Abstract
The FOXO4 transcription factor plays an important role in cell survival in response to oxidative stress. The regulation of FOXO4 is orchestrated by post-translational modifications including phosphorylation, acetylation, and ubiquitination. Here, we demonstrate that O-GlcNAcylation also contributes to the FOXO4-dependent oxidative stress response. We show that hydrogen peroxide treatment of HEK293 cells increases FOXO4 association with OGT, the enzyme that adds O-GlcNAc to proteins, causing FOXO4 O-GlcNAcylation and enhanced transcriptional activity under acute oxidative stress. O-GlcNAcylation is known to be protective for cells under stress conditions, including oxidative stress. Our data provide a mechanism of FOXO4 anti-oxidative protection through O-GlcNAcylation.
Keywords: OGT, NCOAT, hydrogen peroxide, O-GlcNAc, p27Kip1
1. Introduction
FOXO4, together with FOXO1, FOXO3 and FOXO6, belongs to a subset of the Forkhead family of transcription factors [1]. Like other Forkhead box O (FOXO) transcription factors, FOXO4 is able to modulate the expression of genes involved in oxidative stress dependent apoptosis, cell cycle arrest, DNA damage repair and other cellular functions [2,3]. Essers et al showed FOXO4 proteins reduce oxidative stress by directly increasing mRNA and protein levels of manganese superoxide dismutase (MnSOD) and catalase [4]. Furthermore, FOXO4 can up-regulate the cyclin-dependent kinase inhibitor gene, p27Kip1, causing cell cycle arrest, DNA repair, and is thus tumor suppressive [5]. When ineffective DNA repair or excessive damage ensues, cell death is triggered. Thus, FOXO4 may function as a switch between cell death and survival under oxidative stress stimulation.
The FOXO4 transcription factor is regulated in cellular shuttling and DNA binding of target genes by multiple mechanisms. Normally, AKT negatively regulates all FOXO protein activity by direct phosphorylation, causing their nuclear exclusion [6]. However, oxidative stress causes nuclear localization of FOXO4 through Mdm2-dependent mono-ubiquitination [7] and resultant transcriptional activation increase through JNK-mediated phosphorylation at residues 471 and 451 [4]. Furthermore, the longevity protein, SIRT1, deacetylates FOXO proteins and modulates their transcriptional activity in response to stress stimuli [8,9]. Housley and others also found that FOXO1 is O-GlcNAcylated in response to glucose [10,11]. However, O-GlcNAcylation of FOXO4 under oxidative stress has not been studied.
O-GlcNAcylation is an abundant and dynamic post-translational modification (PTM) on serine and threonine residues of nuclear and cytoplasmic proteins. O-GlcNAc Transferase (OGT) and Nuclear Cytoplasmic O-GlcNAcase and Acetyltransferase (NCOAT) catalyze this process of adding and removing the O-GlcNAc groups respectively. O-GlcNAcylation plays a critical role in protein turnover, cell cycle progression, transcription, stress response and other cellular functions [12-14]. Zachara and others showed that a global increase in protein O-GlcNAc levels is critical for cell survival in response to oxidative stress and other stimuli [15,16]. However, a mechanism explaining how O-GlcNAcylation contributes to the oxidative stress response remains complicated.
The regulation of FOXO4 in response to oxidative stress involves many PTMs that include, phosphorylation, acetylation and ubiquitination. Here, we show that FOXO4 is also O-GlcNAcylated and that this modification is increased upon acute oxidative stress treatment. Furthermore, O-GlcNAc modification causes increased transcriptional activity of FOXO4 in stress-related genes. Our work provides another mechanism whereby FOXO4 contributes to the anti-oxidative stress process, through the modulation of O-GlcNAcylation.
2. Materials and methods
2.1. Cell culture, plasmids and transfection
HEK293 and HepG2 cells were maintained in Dulbecco's Modified Eagle medium (1g/L glucose) (Cellgro), supplemented with 10% fetal bovine serum and penicillin/streptomycin. Rat OGT and mouse NCOAT were inserted into pEGFP-C1 (Clontech) as previous described [17]. Mouse FLAG-FOXO4 and myc-FOXO4 plasmids were gifts from Dr. Zhi-Ping Liu [18]. 6× daf-16 protein binding element (DBE)-Luc [19] was a gift from Dr. Boudewijn Burgering. The p27Kip1-DBE-Luc was a gift from Dr. Simon Lees [20]. Transient transfections were performed with Lipofectamine 2000 according to the manufacturer (Invitrogen).
