Abstract
Voriconazole is a broad spectrum antifungal agent for treating life-threatening fungal infections. Its clearance is approximately 3-fold higher in children compared with adults. Voriconazole is cleared predominantly via hepatic metabolism in adults, mainly by CYP3A4, CYP2C19, and flavin-containing monooxygenase 3 (FMO3). In vitro metabolism of voriconazole by liver microsomes prepared from pediatric and adult tissues (n = 6/group) mirrored the in vivo clearance differences in children versus adults, and it showed that the oxidative metabolism was significantly faster in children compared with adults as indicated by the in vitro half-life (T1/2) of 33.8 ± 15.3 versus 72.6 ± 23.7 min, respectively. The Km for voriconazole metabolism to N-oxide, the major metabolite formed in humans, by liver microsomes from children and adults was similar (11 ± 5.2 versus 9.3 ± 3.6 μM, respectively). In contrast, apparent Vmax was approximately 3-fold higher in children compared with adults (120.5 ± 99.9 versus 40 ± 13.9 pmol/min/mg). The calculated in vivo clearance from in vitro data was found to be approximately 80% of the observed plasma clearance values in both populations. Metabolism studies in which CYP3A4, CYP2C19, or FMO was selectively inhibited provided evidence that contribution of CYP2C19 and FMO toward voriconazole N-oxidation was much greater in children than in adults, whereas CYP3A4 played a larger role in adults. Although expression of CYP2C19 and FMO3 is not significantly different in children versus adults, these enzymes seem to contribute to higher metabolic clearance of voriconazole in children versus adults.
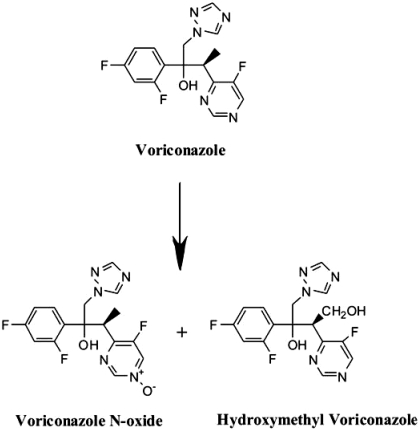
Voriconazole (Vfend; Pfizer, New York, NY) is a potent triazole antifungal agent with broad activity for treating life-threatening fungal infections in children (Steinbach and Benjamin, 2005). In adults, voriconazole is given twice daily: 200 to 300 mg p.o. or 3 to 4 mg/kg i.v. The absolute bioavailability is estimated to be 96%, and its systemic clearance is 2 ml/min/kg (Purkins et al., 2002). Voriconazole is cleared predominantly by oxidative metabolism in the liver (European Public Assessment Report, http://www.emea.europa.eu/humandocs/Humans/EPAR/vfend/vfend.htm, http://www.pfizer.com/pfizer/download/uspi_vfend.pdf; Hyland et al., 2003; Roffey et al., 2003; Yanni et al., 2008). CYP2C19, CYP3A4, flavin-containing monooxygenase (FMO), and to a lesser extent CYP2C9 contribute to the oxidative metabolism of voriconazole by human liver microsomes. The two major metabolites are the N-oxide and the hydroxymethyl derivative as depicted in Fig. 1 (Hyland et al., 2003; Roffey et al., 2003; Theuretzbacher et al., 2006; Murayama et al., 2007). The N-oxide, which accounts for 72% of the circulating metabolites of voriconazole (Roffey et al., 2003), is predominantly formed by CYP3A4 (Hyland et al., 2003) and FMO3 (Yanni et al., 2008); CYP3A5 and CYP3A7 form this metabolite 4- and 15-fold less efficiently than CYP3A4 (Murayama et al., 2007). FMO1 also forms voriconazole N-oxide (Yanni et al., 2008); however, it is only expressed in fetal livers (Hines and McCarver, 2002), and therefore only FMO3 was considered in the present study.
Fig. 1.
Chemical structures of voriconazole and human metabolites. Voriconazole N-oxide, the major circulating metabolite, and hydroxymethyl voriconazole as it has been reported by Hyland et al. (2003) and Murayama et al. (2007).
In multidose studies, the area under the plasma concentration-time curve (AUC) and Cmax of voriconazole in adult subjects increased nonlinearly over a narrow dose range (e.g., 3–5 mg/kg or 200–400 mg), thereby suggesting saturation of clearance (Purkins et al., 2002; Walsh et al., 2004; Levêque et al., 2006). In contrast to adults, the plasma clearance of voriconazole in children age 2 to 11 years was found to be almost 3-fold higher; furthermore, both the AUC and Cmax of voriconazole increased linearly in children (Walsh et al., 2004; Levêque et al., 2006). Recent population pharmacokinetic analyses of voriconazole in children 2 to 12 years of age have shown that an intravenous dosage of 7 mg/kg in young children yields a similar exposure of 3 to 4 mg/kg given to adults. Furthermore, oral bioavailability of voriconazole was found to be much lower (44.6%) in children than in adults (∼96%) (Karlsson et al., 2009).
