Abstract
Platelet-derived growth factor (PDGF) and its receptors (PDGFR) are frequently coexpressed in meningiomas, potentially contributing to their pathogenesis. The North American Brain Tumor Consortium conducted a phase II study to evaluate the therapeutic potential of imatinib mesylate (Gleevec), a PDGFR inhibitor, in patients with recurrent meningiomas. Patients were stratified into benign (WHO grade I) meningiomas or atypical (WHO grade II) and malignant (WHO grade III) meningiomas. The primary end point was 6-month progression-free survival (6M-PFS). Patients requiring enzyme-inducing antiepileptic drugs were ineligible. Patients received imatinib at a dose of 600 mg/day for the first 4-week cycle and then gradually increased to 800 mg/day for subsequent cycles, if there were no unacceptable toxicities. Plasma concentrations of imatinib and its active metabolite, CGP74588, were assessed. Twenty-three heavily pre-treated patients were enrolled into the study (13 benign, 5 atypical, and 5 malignant meningiomas), of whom 22 were eligible. The study was closed prematurely due to slow accrual. Tissue was available only from a minority of patients, but in these specimens there was uniform distribution of PDGFR, the drug target. Imatinib was generally well tolerated. Of 19 patients evaluable for response, 10 progressed at the first scan, and 9 were stable. There were no complete or partial responses. Overall median PFS was 2 months (range, 0.7–34 months); 6M-PFS was 29.4%. For benign meningiomas, median PFS was 3 months (range, 1.1–34 months); 6M-PFS was 45%. For atypical and malignant meningiomas, median PFS was 2 months (range, 0.7–3.7 months); 6M-PFS was 0%. Cycle 1 trough concentrations of imatinib and CGP74588 were 2,129 ± 1,600 ng/ml and 517 ± 326 ng/ml, respectively. Single-agent imatinib was well tolerated but had no significant activity in recurrent meningiomas. Trough plasma concentrations of imatinib exceeded those associated with imatinib activity in chronic myelogenous leukemia.
Keywords: imatinib, meningioma, pharmacokinetics, toxicities
Meningiomas are primary central nervous system (CNS) tumors composed of neoplastic meningothelial cells. They account for more than 20% of primary brain tumors in adults.1,2 The WHO classifies meningiomas into three main categories: benign meningioma (WHO grade I), atypical meningioma (WHO grade II), and anaplastic (malignant) meningioma (WHO grade III) based on the degree of anaplasia, number of mitoses, and presence of necrosis.3 Surgery is the treatment of choice for most meningiomas. Complete surgical resection confers long-term disease-free survival.4 In contrast, following subtotal resection, progression-free survival (PFS) is only 20%–60% at 10 years.4 A significant percentage of meningiomas, especially those involving the skull base, may not be surgically resectable.
Radiotherapy has assumed an increasingly important role in the treatment of meningiomas.5–9 For subtotally resected benign meningiomas that are treated with radiotherapy, the recurrence rate approximates that of completely resected tumors.6 Following radiotherapy for an atypical or malignant meningioma, however, rates of stable disease at 10 years are 13% and 0%, respectively.6 Stereotactic radiosurgery is useful in some cases, but patients with multiply recurrent meningiomas or large diffuse lesions may not be appropriate candidates.5,8
For patients who develop recurrent disease following surgery and radiation therapy, the treatment options are limited. Although meningiomas express high levels of hormonal receptors, hormonal therapy with antiestrogen and progesterone agents has been disappointing.10,11 Similarly, therapy with conventional chemotherapeutic agents is largely ineffective.12–18 Hydroxyurea produces disease stabilization in some patients,14,16–18 and there are anecdotal reports of patients responding to recombinant interferon-α 2b.15 However, for most patients who are refractory to conventional therapies, there are no effective options.