2.2. Transcription activation assay
HEK293 cells were transfected with the indicated constructs plus 10 ng TK-Renilla (Promega) used as a transfection efficiency control. Cells were treated with 200 μM H2O2 for an hour and lysates were measured by the Promega Dual Luciferase reporter assay.
2.3. Protein interaction analysis
Co-immunoprecipitation (co-IP) or IP assays were performed on lysates from HEK293 cells transfected with EGFP-OGT and FLAG-FOXO4 or HepG2 cells. Anti-FLAG M2 Affinity Gel (Sigma) and anti-OGT antibody were used in this Co-IP and IP assay. The immunoprecipitates were washed five times and separated by 10% SDS-PAGE gel electrophoresis and blotted using anti-EGFP (Santa Cruz), anti-FLAG-HRP, anti-O-GlcNAc (RL2, or CTD110.6, Covance), anti-p(Ser193)-FOXO4 (Cell Signaling), anti-OGT (DM-17, Sigma), anti-GAL4 (sc-577), anti-FOXO4 and anti-Nucleolin (Santa Cruz).
2.4. Western blot analysis
O-GlcNAcylated proteins were immunoprecipitated using anti-O-GlcNAc antibody and then immunobloted with anti-FLAG (Sigma), anti-α-TUBULIN (Sigma), anti-FOXO4 (Santa Cruz), anti-FOXO1 (Santa Cruz and Abcam), anti-FOXO3 (Santa Cruz and Abcam) and anti-SIRT1 (Santa Cruz).
2.5. Immunofluorescence
Mouse FLAG-FOXO4 was transfected into HEK293 cells and maintained at normal growth medium for 24 hours. Media was refreshed an hour before the administration of glucosamine, TT06 and LY294002 (OGT and AKT inhibitors). Cells were treated with 500 μM H2O2 for another hour then fixed with 4% paraformaldehyde and stained with anti-FLAG-cy3 antibody and DAPI.
2.6. Statistical analysis
All experiments were performed at least three times. Data are presented as mean ± S.E. and represent three independent experiments. Student's t-test was using for comparing group means, and P values ≤0.05 were accepted as significant.
3. Results
3.1 Oxidative stress modulates FOXO4 protein and O-GlcNAc levels
We first needed to determine if stress stimuli effected O-GlcNAc modification on FOXO proteins. The treatment of transiently transfected Flag-tagged FOXO4 HEK293 cells with hydrogen peroxide resulted in a dramatic increase in O-GlcNAc-modified FOXO4 levels (Fig. 1A). Interestingly, H2O2 treatment of the HEK293 cells reduced total FOXO4 protein levels in the 10% extract. Similarly, heat shock stress also produced a progressive increase in O-GlcNAcylated endogenous FOXO4 protein in HepG2 cells (Fig. 1B and C). Specificity was confirmed by free GlcNAc competition. Although FOXO1 has been reported to be O-GlcNAcylated in response to glucose treatment [10,11], we did not detect O-GlcNAc-FOXO1 in heat-stressed cells. The longevity protein, SIRT1, also remained unchanged in O-GlcNAc and total protein levels (Fig. 1B). Not only is FOXO4 O-GlcNAc-modified in HepG2 cells, but OGT is physically associated with FOXO4 under heat stress (Fig. 1D).
Fig. 1.