Several factors may contribute to observed differences of drug disposition between children and adults. These factors include organ maturation, body composition, changes in liver size, liver blood flow, extent of plasma protein binding, changes in the hormonal levels, and alteration in the expression and/or catalytic activity of some of the drug-metabolizing enzymes (Hines and McCarver, 2002; Kearns et al., 2003; Chen et al., 2006; Kennedy, 2008). For example, drugs that are metabolized by hepatic CYP2C9/19, such as phenytoin, omeprazole, and sirolimus, show higher clearance in children between 2 and 10 years of age than in adults (Litalien et al., 2005; Kennedy 2008; Filler et al., 2008).
Voriconazole, because it is cleared predominantly by hepatic metabolism, may have an increased clearance in children due to differences in the expression or catalytic activity of one or more drug-metabolizing enzymes. Hence, in vitro metabolism of voriconazole by liver microsomes from children between 2 and 10 years of age was compared with that from adults. Results showed that metabolism of voriconazole by liver microsomes from children versus adults mirrored the differences in the clearance of the drug in the two populations. The likely role of CYP2C19, CYP3A4, and FMO3 in altering the metabolic clearance by liver microsomes from adult versus children was evaluated.
Materials and Methods
Chemicals and Reagents.
Voriconazole was generously supplied by Pfizer Global Research and Development (Groton, CT). Cytochrome P450 (P450) and FMO substrates, metabolites, and inhibitors were obtained from commercial sources. Primary antibodies for immunoblotting analysis and recombinant P450 enzymes and FMO3 were purchased from BD Gentest (Woburn, MA). Voriconazole N-oxide was synthesized as described in Yanni et al. (2008). Frozen liver tissues from adults and children were obtained from Comparative Human Tissue Network (Columbus, OH) under an approved UNC-Chapel Hill Institutional Review Board protocol. The ages of children donors were between 2 and 8 years old, whereas the adult donors were >18 years old. The normal liver tissues, free of disease, were snap frozen within 6 h post mortem.
Preparation and Characterization of Liver Microsomes from Adults and Children.
Liver microsomes were prepared from the frozen adult and pediatric tissues by using a standard procedure. Protein concentrations were determined by using the bicinchoninic acid protein assay (Pierce, Rockford, IL) against bovine serum albumin as a standard. All microsomal preparations were prescreened for the overall metabolic capacity, and only those preparations that showed robust oxidative metabolism, as indicated by ability to extensively metabolize 7-ethoxycoumarin (20 μM, >80% metabolized in 60 min, 1 mg microsomal protein/ml) (Shimada et al., 1999), were selected for this study. Six samples of liver microsomes from each group were selected accordingly. The selected microsomal samples were then characterized for P450 activities as described previously (Yanni et al., 2008).
Voriconazole Oxidative Metabolism by Liver Microsomes from Adults and Children.
Initial rate of voriconazole (2 μM) consumption with respect to time and protein was determined as described previously (Yanni et al., 2008). Liver microsomes (1 mg/ml) from each subject were mixed with 2 mM NADPH, 100 mM phosphate buffer (pH 7.4), and 3 mM magnesium chloride. The metabolic reaction was initiated by addition of voriconazole, and aliquots were then removed at defined time points from 0 to 20 min and mixed with ice-cold methanol containing an internal standard (diclofenac) to terminate the reaction. After centrifugation at 10,000g for 10 min, the supernatant was evaporated to dryness, and the residue was then dissolved in 10% acetonitrile in water and analyzed for voriconazole by using high-performance liquid chromatography (HPLC)-UV (254 nm). The percentage of voriconazole remaining was determined by comparing voriconazole/internal standard peak area ratio at each time point to that at time 0 (100%). The slope of the log (percentage remaining) versus incubation time plot was used to determine in vitro half-life (T1/2) (Obach and Reed-Hagen, 2002). For kinetic analysis, voriconazole (0.5 to 500 μM) was metabolized as described above, and the voriconazole N-oxide formed was analyzed by HPLC-MS/MS (Yanni et al., 2008). Nonlinear regression analysis (WinNonlin version 4.0; Pharsight, Cary, NC) was performed by using the Michaelis-Menten equation to determine apparent Km and Vmax for voriconazole N-oxide formation by liver microsomes from each subject.
Role of P450 and FMO Enzymes in the Formation of Voriconazole N-Oxide by Liver Microsomes from Adults and Children.
To compare the contribution of CYP2C19, CYP3A4, or FMO in voriconazole metabolism by liver microsomes from adults and children, metabolism of voriconazole by liver microsomes derived from adults (pool of six samples) or children (pool of six samples) was evaluated in the presence of fluvoxamine (CYP2C19 inhibition, 3 μM), ketoconazole (CYP3A4 inhibition, 3 μM), or with heat-inactivated microsomes (FMO inhibition, 45°C for 5 min), respectively. At the concentrations used, the inhibitors were selective for the respective enzymes and effective under the experimental conditions used (Yanni et al., 2008). Voriconazole N-oxide formed under each condition was measured, and the N-oxidation activity corresponding to CYP2C19, CYP3A4, or FMO3 was estimated by comparing the inhibited activity with the no-inhibition control activity. The percentage contribution of these three enzymes to the overall voriconazole N-oxide formation (100%) by adults and children was also estimated.
