Abstract
Quantitative variation in response to drugs in human populations is multifactorial; genetic factors probably contribute to a significant extent. Identification of the genetic contribution to drug response typically comes from clinical observations and use of classic genetic tools. These clinical studies are limited by our inability to control environmental factors in vivo and the difficulty of manipulating the in vivo system to evaluate biological changes. Recent progress in dissecting genetic contribution to natural variation in drug response through the use of cell lines has been made and is the focus of this review. A general overview of current cell-based models used in pharmacogenomic discovery and validation is included. Discussion includes the current approach to translate findings generated from these cell-based models into the clinical arena and the use of cell lines for functional studies. Specific emphasis is given to recent advances emerging from cell line panels, including the International HapMap Project and the NCI60 cell panel. These panels provide a key resource of publicly available genotypic, expression, and phenotypic data while allowing researchers to generate their own data related to drug treatment to identify genetic variation of interest. Interindividual and interpopulation differences can be evaluated because human lymphoblastoid cell lines are available from major world populations of European, African, Chinese, and Japanese ancestry. The primary focus is recent progress in the pharmacogenomic discovery area through ex vivo models.
I. Introduction
A. Overview of Pharmacogenomics
Pharmacogenetics is the study of variability in drug response, in terms of either drug efficacy or adverse drug reactions, due to variation in genetics. Pharmacogenetics originally evolved from observational studies of rare metabolic abnormalities, particularly within families or between ethnic groups. For example, it was observed that, of soldiers given primaquine for malaria during World War II, approximately 10% of African Americans developed acute hemolytic crises, compared with a very small number of white persons (Clayman et al., 1952). This led to the discovery of genetic deficiencies in glucose 6-phosphate dehydrogenase, an enzyme that alters metabolism in erythrocytes.
Before the advent of the Human Genome Project, pharmacogenetic studies primarily examined candidate genes within well known pharmacokinetic pathways after a clinical observation. A classic example of a successful use of this approach was the discovery of the role of genetic variation in TPMT1 in thiopurine metabolism. TPMT encodes the enzyme thiopurine methyltransferase that metabolizes thiopurines via S-methylation (Weinshilboum and Sladek, 1980). Differences in enzyme activity between individual subjects were first identified in 1980 and were consequently determined to be heritable (Weinshilboum and Sladek, 1980). Almost a decade later, a series of clinical studies showed this activity to be inversely correlated with levels of thiopurine metabolites in red blood cells and drug-induced myelosuppression (Lennard et al., 1987, 1989, 1990; Evans et al., 1991). Subsequent advances in molecular biology led to the cloning of the TPMT locus and identification of polymorphisms in the sequence (Szumlanski et al., 1996; Tai et al., 1996). In white persons, three of these polymorphisms (*3A, *3C, and *2) account for 95% of inherited TPMT deficiencies (McLeod et al., 2000). Consequently, TPMT is a major predictor of therapeutic success and has been incorporated into the dosing parameters for this drug (Lennard et al., 1990; Wang et al., 2005; Schmiegelow et al., 2009).
These results led the FDA to approve use of TPMT genotyping as a biomarker in clinical practice in 2003. Because the term “pharmacogenetics” was first coined 50 years ago, it is surprising that only a handful of examples of incorporation of genetic information into dosage algorithms in clinical practice exist today. In addition to TPMT, variants in UGT1A1, CYP2D6, and VKORC1 have begun to be introduced into clinical practice for dosing of irinotecan, tamoxifen, and warfarin (http://warfarindosing.org), respectively (Maitland et al., 2006; Ratain, 2006; Young, 2006; International Warfarin Pharmacogenetics Consortium et al., 2009). Even with the advances coincident with the genomic era, identification and validation of relevant pharmacogenetic markers has been a slow process for a variety of reasons.
B. Rationale for Use of Cell-Based Models in Pharmacogenomic Discovery
These early pharmacogenetic discoveries were based on observations in clinical populations and were limited to phenotypes in which a single candidate gene variant had a large effect on drug activity. However, this is not a practical approach for pharmacogenetic analysis of most drugs for multiple reasons. First, variation in response to most clinically administered drugs is dependent on the combined contribution of multiple genes with small, independent effects. Comprehensive pharmacogenomic studies of pharmacokinetic and pharmacodynamic genes important in drug response typically precludes use of clinical trials as a means of discovery because these studies are expensive, time-consuming, and require large numbers of patients and infrastructure to obtain reliable clinical phenotype data. Establishing a prospective cohort can take years because of the time required to meet regulatory requirements, to accrue a population of sufficient size, and to conduct follow-up analysis. Although samples from retrospective clinical trials require fewer resources, they generally have neither sufficient statistical power nor the appropriate design to answer specific pharmacogenetic questions. This problem is further compounded by the need for multiple large patient cohorts, to enable both discovery and replication studies. Another complication of clinical pharmacogenetic studies is separating genomic contributions to variation in drug response from confounding factors such as comorbidities, dosage, timing, and diet. Uncontrolled confounders, including population stratification or admixture, can also bias measured effect estimates of genotype-phenotype relationships. Furthermore, pharmacogenetic discovery for highly toxic drugs, such as chemotherapeutics and certain antiviral agents, poses additional challenges that require the development of ex vivo models. For all of the reasons mentioned above, studies have turned to the use of human cell-based models for pharmacogenetic discovery and validation studies. Table 1 summarizes some of the advantages and limitations of pharmacogenetic studies in humans and cell-based models.
TABLE 1.
Advantages and limitations of human and cell models
| Clinical Studies | Cell Models |
|---|---|
Advantages
|
|
Limitations
|
|
Although cell-based models have been used in preclinical drug development for years as a means to evaluate drug-induced cell growth inhibition or apoptosis or to identify interactions of target compounds with drug-metabolizing enzymes and transporter proteins, their use in pharmacogenetics is relatively recent. Cell lines are useful because they can be perturbed easily with drug treatment to examine changes in gene expression and cell growth in response to drugs. Cell lines also facilitate examination of molecular and cellular biomarkers or intermediate phenotypes, which can help elucidate the mechanism of action of the drug and/or etiology of the clinical phenotypes under study. However, there are a limited number of choices of cell types. Although hepatocytes are often the most relevant tissue to identify variant gene function in drug-metabolizing enzymes for many pharmaceutical agents, this tissue is notoriously inaccessible and expensive to obtain. Molecular phenotyping in more accessible cell types, such as red blood cells or lymphocytes, has proven to be a successful alternative but requires that drug response pathways are active within the sampled tissue.
In recent years, there has been increasing utilization of lymphoblastoid cell lines (LCLs) as a model to assess the contribution of genetic or epigenetic variation to drug response (Watters et al., 2004; Shukla and Dolan, 2005; Huang et al., 2007a,b, 2008b,c; Medina et al., 2008). LCLs are typically generated by Epstein-Barr virus (EBV) transformation of peripheral blood mononuclear cells, resulting in a population of immortalized B lymphocytes (Ling and Huls, 2005). One of the first large banks of commercially available LCLs was from the Centre d'Etude du Polymorphisme Humain (CEPH) and Coriell Institute for Medical Research, containing extensive pedigree information of approximately 60 families from France, Utah, and Venezuela, as well as one of Amish origin (Dausset et al., 1990; Cann, 1992). These LCLs, as well as those belonging to the International HapMap project, have been widely studied in genetic and expression analyses and, more recently, in pharmacogenetics. In addition, cell lines from diseased tissue, such as the NCI60 bank of cancer cell lines, have proved to be useful models in pharmacogenetics for examining the direct effects of a drug on a variety of tumor tissue types. Although other publicly available cell panels exist, the extensive genetic data that is publicly available on these two cell collections have made them the primary focus of studies in pharmacogenomics. Therefore, they will also be the focus of discussion in this review.
II. Cell-Based Models for Pharmacogenomic Discovery
A. Developing a Cell-Based Model System
Developing a cell-based system that is most relevant to the clinical phenotype of interest is challenging. One of the most important considerations is whether the cell lines express the relevant pharmacokinetic and pharmacodynamic pathways associated with the drug of interest. Other considerations are the appropriate in vitro assay or phenotype that is measured, the population from which the cell lines are derived, and the relevance of the tissue of origin. Considerations and limitations with the cell-based system must also be taken into account.
1. Selection of Cell System.
The use of cell-based models for pharmacogenomic analysis of drug classes requires consideration of the pathways intact within the system. Many pharmaceutical agents work through modulation of biological pathways that interact only in specialized cell types such as neurons or pancreatic β cells, and these pathways may not be represented within all cell types. In contrast, some drugs act through biological pathways that are universally represented across all cell types. For example, statins act by lowering plasma LDL-cholesterol through competitive inhibition of HMG-CoA reductase (HMGCR) within the liver, blocking endogenous cholesterol biosynthesis. Many cell types, including lymphocytes, express the cholesterol biosynthesis pathway and have been useful models for pharmacogenomic discovery for statins (Medina et al., 2008). Therefore, the cell-model system employed will depend on the expression of genes within pathways most relevant to the drug of interest.
Hepatic cells are often the best model system to study the pharmacogenetic effects of metabolic enzymes like the cytochrome P450 family of enzymes (Zhou et al., 2009). This family of genes, as well as other metabolic enzymes, is crucial for the initial processing of many toxins and drugs in the body. Microsomes from human livers have been used extensively in pharmacogenetics to determine activity of various enzymes in poor metabolizers and extensive metabolizers of a particular drug. For example, microsomes from livers of persons that express low levels of CYP3A5 as a result of the genetic variant CYP3A5*3 metabolized the drug alfentanil poorly compared with those that produce higher levels of CYP3A5 (Klees et al., 2005). Likewise, formation of the 8-hydroxylated form of the antiviral efavirenz, the primary drug metabolite, was associated with both the presence of the CYP2B6*6 allele and CYP2B6 protein levels in a panel of liver microsomes (Desta et al., 2007). Hepatocytes have also been useful in determining genetic control of expression and splicing differences in drug-metabolizing genes. For example, primary hepatocytes from human livers were used to determine that mRNA levels of CYP2A6 were significantly lower in those possessing a deletion polymorphism (CYP2A6*4) or a polymorphism in the TATA box (CYP2A6*9) compared with hepatocytes expressing wild-type CYP2A6 (Kiyotani et al., 2003). These CYP2A6 genotypes were also significantly correlated with decreased protein levels and enzymatic activity in vitro. As useful as primary hepatocytes and microsomes are in pharmacogenetics of cytochrome P450s, they have a finite lifespan with changes occurring with days in culture.
For some drugs, metabolic conversion does not occur; therefore, the PD pathway is more relevant. There are also examples, such as cancer drugs that target EGFR, in which the target gene plays a prominent role in drug activity. Many tumors acquire activating mutations in EGFR, and patients with these tumors respond better to EGFR-targeting drugs, such as erlotinib or gefitinib (Lynch et al., 2004; Paez et al., 2004; Ji et al., 2006a,b). To study how alterations at this gene locus affect sensitivity to these drugs, tumor cells would be the most relevant model system. Tumor cell lines from patients with non–small-cell lung cancer and from patients with head and neck squamous cell carcinoma have been used to determine which mutations in EGFR, as well as EGFR gene amplification and expression, were strong predictors of gefitinib sensitivity (Gandhi et al., 2009; Rogers et al., 2009). Therefore, selection of a cell system requires consideration of the drugs and pathways of interest.
2. Phenotyping Cells.
The selection of endophenotypes, as measured in cell lines after treatment with drug, that accurately reflect clinical drug response is a major challenge. The appropriate phenotype is usually dependent on the mechanism of action of the drug, as well as the clinical phenotype of interest. For example, anticancer drugs are intended to cause growth inhibition, cell death, or apoptosis; therefore, measures of cellular apoptosis or inhibition of cellular proliferation across a range of drug dosages are a reasonable phenotype (Shukla and Dolan, 2005). Another phenotype to consider is measurement of conversion of parent drug to active metabolite. This has been effectively analyzed in the case of methotrexate glutamation (Masson et al., 1996) and the chemotherapeutic AraC, in which the amount of active metabolite (AraCTP) was associated with a specific genotype within an important drug-metabolizing gene (Hartford et al., 2009).