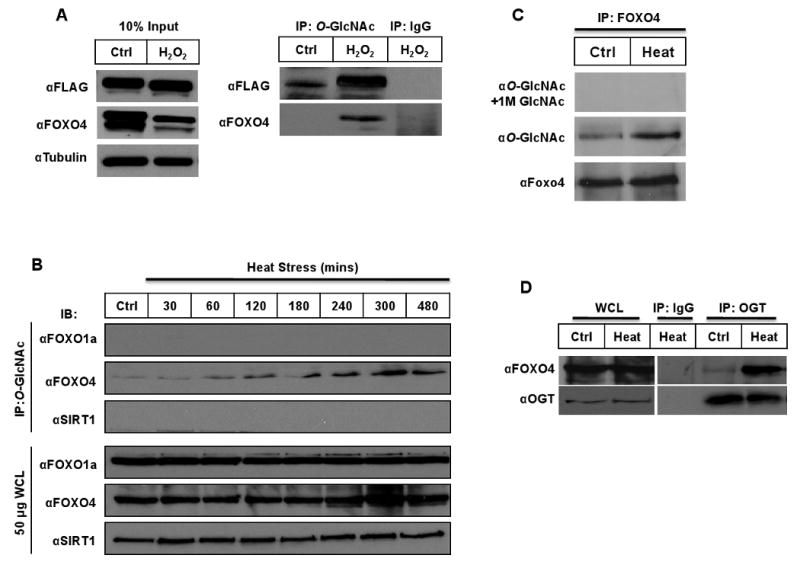
O-GlcNAcylation of FOXO4 protein with H2O2. (A) Western blot of HEK293 cells transfected with FLAG-tagged FOXO4, 24 hours later treated with 500 μM H2O2 for 60 minutes. Cell lysates were immunoprecipitated with antibody (RL2), separated on SDS-PAGE, and immunoblotted with anti-FLAG or anti-FOXO4 antibodies. Total protein levels of FOXO4 and tubulin control are shown with 10% input. (B) HepG2 cells stressed at 42°C for indicated times and lysates IP'd with anti-O-GlcNAc, and immunoblotted with FOXO1a, FOXO4, and SIRT1 antibodies. (C-D) HepG2 cells stressed at 42°C for 2 hours, lysates IP'd with anti-FOXO4 or anti-OGT, and immunoblotted with O-GlcNAc or FOXO4 antibodies.
3.2. OGT interacts and O-GlcNAcylates FOXO4 under oxidative stress
The acute oxidative stress-mediated O-GlcNAcylation of FOXO4 was accentuated with the association between FOXO4 and OGT, demonstrated here in HEK 293 cells in a co-IP assay (Fig. 2). Hydrogen peroxide induced significant binding of transfected EGFP-tagged OGT protein with immunoprecipitated FLAG-tagged FOXO4 (Fig. 2A, panel 1). The O-GlcNAcylation of FOXO4 proteins was confirmed with O-GlcNAc antibody, using 1 M GlcNAc as an O-GlcNAc specific competitor control (Fig 2A, panels 4 and 5). Immunoprecipitation blots with OGT deletion constructs indicated that FOXO4 protein principally binds to the C-terminus of OGT (OGT a.a. 485-944) (Fig. 2C).
Fig. 2.
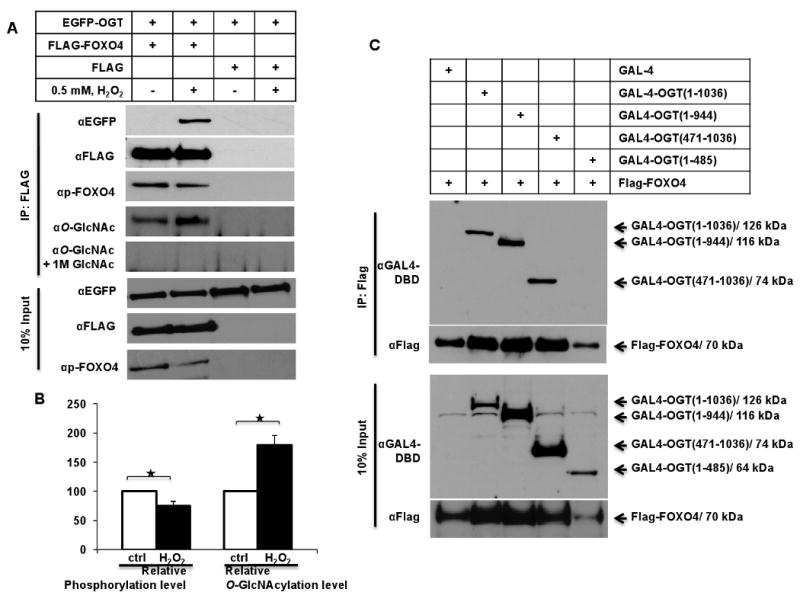
OGT interacts and O-GlcNAcylates FOXO4. (A) Western blot of HEK293 cells that were co-transfected with FLAG-FOXO4 and EGFP-OGT for 24 hours and treated with 500 μM H2O2 for 60 min. Cell lysates were IP'd with anti-FLAG, separated on SDS-PAGE, and immunoblotted with GFP, FLAG, and p(Ser193) FOXO4 antibodies or anti-O-GlcNAc ± 1 M GlcNAc. (B) Relative Ser193 phosphorylation and O-GlcNAcylation of FLAG-tagged FOXO4 of Figure 2A analyzed with “ImageJ”. All values expressed as means ± S.E. for three independent experiments. *P≤0.05 vs. control. (C) Western blot of HEK293 cells that were co-transfected with Flag-FOXO4 and indicated GAL4-tagged OGT deletion constructs for 24 hours and treated with 500 μM H2O2 for 60 min. Cell lysates were IP'd with anti-FLAG, and immunoblotted with Flag and GAL4 antibodies.
