Abstract
Cilengitide is a cyclic peptide antagonist of integrins αvβ3 and αvβ5 that is currently being evaluated as a novel therapeutic agent for recurrent and newly diagnosed glioblastoma. Its mode of action is thought to be mainly antiangiogenic but may include direct effects on tumor cells, notably on attachment, migration, invasion, and viability. In this study we found that, at clinically relevant concentrations, cilengitide (1–100 μM) induces detachment in some but not all glioma cell lines, while the effect on cell viability is modest. Detachment induced by cilengitide could not be predicted by the level of expression of the cilengitide target molecules, αvβ3 and αvβ5, at the cell surface. Glioma cell death induced by cilengitide was associated with the generation of caspase activity, but caspase activity was not required for cell death since ectopic expression of cytokine response modifier (crm)-A or coexposure to the broad-spectrum caspase inhibitor zVAD-fmk was not protective. Moreover, forced expression of the antiapoptotic protein marker Bcl-XL or altering the p53 status did not modulate cilengitide-induced cell death. No consistent effects of cilengitide on glioma cell migration or invasiveness were observed in vitro. Preliminary clinical results indicate a preferential benefit from cilengitide added to temozolomide-based radiochemotherapy in patients with O6-methylguanine DNA methyltransferase (MGMT) gene promoter methylation. Accordingly, we also examined whether the MGMT status determines glioma cell responses to cilengitide alone or in combination with temozolomide. Neither ectopic expression of MGMT in MGMT-negative cells nor silencing the MGMT gene in MGMT-positive cells altered glioma cell responses to cilengitide alone or to cilengitide in combination with temozolomide. These data suggest that the beneficial clinical effects derived from cilengitide in vivo may arise from altered perfusion, which promotes temozolomide delivery to glioma cells.
Keywords: chemotherapy, cilengitide, glioma, integrin, temozolomide
High-grade gliomas represent the most frequent and malignant astroglial tumors in adults. In spite of multimodal therapeutic efforts including surgery, radiotherapy, and chemotherapy, most patients still die within 2 years of diagnosis.1,2 This poor prognosis can be attributed to an intrinsic tumor cell resistance to the genotoxic stress induced by irradiation or classical chemotherapy using DNA-damaging agents, as well as to the diffuse invasion of single glioma cells into the surrounding brain tissue, which makes efforts at curative surgical resection futile. Glioma cell invasiveness requires interaction with specific components of the extracellular matrix (ECM). The ECM of the brain parenchymal tissue predominantly contains glycosaminoglycans. Besides using the host ECM, glioma cells apparently produce their own ECM, including components such as laminin, collagen types I, III, and IV, tenascin, vitronectin, and several types of glycosaminoglycans.3,4
Cell surface receptors of the integrin superfamily play a key role in mediating cell–ECM interactions. Integrins consist of two noncovalently associated type I trans-membrane glycoprotein α- and β-subunits. To date, 19 integrin α-subunits and 8 integrin β-subunits have been described, forming at least 25 different α/β-heterodimers. In addition to regulating cell–cell and cell–ECM adhesion, integrins bidirectionally transmit signals important for cell survival, proliferation, differentiation, and motility.5 For example, ligand receptor interactions between ECM components and integrins activate cytoplasmatic tyrosine kinases, such as focal adhesion kinase, and their downstream effectors. The contribution of specific integrins to the malignant phenotype of numerous types of tumor has become a major area of cancer research.6,7 Two αv integrins recognizing vitronectin through an Arg-Gly-Asp (RGD) binding site, αvβ3 and αvβ5, are expressed by glioma cells and by endothelial cells associated with new blood vessel formation in glioblastomas.8–12 These αv integrins are the primary target of cilengitide (EMD 121974), an RGD-based cyclic peptide developed as an antiangiogenic drug. Based on the concept that αvβ3 and αvβ5 are proangiogenic receptors, two αv antagonists have entered clinical trials:13 cilengitide and vitaxin, a humanized monoclonal anti-αvβ3 antibody. In endothelial cells, blocking integrins αvβ3 and αvβ5 by RGD mimetics induces detachment from vitronectin-coated surfaces and results in a specific type of caspase-dependent apoptosis referred to as anoikis.14,15 Conversely, integrin engagement by vitronectin provides essential survival signals and protects glioma cells from apoptosis.16 There is some evidence for a role of resistance to anoikis in malignancy in that the failure to undergo detachment-induced cell death may confer a selective advantage for tumor cells en route to invasion and metastasis.
Cilengitide is currently being evaluated as a novel therapeutic agent for recurrent and newly diagnosed glioblastoma.17–20 Preliminary results indicate a preferential benefit from cilengitide added to temozolomide-based radiochemotherapy in patients with O6-methyl-guanine DNA methyltransferase (MGMT) promoter methylation.19 In the present study, we characterized the biological effects of cilengitide on glioma cell lines that express different levels of the target molecules, αvβ3 and αvβ5, and that can be modulated regarding their MGMT status and thus sensitivity to temozolomide.