In certain cases, especially when using a candidate gene approach, the nature of the gene-drug relationship may dictate the molecular phenotype that should be evaluated. Promoter SNPs may be tested for allelic imbalance phenotypes, or nonsynonomous coding polymorphisms may be analyzed for changes in substrate affinity or enzymatic activity (Kuehl et al., 2001; Erdman et al., 2006; Ramírez et al., 2006). However, not all clinical phenotypes are amenable to cell-based model systems; for example, certain HIV drugs, such as efavirenz, nevirapine, and abacavir, often cause drug-induced hypersensitivity in patients (Tozzi, 2009); this results from complex interactions between multiple cell types that would be difficult to recapitulate ex vivo.
3. Population of Persons from Whom Cells Are Derived.
Geographic region is becoming increasingly recognized as an important factor accounting for variation in drug response (Huang and Temple, 2008; O'Donnell and Dolan, 2009). For example, there is consistent evidence that persons from East Asia are particularly sensitive to the effects of platinating agents (Millward et al., 2003; Watanabe et al., 2003). Similar differences have been observed in efficacy of statins in African Americans compared with white persons, as well as between Asians, whites, and African Americans in dosing of warfarin (Lee et al., 2005; Engen et al., 2006; Simon et al., 2006). Because studying a population enriched for a particular phenotype (i.e., drug sensitivity) is thought to enhance one's ability to identify genetic variants associated with that phenotype, choosing to study cells derived from an informative population would be helpful in pharmacogenomic discovery.
The International HapMap cell lines representing 11 different ethnic groups have been used to study interethnic differences in drug sensitivity. Pharmacogenomic differences in drug response across ethnicities may represent differences in prevalence of causative polymorphisms and may also act through differences in gene expression. Extensive differences have been described in the genomic regulation of global gene expression between LCLs derived from European versus African donors (Price et al., 2008; Zhang et al., 2008a). Furthermore, genomic regulation of alternatively spliced transcripts is significantly different in LCLs from these two groups (Zhang et al., 2009). Although race plays an important role in drug response and expression phenotypes, race alone is not predictive of genetic heterogeneity (Yen-Revollo et al., 2008), thus specific genetic variants must be examined concomitantly with race. These data suggest that the cell-based model is a meaningful system to assess genomic contributions to differences in cellular response to drugs within and among ethnic populations. Furthermore, ethnicity of the population from which cells are derived is an important consideration when developing a cell-based model system.
4. Experimental Artifacts and Confounders.
To properly assess drug phenotypes and the genetic factors associated with them in cell lines, artifacts of the experimental system must be considered. One study recently attempted to determine some of these factors in LCL model systems and reported that EBV copy number, ATP levels, and growth rate are all nongenetic confounders of response of certain drugs, including 5-fluorouracil, methotrexate, simvastatin, suberoylanilide hydroxamic acid, and 6-mercaptopurine (Choy et al., 2008). Our laboratory found no relationship between EBV copy number or ATP levels and cellular sensitivity to drugs (A. L. Stark, W. Zhang, S. Mi, S. Duan, P. H. O'Donnell, R. S. Huang, M. E. Dolan, submitted). However, we did observe a significant correlation between cellular growth rate and sensitivity to chemotherapy that is not surprising, because many chemotherapeutic drugs are designed against rapidly growing cells (Huang et al., 2008c; Hartford et al., 2009). Therefore, genetic variants associated with sensitivity to chemotherapeutic agents may act through their association with growth rate. More investigation into this area is necessary to properly determine the role of cellular growth rate in drug responses both in vitro and in vivo.
Studies in keratinocytes and B-cell lymphomas have shown that EBV alters apoptosis in response to certain drugs, such as bleomycin, gemcitabine, and doxorubicin, but this was not true of all drugs (i.e., cisplatin, 5-fluorouracil, and 5′-azacytidine) (Feng et al., 2004; Liu et al., 2004). Thus, the effects of EBV seem dependent on cell type, drug, and disease state, and all of these factors should be considered when using EBV-immortalized cell models in pharmacogenetic studies. In addition, it has also been reported that LCLs contain nongermline mutations, including changes in copy number of mitochondrial DNA (Jeon et al., 2007). Because these mutations may alter cellular phenotypes of interest, it is important to check for evidence of Mendelian inheritance of the SNPs of interest in the publicly available HapMap data.
5. Statistical Considerations.
The ability to control some important sources of environmental variation, and the presence of family structure does allow detection of greater differences in drug effect than would be possible in patients or animal models. However, many cell-based pharmacogenomic studies use cell lines derived from fewer than 200 subjects, with resulting limitations in statistical power, particularly when performing genome-wide association studies. Because of this, there is a movement toward studies in larger cell line collections when a priori statistical planning indicates a need for larger sample sizes to increase the power to detect genetic associations.
B. Lymphoblastoid Cell Line Model Systems
Despite the limitations described above, many pharmacogenomic studies have employed LCLs (Table 2). Lymphocytes can be easily obtained by drawing blood, and cell lines can then be established by EBV transformation. In addition, LCLs express approximately half of the known genome (Cheung et al., 2003; Zhang et al., 2008a), allowing for interrogation of many potentially relevant biological pathways.
TABLE 2.
Application of LCL models to pharmacogenomics
| LCL-Based Model | Genetic Approach | Endophenotype | Phenotype | Reference |
|---|---|---|---|---|
| Pregenomic era (before 2003) | ||||
| Patients with HNSCC and unaffected family members | Heritability | Drug sensitivity | Cloos et al., 1999 | |
| Patients with MS and unaffected family members | Heritability | Sensitivity to ionizing radiation | Gipps and Kidson, 1984 | |
| Patients with Wilms' tumor | Candidate gene | Drug sensitivity | Imray et al., 1984 | |
| Patients with Werner syndrome | Candidate gene | Drug sensitivity | Poot et al., 1999 | |
| Postgenomic era (2003 to present) | ||||
| CEPH Pedigrees | Heritability, linkage analysis | Expression | Morley et al., 2004 | |
| HapMap trios | eQTL | Expression | Cheung et al., 2005; Stranger et al., 2005; Spielman et al., 2007; Storey et al., 2007; Stranger et al., 2007a,b; Duan et al., 2008; Huang et al., 2008d; Zhang et al., 2008, 2009; Kudaravalli et al., 2009 | |
| CEPH Pedigrees | Heritability, linkage analysis | Drug sensitivity | Dolan et al., 2004; Watters et al., 2004 | |
| CEPH Pedigrees/HapMap trios | Heritability, linkage-directed association studies | Drug sensitivity | Duan et al., 2007; Shukla et al., 2008, 2009; Bleibel et al., 2009 | |
| CEPH pedigrees | Linkage analysis, association studies | Expression | Response to ionizing radiation | Smirnov et al., 2009 |
| Human Variation Panel | Transcriptional profiling | Drug sensitivity | Li et al., 2008 | |
| Patients with breast cancer, with and without BRCA1/2 mutations | Transcriptional profiling | Response to ionizing radiation | Waddell et al., 2008 | |
| HapMap trios | Transcriptional profiling | Drug sensitivity | Fry et al., 2008 | |
| Subjects with HNSCC and control subjects | Transcriptional profiling | Drug sensitivity | Cloos et al., 2006 | |
| SOPHIE population | Candidate gene | Allelic imbalance | Urban et al., 2006; Tahara et al., 2009 | |
| Polymorphism Discovery Resource | Candidate gene | Allelic imbalance | Poonkuzhali et al., 2008 | |
| HapMap trios | Candidate gene | Allelic imbalance | Johnson et al., 2008 | |
| Subjects with type 2 diabetes and control subjects | Candidate gene | Allelic imbalance | Elbein et al., 2007 | |
| HapMap trios | Candidate gene | Drug sensitivity | Hartford et al., 2009 | |
| Human Variation Panel | Candidate gene | Drug sensitivity | Wang et al., 2008 | |
| Human Variation Panel/CEPH unrelateds | Candidate gene | Drug sensitivity | Innocenti et al., 2009 | |
| Schizophrenia case-control study | Candidate gene | Expression | Chagnon et al., 2008 | |
| HapMap trios | GWAS | Expression | Drug sensitivity | Huang et al., 2007a,b, 2008b,c,; Hartford et al., 2009 |
HNSCC, head and neck squamous cell carcinoma; MS, multiple sclerosis.
1. Application of Lymphoblastoid Cell Line Models in Pharmacogenomics in the Pregenomic Era.
Before the full sequencing of the human genome, pharmacogenetic discoveries within LCLs were made using cell lines established from targeted clinical populations (e.g., diseased persons). For example, LCLs derived from patients who were genetically predisposed to Wilms' tumor showed increased sensitivity to the chemotherapeutic drug mitomycin C, indicating a genetic component to drug sensitivity (Imray et al., 1984). Likewise, LCLs from patients with multiple sclerosis showed significantly higher sensitivity to ionizing radiation compared with normal controls, a trait that showed autosomal-dominant inheritance when examining unaffected first-degree relatives (Gipps and Kidson, 1984). Another study that used lymphocytes from relatives of patients with head and neck squamous cell carcinoma found that approximately 75% of the total variance in rate of bleomycin-induced chromatid breaks was due to genetic factors (Cloos et al., 1999). Finally, LCLs with genetic alterations in the WRN (Werner syndrome ATP-dependent helicase) gene have increased camptothecin-induced apoptosis compared with those with wild-type WRN sequences (Poot et al., 1999), indicating that this gene contributes to drug sensitivity. Most importantly, these initial studies, through the use of LCLs derived from diseased persons, were able to establish links between genetic variation and sensitivity to drug-induced damage or cell kill.
2. Application of Lymphoblastoid Cell Line Models in Pharmacogenomics in the Postgenomic Era.
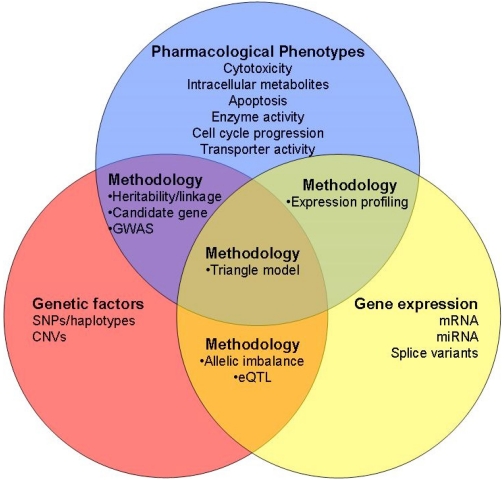
The postgenomic era has allowed a more comprehensive evaluation of the full spectrum of human genetic variation and its role in disease and drug pharmacology (Fig. 1). Of particular relevance was the initiation of the International HapMap project, which uses a large repository of LCLs from a variety of ethnic backgrounds, with a rich set of publicly available genotypic data. These resources have recently been leveraged for discovery and validation of new pharmacogenomic markers.
Fig. 1.
Analysis methods for cell-based models in pharmacogenomics. Schema of how cell models can be applied to pharmacogenomics research. Each circle describes variables that can be measured within cell lines and the overlap describes the methods applied to finding relationships between the two variables. The use of cell lines within pedigrees will allow for heritability and linkage analysis. In addition, candidate gene or genome-wide association (GWA) studies can be used for examining genotype-phenotype relationships in cells of both unrelated and related persons. Gene expression data can be analyzed with pharmacological endpoints in cells to determine expression profiles that are associated with sensitivity or resistance to a drug. The role of genetic variants in regulating gene expression, regardless of drug sensitivity, can also be examined in eQTL and allelic imbalance studies in cell-based models. Integrating genetic, expression, and pharmacological phenotypes can be combined in a “triangle model” to determine genetic markers that are associated with cellular phenotype through their effect on gene expression.
a. International HapMap lymphoblastoid cell lines for pharmacogenomic discovery.
