Abstract
The agonists of μ-opioid receptor (OPRM1) induce extracellular signal-regulated kinase (ERK) phosphorylation through different pathways: morphine uses the protein kinase C (PKC)-pathway, whereas fentanyl functions in a β-arrestin2-dependent manner. In addition, the two pathways result in the different cellular location of phosphorylated ERK and the activation of different sets of transcriptional factors. In the current study, the influence of the two pathways on the expression of microRNAs (miRNAs) was investigated. After treating the primary culture of rat hippocampal neurons and the mouse hippocampi with morphine or fentanyl for 3 days, seven miRNAs regulated by one or two of the agonists were identified. One of the identified miRNAs, miR-190, was down-regulated by fentanyl but not by morphine. This down-regulation was attenuated by 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene (U0126), which blocks the phosphorylation of ERK. When fentanyl-induced but not morphine-induced ERK phosphorylation was blocked in the primary cultures from β-arrestin2(−/−) mouse, fentanyl did not decrease the expression of miR-190. However, a PKC inhibitor that blocked morphine-induced ERK phosphorylation specifically had no effect on the miR-190 down-regulation. Therefore the decrease in miR-190 expression resulted from the agonist-selective ERK phosphorylation. In addition, the expressional changes in one of the miR-190 targets, neurogenic differentiation 1 (NeuroD), correlated with those in miR-190 expression, suggesting the OPRM1 could regulate the NeuroD pathways via the control of miR-190 expression.
An important characteristic of G protein-coupled receptor (GPCR) signaling is that agonists binding to the same receptor can elicit different physiological effects, not only because agonists possess different efficacies but also because they can activate distinct signaling pathways under that particular receptor [i.e., agonist-selective signaling (Urban et al., 2007)]. One of the well studied agonist-selective signaling phenomena is the extracellular signal-regulated kinase (ERK) phosphorylation. Two pathways are used by G protein-coupled receptors to mediate ERK phosphorylation: the protein kinase C (PKC)/protein kinase A pathway and the β-arrestin pathway (DeWire et al., 2007; Violin and Lefkowitz, 2007). The agonists of GPCRs can use both or only one of the two pathways to induce ERK phosphorylation (Gesty-Palmer et al., 2006; Shenoy et al., 2006). For μ-opioid receptor (OPRM1), morphine- and methadone-induced ERK phosphorylation require the activation of PKC, whereas etorphine and fentanyl exert their functions in a β-arrestin2-dependent manner (Zheng et al., 2008b). In addition, ERK phosphorylated via the PKC pathway remains in the cytosol and activates p90 ribosomal S6 kinase. In contrast, ERK phosphorylated via the β-arrestin2 pathway translocates into the nucleus and activates Elk1. These two pathways not only contribute to the agonist-selective ERK phosphorylation but also are involved in other kinds of agonist-selective signaling. For example, agonists differentially induce receptor internalization (Keith et al., 1998) and receptor desensitization (Johnson et al., 2006; Chu et al., 2008) because of their different abilities to activate the β-arrestin2 pathway.
Tolerance (increase in the dose required to achieve the same effect), which develops after long-term or repetitive usage of OPRM1 agonists, limits their application in clinic. Because OPRM1 agonists have different abilities to induce tolerance (Duttaroy and Yoburn, 1995), the agonist-selective signaling mentioned above has been used to explain such difference (Borgland, 2001; Koch et al., 2005). However, signaling usually terminates within seconds and minutes, whereas the development of tolerance requires hours and days. Therefore, the inconsistency between the time courses of the two phenomena needs to be resolved. As hypothesized previously, the changes in gene expression may bridge agonist-selective signaling and agonist-selective tolerance (Zheng et al., 2008a). Differential signaling induced by different agonists leads to the changes in the expression of different sets of genes, which then contribute to the different abilities of agonists to induce tolerance. This hypothesis is supported by fact that etorphine, but not morphine, produced a significant increase in the protein levels of G protein-coupled receptor kinase 2, dynamin II, and β-arrestin2, which are highly related to the signaling of OPRM1 (Narita et al., 2006).
