the light used for the experiment. Each experiment comprised seven repeated procedures. One procedure involved 1 minute of cylinder rotation, which induced the spinning of the goldfish as it tried to follow the rotating patterns it perceived, and 30 seconds of settling down. To prevent the goldfish from adapting to rotation in one direction for the seven trials, the cylinder was spun in alternate directions-clockwise in one trial and counter-clockwise in the next.
Drugs and injections
The drug solutions were prepared by dissolving and diluting muscimol (GABAA receptor agonist), bicuculline (GABAA receptor antagonist), baclofen (GABAB receptor agonist), and CACA (GABAC receptor agonist) in a Ringer's solution (pH 7.40, in mM: 125 NaCl, 2.6 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES). After anesthetization of the goldfish in 0.005% MS 222 for 2~3 minutes, 2.0 µl of a drug solution was intraocularly injected into the goldfish with an IO-KIT intraocular kit (WPI, Sarasota, FL). The post-injection concentrations of the drugs were diluted about 0.05 times the pre-injection concentration in the eye cups, since the volume left after subtracting the lens volume from the total inner eye volume after we injected 2.0 µl into the eye was about 40 µl (The measurements were taken with a measuring microscope; NRM-5XYZ, Nippon Optical Works, Japan). As there were no changes in OMR whether or not the control was injected with the Ringer's solution in a preliminary experiment, the control group was not injected with the Ringer's solution during this experiment.
Data acquisition, treatment, and statistics
OMR data were obtained by subtracting the turns in which the goldfish moved in the opposite direction to that of the pattern's rotation (retrograde) from those in which it moved in the same direction to the pattern's rotation (anterograde), based on the direction of goldfish's head [OMR=(number of goldfish rotations in 1 minute/number of pattern rotation in 1 minute)×100 (%)]. In each experiment, five goldfish were tested. For each goldfish, seven trials were executed but, from the seven measurements obtained, only five were used (the maximum and minimum measurements were disregarded). Thus, 25 measurements were obtained. Measurements were analyzed using Origin 8 (Origin Lab, Northampton, MA) and depicted in a table. In all the text and plots, the data are shown with their respected standard error of mean (mean±S.E.) and represent the number of goldfish. For statistical comparison, differences were considered significant if p<0.05 (*).
RESULTS
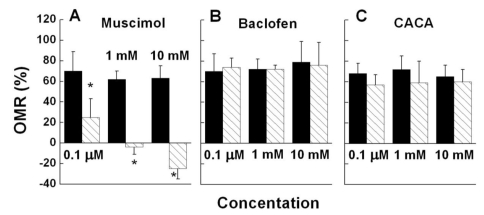
Muscimol, a GABAA receptor agonist, reduced OMR significantly at a concentration of 0.1 µM (p<0.05) and induced negative OMR (reversed direction of rotation of the goldfish to that of the pattern) at concentrations of 1 mM (p<0.05) and 10 mM (p<0.05) (Fig. 1A). Baclofen, a GABAB receptor agonist, did not have any effect on OMR (Fig. 1B). CACA, a GABAC receptor agonist, induced a small reduction that was not significant (p>0.05, Fig. 1C).
Fig. 1.
OMR of a goldfish before (■) and after (▧) administration of three concentrations of muscimol (A), baclofen (B), and CACA (C). All drugs were independently administered five times. Muscimol-induced negative OMR represents the reverse rotation of a goldfish to the direction of pattern rotation. Here and in subsequent figures, unless otherwise indicated the error bar represents±standard error of the mean, *indicates p<0.05 and n represents the number of goldfish.