The International HapMap LCLs constitute an important commercially available resource for genetic studies. These cell lines are used for the study of genotype-phenotype relationships, including pharmacologic phenotypes. The HapMap project was initiated in 2003 to identify common DNA variation across the human genome and to form a haplotype map of these variants (The International HapMap Consortium et al., 2003). In the initial phases I and II of the HapMap project, four populations of apparently healthy persons were included: 30 family trios (father, mother, child) of Yorubas from Ibadan, Nigeria (YRI), 30 family trios of Utah residents of northern and western European descent (CEU), 45 unrelated Han Chinese persons from Beijing, China, and 45 unrelated persons from Tokyo, Japan.
The first phase of the project resulted in genotype data on more than 1 million SNPs in the samples (The International HapMap Consortium et al., 2005); phase II expanded to more than 2 million additional SNPs in each population, with minor allele frequency (MAF) ≥ 0.05 (International HapMap Consortium et al., 2007). This constitutes approximately 25 to 35% of the estimated 9 to 10 million common SNPs thought to exist in the human genome. HapMap phase III is currently under way and will extend the project with genotype information on 11 new ethnically diverse populations (http://ccr.coriell.org/Sections/Collections/NHGRI/hapmap.aspx?PgId=266&col=HG).
In addition to genotype data on more than 4 million SNPs that are currently available for all 270 HapMap LCLs using multiple genotyping platforms (reviewed in Zhang et al., 2008c), copy number variation (CNV) data are also publicly available from the Wellcome Trust Sanger Institute (Stranger et al., 2007a). More recently, data from the international sequencing effort known as the 1000 Genomes Project (http://www.1000genomes.org), with the most detailed catalog of human genetic variation, are emerging on the 270 HapMap LCLs. In addition, basal gene expression data are available for a portion of the 270 LCLs from Affymetrix Focus array (Spielman et al., 2007; Storey et al., 2007), Affymetrix Human Exon 1.0 ST array (exon array) (Duan et al., 2008; Zhang et al., 2009), and Illumina BeadChips (Stranger et al., 2007a,b). Thus, with genotypic and expression data, the HapMap LCLs are a rich resource that requires only pharmacological phenotyping to evaluate genotype-phenotype, expression-phenotype, or genotype-expression-phenotype relationships. In addition, ethnic diversity of the populations from which the cell lines are derived allows for studies of interethnic variation in cellular phenotypes.
b. Candidate gene approaches.
Candidate gene studies continue to have utility in the postgenomic era. This approach has been applied to many pharmacogenetic investigations of membrane transporters, which are important mediators of drug absorption and elimination and are also targets of many drugs. Significant effort has been invested in resequencing transporter genes in LCLs from the Studies of Pharmacogenetics in Ethnically Diverse Populations participants, followed by functional characterization using in vitro assays in Xenopus laevis oocytes or human cell lines such as human embryonic kidney 293 cells (Osato et al., 2003; Gray et al., 2004; Badagnani et al., 2005, 2006; Fujita et al., 2005; Owen et al., 2005, 2006; Urban et al., 2006b, 2007; Abla et al., 2008; Huang et al., 2008a; Sorani et al., 2008; Chen et al., 2009; Miller et al., 2009; Yee et al., 2009). More recently, these LCLs, along with HapMap LCLs, have been used in functional studies as well, particularly involving allelic expression imbalance. Studies of Pharmacogenetics in Ethnically Diverse Populations samples were used to phenotype the consequences of carriers of the promoter SNP −207G>C in the novel organic cation transporter 2 (OCTN2/SLC22A5). LCLs from −207 C/C subjects had lower total and specific substrate transport as well as reduced OCTN2 gene expression (Urban et al., 2006a). This study was later followed up using HapMap LCLs from four ethnically diverse populations, which found a SNP in the neighboring OCTN1/SLC22A4 gene that is in strong linkage disequilibrium with the −207G>C SNP contributing to racial differences in OCTN2 expression (Tahara et al., 2009). Similar studies of allelic expression imbalance in the CEU and Polymorphism Discovery Resource samples led researchers to find a genetic variant in the drug-metabolizing enzyme NQO2 (Johnson et al., 2008) and promoter and intron 1 polymorphisms in the transporter BCRP (Poonkuzhali et al., 2008) that alter expression of each of these genes.
In addition to examining the pharmacogenomics of membrane transporters, the candidate gene approach in LCLs has been used to elucidate significant associations between genes and sensitivity to drugs used to treat a variety of diseases. Examples of these relationships include deoxycytidine kinase (DCK) and AraC used to treat acute myeloid leukemia (AML) (Hartford et al., 2009), TCF7L2 and insulin used to treat diabetes (Elbein et al., 2007), and dystrobrevin binding protein 1 (DTNBP1) and neuregulin 1 (NRG1) and the antipsychotic olanzapine for treatment of schizophrenia (Chagnon et al., 2008). Each of these examples found variants in a candidate gene that operated through alterations in gene expression. In addition, they demonstrate the applicability of LCL models to many different diseases involving tissue types throughout the body. However, association studies using biologically plausible candidate genes have shown variable success. This may be due to the fact that many of the polymorphisms within candidate genes and the surrounding genomic regions are relatively common in the population and are believed to function as low-penetrance alleles influencing response to therapy.
c. Expression quantitative trait loci studies.
LCLs have successfully been applied to the study of genetic regulation of gene expression as well. Gene expression often acts within the causal pathway between genetic variants and more complex phenotypes, such as drug toxicity and efficacy. One study mapped the genomic regions that regulate quantitative expression differences, or expression quantitative trait loci (eQTLs), using 14 CEPH pedigrees and found that gene expression is heritable (Morley et al., 2004). Later studies revealed gene expression varied between populations by evaluating LCLs derived from white persons, Africans, and Asians (Spielman et al., 2007; Storey et al., 2007; Price et al., 2008; Zhang et al., 2008a). These differences in gene expression patterns among populations may have important implications for pharmacogenetics, because there is ample clinical evidence that patients' historical geographic ancestry is often a strong predictor of their response to certain cytotoxic agents (McCollum et al., 2002; Millward et al., 2003; Watanabe et al., 2003; O'Donnell and Dolan, 2009). The eQTL studies in LCLs have found both cis- and trans-acting SNPs associated with gene regulation (Morley et al., 2004; Cheung et al., 2005; Stranger et al., 2005, 2007a; Duan et al., 2008; Zhang et al., 2008a). Potential pharmacogenetic eQTLs have been identified in the HapMap CEU and YRI LCLs as well. Expression of “Very Important Pharmacogenes” as listed on the Pharmacogenomics Knowledge Base (http://www.pharmgkb.org/), was evaluated for association with genetic variants (Huang et al., 2008d). Expression of 12 genes showed association with SNPs constituting eQTLs, two of which were cis-regulated, GSTM1 and GSTT1. Given the importance of these genes to pharmacokinetics and pharmacodynamics, their associated regulatory SNPs may also have potentially important roles in regulating response to drugs.
A limitation inherent to LCL models is the possibility that gene expression (or drug sensitivity) may be altered by EBV-mediated transformation. However, the recent observation that variation in gene expression profiles in LCLs cluster by families (Cheung et al., 2003) indicates that genetic factors drive gene expression, at least to some extent.
d. Heritability and linkage analyses.
In addition to gene expression (Cheung et al., 2003), drug-induced cytotoxicity has also been demonstrated to be heritable and amenable to genetic dissection (Dolan et al., 2004; Watters et al., 2004). Drug-induced cytotoxicity was measured using short-term assays of cell growth inhibition after treatment with increasing drug concentrations (Watters et al., 2004). Cell lines derived from persons within multigenerational, large CEPH pedigrees were used to show that a significant genetic component (38–47%) contributed to cisplatin-induced cytotoxicity (Dolan et al., 2004), as well as docetaxel (26–65%) and 5-fluorouracil (21–70%) toxicity (Watters et al., 2004). Follow-up studies included more pedigrees to provide greater power (Shukla et al., 2008) and additional drugs such as daunorubicin (Duan et al., 2007), etoposide (Bleibel et al., 2009), and carboplatin (Shukla et al., 2009). Table 3 summarizes the estimated heritability of drug-induced cytotoxicity for each of these drugs.
TABLE 3.
Range of heritability measures of drug cytotoxicity
| Drug | Heritability (h2) | Reference |
|---|---|---|
| Carboplatin | 0.17–0.36 | Shukla et al., 2009 |
| Cisplatin | 0.32–0.43 | Shukla et al., 2008 |
| Daunorubicin | 0.18–0.63 | Duan et al., 2007 |
| Docetaxel | 0.21–0.70 | Watters et al., 2004 |
| Etoposide | 0.17–0.25 | Bleibel et al., 2009 |
| 5-Fluorouracil | 0.26–0.65 | Watters et al., 2004 |
With extensive data on microsatellites and SNPs in the public domain for these cell lines, linkage analysis was used to identify genomic regions harboring genetic variants important in drug sensitivity (Dolan et al., 2004; Watters et al., 2004). Even though a LOD score greater than 3 is considered genome-wide significant, drug sensitivity traits are likely multigenic; therefore, peaks with LOD scores above 1.5 were thought to be suggestive of regions harboring genes that contributed to the trait (Dolan et al., 2004; Watters et al., 2004; Duan et al., 2007; Shukla et al., 2008; Bleibel et al., 2009; Shukla et al., 2009). It is noteworthy that in several of these studies there was an inverse relationship between the height of the peak and the concentration of drug used to obtain the phenotype (cell growth inhibition) indicative that some regions of the genome harbor genes that are involved in the delicate balance between cell survival and cell death (low drug concentrations) while others may be involved in cellular apoptosis (higher concentrations of drug).
Many of the linkage regions were unique to each drug; interestingly, however, some were common to multiple drugs. There are likely genes within these regions important in cell survival that are not dependent on the drug used to cause cellular damage. For instance, chromosomes 4q28.2–q32.3, 8q24–24.2, 11p14.3–13, and 16q23.1–24.1 showed strong linkage with percentage survival at different concentrations of cisplatin and etoposide (Duan et al., 2007; Shukla et al., 2008). In addition, 5-FU and docetaxel shared linkage to chromosome 9q13-q22 (Watters et al., 2004). Some analyses included follow-up association studies under the suggestive linkage peaks (Dolan et al., 2004; Duan et al., 2007; Shukla et al., 2009), resulting in identification of novel candidate pharmacogenes, such as INPP4B on chromosome 4 and CDH13 on chromosome 16 contributing to daunorubicin cytotoxicity (Duan et al., 2007).
Similar linkage studies were performed on LCLs from the CEPH pedigrees to analyze gene expression changes in response to ionizing radiation to determine radiation-responsive genes (Smirnov et al., 2009). eQTLs controlling these responsive genes were identified, trans-regulators dominating. Some of these pairs were previously identified, such as TP53BP2 regulating BAX (Samuels-Lev et al., 2001), whereas most others were novel regulators. Functional analyses validated the roles of some of these new pharmacogenes, demonstrating the utility of cell-based models in identifying novel target genes linked to drug-induced expression changes.
There is ample evidence for reasonable LOD scores from linkage analysis of chemotherapeutic-induced cytotoxicity (Dolan et al., 2004; Duan et al., 2007; Shukla et al., 2008, 2009; Bleibel et al., 2009); however, linkage peaks typically cover a large genomic region that contains many genes that require follow-up analysis. Using linkage-directed association studies or evaluating multiple phenotypes for a given drug may narrow the gene list to a reasonable number for follow-up studies.
e. Expression profiling.
Advances in microarray technology have made it possible to examine expression levels across the genome simultaneously, enabling pharmacogenomic researchers to perform eQTL studies as described above but also to determine expression “signatures” that could be predictive of drug sensitivity. For example, Cloos et al., (2006) used LCLs to show that changes in expression of only 37 genes, including many involved in cell growth and maintenance, could be used to distinguish between persons sensitive to bleomycin and those resistant to bleomycin. Likewise, gene expression signatures in response to ionizing radiation were distinct in LCLs from patients with breast cancer who had pathogenic BRCA1 or BRCA2 mutations and those without (Waddell et al., 2008). In some cases, such as the alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine, almost all (94%) of the variation in sensitivity to drug in LCLs can be explained by basal expression levels of a relatively small number of genes (Fry et al., 2008).
