Abstract
Skeletal muscle atrophy is a common phenomenon during the prolonged muscle disuse caused by cast immobilization, extended aging states, bed rest, space flight, or other factors. However, the cellular mechanisms of the atrophic process are poorly understood. In this study, we investigated the involvement of mitogen-activated protein kinase (MAPK) in the expression of muscle-specific RING finger 1 (MuRF1) during atrophy of the rat gastrocnemius muscle. Histological analysis revealed that cast immobilization induced the atrophy of the gastrocnemius muscle, with diminution of muscle weight and cross-sectional area after 14 days. Cast immobilization significantly elevated the expression of MuRF1 and the phosphorylation of p38 MAPK. The starvation of L6 rat skeletal myoblasts under serum-free conditions induced the phosphorylation of p38 MAPK and the characteristics typical of cast-immobilized gastrocnemius muscle. The expression of MuRF1 was also elevated in serum-starved L6 myoblasts, but was significantly attenuated by SB203580, an inhibitor of p38 MAPK. Changes in the sizes of L6 myoblasts in response to starvation were also reversed by their transfection with MuRF1 small interfering RNA or treatment with SB203580. From these results, we suggest that the expression of MuRF1 in cast-immobilized atrophy is regulated by p38 MAPK in rat gastrocnemius muscles.
Keywords: Cast immobilization, MuRF1, p38 MAPK, Skeletal muscle atrophy, Starvation
INTRODUCTION
Skeletal muscle is the major reservoir of body protein, and exhibits very high plasticity in its ability to adapt to changing functional demands. The loss of muscle proteins with reduced muscle activity, malnutrition, metabolic disease, or aging is a universal phenomenon, known as "muscle atrophy" or "wasting" (Jagoe and Goldberg, 2001). The structural and functional consequences of all forms of atrophy are reduced muscle mass and fiber cross-sectional area, reduced contractile force, and increased fatigability and insulin resistance (Booth and Thomason, 1991).
The increased degradation of proteins during skeletal muscle atrophy is commonly coupled to the activation of the ubiquitin-dependent protease pathway. Ubiquitinated proteins are degraded by muscle-specific ubiquitin ligases (Bodine et al., 2001; Tesseraud et al., 2007). Previous studies have suggested that the transcriptional regulation of the E3 protein ligases, atrogin-1/muscle atrophy F-box (MAFbx) and muscle-specific RING finger 1 (MuRF1), is elevated in skeletal muscle and is involved in the initiation and development of skeletal muscle atrophy (Glass, 2005). The expression of MAFbx and MuRF1 is increased in models of skeletal muscle atrophy that involve fasting, denervation, glucocorticoid treatment, or cast immobilization (Gomes et al., 2001; Wray et al., 2003; Li et al., 2005; Tobimatsu et al., 2009). MAFbx expression was elevated in serum-starved skeletal muscle cells (Schulze et al., 2005). The expression of MuRF1 increases in cardiac cells in serum-free medium (Skurk et al., 2005). It has been suggested that MuRF1 is involved in the degradation of functional intracellular proteins, such as troponin I and titin, by their ubiquitination (McElhinny et al., 2002; Kedar et al., 2004). These results indicate that MuRF1 may be used as a marker of skeletal muscle atrophy in the diagnosis and treatment of related diseases.