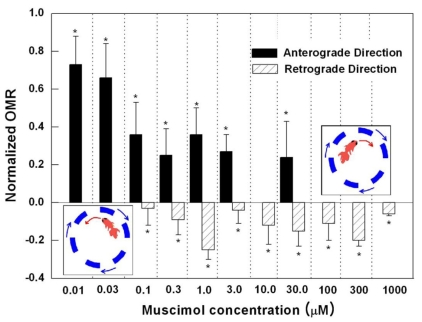
We observed OMR in various concentrations of muscimol (Fig. 2). Muscimol-injected OMRs were normalized to each corresponding control OMRs. Normalized OMR showed three patterns depending on the change of muscimol concentration. At concentrations below 0.03 µM, normalized OMR decreased. From 0.1~30.0 µM, however, normalized OMR decreased and plateaued. But, unlike previous concentrations, negative OMR also appeared. In this concentration range, there was a tendency for the goldfish to rotate persistently in the initial direction and then to rotate without changing its direction in the middle of the procedure. Thus, to obtain enough data for the anterograde rotation and the retrograde, twice as many measurements were taken at this concentration range than at other concentrations. Furthermore, at concentrations above 100 µM, only negative normalized OMR was observed. Based on these results, we inferred that it is possible to induce substantial negative OMR by an exogenous activation of GABAA receptor in goldfish.
Fig. 2.
The relationship between muscimol concentration and normalized OMR. Eleven concentrations of muscimol from 0.01 µM to 1 mM were used (n=5 for each concentration). At certain concentrations, the same goldfish showed both negative and positive OMR. To account for this, each fish treated with concentrations ranging from 0.1~30 µM was measured twice as much. Therefore, the total number of fish used in the experiment was 55, but 30 of were tested and measured twice as much as the remaining 25. As the muscimol concentration increased, there were 3 distinctive OMR results: reduction in anterograde rotation, coexistence of retrograde rotation and reduced anterograde rotation, and retrograde rotation only. The diagram, which shows the anterograde and retrograde rotation, is shown on the graph for reference and clarity.
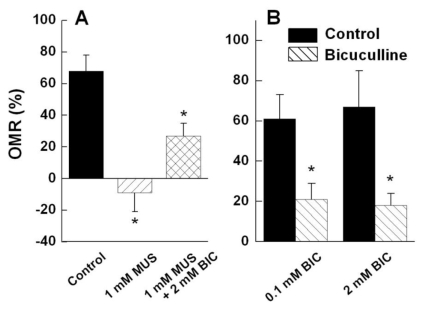
The GABAA receptor antagonist bicuculline seemed to suppress muscimol-induced negative OMR (Fig. 3A). When 1 mM muscimol was infused, OMR was -0.30±0.65%, and a subsequent infusion of a mixture of 1 mM muscimol and 2 mM bicuculline restored OMR to 27.00±0.80%. When only bicuculline was injected, OMR decreased (Fig. 3B), in agreement with those obtained by Mora-Ferrer et al. (2005: Fig. 4D~F).
Fig. 3.
OMR as to GABA receptor related drugs. (A) Co-injection (n=5) of 1 mM muscimol (MUS) and 2 mM bicuculline (BIC) inhibited the negative OMR that muscimol had induced. (B) OMR using the two concentrations of bicuculline. Bicuculline alone markedly reduced anterograde OMR (n=5).
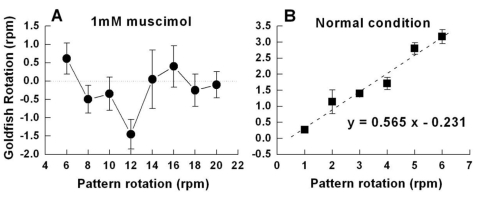
Fig. 4.
Goldfish rotation versus pattern rotation. (A) Number of rotations depending on various pattern velocities after the administration of 1 mM muscimol (n=5 for each velocity). The reversed rotation due to muscimol was the greatest at a pattern velocity of 12 rpm. (B) Rotation of goldfish to low speeds in the control condition (n=5 in different pattern rotation speeds). From this relationship, a linear equation of y=0.565x-0.231 was obtained. Using this, we could predict the pattern's velocity to be 3.12 rpm when a goldfish rotated at a velocity of 1.532 rpm.