Hence, in the current study, morphine and fentanyl were used to activate the PKC pathway and the β-arrestin2 pathway, respectively, and the expression of microRNAs (miRNAs) was measured after agonist treatment. miRNAs are a class of RNA molecules approximately 22 nucleotides long that are widely expressed in organisms ranging from worms to humans. They bind to their target mRNAs to inhibit mRNA translation and/or destabilize the mRNAs (Bartel, 2004). By regulating the expression of numerous genes, miRNAs play critical roles in a variety of biological processes, including those in the central nervous system (Kosik, 2006). For example, miR-134 regulates dendritic spine morphology by controlling actin filament dynamics (Schratt et al., 2006). Given that agonists can control the expression of genes (Zheng et al., 2008b), we hypothesized that OPRM1 agonists have differential influence on miRNA expression. Because of a high expression level of OPRM1 in hippocampus (Arvidsson et al., 1995), primary cultures of rat hippocampal neurons and mouse hippocampi were subjected for miRNA microarray analyses after long-term treatment with morphine or fentanyl.
Materials and Methods
Animal and Primary Cultures.
Six- to 8-week-old CD1 (ICR) male mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA) 2 weeks before experiments. The mice were maintained according to Institutional Animal Care and Use Committee regulations. Tail-flick tests were performed between 1:00 and 4:00 PM. The light intensity was adjusted for a 3- to 5-s baseline latency. Cutoff time of 12 s was used to minimize tail damage. Tail-withdrawal responses were recorded 15, 30, 60, 90, and 120 min after subcutaneous drug injection. Percentage of maximum possible effect (%MPE) was calculated by the following formula: (measured latency − baseline latency) × 100/(cut-off time − baseline latency). Each dose involved 8 to 12 mice. ED50 values were generated by analyzing the dose response curves (fentanyl at 15 min and morphine at 30 min) by nonlinear regression.
For long-term drug treatment, mice were anesthetized by injection of 90 mg/kg ketamine i.p. and 10 mg/kg xylazine i.p., followed by implantation with a micro-osmotic pump (Alzet Corp., Palo Alto, CA). Drugs were administered at a rate of 1 μl/h for 72 h. Mice were divided into three groups: one group was infused with saline, the second with 12 μg/h morphine, and the third with 0.31 μg/h fentanyl. The dosages used for infusion were selected based on the respective ED50 values for their analgesic effect: (2.4 mg/kg)/5 h (for morphine) or (27 μg/kg)/2 h (for fentanyl). Each group involved four mice, and experiments were repeated three times.
Primary cultures of rat or mouse hippocampal neurons were prepared as described previously (Liao et al., 2007). In brief, the hippocampi were collected from the rat (Charles River Laboratories) and wild type/β-arrestin2(−/−) mice (C57BL6 background). After plating, cells were cultured for an additional 3 weeks before being used for experiments.
miRNA Microarrays.
Custom miRNA microarray experiments and data analyses were performed according to Kalscheuer et al. (2008). Microarray data have been deposited into the National Center for Biotechnology Information’s Gene Expression Omnibus database under the series GSE14268. Rat primary cultures and mouse hippocampi samples were prepared in triplicate; mouse cerebella samples were examined in duplicate. The data were normalized against internal control in each chip initially. Then, the expression of miRNAs whose sequences are conserved in human, mouse, and rat were examined.
In the rat primary cultures, the expression of miRNAs in fentanyl-treated or morphine-treated samples were compared with those in control samples. The miRNAs with significant expressional change (>125 or <80%; p < 0.225 by t test) were identified. The miRNAs whose changes were identified in both rat primary cultures and mouse hippocampi but not in mouse cerebella were subjected to real-time PCR (QIAGEN, Valencia, CA).
Immunoblotting.