Insulin-like growth factor 1 induces an increase in muscle mass via the Akt pathway and causes the activation of downstream signals that participate in protein synthesis. Moreover, skeletal muscle atrophy is coupled to the diminution of Akt activation (Sugita et al., 2005), and Akt inhibits the expression of MuRF1 in atrophic muscle (Sandri et al., 2004). Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK) 1/2, p38 MAPK and c-Jun N-terminal kinase (JNK), play important roles in a variety of cells (Lee et al., 2007; Lee et al., 2009). MAPKs are also involved in skeletal muscle atrophy. It has been reported that p38 MAPK activity triggers the development of tumor necrosis factor α-induced, hindlimb-suspension-induced, and immobilization-induced atrophy in skeletal muscle (Hilder et al., 2003; Machida and Booth, 2005). ERK1/2 is involved in the increase in muscle mass and the diminution of MuRF expression (Shi et al., 2009). Moreover, p38 MAPK activity is regulated by Akt in a variety of cells (Yamamoto et al., 2008). It has been suggested that the induction of MAFbx and MuRF1 in atrophied muscle is mediated by the Akt and nuclear factor-κB pathways, respectively (McKinnell and Rudnicki, 2004; Nader, 2005). However, in skeletal muscle, the function of p38 MAPK during cast-immobilization-induced atrophy, and especially in MuRF1 expression, is not yet understood.
Because both MuRF1 and p38 MAPK are important mediators of skeletal muscle atrophy, we hypothesized in this study that the two molecules and their functional interaction are closely involved in the reduction of muscle mass caused by cast immobilization in the rat gastrocnemius muscle. To test this hypothesis, we investigated the effect of p38 MAPK on the expression of MuRF1 in cast-immobilized rat gastrocnemius muscles. The results were confirmed in serum-starved rat L6 skeletal myoblasts.
METHODS
Materials
Anti-p38 MAPK and anti-Akt antibodies were purchased from Cell Signaling (MA, USA). Anti-MuRF1 antibody was purchased from Santa Cruz Biotechnology (CA, USA). Anti-β-actin antibody was purchased from Sigma (MO, USA). SB203580 was purchased from Tocris Cookson Ltd (UK).
Animals and cast immobilization
Our investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All experiments and animal care also conformed to the institutional guidelines established by Konkuk University, Korea. All the animals were housed in a temperature-and humidity-controlled room under a 12-h light/dark cycle and were fed a standard commercial chow, with ad libitum access to both food and water. Male Sprague Dawley rats (190~200 g; Orient Bio, Korea) were anesthetized with an intramuscular injection of ketamine hydrochloride (35 mg/kg) mixed with xylazine hydrochloride (5 mg/kg) before the attachment of a plaster of Paris casting material (Booth and Kelso, 1973).
Measurement of morphological changes
The isolated gastrocnemius muscles were stained with hematoxylin and eosin (H&E) for general histology or incubated with the appropriate fluorescently labeled secondary antibodies (Vectastain universal elite ABC Kit, Vector Laboratories, CA, USA). The samples were mounted on glass slides and the images were captured under a confocal microscope system (FV-1000 spectral, Olympus, Japan). The muscle size was measured using Fluoview software (FV10-ASW, Olympus) and is expressed as the total muscle cell area.
Transfection of MuRF1 siRNA and measurement of cell size
Rat L6 skeletal myoblasts (1×105) from the American Type Culture Collection (MD, USA) were cultured in 60 mm tissue-culture dishes were replenished with FBS-free DMEM and then transfected with small interfering RNA (siRNA) or nonsilencing control MuRF1 siRNA to a final concentration of 500 pM siRNA using a transfection reagent (WelFect-Q™ Gold, Welgene, Korea). The relative expression levels of MuRF1 were examined using immunoblotting analysis with an antibody directed against MuRF1 and β-actin. Two siRNAs were designed to target the rat MuRF1 sequences 5'-UCUCACUCAAAGGCCUAGA-3' (siRNA 1, accession number NM_1775283; Bioneer, Korea) and 5'-CACGAAGACGAGAAAAUCA-3' (siRNA 2, accession number NM_1775284; Bioneer). The nonsilencing control siRNA was purchased from Bioneer. The morphological changes in the cells were visualized with a phase-contrast microscope (DCR-DVD803 NTSC, Carl Zeiss, Japan) and measured with Scion Image 4.03 (Scion Co., MA, USA). The results are given as the area-to-square ratio.