When we observed goldfish rotation at various pattern velocities (6, 8, 10, 12, 14, 16, 18, and 20 rpm) after the intraocular injection of 1 mM muscimol, retrogression was greatest at 12 rpm (Fig. 4A). Based on this result, we supposed that aliasing perceived by goldfish was the smallest at 12 rpm but increased at other pattern velocities.
The discrete Snapshot Model (Purves et al., 1996), which analyzes motion detection using a discrete snapshot, explains the motion detection in the presence of muscimol. In order to obtain the discrete snapshot duration using the Snapshot Model, we tried to find an ideal virtual pattern velocity that the goldfish perceived under the effect of muscimol by using the retrograde rotation of goldfish when the pattern rotated at 12 rpm. We discovered that the average muscimol-induced retrograde rotation velocity of goldfish was about -1.2 rpm (-1.20±0.34 rpm, n=52) and that the adaptation times before the goldfish followed the moving pattern after the pattern began rotating were 5.22±0.88 sec (n=48) for the control and about 13.13±1.2 sec (n=62) for muscimol-affected goldfish respectively. Therefore, the actual retrogression was -1.532 rpm since the goldfish rotated retrograde -1.2 rpm during the 46.87 sec (60-13.13 sec) time interval. By observing OMR at low pattern velocities such as 1, 2, 3, 4, 5, and 6 rpm (Fig. 4B), we obtained a linear relation between velocities of the pattern and that of the goldfish (goldfish rotation=0.565×pattern rotation-0.231). From this relation, we found that 1.532 rpm, the goldfish rotation velocity, corresponded to an ideal virtual pattern velocity of 3.12 rpm (18.72 deg/s). If the goldfish perceived a pattern, whenever the pattern was located in the exact location that the other patterns were located just previously the goldfish would not perceive the change and would not rotate. Yet, whenever the pattern seemed to move 41.28 deg away from the original location, the goldfish perceived the change and rotated -18.72 deg, producing a negative rotation of about -1.532 rpm. The angular velocity of this rotating pattern was 12 rpm (72 deg/s) and, in this condition, the goldfish would have to perceive the movement of the pattern when the pattern rotated 41.28 deg forward for the goldfish to rotate backwards 18.72 deg upon receiving the visual signal from the retina. In this case the discrete snapshot duration was about 0.573s [(41.28 deg)/(72 deg)=573 ms] (For further reference, see discussion).
DISCUSSION
Pharmacological effects used for the OMR of Goldfish are analogous to those seen in the direction selectivity (DS), an electrophysiological experiment using retina of various species of vertebral animals. Of these, antagonists of GABAA receptor inhibit DS (Wyatt and Daw, 1976; Caldwell et al., 1978; Kittila and Massey, 1995; He and Masland, 1997; Mora-Ferrer et al., 2005). Besides, the observations that OMR can be markedly suppressed by bicuculline and picrotoxin (Ferrer et al., 2005) illustrate that GABAA receptors play a key role in motion coding. We presently obtained similar results in goldfish with the use of bicuculline (Fig. 2B). Moreover, use of the GABAA receptor agonist muscimol induced IMR in goldfish.
GABAA receptors are distributed over extensive areas of retinal neurons of animals. In goldfish, they are present on photoreceptors, bipolar cells, and horizontal cell dendrites in the outer retina, and amacrine, amacrine-to-amacrine, and amacrine-to-ganglion cell synapses in the inner retina. These findings have been demonstrated on immunohistochemical staining and measurements of morphology and electrophysiology (Kaneko and Tachibana, 1897; Ishada and Cohen, 1988; Yazulla et al., 1989; Lin and Yazulla, 1994; Watanabe et al., 2000; Paik et al., 2003; Klooster et al., 2004). GABAA receptors are present in nearly all goldfish retina cells. They are also affected by an intraocular administration of muscimol. However, our experimental data could not clarify the exact mechanism by which muscimol induces IMR.