Materials and Methods
Reagents
The RGD peptide cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)21 (cilengitide, EMD 121974) was kindly provided by Merck KGaA (Darmstadt, Germany). Temozolomide was supplied by Schering Plough (Kenilworth, NJ, USA). Ad-p53 was a kind gift from B. Vogelstein (Howard Hughes Medical Institute, Johns Hopkins Oncology Center, Baltimore, MD, USA). Propidium iodide (PI) and the murine IgG1 isotype control were obtained from Sigma (Deisenhofen, Germany); the RAD peptide cyclo-(Arg-Ala-Asp-DPhe-Val), zVAD-fmk (benzyloxy-carbonyl-Val-Ala-DL-Asp-fluoromethylketone), and DEVD-amc (N-acetyl-Asp-Glu-Val-Asp-aminomethyl-coumarin), from Bachem (Weil am Rhein, Germany). Antibodies used were anti-αvβ3 (LM609, Chemicon, Temecula, CA, USA), anti-αvβ5 (P1F6, Chemicon), anti-αv (17E6, Merck), anti-MGMT (Alpha Diagnostic, San Antonio, TX, USA), and anti-actin (sc-1616, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cell Culture
The glioma cell lines were kindly provided by N. de Tribolet (Lausanne, Switzerland) and have been characterized previously.22,23 LNT-229 p53 small interfering RNA (siRNA) cells, MGMT-expressing LNT-229 cells, and Bcl-XL–expressing or cytokine response modifier (crm)-A–expressing LN-18 and T98G cells, as well as neomycin or puromycin-resistant control cells, were generated as described.24–27 NIH-3T3 murine fibroblast cells were from the American Type Culture Collection (Manassas, VA, USA). All cell lines were maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (Gibco, Eggenstein, Germany) supplemented with 10% fetal calf serum, 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Conditioned medium was generated by incubating NIH-3T3 cells in serum-free medium for 48 h and stored at −80°C. Primary ex vivo glioma cell cultures were prepared from surgical samples.28 Adenoviral infection was performed as previously described.29
MGMT Gene Silencing
To silence MGMT gene expression, short-hairpin RNA (shRNA) sequences were cloned into the pSUPER-puro vector.30 Certain sequences below are identified as coding sequences by boldface type; sequences in regular type participate in hairpin formation. The sequences were as follows: MGMT shRNA, 5′-GATCCCCAAGGTTGTGAAATTCGGAGAATTCAAGAGA TTCTCCGAATTTCACAACCTTTTTTTGGAAA-3′ (nucleotides 936–958; Entrez gene ID 11798); and scrambled shRNA, 5′-GATCCCCACTACCGTTGT-TATAGGTCTTCAAGAGAGACCTATAACAACG-GTAGTTTTTTGGAAA-3′ without homology to any known expressed mRNA. LN-18 and T98G glioma cells were transfected with either pSuper-puro-MGMT or pSUPER-puro-scrambled using Metafectene PRO (Biontex, Martinsried, Germany). Stable cell lines were generated by puromycin selection (5 μg/mL). Surviving cells were expanded, and MGMT downregulation in the selected cell pools was controlled by immunoblot.
Growth and Viability Assays
For the evaluation of cell proliferation, glioma cells were plated in 96-well flat-bottom plates and 24 h later treated with serum-free medium alone or with cilengitide. The cells were pulse-labeled with 5-bromo-2-deoxyuridine (BrdU) for the last 4 h and then analyzed using the Amersham Cell Proliferation Biotrak enzyme-linked immunosorbent assay system (GE Healthcare, Buck-inghamshire, UK). To capture overall proliferation and to exclude the detachment effect, labeling medium was removed by air drying as recommended by the manufacturer for suspension cells. Acute cytotoxicity assays involved the exposure of glioma cells seeded at an appropriate density to increasing concentrations of cilengitide (0.1 μM to 1 mM) for different periods of time. Viability was assessed by PI staining and flow cytometry (CyAn ADP flow cytometer, Dako, Cambridge, UK; Summit software version 4.3, Dako, Fort Wayne, IN, USA). Clonogenic survival assays were performed by seeding 500 cells in six-well plates and exposing them to cilengitide or temozolomide for 24 h, followed by centrifugation at 1,200 rpm and further observation in drug-free complete medium for 7–21 days. Cell density or colonies were assessed using crystal violet staining. Colonies of more than 50 cells were counted. For cell cycle analysis, floating cells and adherent cells detached by trypsin treatment were collected, fixed in ethanol (70% vol/vol), and stained with PI (50 μg/mL) diluted in phosphate-buffered saline (PBS) containing RNase A (100 μg/mL). DNA content was analyzed by flow cytometry. In some experiments, cells were irradiated at 0.5, 1, 2, or 8 Gy (137Cs source, Gammacell 40 Exactor, MDS Nordion, Ottawa, ON, Canada). Caspase activity was assessed using the fluorescent substrate DEVD-amc as previously described26 and a Mithras LB 940 microplate reader (Berthold Technologies, Bad Wildbad, Germany). Cells were grown for several time periods in phenol red-free medium containing different cilengitide concentrations or CD95 ligand as a positive control. Subsequently, cells were lysed and exposed to DEVD-amc, both by adding the corresponding solutions.
Quantification of Integrin Expression
Cells were detached with nonenzymatic cell dissociation solution (Sigma-Aldrich, St. Louis, MO, USA) and incubated with primary antibody anti-αvβ3, anti-αvβ5, or isotype control diluted in PBS containing 0.5% bovine serum albumin, 2 mM EDTA, and 1 mM MgCl2. After exposure to the fluorescently conjugated secondary antibody, the cells were analyzed by flow cytometry.