There is accumulating evidence that platelet-derived growth factor (PDGF) is important in meningiomas. PDGF ligands AA and BB and PDGF receptor-β (PDGFR-β) are present in the majority of meningiomas19–30 regardless of grade,23,28 which suggests the existence of an autocrine loop.25,28 Administration of PDGF-BB to meningioma cells in culture stimulates growth and activates mitogen-activated protein kinases22 and c-fos,23 while anti–PDGF-BB antibodies inhibit tumor cell growth.28 These data suggest that PDGFR inhibition may have therapeutic value in patients with recurrent meningiomas.
Imatinib mesylate (Gleevec) is an inhibitor of the Bcr-Abl, PDGFR-α and -β, c-Fms, and c-Kit tyrosine kinases,31 with antitumor activity in chronic myelogenous leukemia32,33 and in gastrointestinal stromal tumors.34 The ability of imatinib to inhibit PDGFR-α or -β (half-maximal inhibitory concentration [IC50] = 0.1 μM [49 ng/ml]) makes it a potentially attractive option for recurrent meningioma therapy. The North American Brain Tumor Consortium (NABTC) conducted a phase II study to evaluate the therapeutic efficacy of imatinib in patients with recurrent meningiomas.
Materials and Methods
Patient Eligibility
Adults (≥18 years old) with histologically confirmed meningiomas with unequivocal tumor recurrence by MRI scans were eligible. A baseline MRI was performed within 21 days of registration. Patients had to be on a stable steroid dosage for ≥5 days. Patients were initially required to have failed prior radiotherapy, but the protocol was later revised to improve accrual and allowed patients who did not have radiotherapy if there was a clear diagnosis of meningioma. Patients must have had an interval of ≥4 weeks from the completion of radio-therapy to study entry. There was no limitation on the number of prior therapies. Additional eligibility criteria included Karnofsky Performance Scale (KPS) score ≥ 60, life expectancy ≥ 8 weeks, adequate bone marrow function (absolute neutrophil count ≥ 2,000/μl, platelet count ≥ 120,000/μl, hemoglobin ≥ 10/dl), adequate liver function (alanine transaminase and alkaline phosphatase ≤2 times the upper limit of normal [ULN], bilirubin < 1.5 mg/dl), and adequate renal function (blood urea nitrogen or creatinine ≤ 1.5 times ULN). Due to potential teratogenicity of imatinib, all patients of child-bearing potential were required to use adequate birth control. Pregnant women, patients with serious intercurrent medical illnesses, and patients with conditions that could alter drug metabolism were excluded. To prevent a potential interaction with imatinib, patients on warfarin were excluded. Because patients on cytochrome P450 enzyme–inducing antiepileptic drugs (EIAEDs) have reduced exposure to imatinib when given at the same dose as patients not taking EIAEDs,35 patients on EIAEDs were ineligible.
The study was approved by the institutional review board (IRB) of each participating institution and was conducted in accordance with institutional and federal guidelines for human investigations. Patients were informed of the investigational nature of the study and signed IRB-approved informed consent forms prior to enrollment.
Stratification
Patients were stratified according to histology: patients with benign meningiomas, and patients with atypical or malignant meningiomas.
Evaluation during Study
Medical history was taken and physical examination was performed at baseline and at the start of each 4-week cycle. MRI was performed at baseline and prior to every other cycle (every 8 weeks). Response to treatment was determined using the Macdonald criteria.36 Responses had to be present for two consecutive scans (8 weeks) and were centrally reviewed at the University of California, San Francisco. Central review of pathology was conducted by a neuropathologist at M. D. Anderson Cancer Center (K.A.).
Treatment Plan
Imatinib was supplied by the National Cancer Institute, Division of Cancer Treatment and Diagnosis, Cancer Therapy Evaluation Program under a Cooperative Research and Development Agreement with Novartis Pharmaceuticals. Patients were treated with imatinib orally once (600 mg/day) or twice daily (800 mg/day).
Patients initially received imatinib at a dose of 600 mg/day for the first 4-week cycle. If there were no significant treatment-related toxicities, the dose of imatinib was increased to 700 mg/day for the second 4-week cycle, and then to 800 mg/day for subsequent cycles. Eight patients received 700 mg/day as the maximum dose, and two patients received 800 mg/day.