Expression of CYP2C19, CYP3A4, and FMO3 in Liver Microsomes from Children and Adults.
A pool of liver microsomes from the six adults or six children was used to determine the differential expression of CYP2C19, CYP3A4, and FMO3 proteins in children versus adults. Liver microsomes and recombinant protein standards for CYP3A4, CYP2C19, and FMO3 were subjected to gel electrophoresis, and the proteins were transferred to nitrocellulose membranes; subsequently, the nitrocellulose membranes were incubated with anti-CYP3A4, anti-CYP2C19, and anti-FMO3 at a dilution of 1:4000, 1:500, and 1:1000, respectively, for 16 h at 4°C. The bound antibody was detected by Bio-Rad VersaDoc Imaging System (Bio-Rad Laboratories, Hercules, CA). The intensity of the bands corresponding to CYP3A4 (57 kDa), CYP2C19 (52 kDa), or FMO3 (57 kDa) was determined, and expression based on the amount of loaded protein was calculated.
Analytical Methods.
Voriconazole in the incubation samples was chromatographically separated by using an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) and C18 Zorbax Eclipse column (150 × 4.6 mm, 5 μ; Agilent Technologies) as previously reported (Yanni et al., 2008). In brief, the analytes were eluted from the column with a linear gradient of mobile phase A/B (v/v) starting from 95:5 at 0 min to 30:70 in 10 min at a flow rate of 1.1 ml/min; the mobile phase A was 5 mM ammonium acetate with 0.1% formic acid, pH 4, and mobile phase B was acetonitrile with 0.1% formic acid. Voriconazole was detected by UV at its λmax of 254 nm; the retention time was 8.2 min. Voriconazole N-oxide in samples from the metabolism studies was analyzed by an HPLC-MS/MS method on an Applied Biosystems API 4000-triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex Instruments, Foster City, CA) fitted with TurbolonSpray interface in conjunction with Shimadzu solvent delivery system (Shimadzu, Kyoto, Japan) and a CTC-PAL autosampler (LEAP Technologies, Carrboro, NC). The HPLC separation was achieved on an Aquasil C18 analytical column (50 × 2.1, 5 μ; Thermo Fisher Scientific, Waltham, MA) using gradient elution of mobile phases from 10% B in A (v/v) at 0.5 min to 95% B in A (v/v) at 3 min at a flow rate of 0.7 ml/min (injection volume was 5 μl); mobile phase A was 0.1% formic acid, and mobile phase B was methanol with 0.1% formic acid. Multiple reaction monitoring was used to monitor voriconazole (m/z transition 350→127), the N-oxide (m/z transition 366→224), and the internal standard (diclofenac, m/z transition 296→215) in positive ion mode. The retention times for the N-oxide and voriconazole were 2.5 min and 2.8 min, respectively. For quantitative determination of the N-oxide formed, a calibration curve was constructed using the peak area ratio of authentic standard of the N-oxide (Yanni et al., 2008) to the internal standard over a concentration range of 1 nM to 5 μM (R2 > 0.99). The quantitative determination of 4-hydroxymephenytoin (m/z transitions of 235→150) and 6β-hydroxytestosterone (m/z transition 305→269), the metabolites of mephenytoin and testosterone formed by CYP2C19 and CYP3A4, respectively, was achieved by a simultaneous HPLC-MS/MS method as described previously (Yanni et al., 2008). Quantitative determination was based on a calibration curve of corresponding hydroxyl metabolite standards.
Data Analysis.
The kinetic parameters Km and Vmax determined for six children and six adults were used to estimate the in vitro intrinsic clearance (Vmax/Km) for voriconazole N-oxidation. The Vmax/Km values were scaled to whole-body intrinsic clearance (CLint) according to eqs. 1 and 2:
where MPPG is milligram microsomal protein per gram liver that was experimentally determined for each subject and liver weight and body weight were obtained from published data of each subject's known age (Björkman, 2004). In vivo clearance of voriconazole was calculated based on in vitro data from children and adults using the well stirred model of hepatic disposition (Björkman, 2006) as shown in eq. 3:
where QH is hepatic blood flow, ƒu is unbound drug fraction, and CLint is the scaled intrinsic clearance determined from in vitro metabolism study.
Statistical Analysis.
All data are expressed as mean ± S.D. of a minimum of three experimental determinations. The statistical significance for the difference between Km, Vmax, scaling factor, intrinsic clearance, or total clearance in children (n = 6) versus adults (n = 6) was determined by the Wilcoxon rank sum test, whereas unpaired t test was used to compare between treated and control in the inhibition studies. In both cases, p < 0.05 was accepted for statistical significance.
Results
In Vitro Oxidative Metabolism of Voriconazole by Liver Microsomes from Adults and Children.
