Abstract
This study prospectively examines the influence of alcohol on neuropsychological functioning in a sample of boys and girls who were characterized prior to initiating drinking (N=76, ages 12–14). Adolescents who transitioned into heavy (n= 25; 11 female, 14 male) or moderate (n=11; 2 female, 9 male) drinking were compared to demographically-matched controls who remained non-users throughout the approximately 3-year follow-up period (n=40; 16 female, 24 male). For girls, more drinking days in the past year predicted a greater reduction in performance on visuospatial tasks from baseline to follow-up, above and beyond performance on equivalent measures at baseline (R2Δ = 10%, p <. 05), particularly on tests of visuospatial memory (R2Δ = 8%, p < .05). For boys, a tendency was seen for more hangover symptoms in the year before follow-up testing to predict a relative worsening of sustained attention (R2Δ = 7%, p < .05). These preliminary longitudinal findings suggest that initiating moderately heavy alcohol use and incurring hangover during adolescence may adversely influence neurocognitive functioning. Neurocognitive deficits linked to heavy drinking during this critical developmental period may lead to direct and indirect changes in neuromaturational course, with effects that would extend into adulthood.
Keywords: Adolescence, alcohol, hangover, neuropsychological assessment, neurocognitive functioning, visuospatial functioning, attention
INTRODUCTION
Adolescence marks a time of significant increases in alcohol use, with 31% of boys and 22% of girls in the 12th grade endorsing heavy episodic drinking in the past two weeks (Johnston, O’Malley, Bachman, & Schulenberg, 2008). These high rates are concerning in regards to potential deleterious effects of alcohol on adolescent brain development (Brown et al., 2008; Squeglia, Jacobus, & Tapert, 2009 for reviews). The adolescent brain incurs rapid anatomical and neurochemical changes (Giedd, 2004; Sowell et al., 2004), including cortical remodeling and hormonal alterations (Crews, He, & Hodge, 2007), with females undergoing these changes 1–2 years before males on average (Giedd et al., 2006). These changes may heighten adolescents’ vulnerability to the neural effects of alcohol (Clark & Tapert, 2008; Dahl, 2004), impeding key processes of cognitive development (Chanraud et al., 2007).
As most studies have been cross-sectional, the effects of adolescent alcohol use on neurocognitive development remain inconclusive. Cross-sectional studies have found disadvantaged neurocognition among adolescents with alcohol use disorders (AUD), including poorer retrieval (Brown, Tapert, Granholm, & Delis, 2000), attention and information processing (Tapert & Brown, 2000; Tarter, Mezzich, Hsieh, & Parks, 1995), visuospatial (Sher, Martin, Wood, & Rutledge, 1997; Tapert & Brown, 1999), and language (Moss, Kirisci, Gordon, & Tarter, 1994) functioning than non-drinkers. Executive functioning deficits have been found in female substance-using teens (Giancola, Shoal, & Mezzich, 2001; Moss et al., 1994). Longitudinal studies found deteriorations in visuospatial functioning and attention in treated teens who continued heavy drinking and reported hangover or withdrawal (Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002).
Post-drinking symptoms (e.g., hangover and withdrawal) indicate relatively intense alcohol use levels, have been linked to brain changes (Glenn, Parsons, Sinha, & Stevens, 1988; Parsons & Stevens, 1986), and are not dependent on quantity, which is difficult to compare across genders. In humans, poorer visuospatial performance, attention (Brown et al., 2000; Tapert et al., 2002), and memory (Tapert et al., 2001), and greater abnormalities in brain response (Tapert et al., 2004) have been seen in adolescents who endorsed more alcohol withdrawal symptoms. Thus, the effects of alcohol withdrawal on adolescent cognition may be more detrimental than the actual quantity of alcohol consumed, or may more accurately mark the extent of a heavy drinking episode than recollections of alcohol quantity. While severe withdrawal is uncommon in adolescents (Martin, Chung, Kirisci, & Langenbucher, 2006), hangover symptoms may index post-drinking effects that could influence neurocognition. Hangovers have been described along a continuum of post-drinking effects that range from withdrawal to mild symptoms (Swift & Davidson, 1998; Wiese, Shlipak, & Browner, 2000). Males tend to report hangover symptoms more commonly than females (Deshmukh et al., 2003), but occurrence in females is more strongly related to heavy drinking (Piasecki, Sher, Slutske, & Jackson, 2005). The long-term effects associated with this milder form of withdrawal have not been examined longitudinally in adolescents first assessed prior to the initiation of substance use.