Pharmacokinetic Studies
Heparinized venous blood samples (7 ml) were collected before and at 1, 2, 4, and 24 h after ingestion of imatinib on day 8 of cycle 1 and, if possible, at the same times on day 8 of cycle 3. If possible, blood samples were also obtained before imatinib ingestion on day 15 of cycles 1 and 3 and day 1 of cycles 2, 3, and 4. Blood samples were centrifuged immediately at 1,200 g for 5 min. The resulting plasma was removed, transferred to poly-propylene screw-cap tubes, and frozen at −20°C until analysis for concentrations of imatinib and its active metabolite, CPG74588. Concentrations of imatinib and CGP74588 in plasma were determined using a validated liquid chromatography/mass spectrometry assay.37
Immunohistochemical Analysis of PDGFR Expression
Available paraffin-embedded tumor tissue was obtained when possible and stained for PDGFR-α and -β. Sections 5 μm thick were cut and immunostained with antibodies to CD31 (Dako, Glostrup, Denmark; prediluted) and to PDGFR-α and PDGFR-β (Cell Signaling Technology, Beverly, MA, USA; 1:100). Epitopes were unmasked by heating the sections in a water bath at 99°C for 20 min in ethylenediaminetetraacetic acid (EDTA) solution (pH 9.0), and endogenous peroxidase activity was blocked by incubating with 0.03% H2O2 in water for 20 min. Primary antibodies were applied overnight at 4°C (with exception of CD31, for which incubation was performed at room temperature for 15 min), and 3,3'-diaminobenzidine (Dako EnVision+ System, Peroxidase) was used for detection. Positive control and negative control (where antibody was omitted) were included in each batch. Semiquantitative analysis was performed scoring the intensity of staining of tumor cells and blood vessels on a scale from 0 (no staining) to 3 (strong staining).
Statistical Considerations
The primary end point was PFS at 6 months (6M-PFS) from the time of registration. The initial plan was to enroll 30 patients into each group. Sample size calculations considered that no effective therapy exists for recurrent meningioma following surgery and radiation therapy. For the atypical and malignant meningioma group, the null hypothesis indicated that 6M-PFS is ≤10%. In order for imatinib to warrant further study in atypical and malignant gliomas, we stipulated that it would need to increase 6M-PFS to ≥30%. With 30 patients enrolled, the study had 92% power to detect such a difference. The α error was set at 8%. Of 30 patients, at least six had to achieve 6M-PFS for the results to be interpreted as favorable. For the benign meningioma group, the null hypothesis indicated that 6M-PFS is ≤50%.11 In order for imatinib to warrant further study in benign meningiomas disease, we stipulated that it would need to increase 6M-PFS to ≥75%. With 30 patients enrolled, the study had 89% power to detect such a difference. The α error was set at 5%. Of 30 patients, at least 20 had to achieve 6M-PFS for the results to be interpreted as favorable.
Pharmacokinetic data were summarized as descriptive statistics. Imatinib and CGP74588 concentrations in day 8 pretreatment and 24-h (day 9 pretreatment) samples were compared using the two-tailed Wilcoxon signed ranks test (SPSS 15.0 for Windows; SPSS Inc., Chicago, IL). A p-value of 0.05 was considered statistically significant.