Based on our experimental results, we inferred that when GABAA receptors in the goldfish retina are activated, continuous stimuli of repeated patterns are likely to make the goldfish perceive patterns at a constant time interval as in a stroboscopic state. The periodicity of perception explained above is considered as discrete snapshot duration. We will refer to this discrete snapshot duration caused by the continuous stimulation of the repetitive pattern as induced discrete snapshot duration (IDSD). Muscimol-induced IDSD requires activation of GABAA receptors, continuous stimulation from the repetitive pattern and an adaptation time of approximately 13 sec prior to the occurrence of IDSD. Although we believe that it is possible to induce IMR without the injection of muscimol under certain conditions, we could not identify this using our experimental conditions. When the goldfish were under the influence of muscimol, however, IDSD was observed with the use of conditions under which IMR and the Snapshot Model occurred. Suppose that a muscimol-injected goldfish receives and, therefore, perceives visual information of the changing pattern at the same time interval to that of IDSD. If so, then when a six-cycle pattern cylinder is rotated at a velocity of 12 rpm (the patterns rotating at a velocity of 72 deg/sec [=(12 rpm)×(360 deg/sec)], the goldfish perceives the stripe as if the adjacent same colored stripe had moved 18.720 degrees [=(3.12 rpm×360 deg)/(60 sec)] backwards. But, in reality the stripe has moved 41.28 degrees forward for the goldfish to perceive the visual information of the rotating pattern as if it was rotating at a velocity of -3.12 rpm. The snapshot duration is, therefore, 573 ms [=(41.28 deg)/(72 deg/sec)].
Human experiments regarding reverse direction coding have shown that when the alternative rate of motion information is approximately 10~15 Hz, the illusory images moving to a reverse direction are visualized most frequently (VanRullen et al., 2006). These results indicate that the frequency of the perception of reverse rotation is the highest when the discrete snapshot duration is around 80 ms, which is much shorter than that caused by muscimol in our experiment. Humans do not always experience IMR under normal conditions (Pakarian and Yasamy, 2003; Kline et al., 2004). These results indicate that humans do not strongly form reverse visual coding. An intraocular injection of muscimol might strongly activate IDSD due to the strength of reverse direction visual coding, which might lead to the experience of IMR. Of course, as shown in Fig. 4A, IMR may show a peak when IDSD is harmonized with optimal environmental conditions.
ACKNOWLEDGEMENTS
We thank R. Vanrullen for critical comments on the manuscript and Hilary Dan Bi Kim for improving the English text. This work was supported by the Korea Research Foundation Grant (KRF-2004-041-E00266).
ABBREVIATIONS
- IDSD
induced discrete snapshot duration
- IMR
illusory motion reversal
- OMR
optomotor response
- WWI
wagon wheel illusion
References
- 1.Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- 2.Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell JH, Daw NW, Wyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Egelhaaf M, Borst A, Pilz B. The role of GABA in detecting visual motion. Brain Res. 1990;509:156–160. doi: 10.1016/0006-8993(90)90325-6. [DOI] [PubMed] [Google Scholar]
- 6.Götz KG. Optomotorische Untersuchung des visuellen Systems einiger Augenmutanten der fruchtfliege Drosophila. Biol Cybern. 1964;2:77–92. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- 7.He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- 8.Ishida AT, Cohen BN. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988;60:381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko A, Tachibana M. GABA mediates the negative feedback from amacrine to bipolar cells. Neurosci Res Suppl. 1987;6:S239–S251. doi: 10.1016/0921-8696(87)90020-x. [DOI] [PubMed] [Google Scholar]
- 10.Kittila CA, Massey SC. Effect of ON pathway blockade on directional selectivity in the rabbit retina. J Neurophysiol. 1995;73:703–712. doi: 10.1152/jn.1995.73.2.703. [DOI] [PubMed] [Google Scholar]