This study prospectively examined neurocognition in adolescents first assessed prior to the onset of any heavy drinking, some of who transitioned into moderately heavy alcohol use during the follow-up. Based on prior work (Brown et al., 2000; Tapert & Brown, 1999, 2000; Tapert et al., 2002), greater alcohol use and hangover over the follow-up were hypothesized to predict worsening scores on tests requiring visuospatial processing, sustained attention, learning and memory, working memory, and planning. Due to the differential influence of alcohol on cognition by sex (Nixon, 1994; Pfefferbaum, Adalsteinsson, & Sullivan, 2006; Sullivan, Fama, Rosenbloom, & Pfefferbaum, 2002), females and males were evaluated separately. It was hypothesized that female drinkers would show worsened visuospatial performance (Tapert et al., 2001) and working memory (Flannery et al., 2007; Sullivan et al., 2002), while male drinkers would show relative decline on sustained attention and planning tasks (Fama, Pfefferbaum, & Sullivan, 2004).
METHODS
Participants
Participants were recruited as part of an ongoing longitudinal neuroimaging study. Flyers were mailed to households of local middle schools describing the project, major inclusion criteria, and compensation. Exclusionary criteria were prenatal alcohol (>2 drinks in a week) or any tobacco or illicit drug use; history of chronic medical illness, any neurological or DSM-IV (APA, 1994) Axis I disorder other than conduct disorder, loss of consciousness (>2 minutes), or learning disabilities; parental history of bipolar, psychotic, or antisocial personality disorder; sensory problems; and left handedness. In all, 13% of respondents met eligibility criteria. Extensive screening and background data were obtained from each participant, one biological parent, and one other parent or close relative. At baseline, this study included 76 participants, ages 12–14 years, who had minimal substance use (≤6 total lifetime drinks with ≤3 drinks on any occasion; ≤3 lifetime uses of marijuana (>0 for n=5) and none in the past three months; ≤10 lifetime cigarette uses (>0 for n=5); and no history of other intoxicant use). The current study included adolescents who transitioned into heavy (n= 25; 11 females) or moderate (n=11; 2 females) drinking, and those who remained non-users through follow-up (n=40; 16 females; see Table 1). Because this study is ongoing, different follow-up time points were examined, ranging from one to five years (mean=3.6).
Table 1.
Descriptive characteristics of adolescents who transitioned into moderate or heavy drinking versus those who did not, at baseline and follow-up
| Females (n=29) M (SD) |
Males (n=47) M (SD) |
|||
|---|---|---|---|---|
| Controls n=16 |
Drinkers n=13 |
Controls n=24 |
Drinkers n=23 |
|
| Age at baseline (range: 12-14) | 13.44 (0.66) |
13.88 (0.76) |
13.52 (0.82) |
13.76 (0.76) |
| Caucasian (%)* | 75% | 85% | 58% | 91% |
| Hollingshead socioeconomic status | 20.38 (11.70) |
22.15 (9.66) |
25.96 (15.75) |
21.83 (11.98) |
| Wide Range Achievement Test-3 Reading standard score |
108.75 (9.50) |
108.85 (9.92) |
108.99 (11.61) |
108.09 (8.56) |
| Family history density of alcohol use disorder (range 0 – 1.5) a e |
0.11 (0.22) |
0.46 (0.51) |
0.18 (0.31) |
0.33 (0.35) |
| Conduct Disorder Questionnaire total score a b c d e | 0.56 (0.89) |
2.15 (2.30) |
1.88 (1.60) |
3.35 (2.35) |
| Conduct disorder diagnosis (%)a d | 0% | 15% | 0% | 39% |
| Lifetime drinking days a d e | 0.00 (0.00) |
1.15 (2.12) |
0.00 (0.00) |
0.83 (1.47) |
| Lifetime marijuana use days a d | 0.00 (0.00) |
0.08 (0.28) |
0.00 (0.00) |
0.39 (0.94) |
| Years until follow-up d | 2.69 (1.08) |
3.54 (1.27) |
2.42 (1.35) |
3.57 (1.08) |
| Number of follow-up assessments a | 1.50 (0.97) |
2.31 (1.18) |
1.54 (0.93) |
1.96 (0.98) |
| Lifetime drinking days a d e | 0.00 (0.00) |
87.92 (87.68) |
1.42 (3.36) |
50.57 (52.22) |
| Average drinks per month, past 3 months a d e | 0.00 (0.00) |
9.85 (10.16) |
0.54 (1.91) |
6.09 (10.38) |
| Past month total drinks a d e | 0.00 (0.00) |
4.46 (7.66) |
0.00 (0.00) |
11.57 (16.92) |
| Lifetime marijuana use days a d | 0.00 (0.00) |
77.69 (184.51) |
1.00 (2.72) |
65.26 (134.42) |
| Lifetime other drug use days d | 0.00 (0.00) |
12.92 (45.40) |
0.00 (0.00) |
2.09 (4.66) |
| Past month cigarette use a | 0.00 (0.00) |
3.31 (7.15) |
0.08 (0.41) |
3.39 (8.63) |
Drinkers ≠ Controls, p<.05
Males ≠ Females, p<.05
Male Controls ≠ Female Controls, p<.05
Male Drinkers ≠ Male Controls, p<.05
Female Drinkers ≠ Female Controls, p<.05
For the full sample, ethnicity was: 15% Hispanic or Latino and 85% Not Hispanic or Latino; race was: 73% Caucasian, 15% multiracial, 5% Asian, and 7% other.