Results
Patient Characteristics
Between June 2003 and August 2005, 23 patients were enrolled into the study, of whom 22 were eligible. One patient had a second malignancy within 3 years of registration and was ineligible. Although the study was initially designed to include a larger number of patients, accrual was stopped earlier than planned because of limited activity and slow accrual. Patient characteristics are summarized in Table 1. There were 13 women and 10 men, with median age of 58 years (range, 26–75 years). Median KPS score was 80 (range, 60–100). One patient had neurofibromatosis. All patients had multiple prior surgeries, and 20 had prior radiotherapy. Seven patients had one prior chemotherapy (three benign meningiomas, two atypical meningiomas, and two malignant meningiomas). Three benign meningioma patients had one prior hormonal therapy.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Patients enrolled, n | 23 |
| Eligible patients, n | 22 |
| Sex, n (%) | |
| Male | 10 (43) |
| Female | 13 (57) |
| Age (years) | |
| Median | 58 |
| Range | 26–75 |
| KPS score | |
| Median | 80 |
| Range | 60–100 |
| Histology, n (%) | |
| Benign (WHO grade I) | 13 (57) |
| Atypical (WHO grade II) | 5 (22) |
| Anaplastic (WHO grade III) | 5 (22) |
| Prior surgeries, n | |
| Median | 3 |
| Range | 1–8 |
| Prior radiation therapies, n | |
| Median | 1 |
| Range | 0–5 |
| Prior chemotherapy regimens, n | |
| Median | 0 |
| Range | 0–2 |
Toxicity Data
Toxicity data are summarized in Table 2. Two patients discontinued treatment as a result of toxicities; otherwise, the drug was generally well tolerated. In addition, one patient had grade 4 neutropenia, and one had a small asymptomatic intratumoral hemorrhage. One patient each had grade 3 anemia, elevated serum glutamic pyruvic transaminase, dizziness, dehydration, neutropenia, and leukopenia, and two patients had grade 3 hypophosphatemia.
Table 2.
Grade 3 or 4 adverse events related to imatinib
| Adverse Event | Grade 3 | Grade 4 |
|---|---|---|
| Hematologic | ||
| Anemia | 1 | 0 |
| Leukopenia | 1 | 0 |
| Neutropenia | 1 | 1 |
| Nonhematologic | ||
| CNS hemorrhagea | 0 | 0 |
| Dehydration | 1 | 0 |
| Dizziness | 1 | 0 |
| Elevated serum glutamic pyruvic transaminase | 1 | 0 |
| Hypophosphatemia | 2 | 0 |
There was one grade 1 intratumoral hemorrhage.
Efficacy Data
Overall, 6M-PFS was 29.4%. For benign meningiomas, 6M-PFS was 45%; for atypical and malignant meningiomas, 6M-PFS was 0%. Overall median PFS was 2 months (range, 0.7–34 months). For benign meningiomas, median PFS was 3 months (range, 1.1–34 months); for atypical and malignant meningiomas, median PFS was 2 months (range, 0.7–3.7 months).
Nineteen patients were evaluable for response. Ten patients progressed at the first scan; nine were stable. There were no partial responses.
Pharmacokinetic Results
Complete cycle 1 day 8 pharmacokinetic data were available for 14 of the 22 eligible patients enrolled into the study (Table 3). Imatinib concentrations in day 8 pretreatment (2,129 ± 1,600 ng/ml) and 24-h (2,248 ± 1,408 ng/ml) plasma samples were not significantly different. This was also true for concentrations of CGP74588 in day 8 pretreatment (517 ± 326 ng/ml) and 24-h (517 ± 275 ng/ml) plasma samples. Maximum plasma concentrations of imatinib (4,452 ± 2,421 ng/ml) were observed at 2 h in 3 patients and at 4 h in the other 11, while maximum plasma concentrations of CGP74588 (906 ± 409 ng/ml) were observed at 2 h in 4 patients and at 4 h in the other 10.
Table 3.
Pharmacokinetic values for imatinib and its active metabolite, CGP74588, on day 8 of cycle 1 (ng/ml)
| Imatinib |
CGP74588 |
|||||
|---|---|---|---|---|---|---|
| Pre | 24 h | Cmax | Pre | 24 h | Cmax | |
| Mean | 2,129 | 2,248 | 4,452 | 517 | 517 | 906 |
| SD | 1,600 | 1,408 | 2,421 | 326 | 275 | 409 |
| Median | 1,702 | 2,066 | 4,361 | 456 | 477 | 847 |
| Minimum | 596 | 626 | 1,942 | 210 | 183 | 323 |
| Maximum | 6,532 | 6,133 | 12,063 | 1,310 | 1,068 | 2,000 |
Abbreviations: Pre, day 8 pretreatment sample; 24 h, 24-h (day 9 pretreatment) sample; Cmax, maximum plasma concentration.