Immunoblotting
Immunoblotting was performed as previously described (Lee et al., 2007; Lee et al., 2009).
Data analysis
The data are expressed as means±standard errors (SE) of the means. The data were statistically evaluated with Student's t test for the comparisons of pairs of groups and with analysis of variance (ANOVA) for multiple comparisons. A p value of <0.05 was considered statistically significant.
RESULTS
Cast-immobilization-induced morphological changes in rat gastrocnemius muscle
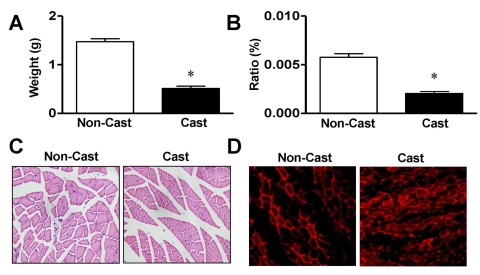
Firstly, we confirmed the effects of cast immobilization, which induces muscle atrophy. The total weight of the gastrocnemius muscle was significantly diminished in rats at day 14 after cast immobilization compared with that of noncasted control rats (0.51±0.046 g vs 1.47±0.061 g, respectively, n=8; Fig. 1A). Similar results were observed for the ratio of the weight of the gastrocnemius muscle to body weight (Fig. 1B). To confirm whether cast immobilization of the limb affects the atrophic response in the rat gastrocnemius muscle, the cross-sectional areas were compared in noncasted and casted gastrocnemius muscles using H&E staining. The cross-sectional areas of the muscle fibers from the cast-atrophic muscles were markedly less than those of the noncasted control group (426.9±4.08 vs 761.2±6.36 µm2, respectively, n=7; Fig. 1C).
Fig. 1.
Morphological characterization of gastrocnemius muscle from cast-immobilized rat hindlimb. Gastrocnemius muscle weights (A, n=8), ratios of muscle weight to body weight (B, n=8), and cross-sectional areas (C, n=7) were measured 14 days after cast immobilization. The muscle fibers were visualized with H&E staining, as described in the Materials and methods. (D) Immunohistological analysis of rats at day 14 after cast immobilization. MuRF1 expression (red) was visualized with a phasecontrast microscope (×400). Representative result of six independent experiments. *Significantly different from the noncasted control (p<0.05).
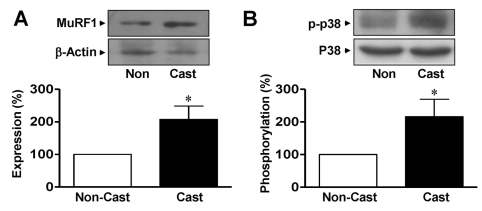
MuRF1 expression is closely related to skeletal muscle atrophy. Therefore, changes in the expression of MuRF1 were determined in casted and noncasted rat gastrocnemius muscles. In a fluorescence study, MuRF1 expression was observed in both noncasted and casted gastrocnemius muscles at day 14 but was greater in the casted group than in the noncasted group (Fig. 1D). Immunoblotting analysis showed that the expression of MuRF1 was increased in the gastrocnemius muscles from cast immobilization group compared with that in the noncasted controls (207.3±40.9% of noncasted control group, n=4; Fig. 2A). To clarify the involvement of p38 MAPK in the elevated expression of MuRF1 in response to cast immobilization , the phosphorylation of p38 MAPK was measured in the gastrocnemius muscle. As shown in Fig. 2B, the phosphorylation of p38 MAPK in the gastrocnemius muscle was significantly increased at day 14 in the hindlimb cast group compared with that in the noncasted control group (216.2±53.3% of the noncasted control, n=4). However, the cast did not influence the total expression levels of β-actin or p38 MAPK in either group (Fig. 2).
Fig. 2.