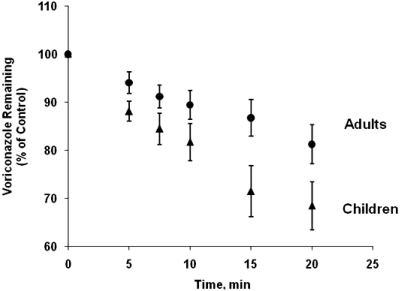
The metabolism of voriconazole by liver microsomes from adults and children as function of time is shown in Fig. 2. The half-life of voriconazole metabolism by liver microsomes from children (33.8 ± 15.3 min) was over 2-fold shorter than that obtained with liver microsomes from adults (72.6 ± 23.7 min), and the difference in the half-life between the two groups was statistically significant (p < 0.05).
Fig. 2.
Voriconazole oxidative metabolism by human liver microsomes from adults and children. Voriconazole oxidative metabolism by liver microsomes prepared from each tissue sample (six adults and six children, 1 mg microsomal protein/ml, 20 min) was determined. The oxidative metabolism of voriconazole was linear with respect to time and protein concentration under the experimental conditions used. Voriconazole remaining as function of time was measured in three separate experiments for each subjects, and mean values of six set of data ± S.D. were plotted for adults (●) or children (▴) as function of time.
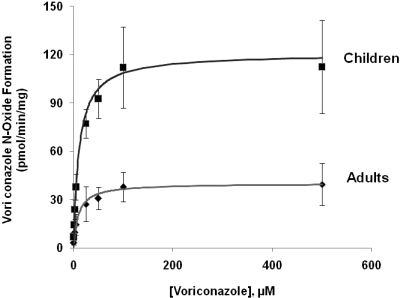
The rate of the N-oxide formation by liver microsomes from adults and children as a function of voriconazole concentration followed Michaelis-Menten kinetics (Fig. 3). The kinetic parameters for the reaction carried out by liver microsomes from individual subjects in the adult and pediatric group are listed in Table 1. The apparent Km value for voriconazole metabolism by liver microsomes from children versus adults is not significantly different (11 ± 5.2 and 9.3 ± 3.6 μM, respectively); however, the mean apparent Vmax was 3-fold higher with liver microsomes from children versus adults (120.5 ± 99.9 and 40 ± 13.9 pmol/min/mg, respectively), and the difference is statistically significant (p < 0.002).
Fig. 3.
Voriconazole N-oxide formation by liver microsomes from adults and children as a function of voriconazole concentration. Voriconazole N-oxide formation by liver microsomes from adults and children, as a function of voriconazole concentration, was determined by incubating microsomal protein (1 mg/ml) from each subject within the two groups with NADPH and voriconazole (0.5–500 μM). The mean rate of N-oxide formation ± S.D. in adults (n = 6) (●) or children (n = 6) (▴), expressed as pmol of N-oxide formed/min/mg protein, was plotted against voriconazole concentration. Nonlinear regression analysis (WinNonlin; Pharsight) was performed by using the Michaelis-Menten kinetic equation for the determination of apparent Km and Vmax.
TABLE 1.
In vitro-in vivo relationship of voriconazole clearance in children and adults
| Group | Subject | Age | Kma | Vmaxa | Scaling Factor | Intrinsic Clearanceb | In Vivo Clearance |
|
|---|---|---|---|---|---|---|---|---|
| Calculatedc | Observedd | |||||||
| yr | μM | pmol/min/mg | mg/kg | ml/min/kg | ml/min/kg | |||
| Adults | A1 | 45 | 8.5 ± 0.9 | 28.0 ± 0.7 | 655.7 | 2.16 | ||
| A2 | 58 | 8.8 ± 1.1 | 44.6 ± 2.3 | 801.4 | 3.6 | |||
| A3 | 24 | 9.8 ± 2.3 | 62.5 ± 1.7 | 600.0 | 3.8 | |||
| A4 | 20 | 8.5 ± 1.0 | 35.6 ± 0.8 | 578.6 | 2.4 | |||
| A5 | 39 | 15.7 ± 4.3 | 45.2 ± 3.0 | 535.7 | 1.5 | |||
| A6 | 46 | 4.5 ± 0.6 | 24.2 ± 0.9 | 728.6 | 3.9 | |||
| 9.3 ± 3.6e | 40.0 ± 13.9e | 650.0 ± 99.8e | 2.9 ± 1.0e | 1.6 ± 0.6e | 2.0e | |||
| Children | C1 | 2 | 3.8 ± 0.4 | 63.0 ± 2.5 | 900.8 | 14.9 | ||
| C2 | 4 | 19.7 ± 2.9 | 315.5 ± 12.7 | 786.7 | 16.2 | |||
| C3 | 8 | 11.9 ± 1.7 | 139.1 ± 4.5 | 844.9 | 10.7 | |||
| C4 | 2 | 12.1 ± 1.5 | 73.1 ± 1.5 | 705.0 | 4.3 | |||
| C5 | 4 | 10.0 ± 1.4 | 68.8 ± 2.3 | 1311.1 | 8.3 | |||
| C6 | 5 | 8.5 ± 1.6 | 63.4 ± 2.3 | 721.1 | 4.8 | |||
| 11.0 ± 5.2e | 120.5 ± 99.9e | 878.3 ± 211.3e | 9.9 ± 5.0e | 5.1 ± 2.6e | 6.7e | |||
The Km and Vmax values for each subject are reported as mean ± S.D.; these values were derived from three determinations of the reaction rate at each substrate concentration.