It has been reported that O-GlcNAcylation may have a reciprocal role with phosphorylation [12]. AKT-mediated phosphorylation of FOXO4 at Ser193 regulates its nuclear exclusion, thus negatively regulating transcription of its target genes. Treatment with H2O2 resulted in an 80% elevation of O-GlcNAcylated FOXO4 (Fig. 2B), with a corresponding 20% reduction in Ser193-specific phosphorylation. However, since the number of potential O-GlcNAc sites on FOXO4 is unknown, reciprocity between phosphorylation and O-GlcNAcylation cannot be directly compared. Our data have shown that acute H2O2 induces OGT-FOXO4 association with O-GlcNAc modification of FOXO4, and oxidative stress also causes a reduction of AKT-dependent phosphorylation of FOXO4 with increased nuclear FOXO4 protein [6]. We now consider if FOXO4 cellular localization is O-GlcNAc-dependent.
3.3. Modulation of O-GlcNAcylation does not affect redistribution of FOXO4 proteins
To investigate the effect of modulation of O-GlcNAcylation on FOXO4 cellular localization, HEK293 cells transfected with FLAG-tagged FOXO4 plasmid were treated with glucosamine and the AKT inhibitor, LY294002, before H2O2 treatment. The increase in total cellular protein-O-GlcNAc was confirmed by Western blot (data not shown). FOXO4, normally distributed throughout the cytoplasm, was largely restricted to the nucleus upon H2O2 treatment (Fig. 3, panel 1). Control cells treated with the AKT inhibitor, LY294002, also showed FOXO4 confined to the nucleus, inferring that AKT activity is required to keep FOXO4 in the cytoplasm (Fig. 3, panel 3). However, increased O-GlcNAcylation by glucosamine treatment did not promote nuclear localization of FOXO4 (Fig. 3, panel 2). Consequently, changing total cellular levels of O-GlcNAcylation does not appear to play a role in cellular distribution of FOXO4 as a reaction to oxidative stress. We now consider the role of O-GlcNAcylation and FOXO4 transcriptional regulation.
Fig. 3.
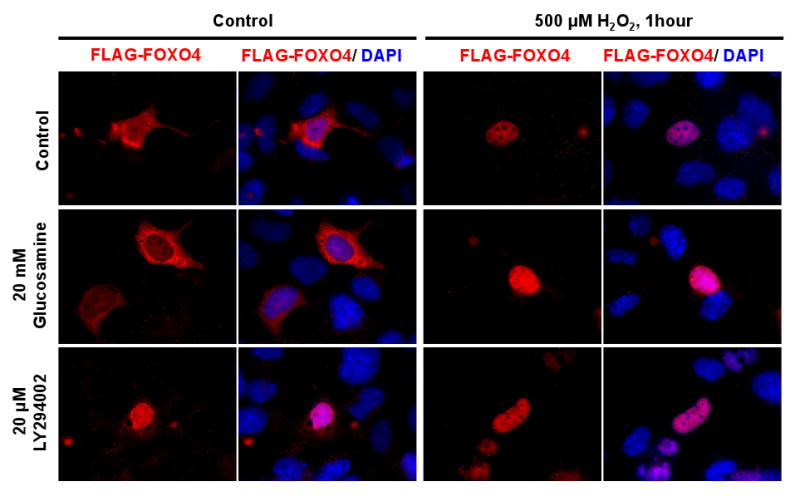
FOXO4 redistribution, unaffected by O-GlcNAcylation. Immunohistochemistry of HEK293 cells transfected with FLAG-FOXO4 and treated with 20 mM glucosamine or 20 μM LY294002 for 60 minutes then stimulated with 500 μM H2O2 for 60 minutes. Cells were fixed and stained with anti-FLAG-cy3 targeting FOXO4 (red), and DAPI for nucleus (blue).