Because microarrays interrogate the entire genome, these analyses allow for identification of novel pathways in drug response. LCLs from the Human Variation Panel were used examine the transcription profiles associated with sensitivity to two cytidine analogs, gemcitabine and AraC, and found that basal transcription of genes within the cytidine metabolic pathway, as well as novel genes, was significantly associated with toxicity (Li et al., 2008). Just as gene expression signatures in disease tissue have been associated with disease prognosis (Bauer et al., 2009; Kim et al., 2009; Walther et al., 2009), these LCL-based studies have shown that transcription profiles have the potential to be equally useful in prediction of drug sensitivity.
f. Integrating genetics, expression, and pharmacologic phenotypes.
With the high-density genotype data available for the HapMap samples, genome-wide association studies to search for novel pharmacogenomic markers can easily be performed. One approach that has evaluated these novel markers for those that are also eQTLs is termed a “triangle” approach, the first arm of the triangle being an evaluation of significant associations between SNPs and drug sensitivity to a specific drug. Then, from this list of SNPs, eQTL analysis is performed to find the subset of SNPs associated with expression of transcripts (second arm of the triangle). In the final arm, the expression of the list of target genes is evaluated for significant linear correlation to drug sensitivity. This type of genome-wide analysis has been successfully used to identify novel genetic variants predicting sensitivity to a variety of chemotherapeutics, including etoposide (Huang et al., 2007a), cisplatin (Huang et al., 2007b), carboplatin (Huang et al., 2008c), daunorubicin (Huang et al., 2008b), and cytarabine (Hartford et al., 2009). However, genome-wide association studies are susceptible to false positives because of the large number of comparisons that are made using data on SNPs or expression across the genome. To address this problem, stringent p values are used, and relationships found in the discovery set have been replicated in an independent set of LCLs (Table 4) (Huang et al., 2008b; Hartford et al., 2009). The ability to replicate these findings in independent sets of cells greatly decreases the likelihood that these associations are spurious but instead could be potentially useful pharmacogenetic markers in a clinical setting.
TABLE 4.
Discovery and validation of novel genes associated with drug sensitivity using cell-based models
| Drugs | Target Gene | Effect | Ref |
|---|---|---|---|
| AraC | SLC25A37 | ↑ expression leads to ↑ drug sensitivity | Hartford et al., 2009 |
| Daunorubicin | CYP1B1 | ↓ expression leads to ↑ drug sensitivity | Huang et al., 2008b |
| Gemcitabine, AraC | NT5C3 | ↓ expression leads to ↑ sensitivity | Li et al., 2008 |
| Gemcitabine, AraC | FKBP5 | ↓ expression leads to ↓ sensitivity | Li et al., 2008 |
| Carboplatin | CD44 | ↓ expression leads to ↑ sensitivity | Shukla et al., 2009 |
| Azacytidine, Inosine-glycodialdehyde | SLC29A1/ENT1 | Treatment with tight binding inhibitor reduces drug sensitivity | Huang et al., 2004 |
| Paclitaxel, bisantrene, geldanamycin analog, Baker's antifol | ABCB1 | ↓ expression leads to ↑ drug sensitivity | Huang et al., 2004 |
| Camptothecin | ABCB5 | ↓ expression leads to ↑ drug sensitivity | Huang et al., 2004 |
In the postgenomic era, LCLs have helped to advance our understanding of how genetic variation alters drug responses. Beyond just candidate gene studies, the extensive genetic variation and gene expression data that are publicly available for these cells, particularly the HapMap cell lines, have enabled interrogation of the entire genome for the discovery of novel pharmacogenomic targets. These model systems have been useful in determining the genetic regulation of expression, as well as in developing sensitivity profiles based on gene expression. Although there are limitations to LCL-based systems, they have the potential to advance the field of pharmacogenomics immensely.
C. NCI-60 Cell-Based Models
Cell lines derived from persons with disease are another important tool for pharmacogenetic discovery. For example, the NCI60 cell line panel has publicly available data on cytotoxicities associated with over 40,000 compounds in a series of 60 cancer cell lines derived from nine different human organs (Shoemaker, 2006). As with the HapMap cell lines, mRNA expression (Ross et al., 2000; Scherf et al., 2000), CNV (Garraway et al., 2005), and SNP genotype data (Garraway et al., 2005) are available for these cell lines. In addition, extensive data on microRNA expression (Blower et al., 2007; Gaur et al., 2007) and proteomic data (Nishizuka et al., 2003) are available (http://discover.nci.nih.gov/cellminer/).
Application of this cell-based disease model to pharmacogenetics discovery has resulted in determination of transcription profiles predicting sensitivity to chemotherapeutics (Weinstein et al., 1997; Staunton et al., 2001; Blower et al., 2002; Huang et al., 2005a; Dai et al., 2006; Salter et al., 2008) as well as proteomic (Ma et al., 2006; Shankavaram et al., 2007; Stevens et al., 2008) and microRNA (Blower et al., 2008; Salter et al., 2008) profiles predicting drug response. Studies using these cells have successfully identified polymorphisms in candidate genes associated with drug response in vitro (Le Morvan et al., 2006; Jarjanazi et al., 2008; Puyo et al., 2008; Sasaki et al., 2008). Methylation of certain promoter CpG islands predicts toxicity to antimetabolites and alkylating agents in the NCI-60 lines (Shen et al., 2007; Sasaki et al., 2008). Like HapMap LCLs, the NCI-60 panel has been important in examining the role of genetic variation in membrane transporters on sensitivity to chemotherapeutics (Huang et al., 2004, 2005b; Szakács et al., 2004; Liu et al., 2007a; Okabe et al., 2008; Pham et al., 2009).
This panel of tumor cell lines can facilitate analysis of the roles of acquired genetic variants in drug responses. For example, one study has assessed the roles of EGFR amplification, mutation, SNPs, a repeat polymorphism, and EGFR mRNA expression in determining resistance or sensitivity to a panel of EGFR inhibitors (Liu et al., 2007b). Other studies have incorporated CNV with gene expression in determining drug sensitivity (Bussey et al., 2006) as well as gene expression and proteomic profiles (Lee et al., 2007; Ma et al., 2009). A combination of SNP and gene expression data for this panel found that cytotoxicity to the alkylphospholipid analog perifosine is associated with MAPK and apopotosis pathways (Zhang et al., 2008b).
As with LCLs, the immense amount of publicly available genetic and expression data for the NCI60 tumor cell line panel have made them an important pharmacogenomic resource. They are tumor cell lines and therefore carry somatic mutations as well, making them quite valuable for studying the effects of chemotherapeutic agents. Beyond the ability to perform transcriptional profiling, candidate gene, and GWA analyses using pharmacological phenotypes in this panel, these cells have the added ability to examine the roles of acquired mutations in drug response.
D. Functional Follow-Up of Genes Identified in Cell-Based Association Studies
A crucial step in bringing genetic variants found in pharmacogenomic studies from the laboratory into clinical practice is functional validation. Functional validation has been used to confirm the GWA results of some cell-based pharmacogenetic studies (Table 4). For example, the role of CD44 in altering sensitivity to carboplatin was functionally validated using siRNA to knock down its expression and sensitize resistant cells (Shukla et al., 2009). Similar siRNA strategies were used to confirm the roles of 5′-nucleotidase, cytosolic III and FK506-binding protein 5 in altering sensitivity to gemcitabine and AraC (Li et al., 2008). The role of SLC29A1/ENT1 in sensitivity to azacytidine and inosine-glycodialdehyde, two nucleoside analogs, was also functionally validated using nitrobenzylmercaptopurine ribonucleoside, a specific, tight-binding inhibitor to SLC29A1/ENT1. Inhibition of this transporter led to reduced sensitivity to both drugs (Huang et al., 2004). In this same study, the roles of other transporters, including ABCB1 and ABCB5, in sensitivity to multiple drugs were functionally confirmed using siRNA (Huang et al., 2004).
Functional studies may be a focal point of future cell model research in pharmacogenetics. Cell lines can be used to functionally validate findings from genome-wide association studies that result in many genes with some false positives. They are also useful as a means to validate clinical observations.
III. Combining Cell-Based Models and Clinical Findings
Traditional approaches to validating clinical genetic associations require identification of the same genotype-to-phenotype relationship in multiple independent populations. Although this standard is also applied to pharmacogenetic associations, these relationships are much more difficult to replicate given the requirement for large clinical studies of patients treated with the same drug regimen. Combined usage of data generated by LCLs and from clinical trials provides a unique opportunity to provide strong evidence for these genotype-phenotype relationships. Examples of such studies are summarized in Table 5.
TABLE 5.
Combining clinical and cell models
| Clinical Problem | Evidence |
|
|---|---|---|
| Clinical | Cell Model | |
| Use of AraC in AML therapy regimens has increased overall survival, but some patients are resistant | Low DCK mRNA levels predict poor survival in patients with AML treated with AraC; low intracellular concentrations of AraC in leukemia cells also predict poorer outcome to therapy | SNPs in DCK are associated with basal expression and drug sensitivity in the HapMap cell lines as well as low intracellular AraC concentrations |
| Development of t-AML occurs in some patients with ALL after etoposide treatment | Presence of MLL translocations that cause t-AML is not associated with etoposide dosage administered to patients with ALL or to toxicity; basal differences in the focal adhesion pathway are associated with etoposide-induced leukemias | No association exists between sensitivity to etoposide and frequency of MLL translocations in HapMap cells; GWAS using HapMap samples also found enrichment of SNPs in the focal adhesion pathway associated with levels of etoposide-induced MLL translocations |
| Reduced response to statins has been observed in some patients | Haplotype H7 (containing rs2846662) and rs2846662 alone associate with reduced response to 2 statins; statin-induced HMGCR13(−) expression inversely correlated with blood lipid measurements after statin treatment | rs2846662 associated with statin-induced expression of alternatively spliced transcript HMGCR13(−); specific siRNA knockdown of full-length HMGCR results in reduced sensitivity to statins |
| About 20% of childhood ALL patients are resistant to therapy, which includes prednisolone | Expression of SMARCB1 contributes to prednisolone sensitivity in leukemic cells | −228 G>T SNP identified which controls SMARCB1 expression via altered binding of PARP1; prednisolone IC50 associated with SMARCB1 expression in cell lines |
| Trimodal distribution of degree of myelosuppression in patients being treated with 6-mercaptopurine | TPMT genotypes are associated with TPMT enzymatic activity and myelosuppression in clinical studies | GWAS performed with HapMap cell lines found TPMT SNPs to be in the top 0.5% of SNPs associated with enzymatic activity |
A. Cytarabine and Deoxycytidine Kinase
Clinical studies have shown that low intracellular concentrations of the chemotherapeutic cytarabine in leukemia cells predict poorer outcome to therapy (Estey et al., 1987; Raza et al., 1992). Likewise, low mRNA levels of DCK, an enzyme involved in the rate-limiting step of cytarabine metabolism, in blast cells predict shorter disease free survival as well as overall survival in an AML population treated with cytarabine (Galmarini et al., 2003). Ex vivo models using LCLs were able to associate these two observations to SNPs in DCK. Examination of LCLs from HapMap populations determined that SNPs within DCK resulted in altered enzyme kinetics, increased ara-CTP intracellular concentrations, higher basal levels of DCK, and increased sensitivity to cytarabine (Lamba et al., 2007; Hartford et al., 2009). Additional SNPs in the 3′untranslated region of DCK (positions +36113 and +35708) were also associated with DCK basal expression and cytarabine sensitivity in the HapMap cell lines and ara-CTP levels leukemic cell samples from patients with AML, respectively (Lamba et al., 2007; Hartford et al., 2009). Thus, for cytarabine pharmacogenomics, clinical studies were successful in identifying a biomarker (ara-CTP levels) and candidate gene (DCK), whereas cell-based models identified candidate SNPs associated with these phenotypes that could be potentially useful in clinical dosing algorithms.