Note: Among drinkers, moderate to heavy drinking was initiated 2.3 years (SD=1.3) after baseline, on average. Drinkers had consumed alcohol at moderate or heavy levels for an average of 2.8 years since initiation (SD=1.3).
Measures
Substance use
The Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) was administered at baseline for self-reported lifetime alcohol, tobacco, and other drug use, and withdrawal/hangover symptoms. The version at annual follow-ups assessed past year and past 3-month use. Breathalyzer and urine screens and parent reports on youth use verified self-report data. The Hangover Symptoms Scale (HSS; Slutske, Thomas M. Piasecki, & Hunt-Carter, 2003) given at each follow-up provided past-year severity ratings on 13 hangover symptoms. HSS scores ranged from 13–36, from a possible range of 13–65, and were internally consistent (Cronbach’s alpha=. 90). Alcohol related blackouts were not examined because of low endorsement (8%).
Neuropsychological (NP) battery
The baseline NP battery lasted ~3 hours and included domains found compromised in prior studies of adolescent alcohol use (Brown et al., 2000; Tapert & Brown, 1999, 2000; Tapert et al., 2002). Measures of visuospatial processing were Complex Figure copy and 30-minute delay accuracy (Rey & Osterrieth, 1993); and Wechsler Abbreviated Scale of Intelligence (WASI) Block Design (Wechsler, 1999). Measures of sustained attention, speeded information processing, and working memory included the Digit Vigilance Test (DVT; Lewis, 1995); Delis Kaplan Executive Function System (D-KEFS) Trail Making (Delis, Kaplan, & Kramer, 2001); and Wechsler Intelligence Scale for Children-III (WISC-III) Digit Span and Coding (Wechsler, 1991). Learning and memory scores were obtained from the California Verbal Learning Test—Children’s Version (CVLT-C; Delis, Kramer, Kaplan, & Ober, 1994); and executive functioning/planning were D-KEFS Color-Word Interference and Towers (Delis et al., 2001). Premorbid functioning and intellectual capacity were estimated from WASI Vocabulary and Similarities and Wide Range Achievement Test-3 Reading scores (WRAT-3; Wilkinson, 1993). The Taylor scoring system (Taylor, 1998) was used for Complex Figures, each independently scored by two trained, reliable raters (intraclass correlation coefficient=.94), then reviewed in consensus meetings. At each follow-up, the DVT target number was alternated, and the Rey-Osterrieth Complex Figure was replaced by a novel figure (Loring & Meador, 2003; Meador et al., 1993; Taylor, 1969) to minimize practice effects. At the 4-year follow-up, WISC-III subtests were replaced with corresponding Wechsler Adult Intelligence Scale-III (Wechsler, 1997) subtests, and the CVLT-II (Delis, Kramer, Kaplan, & Ober, 2000) replaced the CVLT-C.
Covariates
The Family History Assessment Module (FHAM; Rice et al., 1995), administered to youth and parent, ascertained familial density of AUD by adding 0.5 per biological parent and 0.25 per biological grandparent (Zucker, Ellis, & Fitzgerald, 1994) endorsed by either youth or parent. This measure has been shown to be valid and more sensitive than classifications (Stoltenberg, Mudd, Blow, & Hill, 1998). The Conduct Disorder Questionnaire (CDQ; Brown, Gleghorn, Schuckit, Myers, & Mott, 1996), administered to the youth and parent, provided a continuous measure of CD behaviors based on DSM-IV criteria (APA, 1994), with one point added for each item endorsed by either youth or parent (possible range: 0–27; Myers, Stewart, & Brown, 1998). Family socioeconomic status (SES) considered the educational attainment and occupation of each parent (Hollingshead, 1965).
