Abstract
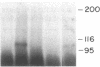
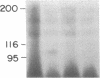
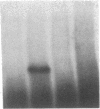
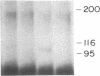
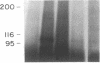
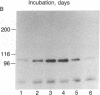
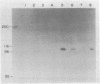
The precise mechanism by which insulin is degraded in mammalian cells is not presently known. Several lines of evidence suggest that degradation is initiated by a specific nonlysosomal insulin-degrading enzyme (IDE). The potential importance of this insulin protease is illustrated by the fact that there is an IDE in Drosophila melanogaster Kc cells that shares both physical and kinetic properties with its mammalian counterpart. We now demonstrate that the IDE is present in other Drosophila cell lines and in the embryo, the larvae, the pupae, and adult tissues of the fruit fly. Further, the level of the IDE is developmentally regulated, being barely detectable in the embryo but elevated approximately 5-fold in the larvae and pupae and approximately 10-fold in the adult fly. The IDE levels in the cell lines are particularly high, at least 10-fold greater than in the adult fly. Analysis of Schneider L3 cells indicates that the addition of the Drosophila hormone ecdysone, which induces differentiation of the cells, causes a small but reproducible increase in the level of the IDE and the insulin-degrading activity. These results demonstrate that the IDE is evolutionarily conserved and that its expression is tightly regulated during differentiation of Drosophila. The particular pattern of developmental regulation suggests that the IDE plays a specific and critical role in the later stages of the life cycle of the fly.