Expression of MuRF1 and p38 MAPK in gastrocnemius muscles from cast-immobilized rats. Immunoblotting analysis of MuRF1 (A) and p38 MAPK (B) in the gastrocnemius muscle. Protein expression and phosphorylation were examined using anti-nonphospho- and anti-phosphospecific antibodies, respectively. The statistical results were obtained from the upper panels. The levels of MuRF1 expression and p38 MAPK phosphorylation in the noncasted muscle group were considered to be 100% (n=4). *Significantly different from the noncasted controls (p<0.05). Non, noncasted.
Properties of L6 myoblasts during serum starvation
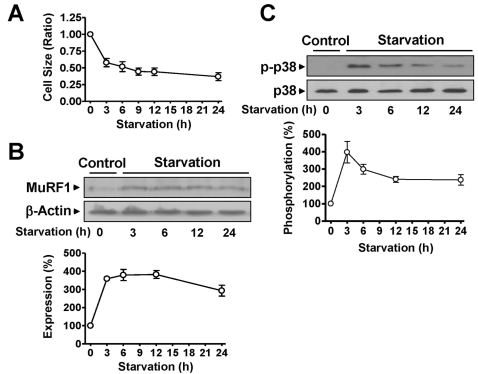
Previous studies have suggested that serum starvation can be used as a cellular model of the atrophy of skeletal muscle caused by cast immobilization. Therefore, the effects of serum starvation on cell sizes were determined. The cells gradually diminished in size during serum starvation in a time-dependent manner (Fig. 3A). The increases in MuRF1 expression observed in cast-immobilized skeletal muscles were confirmed in L6 myoblasts cultured in serum-free medium. The expression of MuRF1 was significantly increased in serum-starved L6 myoblasts (Fig. 3B). Serum starvation of cells significantly increased the phosphorylation of p38 MAPK (Fig. 3C). However, the starvation did not influence the total expression levels of p38 MAPK, β-actin or MuRF1 in either group (Fig. 3).
Fig. 3.
Changes in the characteristics of rat L6 skeletal myoblasts starved in serum-free medium. (A) Changes in the cell size. The cells were cultured in serum-free medium for the indicated times and visualized with H&E staining. The cell sizes were analyzed under a phase-contrast microscope (×100, n=8). (B) The expression of MuRF1. The cells were starved in serum-free medium for 24 h and the lysates were subjected to immunoblotting analysis with anti-MuRF1 and anti-β-actin antibodies. The statistical results were obtained from the upper panel. (C) p38 MAPK phosphorylation in cells starved in serumfree medium. The phosphorylation and expression of p38 AMPK were examined using anti-phospho and anti-nonphospho antibodies, respectively. The basal level of phosphorylation in nonstarved control cells was considered to be 100% (n=5).
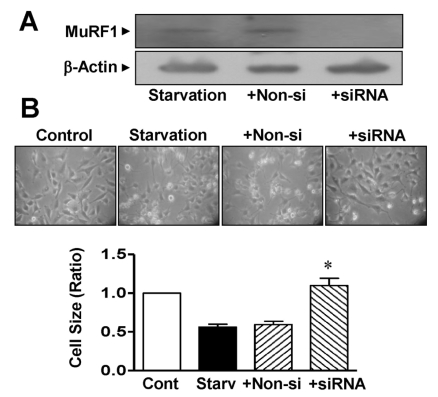
To confirm the role of MuRF1 in atrophied muscle cells, we tested the effects of MuRF1 in L6 myoblasts treated with MuRF1 siRNA. Transfection of L6 myoblasts with MuRF1 siRNA reduced the expression of MuRF1 during L6 cell starvation (to 2.8±1.3% of the nonsilenced control, n=4; Fig. 4A). The reduction in cell size caused by serum starvation was reversed in cells transfected with MuRF1 siRNA (1.1±0.09 vs 0.56±0.04 folds in the siRNA and control starvation groups, respectively, n=8; Fig. 4B). In contrast, transfection with nonsilencing siRNA caused no reduction in cell size (0.59±0.04 folds, n=8) or MuRF1 expression in L6 myoblasts (Fig. 4).