The (whole body) intrinsic clearance for each subject was calculated from the experimentally determined in vitro intrinsic clearance (Vmax/Km) values using eqs. 1 and 2 (see Materials and Methods).
In vivo clearance is calculated based on in vitro data according to the well stirred model of hepatic disposition that is described by eq. 3 (see Materials and Methods).
Clinically observed clearance values in children and adults were reported by Levêque et al. (2006).
Mean ± S.D.
Role of P450 and FMO in Higher Rate of Voriconazole N-Oxide Formation in Children versus Adults.
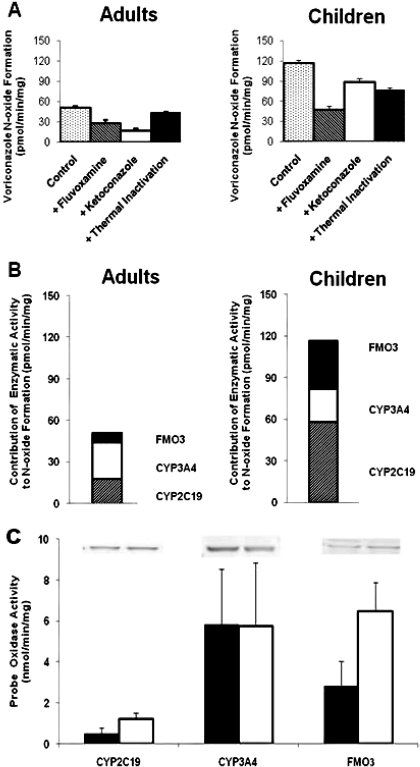
CYP2C19, CYP3A4, and FMO are responsible for most of the voriconazole metabolism by liver microsomes from adults (Yanni et al., 2008). Hence, their role in the quantitative differences in voriconazole N-oxidation by liver microsomes from children versus adults was assessed. This procedure was accomplished by measuring the rate of formation of voriconazole N-oxide by liver microsomes from children and adults in the presence of fluvoxamine (CYP2C19 inhibitor), ketoconazole (CYP3A4 inhibitor), or upon heat inactivation (FMO inhibition) (Fig. 4A). The CYP3A7 activity (estrone 16α-hydroxylation) was assessed in the microsomal preparations from pediatric livers used in this study and was found to be approximately 1000-fold lower than the CYP3A4 activity (testosterone 6β-hydroxylation) (data not shown). Furthermore, Murayama et al. (2007) have showed that the rate of voriconazole N-oxide formation by recombinant CYP3A5 and CYP3A7 is 4- and 15-fold lower, respectively, than that by CYP3A4. For these reasons, the involvement of CYP3A5 and CYP3A7 in voriconazole N-oxide formation was not assessed in this study. The estimated N-oxidation by FMO3 and CYP2C19 is 5- and 3-fold higher, respectively, in children compared with adults, but the estimated N-oxidation by CYP3A4 is not different in both groups (Fig. 4B). Although the results showed that CYP3A4 contributed predominantly to voriconazole metabolism by microsomes from adult livers, CYP2C19 and FMO3 contributed much more than CYP3A4 to voriconazole metabolism by microsomes from pediatric livers (Fig. 4). The expression of CYP2C19 and FMO3 is somewhat higher and that of CYP3A4 is somewhat lower in the liver tissue from children versus adult, as determined by the band densities in the Western blot analysis (Fig. 4C); the densities of the CYP2C19, FMO, and CYP3A4 bands for the liver tissue from children were 140, 170, and 60%, respectively, of that for the liver tissues from adults. When the specific activity of CYP2C19 (S-mepnytoin-4 hydroxylation), CYP3A4 (testosterone 6β-hydroxylation), and FMO (benzydamine N-oxidation) in microsomes from adult and pediatric livers was compared (Fig. 4C), the results showed that the CYP2C19 and FMO activities were higher in children versus adults, whereas the CYP3A4 activity in both groups was comparable.
Fig. 4.
Role of CYP3A4, CYP2C19, and FMO3 in voriconazole metabolism to the N-oxide by liver microsomes from adults and children. A, the N-oxide formation by the pooled liver microsomes from adults and children was inhibited by fluoxamine (CYP2C19), ketoconazole (CYP3A4), and heat treatment (FMO) to determine the contribution of the respective enzymes. The estimated rate of voriconazole N-oxide formation by CYP3A4, CYP2C19, and FMO3 in the liver microsomes from adults and children was calculated from the inhibition experiments in A and is shown in B. The approximate relative contribution of the three enzymes in the formation of N-oxide is as follows: 50% by CYP3A4, 35% by CYP2C19, and 15% by FMO3 in adults; and 20% by CYP3A4, 50% by CYP2C19, and 30% by FMO3 in children. The specific activities of CYP2C19 (S-mephenytoin 4-hydroxylation), CYP3A4 (testosterone 6β-hydroxylation), and FMO3 (benzydamine N-oxidation) in the liver microsomes from adults (filled bar) and children (open bar) are shown in C; the data are the mean of three determinations ± S.D. C, the protein expression of CYP2C19, CYP3A4, and FMO3 in pooled liver microsomes from adults and children is shown as Western blot bands; the band densities in adults 5060 ± 526 (CYP2C19), 13,996 ± 2099 (CYP3A4), and 2310 ± 289 (FMO3), and in children 7084 ± 776 (CYP2C19), 8398 ± 910 (CYP3A4), and 3927 ± 578 (FMO3) were used to estimate relative expression of the respective proteins in adults versus children.