B. 6-Mercaptopurine and Thiopurine Methyltransferase
The ability of GWA studies in cell-based models to recapitulate clinical pharmacogenetic findings has been examined using a classic pharmacogenetics example, TPMT. Past clinical studies have established a strong link between TPMT genotype, enzyme activity, and hematopoietic toxicity in patients receiving thiopurine treatment (Relling et al., 1999; Evans et al., 2001). To determine whether LCLs were useful in recapitulating the relationship between TPMT polymorphisms and TPMT activity, HapMap cell lines were phenotyped for TPMT activity, and both candidate gene analysis and genome-wide association analysis were performed to determine how well SNPs within TPMT predicted enzyme activity relative to other SNPs (Jones et al., 2007). Using the candidate gene approach, five SNPs and four haplotypes predicted TPMT phenotype, two of which were in complete linkage disequilibrium with the known, functional 719A>G SNP in TPMT. Although the genome-wide approach also revealed that a known TPMT haplotype predicted TPMT activity at the p < 0.05 level, haplotypes of 96 other genes ranked higher. Further examination of several of these higher ranked trans-acting variants has confirmed functional links to variation in TPMT activity (M. V. Relling, W. E. Evans, personal communication), providing evidence that some of these are novel variants that regulate TPMT activity. Although TPMT haplotypes were among the top ∼0.5% of genes that predicted TPMT activity, this study points out that GWA studies identify many previously unsuspected genetic associations, probably along with false positives, that require further sifting to determine the true positives. The associations between genetic variants and cell survival after exposure to 6-mercaptopurine, as well as other antileukemic agents, are being tested in these HapMap cell lines as well.
C. Statins and HMG-CoA Reductase
In clinical studies, a haplotype within HMGCR, H7, has been associated with reduced response to both pravastatin and simvastatin in two independent populations (Chasman et al., 2004; Krauss et al., 2008). The mechanism underlying the H7 association was investigated using LCLs derived from participants in The Cholesterol and Pharmacogenetics study. In this particular case, one of the three SNPs contained within the H7 haplotype, rs2846662, was associated with statin-induced expression in LCLs of an alternatively spliced transcript of HMGCR lacking exon 13, HMGCR13(−) (Medina et al., 2008), which was later independently confirmed via expression construct (Burkhardt et al., 2008). Moreover, the magnitude of statin-induced HMGCR13(−) measured ex vivo in the immortalized LCLs was directly correlated with the percentage change of total cholesterol, LDL-cholesterol, apolipoprotein B, and triglycerides measured in vivo in the donor subject after 6 weeks of simvastatin treatment at 40 mg/day (Medina et al., 2008). Furthermore, artificial enrichment of cells with the HMGCR13(−) mRNA via siRNA knockdown of the full-length HMGCR transcript produced an HMGCR enzyme with attenuated sensitivity to statin inhibition, thus identifying a direct mechanism by which rs2846662 might be associated with reduced LDL-cholesterol response to statins (Medina et al., 2008). Indeed, H7 carriers who were also homozygous for rs2846662 had the smaller LDL-C response to statin compared with those who only carried one copy of the variant allele (Krauss et al., 2008). Consequently, this particular case not only exemplifies the utility of the LCLs in functional studies of pharmacogenetically relevant SNPs but also demonstrates their value in the identification of molecular markers of drug response, which may ultimately be tagged by multigenic haplotypes.
D. Etoposide and Myeloid/Lymphoid Leukemia Translocations
LCLs have been used to examine sensitivity to a topoisomerase II-directed agent, etoposide, for treatment of acute lymphoblastic leukemia (ALL) and risk of developing therapy-related AML (t-AML) (Yang et al., 2008). A clinical study found no association between presence of oncogenic translocations that cause t-AML and cumulative etoposide dose or host toxicity. Further validation used LCLs to determine that there was no relationship between inherent sensitivity to etoposide and frequency of translocations. Another study found basal differences in the focal adhesion pathway (i.e., SNPs, CNV, loss of heterozygosity, or expression levels) in patient samples were associated with etoposide-induced leukemias (Hartford et al., 2007). Pathway-based, genome-wide analysis of SNPs in HapMap cell lines with high versus low levels of MLL chimeric fusions after etoposide treatment confirmed this association with the focal adhesion pathway. Thus, cell-based modeling in pharmacogenetics can be useful in recapitulating results found in vivo and providing some insight into mechanism.
E. Prednisolone and Switch/Sucrose Nonfermentable-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily B, Member 1
Genes responsible for prednisolone resistance were identified using an ex vivo screen of leukemic cells from newly diagnosed ALL cases (Holleman et al., 2004). Approximately 33 genes were differentially expressed between sensitive and resistant ALL cells, and this was confirmed in a clinical validation cohort. One of these genes, SMARCB1, was examined further in the CEPH cell lines to determine its role in prednisolone sensitivity. DNA from 90 HapMap cell lines was sequenced to find variants associated with SMARCB1 expression, and a promoter SNP (−228 G>T) was identified that modified basal expression via altered binding of the nuclear protein PARP1 (Pottier et al., 2007). Prednisolone IC50 was inversely related to SMARCB1 expression in CEU cell lines. The −228 SNP was also significantly associated with SMARCB1 mRNA levels (p = 0.046) and protein levels (p = 0.012).
The above studies are excellent examples of how cell-based models can be used to further understand the molecular and cellular underpinnings of pharmacogenetic discoveries made in a clinical setting. LCLs provide a much easier means by which to study the implications of pharmacogenetic findings from clinical studies.
IV. Public Databases for Cell-Based Pharmacogenomic Data
Just as baseline expression (http://www.ncbi.nlm.nih.gov/geo/) and genetic information (http://www.hapmap.org/) is publicly available through large databases for the HapMap samples, so too is important pharmacological and pharmacogenetic data (Table 6). The Pharmacogenetics and Pharmacogenomics Knowledge Base (http://www.pharmgkb.org/) is a central repository for pharmacologic, genomic, and clinical data collected as part of a multitude of pharmacogenomic studies. Organized by the National Institutes of Health Pharmacogenetics Research Network, this database curates primary genotype and drug phenotype data, as well as the gene-drug interactions that are determined by pharmacogenetics research. The site contains information on more than 600 genes in almost 60 pathways and their associations with more than 500 drugs and 500 diseases. Although the immediate purpose of this database is to stimulate pharmacogenetic discovery, the long-term goal is to inform the field of personalized medicine.
TABLE 6.
Useful databases for cell-based pharmacogenetics
| Database | URL | Description |
|---|---|---|
| HapMap | http://www.ncbi.nlm.nih.gov/geo/ | Basal expression data for HapMap samples using various microarray expression platforms |
| http://www.hapmap.org/ | Genetic variation data of >4 million SNPs | |
| PharmGKB | http://www.pharmgkb.org/ | Genotype and drug phenotype data for >600 genes, 500 drugs, and 500 diseases |
| NCI60 | https://dtp.nci.nih.gov/ | SNP, expression, and CNV data on 59 cell lines from various tissues, as well as cytotoxicity data for over 40,000 compounds |
| http://discover.nci.nih.gov/ | Free bioinformatics and data mining tools for use with the genetic and drug phenotype data | |
| SCANdb | http://www.SCANdb.org | Results of genomewide association studies between basal expression of over 13,000 transcripts and genotypes of hundreds of thousands of SNPs in the HapMap CEU and YRI samples |
| Cancer Genome Project | http://www.sanger.ac.uk/genetics/CGP/ | Collection of somatically acquired mutations and other sequence variants in genes that drive oncogenesis |
| VIP Genes and 1000 Genomes | http://genemed1.bsd.uchicago.edu/pharmacodb/thougen/main.php | Organizes the results of high-coverage, deep resequencing of 39 VIP genes in four subjects from the 1000 Genomes project, as well as lower coverage resequencing of 57 other subjects |
Another expansive, publicly available source of pharmacological data is the NCI-60 cancer drug screen (for review, see Shoemaker, 2006). This cell panel currently consists of 59 cell lines (formerly 60) from nine different organ types: breast, colon, central nervous system, leukemia, lung, melanoma, ovarian, prostate, and renal. Originally established to screen for tumor cell growth inhibition of newly identified compounds, this database has evolved into an important resource for pharmacogenetics discovery and validation. More than 40,000 compounds have been characterized in vitro with these cell lines for cytotoxicity, and these data, as well as gene expression, SNP genotypes, and CNV, are publicly available (http://dtp.nci.nih.gov). In addition, helpful bioinformatics software and data mining tools are freely available for use with this data (http://discover.nci.nih.gov), further aiding in application of this data set to advancing pharmacogenetics research.
Because gene expression is often an intermediate phenotype of interest for pharmacogenetic outcomes, a database, called the SNP and CNV Annotation database, or SCAN (http://www.scandb.org), has been developed that catalogs relationships between genetic variants and transcript expression. The data curated in SCAN are the results of GWA studies between Affymetrix Human Exon Array data collected on greater than 13,000 transcript clusters from the 86 CEU and 89 YRI HapMap cell lines and genotype data from the publicly available HapMap resource (release 23a). Additional information on location, linkage disequilibrium, and HapMap frequencies are available for each SNP.
To augment the genetic variation data available through the HapMap Project, the 1000 Genomes Project was initiated to obtain more detailed sequencing of the genomes of 1000 people from all over the world. Rather than focus only on more common variation like HapMap, 1000 Genomes aims to identify >95% of the variants with a MAF >1% in parts of the human genome that can be sequenced, as well as to identify >95% of the variants with a MAF >0.1 to 0.5% in exons (The 1000 Genomes Project, 2007). To assist in applying this new resource to pharmacogenomic research, the “Very Important Pharmacogenes” Genes and 1000 Genomes database (http://genemed1.bsd.uchicago.edu/pharmacodb/thougen/main.php) has emerged to compile the data generated from this project for the “Very Important Pharmacogenes” genes determined by the Pharmacogenetics and Pharmacogenomics Knowledge Base (Gamazon et al., 2009). These data should help inform pharmacogenomic research and introduce novel, potentially relevant variants to be interrogated in association studies.
V. Summary and Future Direction
Interindividual variation in response to drugs among human populations is multifactorial, genetic factors often contributing significantly. Rapid advances in understanding human genetic variation as well as the maturation of high-throughput, cost-effective methods for genotyping and the assay of gene expression continue to provide more powerful research tools for identifying the genetic variants that contribute to drug response. Because of the difficulties in studying drug response in humans, cell-based models have been developed as a means to identify and characterize genetic markers associated with sensitivity to drugs. In particular, with the availability of extensive genotypic (e.g., SNPs and CNVs) and phenotypic (e.g., gene expression) data for the International HapMap cell lines, investigators have begun to analyze pharmacological endpoints within cell lines in efforts to identify clinically important genotype-phenotype relationships. Over the past several years, there has been significant growth in the number of investigators employing cell-based models as a component of their pharmacogenomic research program. Some researchers have used the International HapMap or the Polymorphism Discovery Panel LCLs, whereas other groups have created their own cell lines from individuals with a specific disease for study. There are some nuances of the model system that include the effect of EBV transformation and cellular proliferation rate on gene expression and response to drugs.
The potential utility of stem cell lines remains unexplored, but differentiation into cell types of interest (i.e., hepatocytes or renal cells) could facilitate advances in pharmacogenetics. Furthermore, recent pharmacogenomic studies suggest that common variants can explain only a fraction (<50%) of the variation in drug response (Dolan et al., 2004; Watters et al., 2004). Although other types of genetic variations (e.g., CNVs) and nongenetic factors (e.g., environment) could be responsible for the remaining variation, the unknown, untyped common or rare variants could contribute to the drug response variation. Several large-scale deep resequencing projects such as the SeattleSNPs Project (http://pga.gs.washington.edu/) are working to comprehensively catalog genetic variations, including those relatively rare ones in certain target genes. In contrast, a more recent international sequencing effort, the 1000 Genomes Project (http://www.1000genomes.org) has an ambitious goal to establish a most detailed catalog of human genetic variation in at least 1000 human genomes from world-wide populations using the next-generation sequencing technologies (Mardis, 2008). Once integrated with other public resources, the 1000 Genomes Project data have the potential to greatly benefit pharmacogenomic researches using these samples. Equally important are epigenetic studies or studies incorporating microRNA to add depth to the current pharmacogenomic studies. Most importantly, evaluation of genetic variants, identified originally in cell lines, in large prospective clinical trials will be important to increase confidence in cell-based models.