Adhesion Assays
Cells were detached with nonenzymatic cell dissociation solution (Sigma-Aldrich) and allowed to adhere for 2 h on 96-well plates coated with human vitronectin (0.5 μg/well) or fibronectin (1.0 μg/well; both R&D Systems, Minneapolis, MN, USA) in the presence of different cilengitide concentrations. Attached cells were stained with crystal violet and quantified by measuring the absorbance at 560 nm. Alternatively, the detached cells were incubated with cilengitide, control peptide, or integrin antibodies, and their attachment was monitored by phase-contrast microscopy.
SDS-PAGE and Immunoblotting
For the preparation of protein extracts, floating and attached cells were harvested and lysed in a buffer containing 50 mM Tris-HCl, 120 mM NaCl, 5 mM EDTA, 0.5% Nonidet-P40, 2 μg/mL aprotinin, 10 μg/mL leupeptin, 100 μg/mL phenylmethylsulfonyl fluoride, 50 mM NaF, 200 μM NaVO5, and phosphatase inhibitor cocktails I and II (Sigma-Aldrich). Protein concentrations were determined using a Bradford assay (Bio-Rad, Hercules, CA, USA). Equal amounts of total protein were fractionated under reducing conditions by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted on nitrocellulose (Amersham, Braunschweig, Germany). Membranes were blocked in Tris-buffered saline containing 5% skim milk and 0.1% Tween-20 and incubated with the appropriate primary and secondary antibodies. Immune complexes were detected by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Invasion and Migration Assays
Matrigel invasion assays were performed as described previously,31,32 with some modifications. Briefly, 4 × 105 cells were plated on transwell chambers (12 mm diameter, 12-μm pore size; Corning Costar, Acton, MA, USA) precoated with 10 μg/cm2 Matrigel (Matrigel Basement Membrane Matrix, BD Biosciences, Bedford, MA, USA). NIH-3T3–conditioned medium was used as a chemoattractant. Following a 12-h incubation, noninvading cells were removed with cotton swabs, and invading cells were trypsinized and counted using the Cell-Titer-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA).33 Cell migration toward NIH-3T3– conditioned medium was assayed using modified Boyden chambers (6.5-mm diameter, 8-μm pore size; Corning Costar). After 16 h, migrated cells were fixed, stained, and counted by microscopic examination.
Statistical Analysis
Usually, experiments were performed three times, with similar results. Significance was tested using the two-tailed Student’s t-test. Synergy was assessed by the fractional product method.34
Results
Expression of αvβ3 and αvβ5 Integrins on Glioma Cell Lines
To evaluate the presence of the target molecules of cilengitide on the cell surface, αvβ3 and αvβ5 integrin expression was assessed by flow cytometry. Confirming our previous data,35 αvβ3 expression varied substantially among the cell lines tested. U87MG, LNT-229, and LN-308 cells revealed high αvβ3 levels, whereas LN-18 and LN-319 were negative. αvβ5 was expressed by all glioma cell lines examined (Table 1). As verified in LN-308 cells, αvβ3 and αvβ5 integrin expression did not change in response to prolonged cilengitide exposure (1 μM and 10 μM, 24 h; data not shown).
Table 1.
Cell surface αvβ3 and αvβ5 levels determined by flow cytometry and cilengitide-induced detachment
| Cell Line | αvβ3 (SFI) | αvβ5 (SFI) | OD (1 μM) (%) | OD (10 μM) (%) |
|---|---|---|---|---|
| A172 | 1.54 | 2.13 | 63.9 | 13.2 |
| LN-18 | 1.07 | 1.85 | 98.9 | 64.8 |
| LNT-229 | 25.81 | 1.85 | 91.9 | 84.8 |
| LN-308 | 14.5 | 1.54 | 99.0 | 71.2 |
| LN-319 | 1.07 | 3.29 | 95.5 | 34.3 |
| LN-428 | 2.07 | 2.95 | 86.3 | 55.9 |
| T98G | 4.9 | 1.78 | 96 | 72.8 |
| U87MG | 15.56 | 1.91 | 74 | 57.1 |
Abbreviations: SFI, specific fluorescence index; OD, optical density.
Integrin expression was determined by flow cytometry and is expressed as the mean specific fluorescence index. Residual nondetached cells after exposure to cilengitide at 1 μM or 10 μM for 24 h were stained with crystal violet and are quantified as optical densities relative to untreated cell cultures.