Calculated in Vivo Clearance in Children and Adults.
The calculated in vitro intrinsic clearance (Vmax/Km), determined for metabolism of voriconazole by liver microsomes, was found to be 3-fold higher in children (11.5 ± 6.2 μl/min/mg) than in adults (4.5 ± 1.3 μl/min/mg). The whole-body intrinsic clearance was calculated by multiplying the in vitro metabolic intrinsic clearance by a scaling factor as shown by eqs. 1 and 2. The calculated scaling factor, based on MPPG and liver weight that were normalized to body weight, was somewhat higher for children (878.3 ± 211.3 mg/kg) versus adults (650 ± 99.8 mg/kg), but the statistical significance (p > 0.04) was marginal (Table 1). The MPPG value was determined experimentally for each subject and ranged from 18 to 40 mg/g with a mean value of 26 mg/g in children, whereas in adults MPPG ranged from 27 to 37 mg/g with a mean value of 30.3 mg/g. The mean liver weight in children aged 2 to 8 years was reported as 575 g (470–740 g) by Björkman (2004), whereas an average value of 1500 g was used for the liver weight of adults (Björkman, 2004). The body weight of children of age 2 to 8 years ranged from 12 to 25.4 kg with a mean value of 17.2 kg, whereas 70 kg was set as an average body weight for adults. The whole-body intrinsic clearance was ∼3-fold higher in children (9.9 ± 5 ml/min/kg) compared with adults (2.9 ± 1 ml/min/kg) (p < 0.01). The in vivo clearance of voriconazole was calculated to be 5.1 ml/min/kg in children and 1.6 ml/min/kg in adults using the scaled intrinsic clearance values in children and adults, unbound voriconazole fraction (ƒu) of 0.6 in both adults (Levêque et al., 2006) and children, and the hepatic blood flow value of 37 ml/min/kg in children and 24 ml/min/kg in adults (Björkman, 2004) (eq. 3). These values are approximately 80% of the values observed in the pharmacokinetic studies in children (6.7 ml/min/kg) and adults (2 ml/min/kg) that were previously reported by Levêque et al. (2006).
Discussion
Voriconazole, a frequently prescribed drug for treatment of fungal infection in children, is cleared much more rapidly in children than in adults, and it exhibits distinctly different pharmacokinetic profiles in these two populations (Walsh et al., 2004; Levêque et al., 2006; Karlsson et al., 2009). For example, voriconazole clearance in adults loses linearity over a very small increase in dose from 3 to 5 mg/kg, whereas the clearance remains linear in children over a similar increase in doses. In addition, the oral bioavailability of voriconazole is 2-fold lower in children (44%) than in adults (96%) (Karlsson et al., 2009), suggesting that voriconazole may be subject to significant first-pass metabolism in children but not in adults.
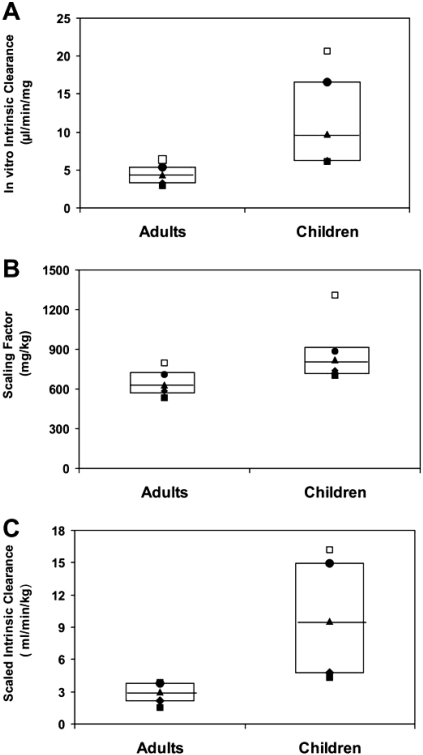
It is interesting to note that results on in vitro metabolism of voriconazole by microsomes, prepared from livers of children aged 2 to 8 years and from adults, showed that oxidative enzymes derived from children metabolized voriconazole at a 3-fold higher rate than those derived from adults. Thus, the microsomal system appears to mimic in vivo metabolic clearance of voriconazole. The in vitro intrinsic clearance (Vmax/Km) of voriconazole N-oxidation by liver microsomes derived from children and adults was used to calculate in vivo clearance (Edginton et al., 2006; Alcorn and McNamara, 2008). Box plot analysis of the intra- and intergroup distribution of values of in vitro intrinsic clearance, scaling factor, and predicted intrinsic clearance generated by six liver microsomes samples from children and adults are shown in Fig. 5, A–C, respectively. These results indicated that the distribution of values among children was much broader than that among adults. However, the median for the in vitro intrinsic clearance and scaled intrinsic clearance in children were significantly greater than in adults. This difference cannot be attributed to differences in the scaling factors because the calculated scaling factor for children was marginally different from that for adults (p > 0.04). The calculated in vivo clearance values were approximately 80% of the observed plasma clearance of voriconazole in children and adults. The underprediction by 20% may be due to the fact that the intrinsic clearance was calculated for the formation of the N-oxide, which is a major but not the only metabolite of voriconazole. These results conclude that the higher clearance of voriconazole in children is the result of higher metabolic clearance of voriconazole in children compared with adults.