Procedures
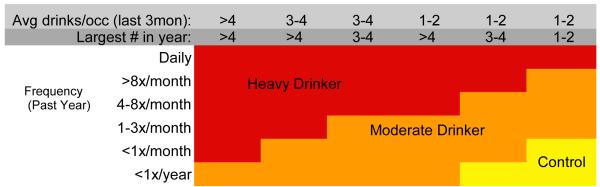
At baseline, screening interviews were administered to youth and parents by trained lab assistants to assess eligibility. Eligible youth and parents independently completed interviews, and participants were administered the standardized NP battery by a trained psychometrist. Participants were followed annually using rigorous follow-up procedures (Twitchell, Hertzog, Klein, & Schuckit, 1992), and contacted by phone to complete questionnaires on current substance use. Those who fit criteria at follow-up (see Figure 1) for heavy or moderate drinking were invited to return and complete assessments, then were matched on gender, familial AUD category (negative, mild, positive), baseline age, and age at follow-up to continuous non-users. Adolescents who were non-drinkers but initiated other drug use were excluded from analyses. Follow-up rates are >95% at each time point. Consent and assent were obtained each year. Study protocol was executed in accordance with standards approved by the University of California, San Diego Human Research Protections Program.
Figure 1.
Outcome drinking classification, based on Cahalan et al., 1969 and modified based on the distribution of drinking characteristics of adolescent males and females observed in the first two years of this project (Schweinsburg et al., 2005; Tapert et al., 2004).
Data Analyses
All substance involvement variables were positively skewed, so logarithmic transformations were applied (Tabachnick & Fidell, 2007). All NP measures were normally distributed and free from outliers. Z-scores for each NP measure at each time point were computed based on scores (raw, unless otherwise noted) of all participants tested at that time point. Change scores were created by subtracting each subject’s baseline z-score from their most recent follow-up z-score for the corresponding NP measure. To reduce the number of dependent variables, composite scores were computed using established cognitive domain groupings, based on tasks’ purported underlying brain systems (Lezak, Howieson, Loring, Hannay, & Fischer, 2004; Medina et al., 2007): (1) Visuospatial Functioning, (2) Attention and Working Memory, (3) Learning and Memory, and (4) Executive Functioning/Planning. Composite scores were computed by averaging change scores across tests within a domain (see Table 2). Age, SES, and familial AUD density, CDQ scores (range: 0–9), and practice effects (Bates, Voelbel, Buckman, Labouvie, & Barry, 2005; Matarazzo & Herman, 1984; White, Campbell, Echeverria, Knox, & Janulewicz, 2009) were used as covariates if they correlated (p<.05) with NP composite change scores. For females, none of these variables correlated with NP measures. For males, SES correlated with Learning and Memory. For females and males, baseline NP scores correlated with their corresponding change scores; to mitigate regression to the mean, baseline scores for each corresponding measure were used as covariates. Females and males were examined separately, as it was hypothesized that alcohol use would have a differential influence by gender. Hierarchical regressions used the four NP composite change scores as dependent variables, entered covariates on Block 1, and transformed past year drinking days on Block 2 (α < .05). Follow-up regressions were run for each significant composite score to determine which NP tasks were predicted by alcohol use, and to see if lifetime drinking, average past 3-month drinking, and HSS scores predicted cognitive change. Exploratory analyses were conducted to examine task specific results, because little is known about associations between neuropsychological performance and alcohol use in adolescents based on prospective data.
Table 2.