Acknowledgments.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants U01-GM61393, U01-GM63340]; the National Institutes of Health National Cancer Institute [Grants CA125183, CA136765]; and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL065899-04, HL065899-05, HL69757].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.001461.
- ABCB
- ATP-binding cassette transporter, sub-family B
- ALL
- acute lymphoblastic leukemia
- AML
- acute myeloid leukemia
- AraC
- cytarabine
- CEPH
- Centre d'Etude du Polymorphisme Humain
- CEU
- CEPH from Utah
- CNV
- copy number variation
- DCK
- deoxycytidine kinase
- EBV
- Epstein-Barr virus
- EGFR
- epidermal growth factor receptor
- eQTL
- expression quantitative trait locus
- GWA
- genome-wide association
- HMGCR
- HMG-CoA reductase
- LCL
- lymphoblastoid cell line
- LDL
- low-density lipoprotein
- LOD
- logarithm of the odds
- MAF
- minor allele frequency
- NCI
- National Cancer Institute
- siRNA
- short interfering RNA
- SLC
- solute carrier
- SMARCB1
- switch/sucrose nonfermentable-related, matrix-associated, actin-dependent regulator of chromatin, subfamily B, member 1
- SNP
- single nucleotide polymorphism
- t-AML
- therapy-related acute myeloid leukemia
- TPMT
- thiopurine methyltransferase
- YRI
- Yoruba people from Ibadan, Nigeria
References
- 1000 Genomes Project (2007) Meeting report: a workshop to plan a deep catalog of human genetic variation; 2007 Sep 17–18; Cambridge, UK Available at http://www.1000genomes.org/files/1000Genomes-MeetingReport.pdf [Google Scholar]
- Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Ferrin TE, et al. (2008) The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther 325: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318: 521–529 [DOI] [PubMed] [Google Scholar]
- Badagnani I, Chan W, Castro RA, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2005) Functional analysis of genetic variants in the human concentrative nucleoside transporter 3 (CNT3; SLC28A3). Pharmacogenomics J 5: 157–165 [DOI] [PubMed] [Google Scholar]
- Bauer JW, Bilgic H, Baechler EC. (2009) Gene-expression profiling in rheumatic disease: tools and therapeutic potential. Nat Rev Rheumatol 5: 257–265 [DOI] [PubMed] [Google Scholar]
- Bleibel WK, Duan S, Huang RS, Kistner EO, Shukla SJ, Wu X, Badner JA, Dolan ME. (2009) Identification of genomic regions contributing to etoposide-induced cytotoxicity. Hum Genet 125: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, et al. (2008) MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther 7: 1–9 [DOI] [PubMed] [Google Scholar]
- Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, Dai Z, Liu CG, Reinhold W, Lorenzi PL, Kaldjian EP, et al. (2007) MicroRNA expression profiles for the NCI-60 cancer cell panel. Mol Cancer Ther 6: 1483–1491 [DOI] [PubMed] [Google Scholar]
- Blower PE, Yang C, Fligner MA, Verducci JS, Yu L, Richman S, Weinstein JN. (2002) Pharmacogenomic analysis: correlating molecular substructure classes with microarray gene expression data. Pharmacogenomics J 2: 259–271 [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Kenny EE, Lowe JK, Birkeland A, Josowitz R, Noel M, Salit J, Maller JB, Pe'er I, Daly MJ, et al. (2008) Common SNPs in HMGCR in micronesians and whites associated with LDL-cholesterol levels affect alternative splicing of exon13. Arterioscler Thromb Vasc Biol 28: 2078–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey KJ, Chin K, Lababidi S, Reimers M, Reinhold WC, Kuo WL, Gwadry F, Jain A, Kouros-Mehr H, Fridlyand J, et al. (2006) Integrating data on DNA copy number with gene expression levels and drug sensitivities in the NCI-60 cell line panel. Mol Cancer Ther 5: 853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann HM. (1992) CEPH maps. Curr Opin Genet Dev 2: 393–399 [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Roy MA, Bureau A, Mérette C, Maziade M. (2008) Differential RNA expression between schizophrenic patients and controls of the dystrobrevin binding protein 1 and neuregulin 1 genes in immortalized lymphocytes. Schizophr Res 100: 281–290 [DOI] [PubMed] [Google Scholar]
- Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP, Jr, Ridker PM. (2004) Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 291: 2821–2827 [DOI] [PubMed] [Google Scholar]
- Chen Y, Teranishi K, Li S, Yee SW, Hesselson S, Stryke D, Johns SJ, Ferrin TE, Kwok P, Giacomini KM. (2009) Genetic variants in multidrug and toxic compound extrusion-1, hMATE1, alter transport function. Pharmacogenomics J 9: 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VG, Conlin LK, Weber TM, Arcaro M, Jen KY, Morley M, Spielman RS. (2003) Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet 33: 422–425 [DOI] [PubMed] [Google Scholar]
- Cheung VG, Spielman RS, Ewens KG, Weber TM, Morley M, Burdick JT. (2005) Mapping determinants of human gene expression by regional and genome-wide association. Nature 437: 1365–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E, Yelensky R, Bonakdar S, Plenge RM, Saxena R, De Jager PL, Shaw SY, Wolfish CS, Slavik JM, Cotsapas C, et al. (2008) Genetic analysis of human traits in vitro: drug response and gene expression in lymphoblastoid cell lines. PLoS Genet 4: e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayman CB, Arnold J, Hockwald RS, Yount EH, Jr, Edgcomb JH, Alving AS. (1952) Toxicity of primaquine in Caucasians. JAMA 149: 1563–1568 [DOI] [PubMed] [Google Scholar]
- Cloos J, de Boer WP, Snel MH, van den Ijssel P, Ylstra B, Leemans CR, Brakenhoff RH, Braakhuis BJ. (2006) Microarray analysis of bleomycin-exposed lymphoblastoid cells for identifying cancer susceptibility genes. Mol Cancer Res 4: 71–77 [DOI] [PubMed] [Google Scholar]
- Cloos J, Nieuwenhuis EJ, Boomsma DI, Kuik DJ, van der Sterre ML, Arwert F, Snow GB, Braakhuis BJ. (1999) Inherited susceptibility to bleomycin-induced chromatid breaks in cultured peripheral blood lymphocytes. J Natl Cancer Inst 91: 1125–1130 [DOI] [PubMed] [Google Scholar]
- Dai Z, Barbacioru C, Huang Y, Sadée W. (2006) Prediction of anticancer drug potency from expression of genes involved in growth factor signaling. Pharm Res 23: 336–349 [DOI] [PubMed] [Google Scholar]
- Dausset J, Cann H, Cohen D, Lathrop M, Lalouel JM, White R. (1990) Centre d'etude du polymorphisme humain (CEPH): collaborative genetic mapping of the human genome. Genomics 6: 575–577 [DOI] [PubMed] [Google Scholar]
- Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, Flockhart DA, Zanger UM. (2007) Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 8: 547–558 [DOI] [PubMed] [Google Scholar]
- Dolan ME, Newbold KG, Nagasubramanian R, Wu X, Ratain MJ, Cook EH, Jr, Badner JA. (2004) Heritability and linkage analysis of sensitivity to cisplatin-induced cytotoxicity. Cancer Res 64: 4353–4356 [DOI] [PubMed] [Google Scholar]
- Duan S, Bleibel WK, Huang RS, Shukla SJ, Wu X, Badner JA, Dolan ME. (2007) Mapping genes that contribute to daunorubicin-induced cytotoxicity. Cancer Res 67: 5425–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Huang RS, Zhang W, Bleibel WK, Roe CA, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, et al. (2008) Genetic architecture of transcript-level variation in humans. Am J Hum Genet 82: 1101–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein SC, Chu WS, Das SK, Yao-Borengasser A, Hasstedt SJ, Wang H, Rasouli N, Kern PA. (2007) Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia 50: 1621–1630 [DOI] [PubMed] [Google Scholar]
- Engen RM, Marsh S, Van Booven DJ, McLeod HL. (2006) Ethnic differences in pharmacogenetically relevant genes. Curr Drug Targets 7: 1641–1648 [DOI] [PubMed] [Google Scholar]
- Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M, et al. (2006) The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol 290: F905–F912 [DOI] [PubMed] [Google Scholar]
- Estey E, Plunkett W, Dixon D, Keating M, McCredie K, Freireich EJ. (1987) Variables predicting response to high dose cytosine arabinoside therapy in patients with refractory acute leukemia. Leukemia 1: 580–583 [PubMed] [Google Scholar]
- Evans WE, Hon YY, Bomgaars L, Coutre S, Holdsworth M, Janco R, Kalwinsky D, Keller F, Khatib Z, Margolin J, et al. (2001) Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 19: 2293–301 [DOI] [PubMed] [Google Scholar]
- Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. (1991) Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr 119: 985–989 [DOI] [PubMed] [Google Scholar]
- Feng WH, Hong G, Delecluse HJ, Kenney SC. (2004) Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol 78: 1893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RC, Svensson JP, Valiathan C, Wang E, Hogan BJ, Bhattacharya S, Bugni JM, Whittaker CA, Samson LD. (2008) Genomic predictors of interindividual differences in response to DNA damaging agents. Genes Dev 22: 2621–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Brown C, Carlson EJ, Taylor T, de la Cruz M, Johns SJ, Stryke D, Kawamoto M, Fujita K, Castro R, et al. (2005) Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet Genomics 15: 201–209 [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Thomas X, Graham K, El Jafaari A, Cros E, Jordheim L, Mackey JR, Dumontet C. (2003) Deoxycytidine kinase and cN-II nucleotidase expression in blast cells predict survival in acute myeloid leukaemia patients treated with cytarabine. Br J Haematol 122: 53–60 [DOI] [PubMed] [Google Scholar]
- Gamazon ER, Zhang W, Huang RS, Dolan ME, Cox NJ. (2009) A pharmacogene database enhanced by the 1000 Genomes Project. Pharmacogenet Genomics 19: 829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi J, Zhang J, Xie Y, Soh J, Shigematsu H, Zhang W, Yamamoto H, Peyton M, Girard L, Lockwood WW, et al. (2009) Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One 4: e4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, et al. (2005) Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436: 117–122 [DOI] [PubMed] [Google Scholar]
- Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. (2007) Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67: 2456–2468 [DOI] [PubMed] [Google Scholar]
- Gipps EM, Kidson C. (1984) Cellular radiosensitivity: expression of an MS susceptibility gene? Neurology 34: 808–811 [DOI] [PubMed] [Google Scholar]
- Gray JH, Mangravite LM, Owen RP, Urban TJ, Chan W, Carlson EJ, Huang CC, Kawamoto M, Johns SJ, Stryke D, et al. (2004) Functional and genetic diversity in the concentrative nucleoside transporter, CNT1, in human populations. Mol Pharmacol 65: 512–519 [DOI] [PubMed] [Google Scholar]
- Hartford C, Yang W, Cheng C, Fan Y, Liu W, Treviño L, Pounds S, Neale G, Raimondi SC, Bogni A, et al. (2007) Genome scan implicates adhesion biological pathways in secondary leukemia. Leukemia 21: 2128–2136 [DOI] [PubMed] [Google Scholar]
- Hartford CM, Duan S, Delaney SM, Mi S, Kistner EO, Lamba JK, Huang RS, Dolan ME. (2009) Population-specific genetic variants important in susceptibility to cytarabine arabinoside cytotoxicity. Blood 113: 2145–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, et al. (2004) Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med 351: 533–542 [DOI] [PubMed] [Google Scholar]
- Huang N, Agrawal V, Giacomini KM, Miller WL. (2008a)Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A 105: 1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wallqvist A, Thanki N, Covell DG. (2005a) Linking pathway gene expressions to the growth inhibition response from the National Cancer Institute's anticancer screen and drug mechanism of action. Pharmacogenomics J 5: 381–399 [DOI] [PubMed] [Google Scholar]