Fig. 5.
Box plot analysis of voriconazole in vitro intrinsic clearance, scaling factor, and scaled intrinsic clearance in children compared with adults. Graphical presentation of in vitro intrinsic clearance, scaling factor, and scaled intrinsic clearance values obtained from analysis of six children and six adults liver microsomes samples is shown by the box plots A, B, C, respectively, where 25% percentile value is indicated by ♦, minimal value is indicated by ■, median value is indicated by ▴, maximal value is indicated by □, and 75% percentile value is indicated by ●. Nonparametric statistical analysis of values in children and adults values using Wilcoxon test showed that two-sided exact p values were 0.0087, 0.041, and 0.0022 for comparison of in vitro intrinsic clearance (Vmax/Km), scaling factor, and predicted intrinsic clearance, respectively.
It has been reported that the oxidative metabolism of voriconazole by liver microsomes from adults generates two major metabolites (see Fig. 1), N-oxidation of the fluoropyrimidine ring and hydroxylation of the adjacent methyl group (Roffey et al., 2003; Murayama et al., 2007). The N-oxide, which is the major circulating metabolite in humans, is formed by CYP3A4, CYP2C19 (Hyland et al., 2003; Murayama et al., 2007), and FMO (FMO3 > FMO1) (Yanni et al., 2008); whereas the hydroxymethyl metabolite is formed exclusively by CYP3A4 (Murayama et al., 2007; Yanni et al., 2008). In the present study, we report that both metabolites are also formed by liver microsomes derived from children. To determine the role of CYP3A4, CYP2C19, and FMO3 in the oxidative metabolism of voriconazole by liver microsomes from adults versus children, the N-oxide formation was measured upon inhibition of CYP2C19 (fluoxamine), CYP3A4 (ketoconazole), and FMO3 (heat inactivation) (Fig. 4A). The results showed that the relative contribution of these enzymes in the formation of N-oxide is considerably different in children versus adults. The estimated rate N-oxidation by FMO3 and CYP2C19 is 5- and 3-fold higher, respectively, in children compared with adults, but the estimated rate of N-oxidation by CYP3A4 is not different in both groups. Consequently, in adults approximately 50% of the N-oxide was formed by CYP3A4, 35% by CYP2C19, and 15% by FMO3, whereas in children 50% of the N-oxide was formed by CYP2C19, 30% by FMO3, and 20% by CYP3A4. To determine whether higher voriconazole metabolism in children versus adults is due to higher expression of hepatic CYP2C19 and/or FMO3 in children, the expression of these two proteins and CYP3A4 was determined in adult versus pediatric livers. The expression of CYP2C19 and FMO3 in the pediatric liver was somewhat higher (<2-fold), whereas the expression of CYP3A4 was somewhat lower (approximately 2-fold) in children compared with adults (Western blot bands at the top of Fig. 4C). In comparison, the activity of CYP2C19 and FMO3 toward their respective probe substrates was approximately 2-fold higher in liver microsomes from children versus adults, with CYP3A4 activity being similar in these two microsomal preparations (Fig. 4C).
This study clearly shows that higher rate of oxidative metabolism of voriconazole in children versus adults contributes to its higher clearance in vivo. The microsomal metabolism of voriconazole is able to predict the in vivo clearance of voriconazole in adults and in children. Thus, results of the in vitro studies to determine the relative contribution of CYP2C19, CYP3A4, and FMO3 should also be indicative of the relative role of these enzymes in vivo. It appears that CYP2C19 and FMO3 play a bigger role in the metabolic clearance of voriconazole in children, whereas CYP3A4 appears to contribute more in adults. The expression of CYP2C19 and FMO is somewhat higher in children versus adults; however, it is not clear whether this difference in expression is sufficient to explain greater metabolic clearance of voriconazole in children versus adults. It is interesting to note that other drugs that are predominantly metabolized by hepatic CYP2C9/19 such as phenytoin, omeprazole, and sirolimus show higher clearance in children between 2 and 10 years of age than in adults (Litalien et al., 2005; Filler et al., 2008; Kennedy, 2008). It would be interesting to identify drugs that are predominantly metabolized by FMO3 and examine their clearance in children versus adults. Taken together, such mechanistic studies may lead to significant improvement in our understanding of why certain drugs are cleared more rapidly in children than in adults, and consequently improve our ability to define optimum doses for children based on pharmacokinetic behavior of these drugs in adults combined with in vitro metabolic studies.