Full text
PDF
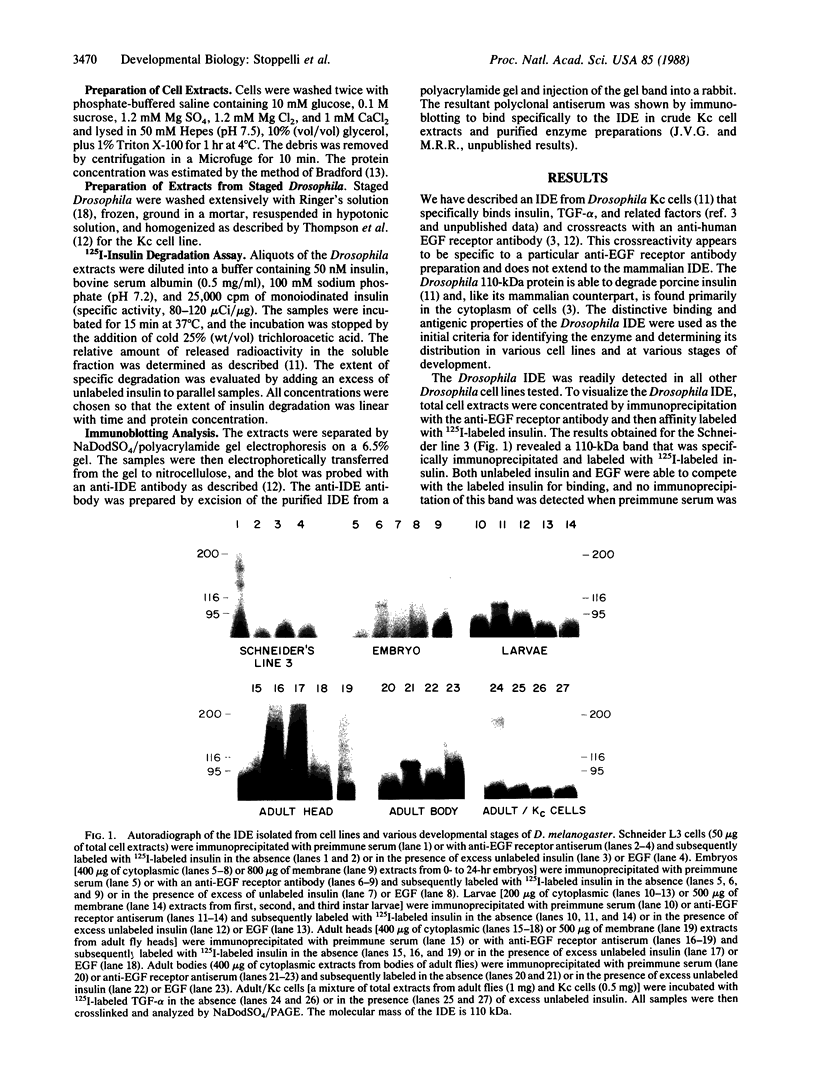
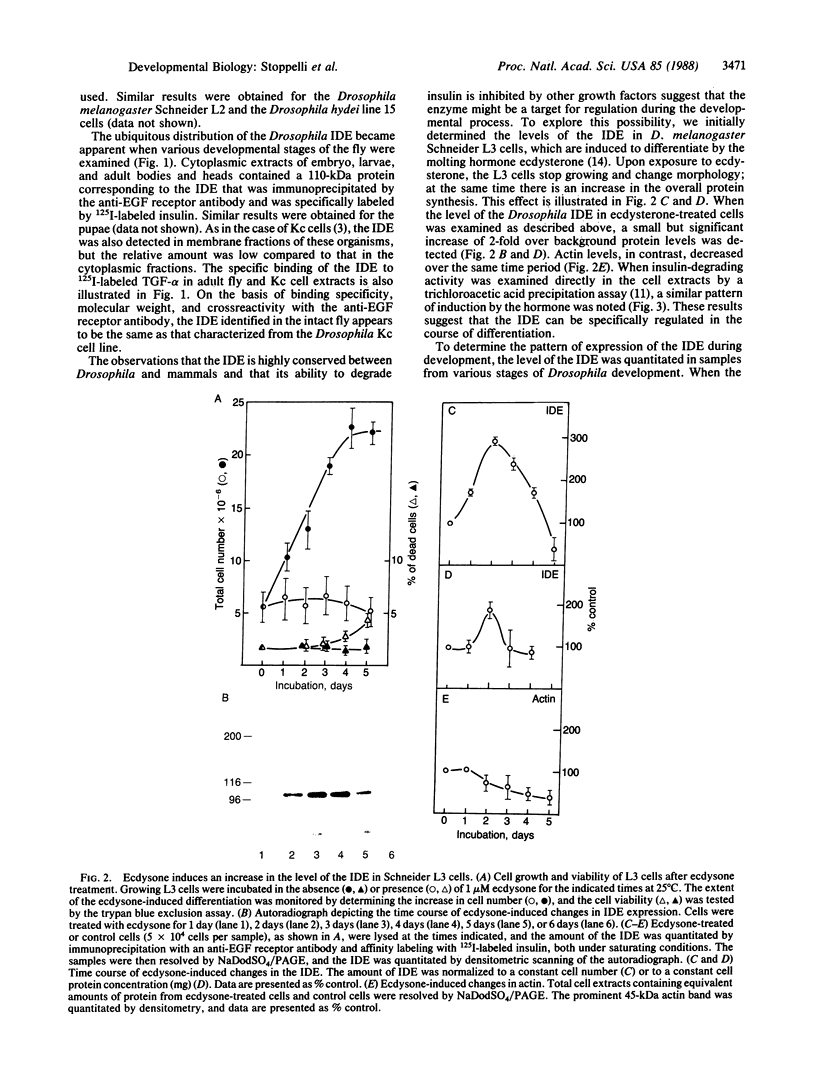
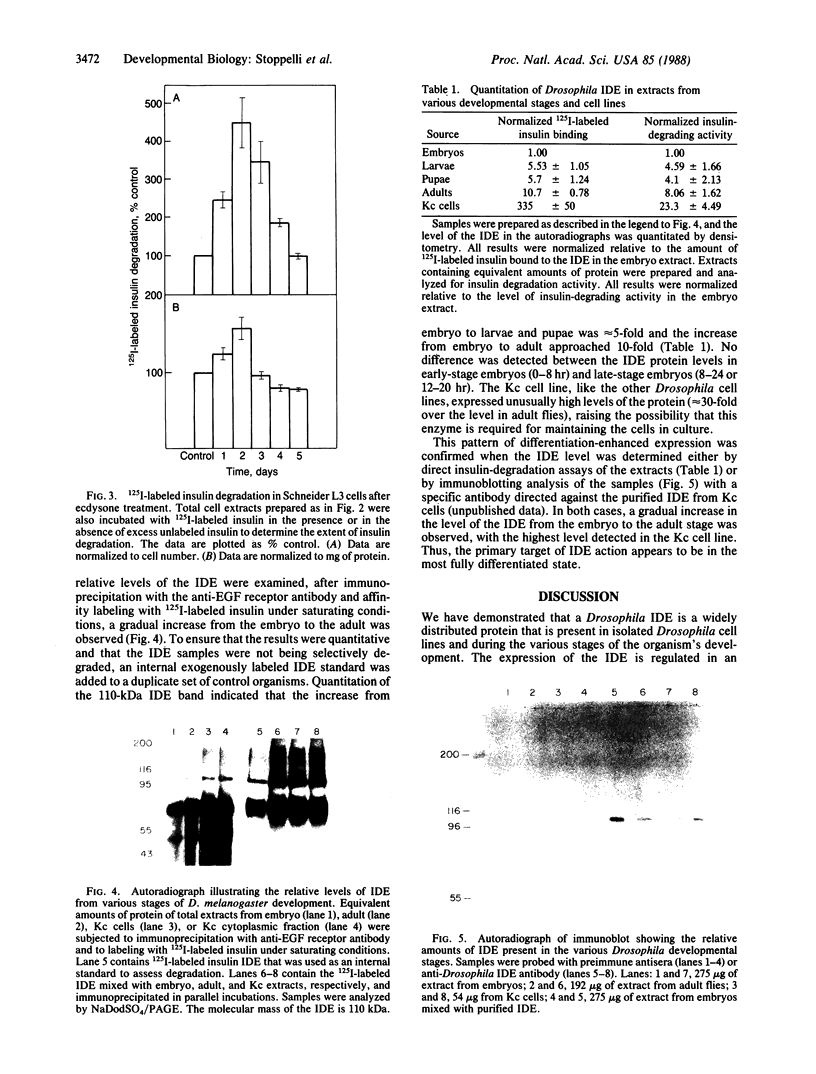

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Courgeon A. M. Action of insect hormones at the cellular level. Morphological changes of a diploid cell line of Drosophila melanogaster, treated with ecdysone and several analogues in vitro. Exp Cell Res. 1972 Oct;74(2):327–336. doi: 10.1016/0014-4827(72)90384-9. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Kitabchi A. E. Insulin metabolism and degradation. Endocr Rev. 1981 Spring;2(2):210–233. doi: 10.1210/edrv-2-2-210. [DOI] [PubMed] [Google Scholar]
- Fernandez-Almonacid R., Rosen O. M. Structure and ligand specificity of the Drosophila melanogaster insulin receptor. Mol Cell Biol. 1987 Aug;7(8):2718–2727. doi: 10.1128/mcb.7.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. V., Stoppelli M. P., Thompson K. L., Decker S. J., Rosner M. R. Characterization of a Drosophila protein that binds both epidermal growth factor and insulin-related growth factors. J Cell Biol. 1987 Jul;105(1):449–456. doi: 10.1083/jcb.105.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel F. G., Peavy D. E., Ryan M. P., Duckworth W. C. High performance liquid chromatographic analysis of insulin degradation by rat skeletal muscle insulin protease. Endocrinology. 1986 Jan;118(1):328–333. doi: 10.1210/endo-118-1-328. [DOI] [PubMed] [Google Scholar]