Fig. 4.
Changes in the size of L6 myoblasts knocked down with MuRF1 siRNA. (A) MuRF1 knockdown using MuRF1 siRNA. The cells were transfected with MuRF1 siRNA. Extracts from the cells were immunoblotted with anti-MuRF1 and anti-β-actin antibodies (n=4). (B) Changes in cell size caused by transfection with MuRF1 siRNA. After transfection of the cells with MuRF1 siRNA, the cell sizes were measured under a phase-contrast microscope (×100). *Significant differences between siRNA-treated cells and nonsilenced controls (n=8; p<0.05). Control, nonstarved control cells; Starv, starvation; +Non-si, nonsilencing; +siRNA, MuRF1 siRNA.
Roles of p38 MAPK in MuRF1 induction and atrophy
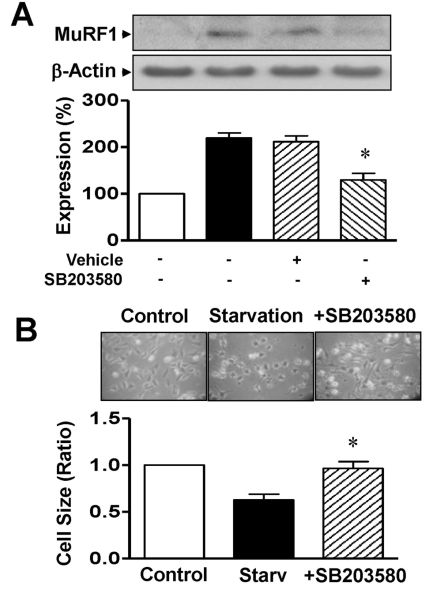
The treatment of L6 cells with 10 µM SB203580, an inhibitor of p38 MAPK, inhibited the MuRF1 expression upregulated in serum-starved L6 myoblasts (129.7±14.2% with SB203580 vs 219.7±10.7% after serum starvation, n=4 in each group; Fig. 5A). DMSO (0.1%) did not affect the expression of MuRF1. The diminished cell size caused by serum starvation was reversed in cells treated with 10 µM SB203580 (0.96±0.07 folds with SB203580 vs 0.63±0.06 folds after starvation, n=8 for each group; Fig. 5B).
Fig. 5.
Effects of p38 MAPK on MuRF1 expression and cell size in serum-starved L6 myoblasts. (A) Effect of p38 MAPK inhibitor on MuRF1 expression. Cells were starved for 12 h in serum-free medium with 0.1% DMSO or 10 µM SB203580. Cell lysates were immunoblotted with anti-MuRF1 and anti-β-actin antibodies. The statistical results were obtained from the upper panel. MuRF1 expression in nonstarved cells was considered to be 100% (n=4). (B) Effect of p38 MAPK inhibitor on cell size. The cells were starved for 12 h in serum-free medium with 0.1% DMSO or 10 µM SB203580 and the cell sizes were measured under a phase-contrast microscope (×100; n=8). *Significant differences between the starved and nonstarved control groups (p<0.05).