Immunoblotting was performed as reported previously (Zheng et al., 2008b). For immunoblotting of NeuroD detected in the nuclear fraction, the nuclear protein was extracted as described previously (Zheng et al., 2008b). Protein concentrations were determined by BCA Assay (Pierce, Rockford, IL). Blotted membranes were developed using the ECF substrate (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). After developing, each band was scanned using a Storm 860 Imager, and its intensity was determined with ImageQuant analysis software (GE Healthcare). The immunoreactivity of total ERK served as the internal control. Antibodies against the following compounds were used: NeuroD, ERK, and phosphorylated ERK (Cell Signaling Technology Inc., Danvers, MA); alkaline phosphatase-conjugated secondary antibody was obtained from Bio-Rad Laboratories (Hercules, CA). U0126, Ro-31-8425, and PP2 were purchased from Calbiochem (San Diego, CA). U73122 was from Sigma (St. Louis, MO).
Luciferase Assay.
To construct a reporter plasmid (3UTR), a ∼300-base-pair fragment of the DNA encoding part of the 3′-untranslated region (UTR) of rat NeuroD mRNA (with the miR-190 binding site in the middle) was amplified by PCR, digested with NheI and XhoI, and inserted downstream of a firefly luciferase gene. Primers used for PCR were 5′-TTAGGCTAGCGATCTATGCAATTTTTAAACTAGTAATGGG-3′ and 5′- ACAACTCGAGTTAGAGAAGAAAGAAGTGCTAAGGCAACGC-3′. A mutated version of the miR-190 binding site (3UTRmu) was constructed using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The miR-190 mimic, miR-190 inhibitor, and control RNAs were obtained from Dharmacon RNA Technologies (Lafayette, CO). HEK293 cells were cotransfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with empty reporter (vector), 3UTR or 3UTRmu, and control RNA, miR-190 mimic, or miR-190 inhibitor. Luciferase activities were determined 24 h after transfection using the Dual-Luciferase Assay System (Promega, Madison, WI). Luciferase activity was normalized against the Renilla reniformis luciferase expressed by pRL-CMV (Promega, WI).
Real-Time PCR and Transfection.
The total RNAs were extracted and reverse transcribed by using the miScript system (QIAGEN). The real-time PCR was performed after instruction in the miScript system, which included a SYBR Green PCR kit (QIAGEN). β-Actin was used as internal control. The following primers were used: NeuroD, 5′-CTTCCCGGTGCATCCCTACTCCTACC-3′ and 5′- GAGGGGTCCGTCAAAGGAAGGGCTGG-3′; β-actin, 5′-TCCTCCCTGGAGAAGAGCTA-3′ and 5′-CCAGACAGCACTGTGTTGGC-3′; miR-184, 5′-TGGACGGAGAACTGATAAGGG-3′; miR-190, 5′-TGATATGTTTGATATATTAGGT-3′; miR-20a, 5′-TAAAGTGCTTATAGTGCAGGTAG-3′; miR-224, 5′-CAAGTCACTAGTGGTTCCGTTTA-3′; miR-301, 5′-CAGTGCAATAGTATTGTCAAAGC-3′; miR-331, 5′-GCCCCTGGGCCTATCCTAGAA-3′; and miR-365, 5′-TAATGCCCCTAAAAATCCTTAT-3′. The transfection was performed by using Lipofectamine 2000 according to the manufacturer’s instructions.
Results
Determine the Dosage of Morphine and Fentanyl.
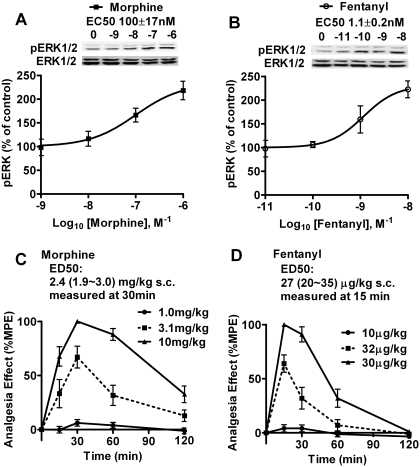
To examine the effects of OPRM1 agonists on miRNA expression, miRNA microarray analyses were performed in the primary cultures of rat hippocampal neurons and in the mouse hippocampi. To compare the effects of morphine and fentanyl, the potencies of these two agonists were determined initially. For the primary cultures of rat hippocampal neurons, ERK phosphorylation was measured after treatment with different concentrations of agonists. As indicated in Fig. 1A, the EC50 of morphine (100 ± 17 nM, n = 4) is approximately 100-fold that of fentanyl (1.1 ± 0.2 nM, n = 4). Therefore, the equivalent concentrations of 1 μM morphine and 10 nM fentanyl were used in the treatment of the rat primary cultures. For the in vivo agonist treatment, the potencies of these two agonists in the antinociceptive test were measured as described under Materials and Methods. The analgesia effect of morphine peaked at 30 min, and the ED50 was 2.4 (1.9∼3.0) mg/kg (Fig. 1C). The analgesia effect of fentanyl peaked at 15 min, and the ED50 was 27 (20∼35) μg/kg (Fig. 1D). Therefore, mice were infused with saline (control), 12 μg/h morphine (1 ED50 dose/5 h), or 0.31 μg/h fentanyl (1 ED50 dose/2 h) for 3 days by using the micro-osmotic pump. The hippocampi and cerebella were dissected from the mouse brains after infusion and subjected to microarray analysis. The mouse cerebella serve as negative control in current study because there is minimal expression of OPRM1 in cerebellum (Arvidsson et al., 1995).