- 11.Kline K, Holcombe AO, Eagleman DM. Illusory motion reversal is caused by rivalry, not by perceptual snapshots of the visual field. Vision Res. 2004;44:2653–2658. doi: 10.1016/j.visres.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Klooster J, Cardozo BN, Yazulla S, Kamermans M. Postsynaptic localization of gamma-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: An ultrastructural study. J Comp Neurol. 2004;474:58–74. doi: 10.1002/cne.20114. [DOI] [PubMed] [Google Scholar]
- 13.Lin ZS, Yazulla S. Heterogeneity of GABAA receptor in goldfish retina. J Comp Neurol. 1994;345:429–439. doi: 10.1002/cne.903450309. [DOI] [PubMed] [Google Scholar]
- 14.Maaswinkel H, Li L. Spatio-temporal frequency characteristics of the optomotor response in zebrafish. Vision Res. 2003;43:21–30. doi: 10.1016/s0042-6989(02)00395-4. [DOI] [PubMed] [Google Scholar]
- 15.Mora-Ferrer C, Hausselt S, Hoffmann RS, Ebisch B, Schick S, Wollenberg K, Schneider C, Teege P, Jürgens K. Pharmacological properties of motion vision in goldfish measured with the optomotor response. Brain Res. 2005;1058:17–29. doi: 10.1016/j.brainres.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Oyster CW, Takahashi E, Collewijn H. Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res. 1972;12:183–193. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- 17.Paik SS, Park NG, Lee SJ, Han HK, Jung CS, Bai SH, Chun MH. GABA receptors on horizontal cells in the goldfish retina. Vision Res. 2003;43:2101–2106. doi: 10.1016/s0042-6989(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 18.Pakarian P, Yasamy MT. Wagon-wheel illusion under steady illumination: real or illusory? Perception. 2003;32:1307–1310. doi: 10.1068/p5133. [DOI] [PubMed] [Google Scholar]
- 19.Pan ZH, Slaughter MM. Control of retinal information coding by GABAB receptors. J Neurosci. 1991;11:1810–1821. doi: 10.1523/JNEUROSCI.11-06-01810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purves D, Paydarfar JA, Andrews TJ. The wagon wheel illusion in movies and reality. Proc Natl Acad Sci USA. 1996;93:3693–3697. doi: 10.1073/pnas.93.8.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichardt W. Evaluation of optical motion information by movement detectors. J Comp Physiol A. 1987;161:533–547. doi: 10.1007/BF00603660. [DOI] [PubMed] [Google Scholar]
- 22.Schaerer S, Neumeyer C. Motion detection in goldfish investigated with the optomotor response is "color blind". Vision Res. 1996;36:4025–4034. doi: 10.1016/s0042-6989(96)00149-6. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan MV, Poteser M, Kral K. Motion detection in insect orientation and navigation. Vision Res. 1999;39:2749–2766. doi: 10.1016/s0042-6989(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 24.VanRullen R. The continuous wagon wheel illusion depends on, but is not identical to neuronal adaptation. Vision Res. 2007;47:2143–2149. doi: 10.1016/j.visres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 25.VanRullen R, Reddy L, Koch C. The continuous wagon wheel illusion is associated with changes in electroencephalogram power at approximately 13 Hz. J Neurosci. 2006;26:502–507. doi: 10.1523/JNEUROSCI.4654-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe S, Koizumi A, Matsunaga S, Stocker JW, Kaneko A. GABA-mediated inhibition between amacrine cells in the goldfish retina. J Neurophysiol. 2000;84:1826–1834. doi: 10.1152/jn.2000.84.4.1826. [DOI] [PubMed] [Google Scholar]
- 27.Werblin FS. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1969;33:342–350. doi: 10.1152/jn.1970.33.3.342. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt HJ, Daw NW. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]
- 29.Yazulla S, Studholme KM, Vitorica J, de Blas AL. Immunocytochemical localization of GABAA receptors in goldfish and chicken retinas. J Comp Neurol. 1989;280:15–26. doi: 10.1002/cne.902800103. [DOI] [PubMed] [Google Scholar]
- 30.Zanker JM, Srinivasan MV, Egelhaaf M. Speed tuning in elementary motion detectors of the correlation type. Biol Cybern. 1999;80:109–116. doi: 10.1007/s004220050509. [DOI] [PubMed] [Google Scholar]