Neuropsychological change (outcome - baseline) z-scores of adolescents who transitioned into moderate or heavy drinking versus those who did not
| Females n=29 M (SD) |
Males n=47 M (SD) |
|||
|---|---|---|---|---|
| Controls n=16 |
Drinkers n=13 |
Controls n=24 |
Drinkers n=23 |
|
| Visuospatial Functioning | ||||
| Complex Figure copy accuracy* | 0.01 (1.43) |
−1.00 (1.61) |
0.12 (0.99) |
0.18 (1.45) |
| Complex Figure delay accuracy a b* | 0.36 (1.26) |
−0.84 (1.42) |
0.29 (1.01) |
−0.10 (0.96) |
| WASI Block Design* | 0.02 (0.61) |
−0.31 (0.45) |
0.22 (0.72) |
0.01 (0.67) |
| Attention and Working Memory | ||||
| Digit Vigilance Test Time† | −0.25 (0.83) |
0.0 (0.70) |
−0.21 (0.71) |
−0.00 (0.96) |
| D-KEFS Trails Condition 4 Time† | −0.25 (0.92) |
0.14 (0.72) |
−0.12 (0.82) |
−0.04 (0.92) |
| Digits Forward* | 0.27 (1.01) |
−0.14 (1.47) |
0.20 (0.81) |
−0.13 (0.93) |
| Digits Backward* | −0.49 (0.75) |
0.10 (0.94) |
−0.10 (0.71) |
0.09 (1.39) |
| Coding* | −0.12 (0.88) |
−0.07 (1.27) |
0.13 (0.84) |
−0.09 (0.61) |
| Learning and Memory | ||||
| CVLT List 1–5 Total* | 0.08 (0.99) |
0.22 (1.38) |
0.09 (0.90) |
0.30 (1.03) |
| CVLT Long Delay Free Recall* | 0.40 (0.96) |
0.17 (1.22) |
−0.13 (0.89) |
0.11 (0.96) |
| Executive Functioning/Planning | ||||
| D-KEFS Color-Word Interference Inhibition/Switching (Completion Time / Combined Naming and Reading Contrast) * |
−0.07 (0.83) |
0.14 (0.83) |
−0.03 (1.29) |
0.21 (1.13) |
| D-KEFS Towers (Total Achievement Score)* | −0.08 (1.61) |
0.09 (0.82) |
0.12 (1.17) |
−0.03 (1.52) |
| Premorbid Functioning, raw scores at baseline | ||||
| WASI Vocabulary* | 53.06 (4.22) | 50.69 (6.21) | 51.63 (8.48) | 52.30 (5.46) |
| WASI Similarities* | 34.31 (5.53) | 34.08 (3.50) | 33.92 (5.83) | 33.22 (5.27) |
| WRAT-3 Reading* | 43.63 (4.57) | 44.08 (3.77) | 44.04 (4.71) | 43.78 (3.88) |
Note: D-KEFS=Delis-Kaplan Executive Function System; WASI=Wechsler Abbreviated Scale of Intelligence; CVLT=California Verbal Learning Test; WRAT-3=Wide Range Achievement Test, 3rd edition. Range of correlations among measures within each NP domain were: Visuospatial functioning (.26−.43); Attention and Working Memory (.01−.31); Learning and Memory (.56); Executive Functioning (.05). Composite scores were based on established cognitive domain groupings (Lezak et al., 2004; Medina et al., 2007).
Drinkers ≠ Controls, p <.05
Female Drinkers ≠ Female Controls, p <.05
Higher scores indicate improved performance
Higher scores indicate worsened performance
RESULTS
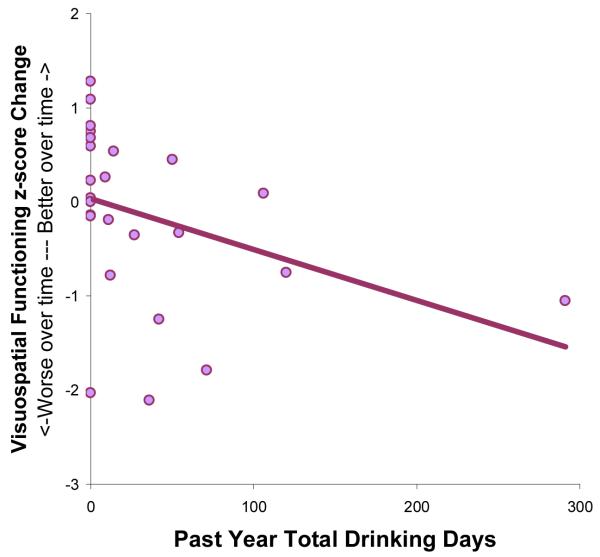
Regression analyses used past year drinking days to predict composite NP change scores. For females (n=29), more drinking days in the year prior to follow-up predicted greater reduction in performance in Visuospatial Functioning from baseline to follow-up, above and beyond baseline Visuospatial Functioning scores (F(2,24)= 11.42, p<.001; R2Δ=10%, p<.05; β=−.33; see Table 3 and Figure 2). For males, follow-up drinking did not predict NP domain change scores.
Table 3.