3.4. OGT enhances FOXO4 dependent transcriptional activity
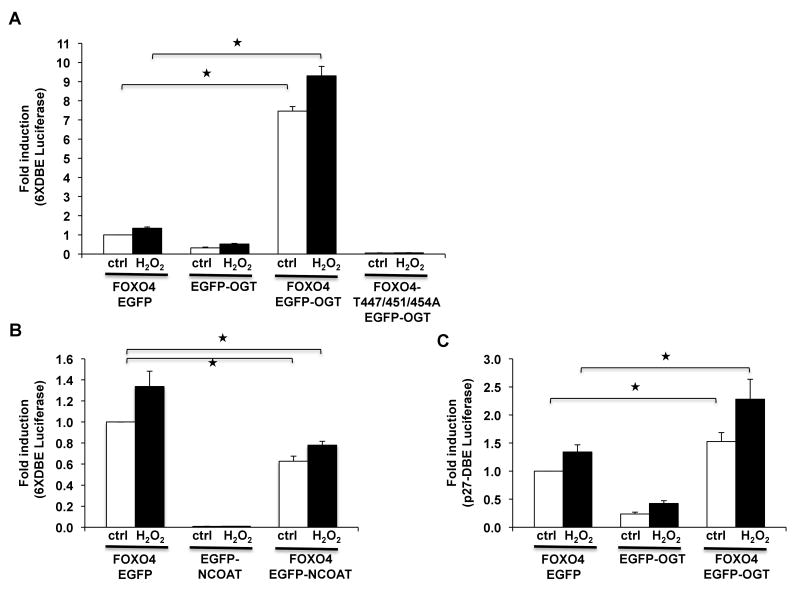
The effect of O-GlcNAcylation on FOXO4 transcriptional activity was examined by reporter assays using FOXO-responsive promoters [19]. When co-transfected with FOXO4, OGT dramatically enhanced FOXO4 transcriptional activity on two different responsive element reporters, 6XDBE-LUC (Fig. 4A) and p27-DBE-LUC (Fig. 4C), and this was further increased with H2O2 treatment. This is also in agreement with previous reports showing H2O2 treatment led to increased FOXO4 transactivation activity in DBE-based luciferase assays [4,7]. OGT alone, or the JNK-mutant FOXO4-T447/451/454A had insignificant transcriptional activity (Fig. 4A&C). In contrast to OGT, the O-GlcNAcylase enzyme, NCOAT, decreased FOXO4 transcriptional activity (Fig. 4B), emphasizing the relevance of the role of O-GlcNAcylation in FOXO4 transcription.
Fig. 4.
OGT enhances FOXO4-dependent transcriptional activity. HEK293 cells were transfected with the FOXO responsive 6XDBE-Luciferase plasmid together with, (A) FOXO4, FOXO4-T447/451/454A mutant and OGT plasmids, or (B) FOXO4 and NCOAT plasmids, and treated with 200 μM H2O2 for 60 min. (C) Similar to Fig. 4A using p27-DBE-Luciferase. Data expressed as RLU of 6XDBE-Luciferase or p27-DBE-Luciferase divided by Renilla luciferase control, from three experiments shown as mean ± S.E. of triplicates. *P≤0.05 vs. control.
4. Discussion
Signaling pathways that decide the “survival vs die” fate of a cell often depend on post-translational modifications of cellular proteins. The response to oxidative stress involves members of the Forkhead family of transcription factors and in particular, FOXO4 can regulate anti-oxidant genes through phosphorylation, acetylation, and ubiquitination [4,8,22,23]. Here we demonstrate O-GlcNAcylation involvement in the oxidative stress response. Hydrogen peroxide caused increased association between OGT and FOXO4 proteins leading to O-GlcNAcylation of FOXO4 with enhanced transcriptional activity. Specifically, OGT and O-GlcNAc, promotes FOXO4-dependent transcriptional activity of p27Kip1, causing cell cycle arrest [24], and enhanced cell survival (Fig 4C). Disruption of O-GlcNAcylation has also been shown to involve cell cycle arrest [25]. Conversely, the decrease in FOXO4 transcriptional activity by the O-GlcNAcase, (NCOAT, Fig.4B) could lead to a relaxing of cell cycle regulation and favor tumor growth.