Results of Immunohistochemical Studies
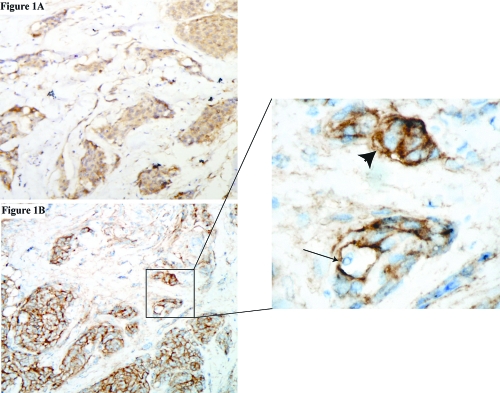
Tumor specimens were available from only seven patients. Five of these yielded adequate tissue to validate PDGFR-α and -β receptor expression status. Results are summarized in Table 4. Both receptor proteins were expressed on tumor cells in all patients (Fig. 1). Positive staining in tumor cells was diffusely present in >90% of tumor cells in all patients for both receptors, consistent with uniform distribution of the drug target in tumor cells. Overall levels of receptor expression were generally mild to moderate. Staining in vessels was also evaluated. PDGF-α receptor expression was present in most vessels in two patients and less than 5% of vessels in three patients. PDGF-β receptor expression was present in most vessels. There were insufficient samples to allow PDGFR-α and -β expression to be correlated with treatment outcome with imatinib.
Table 4.
Results of immunohistochemical studies
| PDGFR-α Staining |
PDGFR-β Staining |
|||||
|---|---|---|---|---|---|---|
| Specimen | Tumor Cellsa | Blood Vesselsb | Tumor Cellsa | Blood Vesselsb | Response | PFS (Months) |
| 1 | 2+ | Few | 3+ | Some | PD | 2 |
| 2 | 3+ | All | 3+ | All | SD | 9 |
| 3 | 3+ | Few | 3+ | Some | SD | 6 |
| 4 | 1+ | All | 1+ | All | PD | <1 |
| 5 | 3+ | Few | 3+ | Some | SD | 7 |
Abbreviations: PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; PD, progressive disease; SD, stable disease.
Expression-level intensity in tumor vessels: 1+, faint but distinct; 2+, strong; 3+, very strong.
Percentage of tumor cells expressing protein: few, less than 5%; some, 10%–20%; all, >60%.
Fig. 1.
PDGFR Immunohistochemistry. (A) Staining for PDGFR-α. (B) Staining for PDGFR-β. In the inset, the arrow shows a blood vessel; the arrowhead shows positive tumor cells. Magnification: A and B, ×10; inset, ×40.
Discussion
Coexpression of PDGF and PDGFR in meningiomas suggests the possibility that autocrine or paracrine loops drive meningioma growth. In this small phase II study, the NABTC evaluated the therapeutic efficacy of imatinib mesylate, a PDGFR inhibitor, in patients with recurrent meningiomas. The drug was generally well tolerated, with only one grade 4 toxicity. Imatinib has been associated with an increased incidence of intratumoral hemorrhages in previous trials in patients with glioblastomas35 and brainstem gliomas,38 but in this study only one patient developed an asymptomatic intratumoral hemorrhage. However, imatinib showed no significant evidence of activity. No patients achieved a radiographic response. 6M-PFS for benign meningiomas was 45%, and for atypical and malignant meningiomas, it was 0%. Because data on the natural history of untreated recurrent meningiomas are limited, our results are difficult to interpret. The present results with imatinib appear inferior to those reported with RU48611 and hydroxyurea.16,17 However, the population studied here includes more patients with atypical and malignant meningiomas and more extensive pretreatment. These factors potentially account for poorer outcomes.
The pharmacokinetic data in this trial are consistent with previously published descriptions of imatinib pharmacokinetics.39,40 In only two patients were trough imatinib concentrations less than the 1,000 ng/ml value being targeted in patients with chronic myelogenous leukemia,41,42 and in eight patients, trough concentrations were >1,500 ng/ml. The range of plasma trough imatinib concentrations (596–6,532 ng/ml) is approximately 12- to 133-fold greater than the reported IC50 for imatinib inhibition of PDGFR (49 ng/ml); however, such data need to be considered in light of the high protein binding of imatinib in plasma compared with in vitro studies, where there is a much lower protein concentration.