Acknowledgments.
We thank Pfizer for providing voriconazole, Dr. Arlene Bridges for her support with LC-MS/MS analysis, and Dr. Ronald Hines for valuable discussion.
This work was supported by the National Institutes of Health National Institute of Child Health and Human Development [Grant NCC-PPRU 5U10 HD045962-06]. D.K.B. was supported by the National Institutes of Health [Grants 1RO1-HD05795602, 1RO1-FD00351901, 1U10-HD4596206, 1K24-HD05873501, Government Contract HHSN267200700051C] (for work in pediatric and neonatal clinical pharmacology); the nonprofit organization Thrasher Research Foundation for work in neonatal candidiasis (http://www.thrasherresearch.org); and the industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.029769
- FMO
- flavin-containing monooxygenase
- AUC
- area under the plasma concentration-time curve
- P450
- cytochrome P450
- HPLC
- high-performance liquid chromatography
- HPLC-MS/MS
- high-performance liquid chromatography coupled with tandem mass spectrometry
- CLint
- intrinsic clearance
- MPPG
- milligram microsomal protein per gram liver
- ƒu
- unbound fraction of drug.
References
- Alcorn J, McNamara PJ. (2008) Using ontogeny information to build predictive models for drug elimination. Drug Discov Today 13:507–512 [DOI] [PubMed] [Google Scholar]
- Björkman S. (2004) Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol 59:691–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman S. (2006) Prediction of cytochrome P450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods? Clin Pharmacokinet 45: 1–11 [DOI] [PubMed] [Google Scholar]
- Chen N, Aleksa K, Woodland C, Rieder M, Koren G. (2006) Ontogeny of drug elimination by the human kidney. Pediatr Nephrol 21:160–168 [DOI] [PubMed] [Google Scholar]
- Edginton AN, Schmitt W, Voith B, Willmann S. (2006) A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 45:683–704 [DOI] [PubMed] [Google Scholar]
- Filler G, Bendrick-Peart J, Christians U. (2008) Pharmacokinetics of mycophenolate mofetil and sirolimus in children. Ther Drug Monit 30:138–142 [DOI] [PubMed] [Google Scholar]
- Hines RN, McCarver DG. (2002) The ontogeny of human drug-metabolizing enzymes: phase 1 oxidative enzymes. J Pharmacol Exp Ther 300:355–360 [DOI] [PubMed] [Google Scholar]
- Hyland R, Jones BC, Smith DA. (2003) Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 31:540–547 [DOI] [PubMed] [Google Scholar]
- Johnson TN, Rostami-Hodjegan A, Tucker GT. (2006) Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet 45:931–956 [DOI] [PubMed] [Google Scholar]
- Karlsson MO, Lutsar I, Milligan PA. (2009) Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob Agents Chemother 53:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. (2008) Hormonal regulation of hepatic drug-metabolizing enzyme activity during adolescence. Clin Pharmacol Ther 84:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. New Engl J Med 349:1157–1167 [DOI] [PubMed] [Google Scholar]
- Levêque D, Nivoix Y, Jehl F, Herbrecht R. (2006) Clinical pharmacokinetics of voriconazole. Int J Antimicrob Agents 27:274–284 [DOI] [PubMed] [Google Scholar]
- Litalien C, Théorêt Y, Faure C. (2005) Pharmacokinetics of proton pump inhibitors in children. Clin Pharmacokinet 44:441–466 [DOI] [PubMed] [Google Scholar]
- Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. (2007) Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol 73:2020–2026 [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE. (2002) Measurement of Michaelis constants for cytochrome P450-mediated biotransformation reactions using a substrate depletion approach. Drug Metab Dispos 30:831–837 [DOI] [PubMed] [Google Scholar]
- Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. (2002) Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother 46:2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. (2003) The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 31:731–741 [DOI] [PubMed] [Google Scholar]
- Shimada T, Tsumura F, Yamazaki H. (1999) Prediction of human liver microsomal oxidations of 7-ethoxycoumarin and chlorzoxazone with kinetic parameters of recombinant cytochrome P-450 enzymes. Drug Metab Dispos 27:1274–1280 [PubMed] [Google Scholar]
- Steinbach WJ, Benjamin DK. (2005) New antifungal agents under development in children and neonates. Curr Opin Infect Dis 18:484–489 [DOI] [PubMed] [Google Scholar]
- Theuretzbacher U, Ihle F, Derendorf H. (2006) Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet 45:649–663 [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez-Llorens X, Vora AJ, Arrieta AC, Blumer J, Lutsar I, et al. (2004) Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother 48:2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni SB, Annaert PP, Augustijns P, Bridges A, Gao Y, Benjamin DK, Jr, Thakker DR. (2008) Role of flavin-containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab Dispos 36:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]