Standardized betas showing extent to which adolescent drinking days in the past year predicted change in neuropsychological performances
| Dependent variable: | Females | Males |
|---|---|---|
| Visuospatial Functioning | −.33* | n.s. |
| Attention and Memory | n.s. | n.s. |
| Learning and Memory | n.s. | n.s. |
| Executive Functioning/Planning | n.s. | n.s. |
p <.05
Note: All analyses controlled for baseline neuropsychological functioning
Figure 2.
For female adolescents, more past year drinking days predicted a greater reduction in performance on the Visuospatial Functioning composite score (R2Δ =10%, β = −.33, p < .05).
Exploratory hierarchical regressions used task-specific NP change scores as dependent variables (i.e., the 12 non-premorbid functioning measures in Table 2), the corresponding baseline NP score and any covariates on Block 1, and substance involvement variables on Block 2. Variables that correlated (p<.05) with outcome change scores were used as covariates in analyses. For females, age was used as a covariate for Coding, Block Design, and CVLT Long Delay Free Recall change scores. For males, age was a covariate for Coding and Color Word Interference Inhibition/Switching; CDQ total for Complex Figure copy; family history density for Coding and Block Design; and SES for CVLT Long Delay Free Recall change scores. For females, past year drinking days predicted Complex Figure change scores, similar as above (F(2,20)= 19.04, p<.001; R2Δ=8%, p<.05; β=−.32). For males, higher follow-up HSS scores predicted slowed DVT completion times from baseline to follow-up, above and beyond baseline DVT time (F(3,39)=8.65, p<.001; R2Δ=7%, p<.05; β=.27). DVT omission and commission errors were not significantly related to HSS scores, suggesting that the decrements in time completion were due to processing speed rather than accuracy.
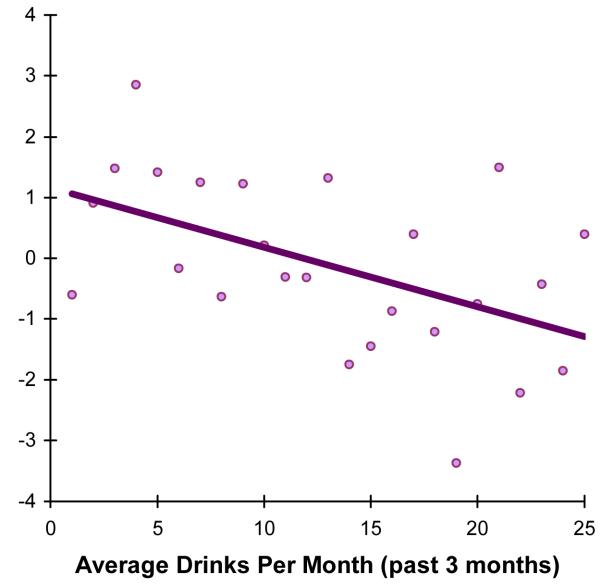
Follow-up regressions using lifetime drinking, average past 3-month drinking, and HSS scores were used to predict individual NP test change scores within the Visuospatial Functioning domain for females. More past 3-month drinking predicted greater reduction in Complex Figure delay performance from baseline to follow-up, above and beyond baseline Complex Figure delay scores (F(2,20)= 19.17, p<.001; R2Δ=8%, p<.05; β=−.32; see Figure 3). Lifetime drinking days and HSS scores did not predict reductions on Block Design or Complex Figure copy.
Figure 3.
For female adolescents, more drinks per month predicted a greater reduction in performance on complex figure delay (R2Δ = 8%, β = −.32, p < .05).
Results above remained unchanged when using non-transformed alcohol involvement variables. After controlling for alcohol consumption, lifetime and past month tobacco and marijuana use reported at follow-up did not significantly predict any follow-up NP change score. Recency of drinking did not predict follow-up NP change measures, and results above remained unchanged when controlling for days since last alcohol use.
DISCUSSION
This study prospectively examined the effects of alcohol use on neurocognition in adolescent girls and boys. For girls, more drinking days in the year before the follow-up NP assessment predicted a relative worsening in visuospatial functioning. For boys, greater hangover symptoms in the year before the follow-up was linked to relative worsening in sustained attention. This is consistent with past research reporting visuospatial (Brown et al., 2000; Sher et al., 1997; Tapert et al., 2001; Tapert & Brown, 1999) and attentional (Tapert & Brown, 1999; Tarter et al., 1995) decrements in adolescent substance users. Contrary to hypotheses, planning scores did not relate to drinking. Our findings are part of a longitudinal study in which subjects had little to no substance exposure at baseline, reducing the likelihood that decrements are attributable to premorbid traits.
