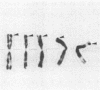Abstract
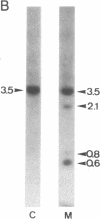
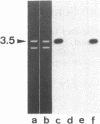
The gene coding for the mouse cell adhesion molecule uvomorulin has been mapped to chromosome 8. Uvomorulin cDNA clone F5H3 identified restriction fragment length polymorphisms in Southern blots of genomic DNA from mouse species Mus musculus domesticus and Mus spretus. By analyzing the segregation pattern of the gene in 75 offspring from an interspecific backcross a single genetic locus, Um, was defined on chromosome 8. Recombination frequency between Um and the co-segregating loci serum esterase 1 (Es-1) and tyrosine aminotransferase (Tat) places Um about 14 centimorgan (cM) distal to Es-1, and 5 cM proximal to Tat. In situ hybridization of uvomorulin [3H]cDNA to mouse metaphase chromosomes located the Um locus close to the distal end of chromosome 8 (bands C3-E1). Since uvomorulin is evolutionarily highly conserved, its chromosomal assignment adds an important marker to the mouse genetic map.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph S., Bartram C. R., Hameister H. Mapping of the oncogenes Myc, Sis, and int-1 to the distal part of mouse chromosome 15. Cytogenet Cell Genet. 1987;44(2-3):65–68. doi: 10.1159/000132345. [DOI] [PubMed] [Google Scholar]
- Avner P., Amar L., Dandolo L., Guénet J. L. Genetic analysis of the mouse using interspecific crosses. Trends Genet. 1988 Jan;4(1):18–23. doi: 10.1016/0168-9525(88)90123-0. [DOI] [PubMed] [Google Scholar]
- Birsner U., Gilles U., Nielsen P., McMaster G. K. Reversed-phase high-performance liquid chromatographic system for the rapid, automated purification of oligonucleotides. J Chromatogr. 1987 Jul 31;402:381–386. doi: 10.1016/0021-9673(87)80043-2. [DOI] [PubMed] [Google Scholar]
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bućan M., Yang-Feng T., Colberg-Poley A. M., Wolgemuth D. J., Guenet J. L., Francke U., Lehrach H. Genetic and cytogenetic localisation of the homeo box containing genes on mouse chromosome 6 and human chromosome 7. EMBO J. 1986 Nov;5(11):2899–2905. doi: 10.1002/j.1460-2075.1986.tb04585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capanna E., Gropp A., Winking H., Noack G., Civitelli M. V. Robertsonian metacentrics in the mouse. Chromosoma. 1976 Nov 29;58(4):341–353. doi: 10.1007/BF00292842. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P., Owens G. C., Edelman G. M., Cunningham B. A. Chromosomal location of the gene encoding the neural cell adhesion molecule (N-CAM) in the mouse. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7631–7635. doi: 10.1073/pnas.82.22.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Richa J., Solter D., Knudsen K., Buck C. A. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Dandoy F., De Maeyer E., Bonhomme F., Guenet J. L., De Maeyer-Guignard J. Segregation of restriction fragment length polymorphism in an interspecies cross of laboratory and wild mice indicates tight linkage of the murine IFN-beta gene to the murine IFN-alpha genes. J Virol. 1985 Oct;56(1):216–220. doi: 10.1128/jvi.56.1.216-220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénet J. L. The contribution of wild derived mouse inbred strains to gene mapping methodology. Curr Top Microbiol Immunol. 1986;127:109–113. doi: 10.1007/978-3-642-71304-0_13. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Buonanno A., Geoffroy B., Robert B., Guénet J. L., Merlie J. P., Changeux J. P. Chromosomal localization of muscle nicotinic acetylcholine receptor genes in the mouse. Science. 1986 Nov 14;234(4778):866–868. doi: 10.1126/science.3022377. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Imhof B. A., Vollmers H. P., Goodman S. L., Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983 Dec;35(3 Pt 2):667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Kemler R., Babinet C., Eisen H., Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Scherer G., Zentgraf H., Ruppert S., Herrmann B., Lehrach H., Schütz G. Isolation, characterization and chromosomal mapping of the mouse tyrosine aminotransferase gene. J Mol Biol. 1985 Aug 5;184(3):367–373. doi: 10.1016/0022-2836(85)90287-6. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Schuh R., Vestweber D., Riede I., Ringwald M., Rosenberg U. B., Jäckle H., Kemler R. Molecular cloning of the mouse cell adhesion molecule uvomorulin: cDNA contains a B1-related sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1364–1368. doi: 10.1073/pnas.83.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Hatta K., Hosoda M., Tsunasawa S., Sakiyama F., Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986 Oct;5(10):2485–2488. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984 May;152(1):169–178. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- Weydert A., Daubas P., Lazaridis I., Barton P., Garner I., Leader D. P., Bonhomme F., Catalan J., Simon D., Guénet J. L. Genes for skeletal muscle myosin heavy chains are clustered and are not located on the same mouse chromosome as a cardiac myosin heavy chain gene. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7183–7187. doi: 10.1073/pnas.82.21.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]