Modulation of Glioma Cell Attachment and Viability by Cilengitide
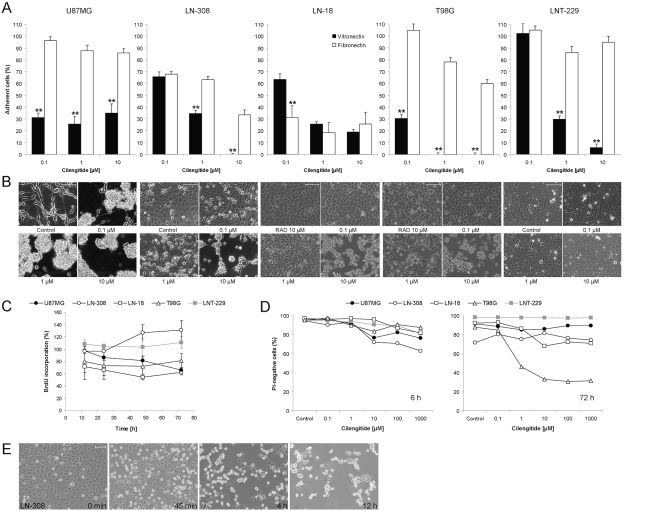
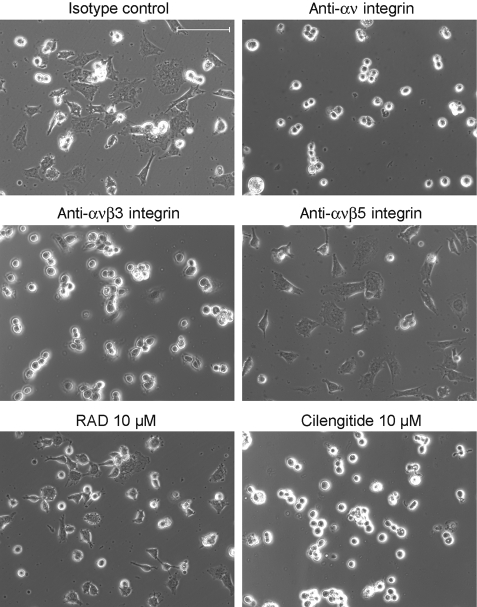
We confirmed that the adhesion of U87MG, LN-308, LN-18, T98G, and LNT-229 cells to vitronectin and, to a lesser extent, to fibronectin was concentration- dependently impaired by cilengitide (Fig. 1A). Both vitronectin and fibronectin are ligands for integrin αvβ3, whereas integrin αvβ5 exclusively binds to vitronectin. The lesser detachment on fibronectin-coated wells versus vitronectin can be explained by the fact that, apart from αvβ3, other integrins expressed by glioma cells, such as α5β1, may bind to fibronectin as well. Representative photomicrographs of cilengitide-treated U87MG, LN-308, LN-18, T98G, and LNT-229 cells are shown in Fig. 1B. The time course of cilengitide induced in LN-308 cells is exemplified in Fig. 1E. Whereas there was differential sensitivity to cilengitide-induced detachment, the control peptide RAD never induced detachment in any cell line tested. A comparative analysis of the data summarized in Table 1 and Fig. 1 shows no apparent link between the sensitivity to cilengitide-induced detachment and integrin expression. In contrast to cilengitide, antibodies to integrins αvβ3 or αvβ5 did not detach monolayer cultures. This was probably due to steric inability for the antibodies to reach their target. When the paradigm was changed to expose detached cells to cilengitide or the antibodies and then monitor their attachment, either cilengitide or antibodies to αv or αvβ3, but not the control peptide or the antibody to αvβ5, prevented the attachment of LN-308 cells (Fig. 2).
Fig. 1.
Effects of cilengitide on the adhesion, growth, and viability of cultured human glioma cells. (A) U87MG, LN-308, LN-18, T98G, and LNT-229 cells were cultured in the absence or presence of cilengitide for 2 h. Adherence was measured by crystal violet staining and is expressed in percentages relative to untreated cells. **p < 0.01, vitronectin versus fibronectin. (B) Cells were exposed to cilengitide at 0.1 μM, 1 μM, or 10 μM for 24 h and monitored by phase-contrast microscopy. Scale bars, 100 μm. For LN-18 and T98G cells, an alanine-substituted peptide (RAD) served as an additional nonbinding control. (C) U87MG, LN-308, LN-18, T98G, and LNT-229 cells treated with cilengitide (10 μM) for 12 h, 24 h, 48 h, or 72 h were assessed for proliferation by BrdU incorporation (mean ± SEM; n = 3). (D) The relative proportion of living (PI-negative) pooled adherent and detached cells at 6 h and 72 h after cilengitide exposure. (E) The time course of detachment in the presence of cilengitide (10 μM) in LN-308 cells. Scale bar, 100 μm.
Fig. 2.
Cilengitide and antibodies to αv or αvβ3 integrin, but not control peptide or antibodies to αvβ5, prevent the attachment of LN-308 cells. LN-308 cells were detached nonenzymatically, plated in the presence of cilengitide, RAD control peptide (10 μM), or antibodies to αv (10 μg/mL), αvβ3 (10 μg/mL), αvβ5 (10 μg/mL), or IgG1 isotype control (10 μg/mL) and monitored for attachment for 24 h. Scale bar, 100 μm.
To confirm that the effects of cilengitide were not restricted to long-term cultured cell lines, we performed similar studies in five primary ex vivo glioma cell cultures. There was strong detachment in three cell lines, whereas two did not detach (data not shown). BrdU incorporation assays performed over a time span of 72 h revealed that proliferation in U87MG, LN-308, LN-18, T98G, and LNT-229 cells was differently modulated by cilengitide: at 72 h posttreatment, BrdU incorporation was decreased by 35% in U87MG cells but increased by 30% in LN-308 cells (Fig. 1C). Flow cytometric analysis of cell cycle progression failed to identify any specific change of cell cycle distribution in either cell line in association with these changes in proliferation (data not shown).
We assessed whether cilengitide exposure resulted not only in detachment but also in a loss of viability. At 6–8 h after exposure, a PI-positive cell population of up to 15%–35% was detected. At later time points up to 120 h after exposure, there was no further increase in dead cells in either U87MG, LN-308, or LNT-229 cells. In contrast, a rising percentage of cells taking up PI was observed in T98G and LN-18 cells (Fig. 1D). In contrast, the viability of cells treated with the control peptide RAD did not differ from untreated controls; for example, there were 93.1% PI-negative LN-18 cells at 72 h after treatment with 10 μM RAD peptide versus 92.2% PI-negative untreated controls, and 80.5% versus 81.2% for T98G cells. To exclude a reduced stability of cilengitide in the cell culture in prolonged exposure assays, LN-308 cells were treated with cilengitide pre-incubated in medium at 37°C for 24 h. In these experiments, cellular viability and detachment did not differ from previous experiments with freshly dissolved substance (data not shown).