DISCUSSION
In this study, we have demonstrated that the cast immobilization of the rat hindlimb increased the expression of MuRF1 in the gastrocnemius muscle. MuRF1 contains a RING-domain-related ubiquitination activity at its N-terminal end, which probably acts as an E3 ligase to regulate protein degradation, and is the major factor mediating the atrophy of a variety of cells, including skeletal muscle cells (Freemont, 2000; Centner et al., 2001). Ankle joint immobilization and the injection of dexamethasone in vivo increase the expression of MuRF1 mRNA and protein in skeletal muscle (Bodine et al., 2001; Benveniste et al., 2005). Both MuRF1 and MAFbx have roles as ligases and their expression is involved in the development of skeletal muscle atrophy (Glass, 2005). MuRF1 ubiquitinates and degrades the contractile apparatus involving troponin I and titin in the M-line region of muscles (Centner et al., 2001; Kedar et al., 2004). These data indicate that cast immobilization can lead to the expression of MuRF1, which in turn facilitates the degradation of muscle proteins and muscle atrophy. In this study, we also confirmed that cast immobilization reduces the weight of the gastrocnemius muscle. It has been widely reported that skeletal muscle atrophy is related to a reduction in nutrients, including cell starvation, the depletion of amino acids, and other phenomena (Samarel et al., 1987; Medina et al., 1995; Thomas, 2007). It has also been reported that the expression of MAFbx and MuRF1 is increased in cardiac cells cultured in serum-free medium (Schulze et al., 2005; Skurk, et al., 2005). Moreover, mouse fasting leads to the expression of proteins involved in protein degradation (e.g., MuRF1) in the gastrocnemius muscle (Lecker et al., 2004). In this study, we demonstrated for the first time that the increased expression of MuRF1 and the phosphorylation of p38 MAPK caused in cells by starvation are similar in pattern to the changes observed in gastrocnemius muscles immobilized by casts. In this study, we also found that the size of L6 myoblasts decreased significantly in serum-free medium, but this reduction was reversed in cells transfected with MuRF1 siRNA. These results show that the changes in cells starved in serum-free medium mimic the morphological and functional characteristics typical of gastrocnemius muscle treated with cast immobilization. These results therefore imply that the starvation of L6 cells in serum-free medium reflects the atrophy caused by cast immobilization via a similar pathway and should be useful in the analysis of the mechanism of atrophy in the gastrocnemius muscle at the cellular level.
It has been reported that muscle atrophy is regulated by both the Akt and p38 MAPK pathways and that the induction of MuRF1 induced by atrophy is regulated by the inhibition of Akt activation (Machida and Booth, 2005). Moreover, the upregulation of MAFbx and MuRF1 expression in atrophied muscles is mediated by p38 MAPK and nuclear factor-κB, respectively. Nuclear factor-κB is a ubiquitous transcription factor involved in a wide variety of inflammatory and pathological processes (Zhou et al., 2001; Cai et al., 2004). It has been widely reported that the p38 MAPK and JNK pathways are responsive to stress, such as ischemic injury, osmotic shock, ionizing radiation, cytokines, hormones, growth factors, and other stressors (Chang and Karin, 2001; Kyriakis and Avruch, 2001). However, the regulation of MuRF1 expression by the p38 MAPK pathway in response to skeletal muscle atrophy had not been demonstrated. In this study, we observed increased p38 MAPK activity in both the cast immobilization of the gastrocnemius muscle and serum-starved L6 myoblasts, and a p38 MAPK inhibitor abolished this increase in MuRF1 in response to serum starvation in L6 myoblasts. These results strongly suggest that the expression of MuRF1 in casted gastrocnemius muscle is mediated by the activation of p38 MAPK. Although it has been reported that an inhibitor of ERK1/2 reduced the muscle mass and elicited MuRF expression and skeletal muscle atrophy (Shi et al., 2009), the present results demonstrate for the first time that the expression of MuRF1 in atrophic muscle is regulated by p38 MAPK. Our findings suggest that the regulator p38 MAPK may act in preventing skeletal muscle disorders, including muscle atrophy, which should constitute useful information in the development of therapeutic strategies.
In conclusion, the increase in MuRF1 expression in serum-starved L6 myoblasts was significantly attenuated by the inhibition of p38 MAPK. Moreover, the size changes in L6 myoblasts induced by serum starvation were reversed in cells transfected with MuRF1 siRNA or treated with a p38 MAPK inhibitor. From these results, we infer that the MuRF1-mediated atrophy observed in cast-immobilized rat gastrocnemius muscles is regulated by p38 MAPK.