Several factors may contribute to the disappointing results with single-agent imatinib in meningiomas. There is a paucity of data regarding the molecular changes that drive meningioma growth. Although PDGFR autocrine/paracrine loops contribute to meningioma proliferation, it is unclear whether PDGFRs are the critical tyrosine kinases for tumor maintenance. It is possible that inhibition of PDGFR alone is insufficient to prevent growth of meningiomas and that other tyrosine kinases, such as the epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor, may play a greater role in maintaining the transformed phenotype. In addition, most of the patients in this study had very advanced disease and were heavily pretreated. Molecular alterations in these patients are highly complex, and single-agent tyrosine kinase inhibitors such as imatinib may be effective only in patients with less advanced disease. An attempt was made to correlate tumor genotype with response. Although expression of both receptors was validated in all five tumors, because only five specimens were available for analysis, no correlation was observed between expression of PDGFR-α and -β on meningiomas and PFS following therapy with imatinib. In this context, it is important to note that PDGFRs are also present on the pericytes of blood vessels, and blocking PDGFR signaling in these cells makes them dysfunctional and can make blood vessels more leaky.43 This enhanced leakiness could potentially increase edema and compromise the efficacy of agents that primarily target PDGFR.44
With other brain tumors such as malignant gliomas, limited penetration of imatinib across the blood–brain barrier (BBB) may limit efficacy. Because meningiomas are usually supplied by meningeal vessels and do not have an intact BBB, this is less likely to be an issue for meningioma. Nonetheless, the extent to which imatinib penetrates meningiomas and effectively inhibits PDGFR in vivo is unknown. More potent PDGFR inhibitors with improved penetration, or the combination of imatinib with drugs that inhibit P-glycoprotein or other drug efflux pumps, might improve efficacy.
Several studies suggest that the combination of imatinib with hydroxyurea may be more effective against recurrent malignant gliomas than either agent alone.45,46 Factors that may contribute to this increased activity include complementary antiangiogenic activity, enhanced chemotherapy delivery related to reduction of tumor interstitial pressure by imatinib, or enhanced drug delivery related to modulation of ATP-dependent transporter proteins. Because hydroxyurea itself has modest single-agent activity in meningiomas, a trial evaluating the combination of imatinib with hydroxyurea in recurrent meningiomas is under way.
This trial represents one of the first studies of a targeted molecular agent in meningiomas. Although imatinib itself showed no significant activity, the use of targeted agents for meningiomas represents a promising and largely unexplored therapeutic area.47 EGFRs are expressed in more than 60% of meningiomas 48,49 and represent a possible target. The NABTC recently completed two small studies of EGFR inhibitors (gefitinib and erlotinib) in patients with recurrent meningiomas. The results of these studies should become available in the near future. There is also increasing evidence that vascular EGFR and the mitogen-activated protein kinase and phosphoinositide-3-kinase/Akt pathways are upregulated in meningiomas, making them potential targets of therapy.47 However, the rational use of the growing armamentarium of targeted agents in meningiomas will depend on improved understanding of the critical molecular changes that drive the growth of these tumors.
In summary, single-agent imatinib was reasonably well tolerated but has minimal activity in recurrent meningiomas. Treatment of meningiomas with imatinib in combination with hydroxyurea or with other targeted molecular agents may be more effective.
