Abstract
By using an immunological and peptide mapping approach two calcium-dependent cell-cell adhesion molecules (calCAMs) in the embryonic chicken are compared. A third closely related molecule is identified and compared to the two calCAMs. One of the calCAMs appears to be identical to the previously identified adhesion molecule N-cadherin, originally identified in chicken retina and localized to neural tissues. The second is the same as L-CAM, originally identified in chicken liver but localized to a variety of epithelial tissues. The third, also found in liver, is similar to L-CAM but is much closer in structure to N-cadherin. It is, however, immunologically distinct from N-cadherin. We therefore refer to this newly identified molecule as CRM-L for cadherin-related molecule in liver. CRM-L, N-cadherin, and L-CAM are all cell-surface proteins with a similar stability to tryptic digestion in the presence of calcium. CRM-L has the same molecular mass and isoelectric point as N-cadherin but is distinct from L-CAM in these properties. Two-dimensional peptide maps of complete tryptic digests reveal that CRM-L shares 69% of its peptides with N-cadherin and 20% with L-CAM. On the basis of these data, we suggest that there are at least two distinguishable types of calCAMs in the chicken embryo: one represented by the closely related molecules N-cadherin and CRM-L, and another represented by L-CAM.
Full text
PDF
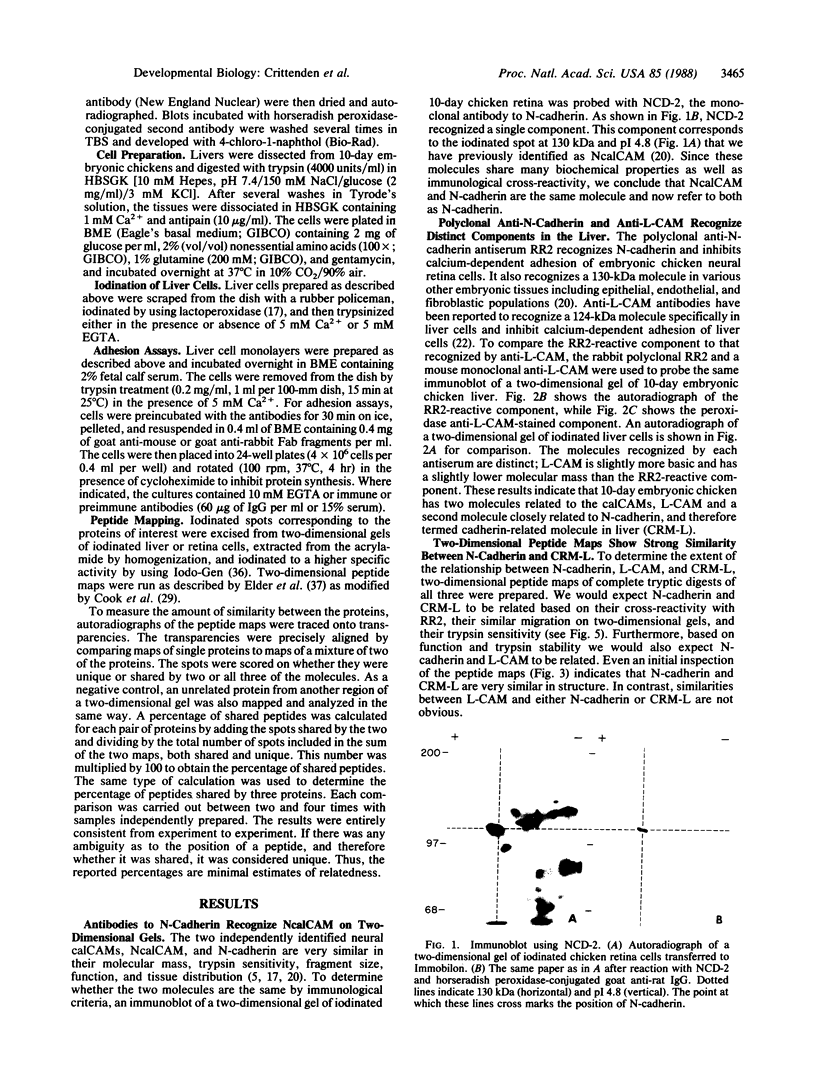
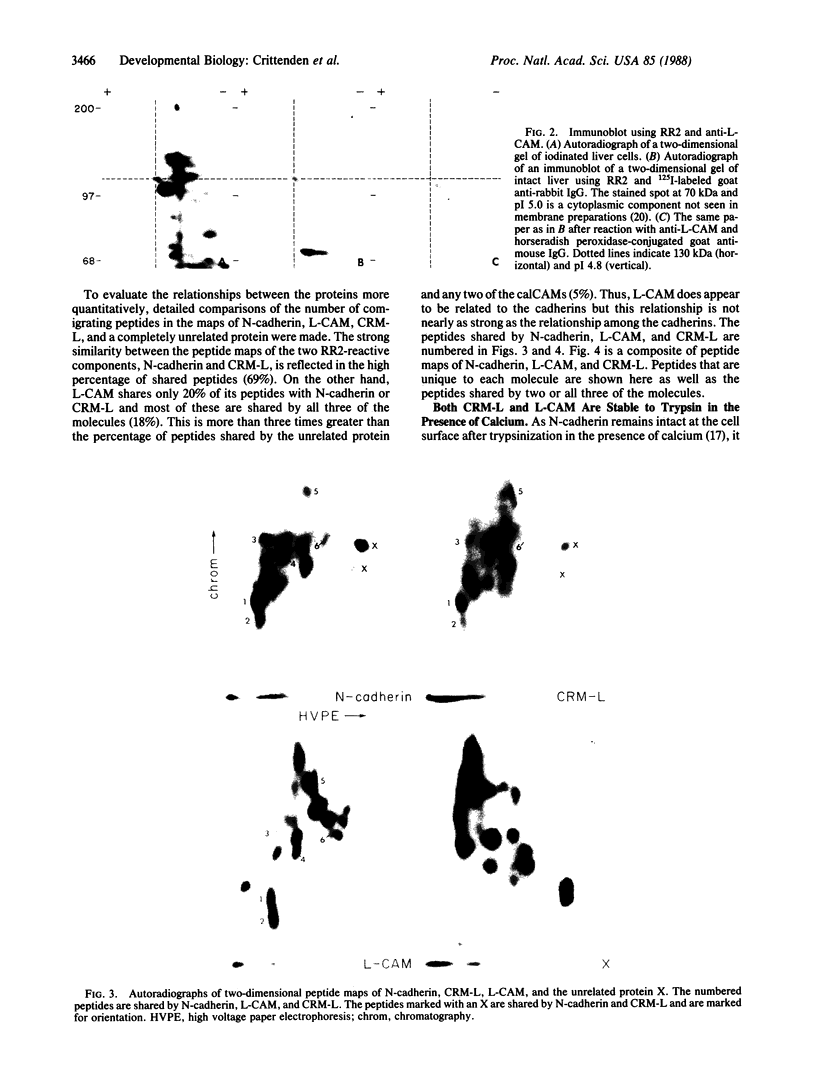

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolotti R., Rutishauser U., Edelman G. M. A cell surface molecule involved in aggregation of embryonic liver cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4831–4835. doi: 10.1073/pnas.77.8.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Pratt R. S., Lilien J., Reichardt L. F. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca2+-dependent and -independent cell adhesion molecules. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. H., Lilien J. The accessibility of certain proteins on embryonic chick neural retina cells to iodination and tryptic removal is altered by calcium. J Cell Sci. 1982 Jun;55:85–103. doi: 10.1242/jcs.55.1.85. [DOI] [PubMed] [Google Scholar]
- Cook J. H., Pratt R. S., Lilien J. Turnover and orientation of the major neural retina cell surface protein protected from tryptic cleavage by calcium. Biochemistry. 1984 Feb 28;23(5):899–904. doi: 10.1021/bi00300a016. [DOI] [PubMed] [Google Scholar]
- Crittenden S. L., Pratt R. S., Cook J. H., Balsamo J., Lilien J. Immunologically unique and common domains within a family of proteins related to the retina Ca2+-dependent cell adhesion molecule, NcalCAM. Development. 1987 Dec;101(4):729–740. doi: 10.1242/dev.101.4.729. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Leutzinger Y., Gallin W. J., Sorkin B. C., Edelman G. M. Linear organization of the liver cell adhesion molecule L-CAM. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5787–5791. doi: 10.1073/pnas.81.18.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Richa J., Solter D., Knudsen K., Buck C. A. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Dufour S., Hatta K., Takeichi M., Edelman G. M., Thiery J. P. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987 May;104(5):1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Chuong C. M., Finkel L. H., Edelman G. M. Antibodies to liver cell adhesion molecule perturb inductive interactions and alter feather pattern and structure. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8235–8239. doi: 10.1073/pnas.83.21.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Prediger E. A., Edelman G. M., Cunningham B. A. Isolation of a cDNA clone for the liver cell adhesion molecule (L-CAM). Proc Natl Acad Sci U S A. 1985 May;82(9):2809–2813. doi: 10.1073/pnas.82.9.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller R. L., Lilien J. Repair of a calcium-dependent adhesive mechanism of embryonic neural retina cells: kinetic and molecular analysis. J Cell Sci. 1983 Mar;60:29–49. doi: 10.1242/jcs.60.1.29. [DOI] [PubMed] [Google Scholar]
- Gros D. B., Nicholson B. J., Revel J. P. Comparative analysis of the gap junction protein from rat heart and liver: is there a tissue specificity of gap junctions? Cell. 1983 Dec;35(2 Pt 1):539–549. doi: 10.1016/0092-8674(83)90188-5. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B., Geller R. L., Lilien J. Enzymatic dissection of embryonic cell adhesive mechanisms. J Cell Biol. 1980 Jun;85(3):766–776. doi: 10.1083/jcb.85.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald G. B., Pratt R. S., Lilien J. Enzymic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retina cells. J Cell Sci. 1982 Jun;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Okada T. S., Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985 May;82(9):2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987 Mar;120(1):215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986 Apr 3;320(6061):447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Gros D. B., Kent S. B., Hood L. E., Revel J. P. The Mr 28,000 gap junction proteins from rat heart and liver are different but related. J Biol Chem. 1985 Jun 10;260(11):6514–6517. [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987 Dec 1;6(12):3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogou S. I., Yoshida-Noro C., Takeichi M. Calcium-dependent cell-cell adhesion molecules common to hepatocytes and teratocarcinoma stem cells. J Cell Biol. 1983 Sep;97(3):944–948. doi: 10.1083/jcb.97.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Hatta K., Hosoda M., Tsunasawa S., Sakiyama F., Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986 Oct;5(10):2485–2488. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Okada T. S., Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983 Dec;35(3 Pt 2):631–638. doi: 10.1016/0092-8674(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Delouvée A., Gallin W. J., Cunningham B. A., Edelman G. M. Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev Biol. 1984 Mar;102(1):61–78. doi: 10.1016/0012-1606(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R., Ekblom P. Cell-adhesion molecule uvomorulin during kidney development. Dev Biol. 1985 Nov;112(1):213–221. doi: 10.1016/0012-1606(85)90135-6. [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C., Suzuki N., Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984 Jan;101(1):19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]