As females and males drank at similar rates in this sample, the different manifestations of cognitive decrements could relate to divergent neurodevelopmental trajectories, physiological responses to alcohol, and social factors influencing drinking onset. Generally, females outperform males on tasks of psychomotor speed and accuracy, while males perform better on visuospatial tests (Lezak et al., 2004). Areas of relative weakness may be vulnerable to effects of alcohol and hangover. For girls, decrements seen in complex non-verbal productive memory, but not visual reproduction or verbal memory, are consistent with findings in adult female alcoholics (Sullivan et al., 2002), and may suggest affected frontoparietal circuitry (Selemon & Goldman-Rakic, 1988; Tapert et al., 2001). Girls consuming ≥12 drinks per month at follow-up showed a relative decline in visuospatial memory (see Figure 3), while females consuming <12 drinks per month did not. For males, decrements were found on DVT completion time but not other tasks of processing speed (i.e., Trails or Digit Symbol), suggesting reduced maturation in sustained attention and not simply processing speed, consistent with findings in adult alcoholics (Tedstone & Coyle, 2004). These results are concerning, as the severity of alcohol use is relatively low (mean drinks per month = 7.4) and most drinking is subclinical (only 4 of the 76 subjects met criteria for an AUD at follow-up).
While only some behavioral disadvantages may be apparent yet, it is possible that underlying neural structures may be compromised. White matter coherence may drive the spatial decrements found in females, as subclinical adolescent drinking has been linked to reduced white matter integrity (McQueeny et al., in press), and coherence positively relates to visuospatial skills (Fryer et al., 2008). fMRI studies have detected abnormalities in brain response to spatial working memory tasks despite intact performance in youth with short drinking histories (Tapert et al., 2004), but young adult females with longer drinking courses showed decreased frontal and parietal activation and attenuated performance (Tapert et al., 2001).
Limitations of this study include the relatively low drinking level and sample size. We used different follow-up durations across participants; ideally, each individual would be examined after the same follow-up duration. As the z-scores represent a ranking of scores at each time point, the results are relative to those assessed at a given assessment period. However, with the nature of longitudinal pediatric cognitive data, raw scores cannot be used because of differences between child and adult versions and alternate forms of tests at follow-ups. Future studies are needed to replicate the above findings, given the exploratory nature of some analyses.
The current findings present important clinical and public health implications. Visuospatial deficits could negatively impact females’ capacity to recall previously encoded spatial information, which could potentially have consequences for success in driving and figural reasoning. Deficits in males’ sustained attention adversely influence academic achievement and behavioral functioning. While the effects sizes reported in this study are relatively modest, negative NP consequences from drinking could affect large numbers of youth who engage in moderate to heavy levels of alcohol consumption.
Acknowledgements
This research was supported by NIAAA grant R01 AA13419 (PI: Tapert).
The authors thank Valerie Barlett, Veronique Boucquey, Lisa Caldwell, Sonja Eberson, Jesse Feng, Sonia Lentz, Andria Norman, Dr. Carmen Pulido, Dr. Sarah Mattson, and Dr. Sandra A. Brown, and the participating families and schools.
Portions of this study were presented at the 2008 meeting of the Research Society on Alcoholism. This work represents the master’s thesis of the first author.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/adb
References
- APA . Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcoholism: Clinical & Experimental Research. 2005;29(3):367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Supplement 4):S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive Functioning of Adolescents: Effects of Protracted Alcohol Use. Alcoholism: Clinical and Experimental Research. 2000;24(2):164–171. [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clark DB, Tapert SF. Introduction to alcohol and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32(3):373–374. doi: 10.1111/j.1530-0277.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test-Children’s Version. The Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-Second Edition. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Sassoon S, O’Reilly A, Pfefferbaum A, Sullivan EV. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. Journal of Studies on Alcohol. 2003;64(3):375–379. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcoholism: Clinical and Experimental Research. 2004;28(11):1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Flannery B, Fishbein D, Krupitsky E, Langevin D, Verbitskaya E, Bland C, et al. Gender differences in neurocognitive functioning among alcohol-dependent Russian patients. Alcoholism: Clinical and Experimental Research. 2007;31(5):745–754. doi: 10.1111/j.1530-0277.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Fryer SF, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg BJ, et al. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and Cognition. 2008;67(2):225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Shoal GD, Mezzich AC. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Experimental and Clinical Psychopharmacology. 2001;9(2):215–227. doi: 10.1037//1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021(77–85) doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Glenn S, Parsons O, Sinha R, Stevens L. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol & Alcoholism. 1988;23(5):337–342. doi: 10.1093/oxfordjournals.alcalc.a044826. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2007. National Institute on Drug Abuse; Bethesda, MD: 2008. NIH Publication No. 08-6418. [Google Scholar]