Acknowledgments
Support for this article, which is designated the NABTC Study 01–08, was received through prime award CA 62399 from the National Institutes of Health to the North American Brain Tumor Consortium (NABTC) investigators who coauthored this article and through the individual NABTC and General Clinical Research Center (GCRC) grants listed below.
| Institution | Investigator | NABTC Grant | GCRC Grant |
|---|---|---|---|
| Dana-Farber Cancer Center | Patrick Y. Wen* | CA 62407 | None |
| Memorial Sloan-Kettering Cancer Center | Lisa M. DeAngelis* | CA 105663 | None |
| University of California, Los Angeles | Timothy F. Cloughesy* | CA 62399 | M01-RR0865 |
| University of California, San Francisco | Michael D. Prados* Susan M. Chang | CA 62422 | M01-RR00079 |
| University of Texas M. D. Anderson Cancer Center | W.K.A. Yung* Mark Gilbert | CA 62412 | None |
| University of Texas Southwestern Medical Center | Karen Fink* | CA 62455 | M01-RR00633 |
| University of Pittsburgh Cancer Institute | Frank Lieberman* | CA 62404 | M01-RR00056 |
| University of Texas, San Antonio | John Kuhn* | CA 62426 | M01-RR0134 |
| University of Wisconsin, Madison | Minesh Mehta* H. Ian Robins | CA 62421 | M01-RR03186 |
Principal investigator
References
- 1.Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197–205. doi: 10.1007/BF00165649. [DOI] [PubMed] [Google Scholar]
- 2.DeMonte F, Marmor E, Al-Mefty O. Meningiomas. In: Kaye AH, Laws ER Jr, editors. Brain Tumors. London: Churchill Livingstone; 2001. pp. 719–750. [Google Scholar]
- 3.Kleihues P, Cavenee WK, editors. WHO Classification of Tumors of the Nervous System. Lyon, France: International Agency for Research on Cancer; 2000. Meningeal tumors; pp. 175–196. [Google Scholar]
- 4.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Levy EI, Niranjan A, Flickinger JC, Lunsford LD. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44–50. doi: 10.3171/jns.1999.91.1.0044. [DOI] [PubMed] [Google Scholar]
- 6.Maor MH. Radiotherapy for meningiomas. J Neurooncol. 1996;29:261–267. doi: 10.1007/BF00165656. [DOI] [PubMed] [Google Scholar]
- 7.Miralbell R, Linggood RM, de la Monte S, Convery K, Munzenrider JE, Mirimanoff RO. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:157–164. doi: 10.1007/BF00172765. [DOI] [PubMed] [Google Scholar]
- 8.Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029–1038. doi: 10.1097/00006123-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BW, Jr, Marcus RB, Jr, Friedman WA, Ballinger WE, Jr, Million RR. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299–304. doi: 10.1016/s0360-3016(98)90008-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15:75–77. doi: 10.1007/BF01050266. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg SM, Rankin C, Townsend J, et al. Phase III double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unresectable meningioma. Proc Am Soc Clin Oncol. 2001;20:222. [Google Scholar]
- 12.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210–1212. doi: 10.1212/01.wnl.0000118300.82017.f4. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78:271–276. doi: 10.1007/s11060-005-9093-x. [DOI] [PubMed] [Google Scholar]
- 14.Cusimano MD. Hydroxyurea for treatment of meningioma. J Neurosurg. 1998;88:938–939. doi: 10.3171/jns.1998.88.5.0938. [DOI] [PubMed] [Google Scholar]
- 15.Kaba SE, DeMonte F, Bruner JM, et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40:271–275. doi: 10.1097/00006123-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341–346. doi: 10.3171/jns.2002.97.2.0341. [DOI] [PubMed] [Google Scholar]
- 17.Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18:495–499. doi: 10.1080/02688690400012392. [DOI] [PubMed] [Google Scholar]
- 18.Schrell UM, Rittig MG, Anders M, et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840–844. doi: 10.3171/jns.1997.86.5.0840. [DOI] [PubMed] [Google Scholar]
- 19.Black PM, Carroll R, Glowacka D, Riley K, Dashner K. Platelet-derived growth factor expression and stimulation in human meningiomas. J Neurosurg. 1994;81:388–393. doi: 10.3171/jns.1994.81.3.0388. [DOI] [PubMed] [Google Scholar]