We sought to define the biochemical mode of the limited amount of cell death induced by cilengitide. Cilengitide did not induce DEVD-amc–cleaving caspase activity in U87MG, LN-308, or LNT-229 cells and induced little activity in LN-18 cells. In T98G cells, caspase activity was detectable at 24 h and 48 h, although not at 6 h, after cilengitide exposure (Table 2). Ectopic expression of crm-A in LN-18 or T98G cells or of the antiapoptotic protein marker Bcl-XL in LN-18 cells did not modify detachment, viability, or cell cycle distribution after cilengitide treatment (0.1 μM, 1 μM, 10 μM, 100 μM, or 1 mM, for 8 h, 24 h, 72 h, or 120 h; Table 3, data not shown). Similarly, although caspase activity was nullified, the broad-spectrum caspase inhibitor zVAD-fmk failed to prevent cell death (Table 3).
Table 2.
DEVD-amc (N-acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin)-cleaving caspase activity after 24 h of cilengitide treatment
| Cilengitide Concentration |
||||||
|---|---|---|---|---|---|---|
| Cell Line | Time Period | 0.1 μM | 1 μM | 10 μM | 100 μM | 1 mM |
| U87MG | 24 h | 100.9 | 102.4 | 101.4 | 100.7 | 102.0 |
| LN-308 | 24 h | 98.6 | 102.9 | 102.3 | 101.8 | 102.5 |
| LN-18 | 24 h | 99.5 | 102.6 | 107.1 | 106.7 | 110.0 |
| T98G | 6 h | 97.2 | 100.5 | 99.8 | 97.9 | 101.5 |
| 24 h | 105.6 | 131.9 | 144.5 | 140.8 | 154.4 | |
| 48 h | 92.4 | 144.1 | 192.2 | 202.1 | 226.2 | |
| LNT-229 | 24 h | 94.7 | 93.1 | 89.2 | 90.2 | 87.6 |
Caspase activity in all floating and adherent cells was assessed by DEVD-amc cleavage. Data are expressed as percentages relative to untreated control cells.
Table 3.
No protection from cell death afforded by cytokine response modifier (crm)-A, the antiapoptotic protein marker Bcl-XL, or the broad-spectrum caspase inhibitor zVAD-fmk
| Cilengitide Concentration |
||||||
|---|---|---|---|---|---|---|
| Cell Line | Control | 0.1 μM | 1 μM | 10 μM | 100 μM | 1 mM |
| T98G puromycin | 96.1 | 92.7 | 86.7 | 76.6 | 75.9 | 73.5 |
| T98G crm-A | 93.4 | 91.5 | 84.2 | 78.2 | 80.5 | 79.1 |
| T98G | 96.2 | 95.8 | 88.5 | 80.6 | 85.1 | 84.3 |
| T98G zVAD-fmk | 94.8 | 93.2 | 83.4 | 78.2 | 84.7 | 84.0 |
| LN-18 puromycin | 95.4 | 94.0 | 90.6 | 88.7 | 76.4 | 76.2 |
| LN-18 crm-A | 93.5 | 92.9 | 79.5 | 79.3 | 57.7 | 50.1 |
| LN-18 neomycin | 92.5 | 88.1 | 88.3 | 61.0 | 60.3 | 50.5 |
| LN-18 Bcl-XL | 91.4 | 90.1 | 82.8 | 57.1 | 56.9 | 41.3 |
| LN-18 | 92.8 | 87.9 | 84.0 | 53.9 | 51.4 | 45.1 |
| LN-18 zVAD-fmk | 91.7 | 89.1 | 84.3 | 65.5 | 61.4 | 57.9 |
The percentage of living (propidium iodide negative) cells at 24 h after cilengitide exposure was determined in glioma cell cultures engineered to express crm-A or Bcl-XL or coexposed to zVAD-fmk (50 μM).
The spectrum of cell lines used had already indicated that the effects of cilengitide were independent of the endogenous p53 status of the cell lines22 (Table 1) because both p53 wild-type and p53-deficient cell lines were susceptible to cilengitide-induced detachment. To formally confirm this, we took a twofold approach: we assessed the effects of cilengitide by phase-contrast microscopy and cell cycle analysis in p53 wild-type LNT-229 cells depleted of p53 by siRNA or p53 null LN-308 cells transduced with an adenoviral vector expressing wild-type p53.24,29 Neither intervention altered the cellular sensitivity to cilengitide in these assays (data not shown).
Modulation of Glioma Cell Motility and Invasiveness by Cilengitide
The infiltrative behavior of glioma cells is a function of two phenotypes: migration and invasiveness. Migration refers to the capacity of locomotion, whereas invasion involves migration plus a degradative function achieved by the liberation of proteolytic enzymes. Using a classical migration assay, cilengitide induced a concentration-dependent increase in migrated tumor cell numbers in U87MG and LNT-229 cells. The migration of LN-308 cells in that assay was unaffected by cilengitide. Using a classical Matrigel invasion assay, the invasiveness of LN-308 glioma cells was significantly reduced by cilengitide, to an extent that could not be attributed to the small cytotoxic effect observed after short-term incubation. In contrast, in U87MG or LNT-229 cells, there was no such effect (Table 4).