- Huang RS, Duan S, Bleibel WK, Kistner EO, Zhang W, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, et al. (2007a)A genome-wide approach to identify genetic variants that contribute to etoposide-induced cytotoxicity. Proc Natl Acad Sci U S A 104: 9758–9763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Duan S, Kistner EO, Bleibel WK, Delaney SM, Fackenthal DL, Das S, Dolan ME. (2008b) Genetic variants contributing to daunorubicin-induced cytotoxicity. Cancer Res 68: 3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Duan S, Kistner EO, Hartford CM, Dolan ME. (2008c) Genetic variants associated with carboplatin-induced cytotoxicity in cell lines derived from Africans. Mol Cancer Ther 7: 3038–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Duan S, Kistner EO, Zhang W, Bleibel WK, Cox NJ, Dolan ME. (2008d) Identification of genetic variants and gene expression relationships associated with pharmacogenes in humans. Pharmacogenet Genomics 18: 545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Duan S, Shukla SJ, Kistner EO, Clark TA, Chen TX, Schweitzer AC, Blume JE, Dolan ME. (2007b) Identification of genetic variants contributing to cisplatin-induced cytotoxicity by use of a genomewide approach. Am J Hum Genet 81: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Temple R. (2008) Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther 84: 287–294 [DOI] [PubMed] [Google Scholar]
- Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadée W. (2004) Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res 64: 4294–301 [DOI] [PubMed] [Google Scholar]
- Huang Y, Dai Z, Barbacioru C, Sadée W. (2005b) Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res 65: 7446–7454 [DOI] [PubMed] [Google Scholar]
- Imray FP, Smith PJ, Relf W, Kidson C. (1984) Wilms' tumour: association with cellular sensitivity to mitomycin C in patients and first-degree relatives. Lancet 1: 1148–1151 [DOI] [PubMed] [Google Scholar]
- Innocenti F, Mirkov S, Nagasubramanian R, Ramírez J, Liu W, Bleibel WK, Shukla SJ, Hennessy K, Rosner GL, Cook E, Jr, et al. (2009) The Werner's syndrome 4330T>C (Cys1367Arg) gene variant does not affect the in vitro cytotoxicity of topoisomerase inhibitors and platinum compounds. Cancer Chemother Pharmacol 63: 881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium (2003) The International HapMap Project. Nature 426: 789–796 [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437: 1299–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium. Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, et al. (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449: 851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Warfarin Pharmacogenetics Consortium. Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, et al. (2009) Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 360: 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjanazi H, Kiefer J, Savas S, Briollais L, Tuzmen S, Pabalan N, Ibrahim-Zada I, Mousses S, Ozcelik H. (2008) Discovery of genetic profiles impacting response to chemotherapy: application to gemcitabine. Hum Mutat 29: 461–467 [DOI] [PubMed] [Google Scholar]
- Jeon JP, Shim SM, Nam HY, Baik SY, Kim JW, Han BG. (2007) Copy number increase of 1p36.33 and mitochondrial genome amplification in Epstein-Barr virus-transformed lymphoblastoid cell lines. Cancer Genet Cytogenet 173: 122–130 [DOI] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, et al. (2006a) The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 9: 485–495 [DOI] [PubMed] [Google Scholar]
- Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, Jung BL, McNamara K, McNamara, Xia H, et al. (2006b)Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci U S A 103: 7817–7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Zhang Y, Papp AC, Pinsonneault JK, Lim JE, Saffen D, Dai Z, Wang D, Sadée W. (2008) Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: detection through allelic expression imbalance in human target tissues. Pharmacogenet Genomics 18: 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TS, Yang W, Evans WE, Relling MV. (2007) Using HapMap tools in pharmacogenomic discovery: the thiopurine methyltransferase polymorphism. Clin Pharmacol Ther 81: 729–734 [DOI] [PubMed] [Google Scholar]
- Kim C, Taniyama Y, Paik S. (2009) Gene expression-based prognostic and predictive markers for breast cancer: a primer for practicing pathologists. Arch Pathol Lab Med 133: 855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyotani K, Yamazaki H, Fujieda M, Iwano S, Matsumura K, Satarug S, Ujjin P, Shimada T, Guengerich FP, Parkinson A, et al. (2003) Decreased coumarin 7-hydroxylase activities and CYP2A6 expression levels in humans caused by genetic polymorphism in CYP2A6 promoter region (CYP2A6*9). Pharmacogenetics 13: 689–695 [DOI] [PubMed] [Google Scholar]
- Klees TM, Sheffels P, Thummel KE, Kharasch ED. (2005) Pharmacogenetic determinants of human liver microsomal alfentanil metabolism and the role of cytochrome P450 3A5. Anesthesiology 102: 550–556 [DOI] [PubMed] [Google Scholar]
- Krauss RM, Mangravite LM, Smith JD, Medina MW, Wang D, Guo X, Rieder MJ, Simon JA, Hulley SB, Waters D, et al. (2008) Variation in the 3-hydroxyl-3-methylglutaryl coenzyme a reductase gene is associated with racial differences in low-density lipoprotein cholesterol response to simvastatin treatment. Circulation 117: 1537–1544 [DOI] [PubMed] [Google Scholar]
- Kudaravalli S, Veyrieras JB, Stranger BE, Dermitzakis ET, Pritchard JK. (2009) Gene expression levels are a target of recent natural selection in the human genome. Mol Biol Evol 26: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, et al. (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27: 383–391 [DOI] [PubMed] [Google Scholar]
- Lamba JK, Crews K, Pounds S, Schuetz EG, Gresham J, Gandhi V, Plunkett W, Rubnitz J, Ribeiro R. (2007) Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther 323: 935–945 [DOI] [PubMed] [Google Scholar]
- Le Morvan V, Bellott R, Moisan F, Mathoulin-Pélissier S, Bonnet J, Robert J. (2006) Relationships between genetic polymorphisms and anticancer drug cytotoxicity vis-à-vis the NCI-60 panel. Pharmacogenomics 7: 843–852 [DOI] [PubMed] [Google Scholar]
- Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, Lee C, Chen Y, Schneck D. (2005) Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 78: 330–341 [DOI] [PubMed] [Google Scholar]
- Lee JK, Havaleshko DM, Cho H, Weinstein JN, Kaldjian EP, Karpovich J, Grimshaw A, Theodorescu D. (2007)A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A 104: 13086–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. (1990) Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet 336: 225–229 [DOI] [PubMed] [Google Scholar]
- Lennard L, Van Loon JA, Lilleyman JS, Weinshilboum RM. (1987) Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther 41: 18–25 [DOI] [PubMed] [Google Scholar]
- Lennard L, Van Loon JA, Weinshilboum RM. (1989) Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther 46: 149–154 [DOI] [PubMed] [Google Scholar]
- Li L, Fridley B, Kalari K, Jenkins G, Batzler A, Safgren S, Hildebrandt M, Ames M, Schaid D, Wang L. (2008) Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Res 68: 7050–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling PD, Huls HM. (2005) Isolation and immortalization of lymphocytes. Curr Protoc Mol Biol Chapter 28:Unit 28 2 [DOI] [PubMed] [Google Scholar]
- Liu MT, Chen YR, Chen SC, Hu CY, Lin CS, Chang YT, Wang WB, Chen JY. (2004) Epstein-Barr virus latent membrane protein 1 induces micronucleus formation, represses DNA repair and enhances sensitivity to DNA-damaging agents in human epithelial cells. Oncogene 23: 2531–2539 [DOI] [PubMed] [Google Scholar]
- Liu R, Blower PE, Pham AN, Fang J, Dai Z, Wise C, Green B, Teitel CH, Ning B, Ling W, et al. (2007a) Cystine-glutamate transporter SLC7A11 mediates resistance to geldanamycin but not to 17-(allylamino)-17-demethoxygeldanamycin. Mol Pharmacol 72: 1637–1646 [DOI] [PubMed] [Google Scholar]
- Liu W, Wu X, Zhang W, Montenegro RC, Fackenthal DL, Spitz JA, Huff LM, Innocenti F, Das S, Cook EH, Jr, et al. (2007b) Relationship of EGFR mutations, expression, amplification, and polymorphisms to epidermal growth factor receptor inhibitors in the NCI60 cell lines. Clin Cancer Res 13: 6788–6795 [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139 [DOI] [PubMed] [Google Scholar]
- Ma Y, Ding Z, Qian Y, Shi X, Castranova V, Harner EJ, Guo L. (2006) Predicting cancer drug response by proteomic profiling. Clin Cancer Res 12: 4583–4589 [DOI] [PubMed] [Google Scholar]
- Ma Y, Ding Z, Qian Y, Wan YW, Tosun K, Shi X, Castranova V, Harner EJ, Guo NL. (2009) An integrative genomic and proteomic approach to chemosensitivity prediction. Int J Oncol 34: 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland ML, Vasisht K, Ratain MJ. (2006) TPMT, UGT1A1 and DPYD: genotyping to ensure safer cancer therapy? Trends Pharmacol Sci 27: 432–437 [DOI] [PubMed] [Google Scholar]
- Mardis ER. (2008) The impact of next-generation sequencing technology on genetics. Trends Genet 24: 133–141 [DOI] [PubMed] [Google Scholar]
- Masson E, Relling MV, Synold TW, Liu Q, Schuetz JD, Sandlund JT, Pui CH, Evans WE. (1996) Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. A rationale for high-dose methotrexate. J Clin Invest 97: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, Fuchs CS. (2002) Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst 94: 1160–1167 [DOI] [PubMed] [Google Scholar]
- McLeod HL, Krynetski EY, Relling MV, Evans WE. (2000) Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia 14: 567–572 [DOI] [PubMed] [Google Scholar]
- Medina MW, Gao F, Ruan W, Rotter JI, Krauss RM. (2008) Alternative splicing of 3-hydroxy-3-methylglutaryl coenzyme A reductase is associated with plasma low-density lipoprotein cholesterol response to simvastatin. Circulation 118: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Huang N, Agrawal V, Giacomini KM. (2009) Genetic variation in human P450 oxidoreductase. Mol Cell Endocrinol 300: 180–184 [DOI] [PubMed] [Google Scholar]
- Millward MJ, Boyer MJ, Lehnert M, Clarke S, Rischin D, Goh BC, Wong J, McNeil E, Bishop JF. (2003) Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol 14: 449–454 [DOI] [PubMed] [Google Scholar]
- Morley M, Molony CM, Weber TM, Devlin JL, Ewens KG, Spielman RS, Cheung VG. (2004) Genetic analysis of genome-wide variation in human gene expression. Nature 430: 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka S, Charboneau L, Young L, Major S, Reinhold WC, Waltham M, Kouros-Mehr H, Bussey KJ, Lee JK, Espina V, et al. (2003)Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proc Natl Acad Sci U S A 100: 14229–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PH, Dolan ME. (2009) Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 15: 4806–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Szakács G, Reimers MA, Suzuki T, Hall MD, Abe T, Weinstein JN, Gottesman MM. (2008) Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther 7: 3081–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osato DH, Huang CC, Kawamoto M, Johns SJ, Stryke D, Wang J, Ferrin TE, Herskowitz I, Giacomini KM. (2003) Functional characterization in yeast of genetic variants in the human equilibrative nucleoside transporter, ENT1. Pharmacogenetics 13: 297–301 [DOI] [PubMed] [Google Scholar]
- Owen RP, Gray JH, Taylor TR, Carlson EJ, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, Giacomini KM. (2005) Genetic analysis and functional characterization of polymorphisms in the human concentrative nucleoside transporter, CNT2. Pharmacogenet Genomics 15: 83–90 [DOI] [PubMed] [Google Scholar]
- Owen RP, Lagpacan LL, Taylor TR, De La Cruz M, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, Giacomini KM. (2006) Functional characterization and haplotype analysis of polymorphisms in the human equilibrative nucleoside transporter, ENT2. Drug Metab Dispos 34: 12–15 [DOI] [PubMed] [Google Scholar]
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–500 [DOI] [PubMed] [Google Scholar]
- Pham AN, Wang J, Fang J, Gao X, Zhang Y, Blower PE, Sadée W, Huang Y. (2009) Pharmacogenomics approach reveals MRP1 (ABCC1)-mediated resistance to geldanamycins. Pharm Res 26: 936–945 [DOI] [PubMed] [Google Scholar]
- Poonkuzhali B, Lamba J, Strom S, Sparreboom A, Thummel K, Watkins P, Schuetz E. (2008) Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms. Drug Metab Dispos 36: 780–795 [DOI] [PubMed] [Google Scholar]
- Poot M, Gollahon KA, Rabinovitch PS. (1999) Werner syndrome lymphoblastoid cells are sensitive to camptothecin-induced apoptosis in S-phase. Hum Genet 104: 10–14 [DOI] [PubMed] [Google Scholar]