- 20.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60:168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 21.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 22.Johnson MD, Woodard A, Kim P, Frexes-Steed M. Evidence for mitogen-associated protein kinase activation and transduction of mitogenic signals by platelet-derived growth factor in human meningioma cells. J Neurosurg. 2001;94:293–300. doi: 10.3171/jns.2001.94.2.0293. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch M, Wilson JC, Black P. Platelet-derived growth factor in human brain tumors. J Neurooncol. 1997;35:289–301. doi: 10.1023/a:1005872718547. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell M, Galanopoulos T, Hedley-Whyte ET, Black PM, Antoniades HN. Human meningiomas co-express platelet-derived growth factor (PDGF) and PDGF-receptor genes and their protein products. Int J Cancer. 1990;46:16–21. doi: 10.1002/ijc.2910460106. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell M, Naber SP, Wolfe HJ, et al. Coexpression of platelet-derived growth factor (PDGF) and PDGF-receptor genes by primary human astrocytomas may contribute to their development and maintenance. J Clin Invest. 1990;86:131–140. doi: 10.1172/JCI114675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagashima G, Asai J, Suzuki R, Fujimoto T. Different distribution of c-myc and MIB-1 positive cells in malignant meningiomas with reference to TGFs, PDGF, and PgR expression. Brain Tumor Pathol. 2001;18:1–5. doi: 10.1007/BF02478918. [DOI] [PubMed] [Google Scholar]
- 27.Nister M, Claesson-Welsh L, Eriksson A, Heldin CH, Westermark B. Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem. 1991;266:16755–16763. [PubMed] [Google Scholar]
- 28.Todo T, Adams EF, Fahlbusch R, Dingermann T, Werner H. Autocrine growth stimulation of human meningioma cells by platelet-derived growth factor. J Neurosurg. 1996;84:852–859. doi: 10.3171/jns.1996.84.5.0852. [DOI] [PubMed] [Google Scholar]
- 29.Wang JL, Nister M, Hermansson M, Westermark B, Ponten J. Expression of PDGF beta-receptors in human meningioma cells. Int J Cancer. 1990;46:772–778. doi: 10.1002/ijc.2910460504. [DOI] [PubMed] [Google Scholar]
- 30.Yang SY, Xu GM. Expression of PDGF and its receptor as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J Clin Neurosci. 2001;8(suppl 1):49–53. doi: 10.1054/jocn.2001.0877. [DOI] [PubMed] [Google Scholar]
- 31.Buchdunger E, Zimmermann J, Mett H, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. [PubMed] [Google Scholar]
- 32.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 33.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 34.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 35.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 37.Parise RA, Ramanathan RK, Hayes MJ, Egorin MJ. Liquid chromatographic-mass spectrometric assay for quantitation of imatinib and its main metabolite (CGP 74588) in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:39–44. doi: 10.1016/s1570-0232(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 38.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro-Oncology. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44:879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 40.Widmer N, Decosterd LA, Csajka C, et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol. 2006;62:97–112. doi: 10.1111/j.1365-2125.2006.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 42.Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 43.Jain RK, Lahdenranta L, Fukumura D. Targeting platelet-derived growth factor signaling in carcinoma-associated fibroblasts controls cervical cancer in mouse model. PLoS Med. 2008;5(1):e24. doi: 10.1371/journal.pmed.0050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain RK. Normalization of the tumor vasculature: an emerging concept in anti-angiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 45.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005;16:1702–1708. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 46.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 47.Norden AD, Drappatz J, Wen PY. Targeted drug therapy for meningiomas. Neurosurg Focus. 2007;23:E12. doi: 10.3171/FOC-07/10/E12. [DOI] [PubMed] [Google Scholar]
- 48.Andersson U, Guo D, Malmer B, et al. Epidermal growth factor receptor family (EGFR, ErbB2–4) in gliomas and meningiomas. Acta Neuropathol. 2004;108:135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 49.Carroll RS, Black PM, Zhang J, et al. Expression and activation of epidermal growth factor receptors in meningiomas. J Neurosurg. 1997;87:315–323. doi: 10.3171/jns.1997.87.2.0315. [DOI] [PubMed] [Google Scholar]