Table 4.
Migration and Matrigel invasion: differential modulation by cilengitide
| Measure | U87MG | LN-308 | LNT-229 |
|---|---|---|---|
| Migration | 256 ± 19** | 114 ± 13 | 408 ± 49* |
| Invasiveness | 114 ± 17 | 12 ± 6* | 79 ± 20 |
U87MG, LN-308, and LNT-229 cells were exposed to cilengitide (10 μM) and migration and invasiveness were assessed. Data are expressed as mean percentages relative to untreated control cultures and SEM, with triplicate samples from a representative experiment.
p < 0.05,
p < 0.01 relative to control.
Targeted Alterations of the MGMT Status Do Not Modulate Glioma Cell Sensitivity to Cilengitide
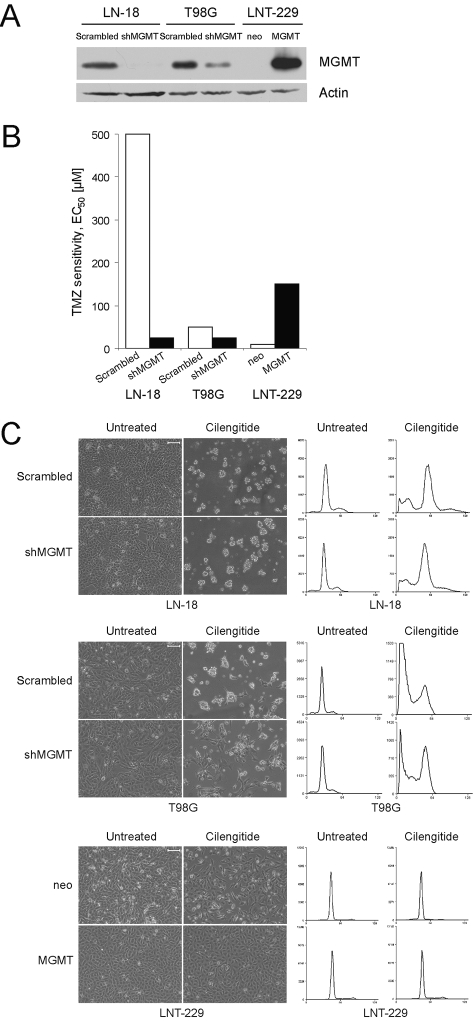
The apparent benefit derived from cilengitide when combined with radiotherapy and temozolomide specifically for patients with MGMT promoter methylation in study EMD 121974-01019 necessitated further studies on the relation between MGMT gene promoter status and cilengitide sensitivity in our cell culture paradigms. To this end, we studied either MGMT-positive T98G and LN-18 cells depleted of endogenous MGMT by shRNA or MGMT-negative LNT-229 cells transfected with an MGMT plasmid (Fig. 3A). MGMT depletion shifted the half-maximal effective concentration (EC50) for temozolomide in clonogenic cell death assays from 500 to 25 μM in LN-18 and from 50 to 25 μM in T98G cells, whereas the MGMT gene transfer into LNT-229 cells shifted the EC50 concentration from 10 to 150 μM (Fig. 3B).27 These paired cell lines were exposed to increasing cilengitide concentrations (0.1 μM, 1 μM, 10 μM, 100 μM, 1 mM) for different periods of time (6 h, 24 h, 72 h, 120 h) and assayed by phase-contrast microscopy and cell cycle analysis. In summary, neither changes induced by cilengitide in cell cycle distribution nor cell viability depended on the MGMT expression levels. Overall, these studies did not reveal a modulation of cilengitide sensitivity by altering MGMT expression. Representative data are shown in Fig. 3C. Appropriate control experiments were performed to ascertain that the modulation of MGMT expression in these glioma cell lines did not affect the cell surface expression of the target molecules of cilengitide, αvβ3, and αvβ5 (data not shown).
Fig. 3.