- Pottier N, Cheok MH, Yang W, Assem M, Tracey L, Obenauer JC, Panetta JC, Relling MV, Evans WE. (2007) Expression of SMARCB1 modulates steroid sensitivity in human lymphoblastoid cells: identification of a promoter SNP that alters PARP1 binding and SMARCB1 expression. Hum Mol Genet 16: 2261–2271 [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson N, Hancks DC, Myers S, Reich D, Cheung VG, Spielman RS. (2008) Effects of cis and trans genetic ancestry on gene expression in African Americans. PLoS Genet 4: e1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyo S, Le Morvan V, Robert J. (2008) Impact of EGFR gene polymorphisms on anticancer drug cytotoxicity in vitro. Mol Diagn Ther 12: 225–234 [DOI] [PubMed] [Google Scholar]
- Ramírez J, Komoroski BJ, Mirkov S, Graber AY, Fackenthal DL, Schuetz EG, Das S, Ratain MJ, Innocenti F, Strom SC. (2006) Study of the genetic determinants of UGT1A1 inducibility by phenobarbital in cultured human hepatocytes. Pharmacogenet Genomics 16: 79–86 [DOI] [PubMed] [Google Scholar]
- Ratain MJ. (2006) From bedside to bench to bedside to clinical practice: an odyssey with irinotecan. Clin Cancer Res 12: 1658–1660 [DOI] [PubMed] [Google Scholar]
- Raza A, Gezer S, Anderson J, Lykins J, Bennett J, Browman G, Goldberg J, Larson R, Vogler R, Preisler HD. (1992) Relationship of [3H]Ara-C incorporation and response to therapy with high-dose Ara-C in AML patients: a Leukemia Intergroup study. Exp Hematol 20: 1194–200 [PubMed] [Google Scholar]
- Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE. (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91: 2001–2008 [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Box C, Chambers P, Barbachano Y, Nutting CM, Rhŷs-Evans P, Workman P, Harrington KJ, Eccles SA. (2009) Determinants of response to epidermal growth factor receptor tyrosine kinase inhibition in squamous cell carcinoma of the head and neck. J Pathol 218: 122–130 [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, et al. (2000) Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24: 227–235 [DOI] [PubMed] [Google Scholar]
- Salter KH, Acharya CR, Walters KS, Redman R, Anguiano A, Garman KS, Anders CK, Mukherjee S, Dressman HK, Barry WT, et al. (2008) An integrated approach to the prediction of chemotherapeutic response in patients with breast cancer. PLoS ONE 3: e1908. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X. (2001) ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8: 781–794 [DOI] [PubMed] [Google Scholar]
- Sasaki S, Kobunai T, Kitayama J, Nagawa H. (2008) DNA methylation and sensitivity to antimetabolites in cancer cell lines. Oncol Rep 19: 407–412 [PubMed] [Google Scholar]
- Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, et al. (2000) A gene expression database for the molecular pharmacology of cancer. Nat Genet 24: 236–244 [DOI] [PubMed] [Google Scholar]
- Schmiegelow K, Forestier E, Kristinsson J, Söderhäll S, Vettenranta K, Weinshilboum R, Wesenberg F, Nordic Society of Paediatric Haematology and Oncology (2009) Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia 23: 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK, Reimers MA, Scherf U, Kahn A, Dolginow D, et al. (2007) Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther 6: 820–832 [DOI] [PubMed] [Google Scholar]
- Shen L, Kondo Y, Ahmed S, Boumber Y, Konishi K, Guo Y, Chen X, Vilaythong JN, Issa JP. (2007) Drug sensitivity prediction by CpG island methylation profile in the NCI-60 cancer cell line panel. Cancer Res 67: 11335–43 [DOI] [PubMed] [Google Scholar]
- Shoemaker RH. (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6: 813–823 [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Dolan ME. (2005) Use of CEPH and non-CEPH lymphoblast cell lines in pharmacogenetic studies. Pharmacogenomics 6: 303–310 [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Duan S, Badner JA, Wu X, Dolan ME. (2008) Susceptibility loci involved in cisplatin-induced cytotoxicity and apoptosis. Pharmacogenet Genomics 18: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SJ, Duan S, Wu X, Badner JA, Kasza K, Dolan ME. (2009) Whole-genome approach implicates CD44 in cellular resistance to carboplatin. Hum Genomics 3: 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, Rotter JI, Nickerson DA, Yang H, Saad M, et al. (2006) Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol 97: 843–850 [DOI] [PubMed] [Google Scholar]
- Smirnov DA, Morley M, Shin E, Spielman RS, Cheung VG. (2009) Genetic analysis of radiation-induced changes in human gene expression. Nature 459: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorani MD, Zador Z, Hurowitz E, Yan D, Giacomini KM, Manley GT. (2008) Novel variants in human Aquaporin-4 reduce cellular water permeability. Hum Mol Genet 17: 2379–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, Bastone LA, Burdick JT, Morley M, Ewens WJ, Cheung VG. (2007) Common genetic variants account for differences in gene expression among ethnic groups. Nat Genet 39: 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton JE, Slonim DK, Coller HA, Tamayo P, Angelo MJ, Park J, Scherf U, Lee JK, Reinhold WO, Weinstein JN, et al. (2001) Chemosensitivity prediction by transcriptional profiling. Proc Natl Acad Sci U S A 98: 10787–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EV, Nishizuka S, Antony S, Reimers M, Varma S, Young L, Munson PJ, Weinstein JN, Kohn EC, Pommier Y. (2008) Predicting cisplatin and trabectedin drug sensitivity in ovarian and colon cancers. Mol Cancer Ther 7: 10–18 [DOI] [PubMed] [Google Scholar]
- Storey JD, Madeoy J, Strout JL, Wurfel M, Ronald J, Akey JM. (2007) Gene-expression variation within and among human populations. Am J Hum Genet 80: 502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Forrest MS, Clark AG, Minichiello MJ, Deutsch S, Lyle R, Hunt S, Kahl B, Antonarakis SE, Tavaré S, et al. (2005) Genome-wide associations of gene expression variation in humans. PLoS Genet 1: e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Forrest MS, Dunning M, Ingle CE, Beazley C, Thorne N, Redon R, Bird CP, de Grassi A, Lee C, et al. (2007a) Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315: 848–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, et al. (2007b) Population genomics of human gene expression. Nat Genet 39: 1217–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakács G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, et al. (2004) Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell 6: 129–137 [DOI] [PubMed] [Google Scholar]
- Szumlanski C, Otterness D, Her C, Lee D, Brandriff B, Kelsell D, Spurr N, Lennard L, Wieben E, Weinshilboum R. (1996) Thiopurine methyltransferase pharmacogenetics: human gene cloning and characterization of a common polymorphism. DNA Cell Biol 15: 17–30 [DOI] [PubMed] [Google Scholar]
- Tahara H, Yee SW, Urban TJ, Hesselson S, Castro RA, Kawamoto M, Stryke D, Johns SJ, Ferrin TE, Kwok PY, et al. (2009) Functional genetic variation in the basal promoter of the organic cation/carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5). J Pharmacol Exp Ther 329: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HL, Krynetski EY, Yates CR, Loennechen T, Fessing MY, Krynetskaia NF, Evans WE. (1996) Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet 58: 694–702 [PMC free article] [PubMed] [Google Scholar]
- Tozzi V. (2009) Pharmacogenetics of antiretrovirals. Antiviral Res doi: 10.1016/j.antiviral.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Gallagher RC, Brown C, Castro RA, Lagpacan LL, Brett CM, Taylor TR, Carlson EJ, Ferrin TE, Burchard EG, et al. (2006a) Functional genetic diversity in the high-affinity carnitine transporter OCTN2 (SLC22A5). Mol Pharmacol 70: 1602–1611 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Sebro R, Hurowitz EH, Leabman MK, Badagnani I, Lagpacan LL, Risch N, Giacomini KM. (2006b) Functional genomics of membrane transporters in human populations. Genome Res 16: 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, Huang CC, Stryke D, Johns SJ, Kawamoto M, et al. (2007) Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4). Pharmacogenet Genomics 17: 773–782 [DOI] [PubMed] [Google Scholar]
- Waddell N, Ten Haaf A, Marsh A, Johnson J, Walker LC, Investigators K, Gongora M, Brown M, Grover P, Girolami M, et al. (2008) BRCA1 and BRCA2 missense variants of high and low clinical significance influence lymphoblastoid cell line post-irradiation gene expression. PLoS Genet 4: e1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. (2009) Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer 9: 489–499 [DOI] [PubMed] [Google Scholar]
- Wang L, Kumar S, Fridley BL, Kalari KR, Moon I, Pelleymounter LL, Hildebrandt MA, Batzler A, Eckloff BW, Wieben ED, et al. (2008) Proteasome beta subunit pharmacogenomics: gene resequencing and functional genomics. Clin Cancer Res 14: 3503–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Nguyen TV, McLaughlin RW, Sikkink LA, Ramirez-Alvarado M, Weinshilboum RM. (2005) Human thiopurine S-methyltransferase pharmacogenetics: variant allozyme misfolding and aggresome formation. Proc Natl Acad Sci U S A 102: 9394–9399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Taniguchi M, Sasaki S. (2003) Induction chemotherapy with docetaxel, cisplatin, fluorouracil and l-leucovorin for locally advanced head and neck cancers: a modified regimen for Japanese patients. Anticancer Drugs 14: 801–807 [DOI] [PubMed] [Google Scholar]
- Watters JW, Kraja A, Meucci MA, Province MA, McLeod HL. (2004) Genome-wide discovery of loci influencing chemotherapy cytotoxicity. Proc Natl Acad Sci U S A 101: 11809–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum RM, Sladek SL. (1980) Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 32: 651–662 [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, et al. (1997) An information-intensive approach to the molecular pharmacology of cancer. Science 275: 343–349 [DOI] [PubMed] [Google Scholar]
- Yang J, Bogni A, Cheng C, Bleibel WK, Cai X, Fan Y, Yang W, Rocha JC, Pei D, Liu W, et al. (2008) Etoposide sensitivity does not predict MLL rearrangements or risk of therapy-related acute myeloid leukemia. Clin Pharmacol Ther 84: 691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SW, Shima JE, Hesselson S, Nguyen L, De Val S, Lafond RJ, Kawamoto M, Johns SJ, Stryke D, Kwok PY, et al. (2009) Identification and characterization of proximal promoter polymorphisms in the human concentrative nucleoside transporter 2 (SLC28A2). J Pharmacol Exp Ther 328: 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen-Revollo JL, Auman JT, McLeod HL. (2008) Race does not explain genetic heterogeneity in pharmacogenomic pathways. Pharmacogenomics 9: 1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. (2006) Genetics examined in tamoxifen's effectiveness: recurrence warning urged for labeling. Am J Health Syst Pharm 63: 2286–2296 [DOI] [PubMed] [Google Scholar]
- Zhang W, Duan S, Bleibel WK, Wisel SA, Huang RS, Wu X, He L, Clark TA, Chen TX, Schweitzer AC, et al. (2009) Identification of common genetic variants that account for transcript isoform variation between human populations. Hum Genet 125: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Duan S, Kistner EO, Bleibel WK, Huang RS, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, et al. (2008a) Evaluation of genetic variation contributing to differences in gene expression between populations. Am J Hum Genet 82: 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu W, Poradosu E, Ratain MJ. (2008b) Genome-wide identification of genetic determinants for the cytotoxicity of perifosine. Hum Genomics 3: 53–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ratain MJ, Dolan ME. (2008c) The HapMap Resource is Providing New Insights into Ourselves and its Application to Pharmacogenomics. Bioinform Biol Insights 2: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF, Liu JP, Chowbay B. (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41: 89–295 [DOI] [PubMed] [Google Scholar]