Fig. 1.
Morphine and fentanyl are used at equivalent doses. A and B, dose-dependent curves of morphine (A) and fentanyl (B) to induce ERK phosphorylation in rat primary cultures. Cultures were treated with indicated concentrations of agonists for 5 min. The immunoreactivities of phosphorylated ERK were normalized against those of total ERK. Then the normalized results were compared with those obtained from untreated rat primary cultures (control). EC50 was calculated by averaging the EC50 values generated in each repetition of the experiments. Experiments were repeated for four times. The error bar represents S.D. C and D, dose- and time-dependent curves of morphine (C) and fentanyl (D) to induce analgesia effect in CD1 mouse. Mice were subcutaneously injected with the indicated doses of agonists for indicated times. The %MPE and ED50 were determined as described under Materials and Methods. The ED50 values of morphine and fentanyl were calculated depending on the %MPEs at 30 min and at 15 min (the peaks of analgesia effect), respectively. Each point in the graph included 8 to 12 mice. The error bar presented the S.E.
Morphine and Fentanyl Modulate the Expression of miRNAs Differently.
As described under Materials and Methods, the microarray data were normalized against the internal control first. Then the results in agonist-treated samples were compared with those in control samples. The miRNAs modulated by the agonists in the rat primary cultures and in mouse hippocampi, but not in mouse cerebella, were further monitored by using real-time PCR. As listed in Table 1, three miRNAs, miR-224, miR-331, and miR-365, were modulated by both morphine and fentanyl. One miRNA, miR-20a, was modulated only by morphine. Another three miRNAs, miR-184, miR-190, and miR-301, were modulated by fentanyl only.
TABLE 1.
Morphine and fentanyl differentially modulate the expression of miRNAs
Rat primary cultures were treated with PBS, 1 μM morphine, or 10 nM fentanyl for 3 days. CD1 mice were infused with saline (control), 12 μg/h morphine (1 ED50 dose/ 5 h), or 0.31 μg/h fentanyl (1 ED50 dose/2 h) for 3 days by using a micro-osmotic pump. Microarray experiments were performed in rat primary cultures (three times), mouse hippocampi (three times), and mouse cerebella (Cere. two times). The results were analyzed as described under Materials and Methods. The identified miRNAs have conserved sequences in human, mouse, and rat. Their expression was modulated by agonists in rat primary cultures and mouse hippocampi but not in mouse cerebella. The expression of these miRNAs was also measured by using real-time PCR in rat primary cultures and mouse hippocampi. Real-time PCR results were repeated four times and had P values less than 0.05. Data were analyzed by one-way ANOVA with post hoc Dunnett test for comparisons. Values presented are percentages of control values.