Glioma cell sensitivity to cilengitide is not related to methylguanine DNA methyltransferase (MGMT) expression. (A) Short-hairpin RNA-mediated MGMT (shMGMT) gene silencing (LN-18, T98G) or MGMT transgene expression (LNT-229) was verified by immunoblot. (B) MGMT depletion shifted EC50 for TMZ in clonogenic cell death assays from 500 to 25 μM in LN-18, from 50 to 25 μM in T98G cells, but the MGMT gene transfer into LNT-229 cells shifted the EC50 concentration from 10 to 150 μM. (C) After 72 h incubation with cilengitide (10 μM), LN-18, T98G, and LNT-229 cells were examined morphologically (left; scale bars, 100 μm) or stained with PI for the analysis of DNA content (right). Abbreviation: TMZ, temozolomide
Combined Modality Treatment Using Cilengitide and Irradiation or Temozolomide: Role of the MGMT Status
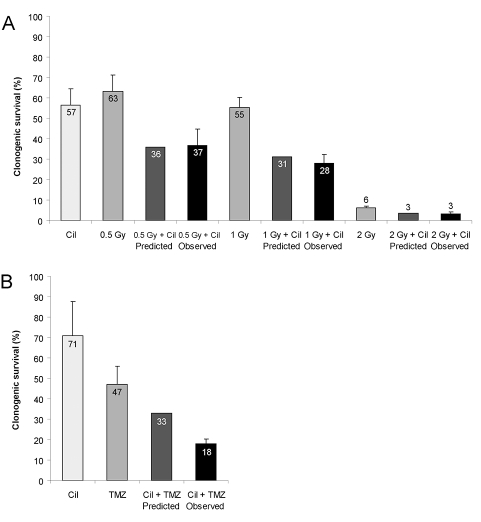
The presence of ECM increases resistance to cell- damaging agents such as ionizing radiation.36,37 In LN-308 glioma cells irradiated at 8 Gy, the irradiation-induced G2/M arrest was unaffected by cilengitide. Clonogenic survival assays indicated an additive effect of irradiation at 0.5, 1, or 2 Gy when combined with cilengitide at 10 μM in LN-308 cells (Fig. 4A). We also assessed the effects of combining cilengitide and temozolomide in clonogenic survival assays. At certain combinations of concentrations of both agents, there was a synergistic suppression of clonogenic survival in LN-308 cells as defined by the fractional product method (Fig. 4B).34 We examined whether a similar synergistic effect may be detected depending on the targeted MGMT alterations in LN-18, T98G, and LNT-229 cells. Using fixed concentrations of cilengitide and either equimolar or equipotent concentrations of temozolomide, we commonly observed additive but rarely synergistic activity of the combination (Fig. 5).
Fig. 4.
Suppression of clonogenic survival by temozolomide or irradiation in combination with cilengitide. LN-308 cells were irradiated (0.5 Gy, 1 Gy, 2 Gy) and exposed to cilengitide (Cil, 10 μM; A) or cotreated with temozolomide (TMZ, 10 μM; B) and assessed for clonogenic survival (mean and SEM, n = 3). According to the fractional product method,34 the predicted effect of cotreatment expressed as the product of the surviving fractions with single agent treatment is compared with the observed effect.
Fig. 5.
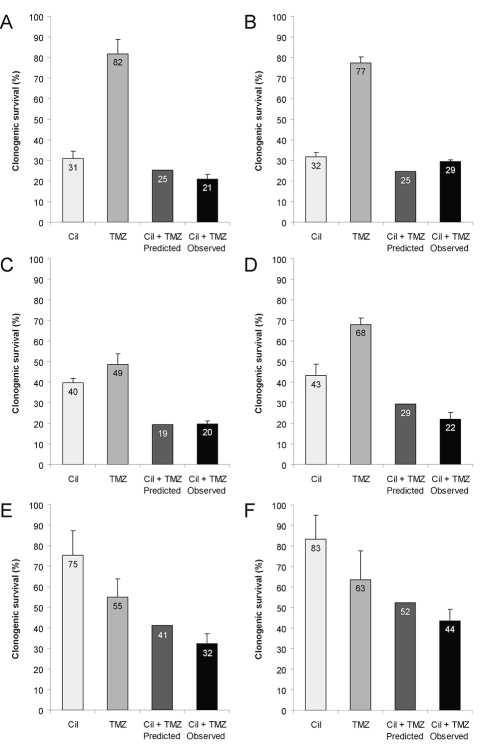
Methylguanine DNA methyltransferase (MGMT)-independent coinhibition of glioma cell growth by temozolomide (TMZ) and cilengitide. LN-18 scrambled short-hairpin RNA (shRNA) control (A), T98G scrambled shRNA control (C), LNT-229 neomycin (E), or LN-18 and T98G MGMT-knockdown (B, D) or LNT-229 MGMT-overexpressing (F) cells were treated with temozolomide (300 μM for LN-18 scrambled, 10 μM for LN-18 shMGMT, 50 μM for T98G scrambled, 25 μM for T98G shMGMT, 8 μM for LNT-229 neo, 100 μM for MGMT-overexpressing LNT-229 cells) or cilengitide (10 μM) or both and assessed for clonogenic survival (mean and SEM, n = 3).
Discussion
Current efforts at improving the progression-free survival for patients affected by glioblastoma include the addition of novel agents to the standard of care of involved field radiotherapy plus concomitant and adjuvant temozolomide.1 Among these, antiangiogenic agents such as bevacizumab, enzastaurin, or cilengitide have received particular attention. To understand how such agents may contribute to a favorable clinical outcome in patients with glioblastoma, it is important to dissect the molecular effects of such agents on glioma cells versus various host target cell populations, notably endothelial cells.
We here characterize strong detaching properties of clinically relevant concentrations of cilengitide in the majority of human glioma cell lines, associated with a moderate loss of viability (Fig. 1). This moderate loss of viability was unaffected by caspase inhibition or ectopic expression of Bcl-XL, suggesting that cilengitide-induced glioma cell death does not involve death-receptor– dependent or mitochondrial apoptosis pathways (Tables 2 and 3). This contrasts with human endothelial cells, which were reported to detach, to activate caspases, and to undergo apoptosis following cilengitide treatment.14 The effects on migration and invasiveness were highly variable across the three cell lines examined in more detail (Table 4). It remains a matter of controversy whether these assays are suitable to preclinically assess the clinical potential of agents such as cilengitide in vitro.