- Lewis RF. Digit Vigilance Test. Psychological Assessment Resources; Odessa, FL: 1995. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed. Oxford University Press; New York: 2004. [Google Scholar]
- Loring DW, Meador KJ. The Medical College of Georgia (MCG) Complex Figures: Four forms for follow-up. In: Knight J, Kaplan E, editors. Rey-Osterrieth handbook. Psychological Assessment Resources; Odessa, Fl: 2003. [Google Scholar]
- Martin CS, Chung T, Kirisci L, Langenbucher JW. Item response theory analysis of diagnostic criteria for alcohol and cannabis use disorders in adolescents: Implications for DSM-V. Journal of Abnormal Psychology. 2006;115:807–814. doi: 10.1037/0021-843X.115.4.807. [DOI] [PubMed] [Google Scholar]
- Matarazzo JD, Herman DO. Base rate data for the WAIS-R: Test-retest stability and VIQ-PIQ differences. Journal of Clinical and Experimental Neuropsychology. 1984;6(4):351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. doi: 10.1111/j.1530-0277.2009.00953.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Moore EE, Nichols ME, Abney OL, Taylor HS, Zamrini EY, et al. The role of cholinergic systems in visuospatial processing and memory. Journal of Clinical & Experimental Neuropsychology. 1993;15(5):832–842. doi: 10.1080/01688639308402599. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson K, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after 30 days of abstinence. Journal of International Neuropsychological Society. 2007;13:207–220. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism: Clinical & Experimental Research. 1994;18(159–163) doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry. 1998;155(4):479–485. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Cognitive deficits in alcoholic women. Alcohol Health & Research World. 1994;18:228–232. [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Stevens L. Previous alcohol intake and residual cognitive deficits in detoxified alcoholics and animals. Alcohol & Alcoholism. 1986;21:137–157. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006;59(4):364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. Journal of Abnormal Psychology. 2005;114(2):223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Rey A, Osterrieth PA. Corwin J, Bylsma FW, editors. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and P.A. Osterreich’s “The complex figure copy test”. The Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug & Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Experimental and Clinical Psychopharmacology. 1997;5(3):304–315. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Thomas M, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcoholism: Clinical & Experimental Research. 2003;27(9):1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. C.M., L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Journal of Clinical EEG & Neuroscience. 2009;40(1):31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: Density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16(1):74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Swift R, Davidson D. Alcohol hangover: Mechanisms and mediators. Alcohol Health & Research World. 1998;22(1):54–60. [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Pearson; Boston: 2007. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical & Experimental Research. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical & Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh Y-C, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug and Alcohol Dependence. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Taylor LB. Localisation of cerebral lesions by psychological testing. Clinical Neurosurgery. 1969;16:269–287. doi: 10.1093/neurosurgery/16.cn_suppl_1.269. [DOI] [PubMed] [Google Scholar]
- Taylor LB. Scoring criteria for the Rey-Osterrieth Complex Figure Test. In: Spreen O, Strauss E, editors. A compendium of neuropsychological tests. Administration, norms, and commentary. Oxford University Press; New York (NY): 1998. pp. 350–351. [Google Scholar]
- Tedstone D, Coyle K. Cognitive performance in sober alcoholics: Performance on selected and divided attention tasks. Drug and Alcohol Dependence. 2004;75(3):277–286. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Twitchell G, Hertzog C, Klein J, Schuckit M. The anatomy of a follow-up. Addiction. 1992;87(9):1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. Psychological Corporation; New York: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- White RF, Campbell R, Echeverria D, Knox SS, Janulewicz P. Assessment of neuropsychological trajectories in longitudinal population-based studies of children. Journal of Epidemiology and Community Health. 2009;63(Suppl 1):i15–i26. doi: 10.1136/jech.2007.071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese J, Shlipak M, Browner W. The Alcohol Hangover. Annals of Internal Medicine. 2000;132:897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test administration manual. 3rd ed. Western Psychological Services; Wilmington, DE: 1993. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopyschosocial variation among pathways into symptomatic difficulty. In: Babor TF, Hesselbrock V, Meyer RE, editors. Types of Alcoholics: Evidence from Clinical, Experimental and Genetic Research. The New York Academy of Sciences; New York: 1994. pp. 134–146. S. W. [DOI] [PubMed] [Google Scholar]