Many previous studies implicate the elevation of O-GlcNAc as providing survival signals in response to various acute stress stimuli [15,26,27]. Specifically, O-GlcNAcylation of FOXO1 and FOXO3 proteins occur in response to nutrition and stress stimuli [10,11]. However, we did not detect O-GlcNAc modified FOXO1 (Fig. 1B) and FOXO3 (data not shown) in HEK293 cells under oxidative stress conditions. Housley et al [10] showed there are at least five potential O-GlcNAcylation sites on FOXO1 protein. Interestingly, H2O2 caused relatively larger increases in O-GlcNAcylation than decreases in phosphorylation of FOXO4 (Ser193) (Fig. 2B), suggesting multiple O-GlcNAc sites. However, since we (Fig. 3, panels 2&4), and others [10] now show that O-GlcNAcylated FOXO proteins does not influence their AKT-dependent cellular redistribution, regulation of FOXO4 under oxidative stress may occur through its direct association with OGT, the exact mechanism yet to be determined.
In summary, our data describe a mechanism by which O-GlcNAc provides cell survival signaling in response to acute oxidative stress. OGT enhances FOXO4 transcriptional activity upon oxidative stress, causing the up-regulation of FOXO4 dependent target genes, such as cell cycle arrest and cell longevity genes, thereby promoting oxidative resistance.
Acknowledgments
We appreciate the generous gifts of, FLAG-FOXO4 and myc-FOXO4 plasmids from Dr. Zhi-Ping Liu, HA-FOXO4-T447/451/454A, 6XDBE-Luc and pSODLUC-3340 from Dr. Boudewijn Burgering, p27Kip1-DBE-Luc from Dr. Simon Lees, anti-OGT antibody (AL28) from Dr. Gerald Hart. This work was supported in full by a grant from the NIH (DK043652).
Footnotes
Structured summary:
MINT-7299700, MINT-7299716:
Foxo4 (uniprotkb:Q9WVH3) physically interacts (MI:0915) with Ogt (uniprotkb:P56558) by anti tag communoprecipitation (MI:0007)
MINT-7299691:
Ogt (uniprotkb:015294) physically interacts (MI:0915) with Foxo4 (uniprotkb:P98177) by anti bait coimmunoprecipitation (MI:0006)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- 2.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–6. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 4.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenkman AB, de Keizer PL, van den Broek NJ, van der Groep P, van Diest PJ, van der Horst A, Smits AM, Burgering BM. The peptidyl-isomerase Pinl regulates p27kip1 expression through inhibition of Forkhead box O tumor suppressors. Cancer Res. 2008;68:7597–605. doi: 10.1158/0008-5472.CAN-08-1059. [DOI] [PubMed] [Google Scholar]
- 6.Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Molecular and Cellular Biology. 2001;21:3534–46. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRTl) J Biol Chem. 2004;279:28873–9. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 10.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–92. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-glycosylation of Fox01 increases its transcriptional activity towards the glucose 6-phosphatase gene. FEBS Lett. 2008;582:829–34. doi: 10.1016/j.febslet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 13.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 14.Kudlow JE. Post-translational modification by O-GlcNAc: another way to change protein function. J Cell Biochem. 2006;98:1062–75. doi: 10.1002/jcb.20926. [DOI] [PubMed] [Google Scholar]
- 15.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 16.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–9. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–63. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- 18.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9:261–70. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–34. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lees SJ, Childs TE, Booth FW. Age-dependent FOXO regulation of p27Kip1 expression via a conserved binding motif in rat muscle precursor cells. Am J Physiol Cell Physiol. 2008;295:C1238–46. doi: 10.1152/ajpcell.00349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–9. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 22.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–4. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 23.van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–73. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 24.Dijkers PF, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–56. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–12. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Guinez C, Lemoine J, Michalski JC, Lefebvre T. 70-kDa-heat shock protein presents an adjustable lectinic activity towards O-linked N-acetylglucosamine. Biochem Biophys Res Commun. 2004;319:21–6. doi: 10.1016/j.bbrc.2004.04.144. [DOI] [PubMed] [Google Scholar]