A phase II trial of cilengitide added to radiotherapy and temozolomide in patients with newly diagnosed glioblastoma appeared to provide a progression-free survival advantage specifically in patients with MGMT gene promoter methylation in the tumor19 who are most likely to benefit from temozolomide.38 The mechanisms underlying this apparent interrelation between the response to cilengitide and the MGMT status in the tumor tissue have remained obscure. Using genetically engineered cell lines, we determined that targeted alterations in MGMT expression do not alter cellular responses to cilengitide (Fig. 3). Depending on the cell line studied, temozolomide and irradiation had synergistic or additive, but never antagonistic, effects when combined with cilengitide in clonogenic survival assays (Fig. 4). Moreover, when equipotent concentrations of temozolomide were used in parallel assays of MGMT-deficient and MGMT-proficient LNT-229 cells, there were similar interactions with cilengitide (Fig. 5). Altogether, these studies did not lead to the identification of specific pharmacological interactions of temozolomide and cilengitide in vitro. Alternative explanations for the beneficial clinical effects derived from cilengitide in patients with MGMT-promoter–methylated tumors must therefore be sought. Likely, cilengitide will inhibit angiogenesis and therefore induce a more mature vessel phenotype that improves tumor perfusion and thus promotes temozolomide delivery to glioma cells in vivo. Accordingly, more temozolomide will benefit those patients likely to be responsive to temozolomide anyway (the “methylators”) but not those exhibiting primary resistance to temozolomide (the majority of the “nonmethylators”). If cilengitide eventually does find a place in the standard of care of glioblastoma, it will become a challenging task to dissect to what extent effects on the glioma cells contribute to the clinical activity of this agent.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Goldbrunner RH, Bernstein JJ, Tonn JC. ECM-mediated glioma cell invasion. Microsc Res Tech. 1998;43:250–257. doi: 10.1002/(SICI)1097-0029(19981101)43:3<250::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 6.Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 7.Bogler O, Mikkelsen T. Angiogenesis and apoptosis in glioma: two arenas for promising new therapies. J Cell Biochem. 2005;96:16–24. doi: 10.1002/jcb.20475. [DOI] [PubMed] [Google Scholar]
- 8.Bello L, Francolini M, Marthyn P, et al. Alpha(v)beta3 and alpha(v) beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–390. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Gladson CL, Wilcox JN, Sanders L, Gillespie GY, Cheresh DA. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci. 1995;108(pt 3):947–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- 10.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattern RH, Read SB, Pierschbacher MD, et al. Glioma cell integrin expression and their interactions with integrin antagonists: research article. Cancer Ther. 2005;3A:325–A340. [PMC free article] [PubMed] [Google Scholar]
- 12.Gladson CL. Expression of integrin alpha v beta 3 in small blood vessels of glioblastoma tumors. J Neuropathol Exp Neurol. 1996;55:1143–1149. doi: 10.1097/00005072-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Tucker GC. Alpha v integrin inhibitors and cancer therapy. Curr Opin Investig Drugs. 2003;4:722–731. [PubMed] [Google Scholar]
- 14.Maubant S, Saint-Dizier D, Boutillon M, et al. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108:3035–3044. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 15.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 16.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 17.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Goldbrunner R, Neyns B, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2007;25:2000. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 20.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dechantsreiter MA, Planker E, Matha B, et al. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 22.Weller M, Rieger J, Grimmel C, et al. Predicting chemoresistance in human malignant glioma cells: the role of molecular genetic analyses. Int J Cancer. 1998;79:640–644. doi: 10.1002/(sici)1097-0215(19981218)79:6<640::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Ishii N, Maier D, Merlo A, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22:8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- 25.Glaser T, Wagenknecht B, Weller M. Identification of p21 as a target of cycloheximide-mediated facilitation of CD95-mediated apoptosis in human malignant glioma cells. Oncogene. 2001;20:4757–4767. doi: 10.1038/sj.onc.1204498. [DOI] [PubMed] [Google Scholar]
- 26.Wagenknecht B, Schulz JB, Gulbins E, Weller M. Crm-A, bcl-2 and NDGA inhibit CD95L-induced apoptosis of malignant glioma cells at the level of caspase 8 processing. Cell Death Differ. 1998;5:894–900. doi: 10.1038/sj.cdd.4400435. [DOI] [PubMed] [Google Scholar]
- 27.Hermisson M, Klumpp A, Wick W, et al. O6-Methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 28.Bähr O, Rieger J, Duffner F, et al. P-glycoprotein and multidrug resistance-associated protein mediate specific patterns of multidrug resistance in malignant glioma cell lines, but not in primary glioma cells. Brain Pathol. 2003;13:482–494. doi: 10.1111/j.1750-3639.2003.tb00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naumann U, Kügler S, Wolburg H, et al. Chimeric tumor suppressor 1, a p53-derived chimeric tumor suppressor gene, kills p53 mutant and p53 wild-type glioma cells in synergy with irradiation and CD95 ligand. Cancer Res. 2001;61:5833–5842. [PubMed] [Google Scholar]
- 30.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 31.Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 32.Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- 33.de la Monte SM, Lahousse SA, Carter J, Wands JR. ATP luminescence-based motility-invasion assay. Biotechniques. 2002;33:98–100. 102. doi: 10.2144/02331rr01. 104 passim. [DOI] [PubMed] [Google Scholar]
- 34.Webb J. Effects of more than one inhibitor. In: Webb JL, editor. Enzymes and Metabolic Inhibitors. Vol. 1. New York: Academic Press; 1963. pp. 487–512. [Google Scholar]
- 35.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg. 2001;94:978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- 36.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89:2122–2132. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]