- Hammons G. T., Jarett L. Lysosomal degradation of receptor-bound 125I-labeled insulin by rat adipocytes: its characterization and dissociation from the short-term biologic effects of insulin. Diabetes. 1980 Jun;29(6):475–486. doi: 10.2337/diab.29.6.475. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Lane M. D. Evidence for different pathways for the degradation of insulin and insulin receptor in the chick liver cell. J Biol Chem. 1982 Feb 10;257(3):1372–1377. [PubMed] [Google Scholar]
- LeRoith D., Lesniak M. A., Roth J. Insulin in insects and annelids. Diabetes. 1981 Jan;30(1):70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- Meneses P., De Los Angeles Ortíz M. A protein extract from Drosophila melanogaster with insulin-like activity. Comp Biochem Physiol A Comp Physiol. 1975 Jun 1;51(2):483–485. doi: 10.1016/0300-9629(75)90398-9. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Garcia-Arenas R., Rosen O. M. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. J Biol Chem. 1985 Dec 25;260(30):16072–16075. [PubMed] [Google Scholar]
- Shii K., Roth R. A. Inhibition of insulin degradation by hepatoma cells after microinjection of monoclonal antibodies to a specific cytosolic protease. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4147–4151. doi: 10.1073/pnas.83.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shii K., Yokono K., Baba S., Roth R. A. Purification and characterization of insulin-degrading enzyme from human erythrocytes. Diabetes. 1986 Jun;35(6):675–683. doi: 10.2337/diab.35.6.675. [DOI] [PubMed] [Google Scholar]
- Thompson K. L., Decker S. J., Rosner M. R. Identification of a novel receptor in Drosophila for both epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8443–8447. doi: 10.1073/pnas.82.24.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]