ACKNOWLEDGEMENTS
We thank Zhi Fang for technical assistant. This work was supported by a grant from the Korea Research Foundation funded by the Korea Government (MOEHRD; KRF-2006-331-E00275), Bio Food and Drug Research Center, the Regional Research Universities Program/Chungbuk BIT Research-Oriented University Consortium, and Specific Joint Agricultural Research promoting Projects (No. 20070101-033159) of RDA, Republic of Korea.
ABBREVIATIONS
- Akt
phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B
- ERK1/2
extracellular signal-regulated kinase 1/2
- JNK
c-Jun N-terminal kinase
- MAFbx
atrogin-1/muscle atrophy F-box
- MAPK
mitogen-activated protein kinase
- MuRF1
muscle-specific RING finger 1
- siRNA
small interfering RNA
References
- 1.Benveniste O, Jacobson L, Farrugia ME, Clover L, Vincent A. MuSK antibody positive myasthenia gravis plasma modifies MuRF-1 expression in C2C12 cultures and mouse muscle in vivo. J Neuroimmunol. 2005;170:41–48. doi: 10.1016/j.jneuroim.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Kelso JR. Production of rat muscle atrophy by cast fixation. J Appl Physiol. 1973;34:404–406. doi: 10.1152/jappl.1973.34.3.404. [DOI] [PubMed] [Google Scholar]
- 4.Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- 5.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Karin K. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 8.Freemont PS. RING for destruction? Curr Biol. 2000;10:R84–R87. doi: 10.1016/s0960-9822(00)00287-6. [DOI] [PubMed] [Google Scholar]
- 9.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilder TL, Tou JC, Grindeland RE, Wade CE, Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Lett. 2003;553:63–67. doi: 10.1016/s0014-5793(03)00972-4. [DOI] [PubMed] [Google Scholar]
- 12.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy. Curr Opin Clin Nutr Metab Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 15.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 16.Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009;104:219–227. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 17.Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 2007;105:74–81. doi: 10.1254/jphs.fp0070770. [DOI] [PubMed] [Google Scholar]
- 18.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machida S, Booth FW. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol Scand. 2005;183:171–179. doi: 10.1111/j.1365-201X.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 20.McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 2002;157:125–136. doi: 10.1083/jcb.200108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Medina R, Wing SS, Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995;307:631–637. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nader GA. Molecular determinants of skeletal muscle mass: getting the "AKT" together. Int J Biochem Cell Biol. 2005;37:1985–1996. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Samarel AM, Parmacek MS, Magid NM, Decker RS, Lesch M. Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Circ Res. 1987;60:933–941. doi: 10.1161/01.res.60.6.933. [DOI] [PubMed] [Google Scholar]
- 25.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, Lee RT, Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005;97:418–426. doi: 10.1161/01.RES.0000179580.72375.c2. [DOI] [PubMed] [Google Scholar]
- 27.Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009;296:C1040–C1048. doi: 10.1152/ajpcell.00475.2008. [DOI] [PubMed] [Google Scholar]
- 28.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugita H, Kaneki M, Sugita M, Yasukawa T, Yasuhara S, Martyn JA. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E585–E591. doi: 10.1152/ajpendo.00321.2004. [DOI] [PubMed] [Google Scholar]
- 30.Tesseraud S, Métayer-Coustard S, Boussaid S, Crochet S, Audouin E, Derouet M, Seiliez I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochem Biophys Res Commun. 2007;357:181–186. doi: 10.1016/j.bbrc.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Tobimatsu K, Noguchi T, Hosooka T, Sakai M, Inagaki K, Matsuki Y, Hiramatsu R, Kasuga M. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun. 2009;378:399–403. doi: 10.1016/j.bbrc.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 33.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 2003;35:698–705. doi: 10.1016/s1357-2725(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y, Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. doi: 10.1093/cvr/cvn076. [DOI] [PubMed] [Google Scholar]
- 35.Zhou LZ, Johnson AP, Rando TA. NFκB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]