| Long-Term Treatment | Microarray for miRNAs |
Real-Time PCR |
|||
|---|---|---|---|---|---|
| Primary | Hippocampi | Cerebella | Primary | Hippocampi | |
| % | % | % | % | % | |
| Morphine | |||||
| miR-224 | 137 | 162 | 109 | 131 ± 12 | 127 ± 7 |
| miR-331 | 131 | 127 | 95 | 176 ± 23 | 152 ± 8 |
| miR-365 | 129 | 126 | 101 | 125 ± 13 | 120 ± 9 |
| miR-20a | 132 | 132 | 110 | 143 ± 21 | 128 ± 9 |
| Fentanyl | |||||
| miR-224 | 147 | 172 | 101 | 135 ± 14 | 154 ± 23 |
| miR-331 | 128 | 131 | 91 | 120 ± 11 | 131 ± 11 |
| miR-365 | 194 | 129 | 98 | 176 ± 33 | 143 ± 27 |
| miR-184 | 76 | 56 | 88 | 84 ± 8 | 63 ± 23 |
| miR-190 | 68 | 65 | 103 | 55 ± 18 | 70 ± 6 |
| miR-301 | 79 | 49 | 109 | 70 ± 8 | 53 ± 17 |
Morphine and Fentanyl Modulate the Expression of miR-190 Differently.
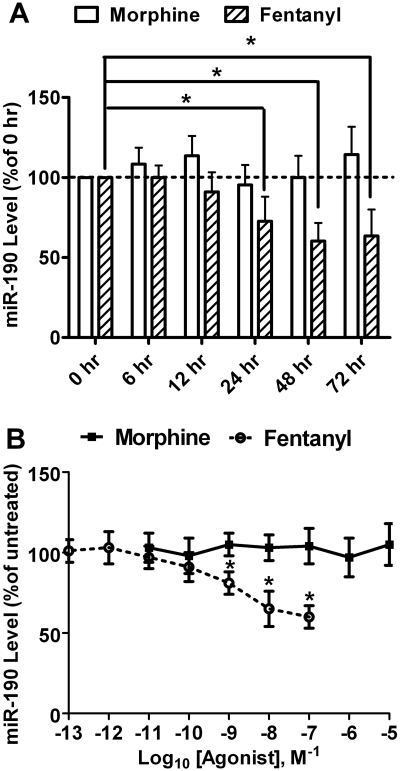
The changes in miR-190 expression in rat primary culture and in mouse hippocampi were not only highly consistent but also larger than those of other miRNAs (Table 1). In addition, only fentanyl but not morphine could decrease the expression of miR-190. Hence, the effect of agonists on miR-190 expression was further analyzed. miR-190 expression in rat primary cultures was monitored by real-time PCR at different time points after initiation of agonist treatment. Morphine did not reduce miR-190 expression at any time point tested (Fig. 2A). In contrast, a significant decrease in miR-190 expression was observed 24 h after initiation of fentanyl treatment (73 ± 15%, n = 4, q = 3.249, p < 0.01). The decrease reached the status state 48 h after initiation (60 ± 11%, n = 4, q = 4.728, p < 0.001) and persisted at least until 72 h after initiation of fentanyl treatment (63 ± 17%, n = 4, q = 4.356, p < 0.01) (Fig. 2A).
Fig. 2.
Agonist-selective regulation on miR-190 expression. A, time-dependent abilities of morphine and fentanyl to modulate the expression of miR-190 in rat primary cultures. Cultures were treated with 1 μM morphine or 10 nM fentanyl for indicated times. The expression of miR-190 was determined by real-time PCR and normalized against the mRNA level of β-actin as described under Materials and Methods. The normalized results were further normalized against the results in untreated cultures (0 h). B, dose-dependent curves of morphine and fentanyl to modulate the expression of miR-190 in rat primary cultures. Cultures were treated with indicated doses of agonists for 3 days. The expression of miR-190 was determined as in A. Experiments were repeated four times. Data were analyzed by one-way ANOVA with post hoc Dunnett test for comparisons. Error bars, S.D.; *, significant changes.
Although morphine did not decrease miR-190 expression at any dose tested (Fig. 2B), fentanyl decreased miR-190 expression at 100 nM (60 ± 7%, n = 4, q = 7.285, p < 0.001), 10 nM (65 ± 11%, n = 4, q = 6.375, p < 0.001), and 1 nM (81 ± 7%, n = 4, q = 3.461, p < 0.05) (Fig. 2B). The EC50 of fentanyl to regulate miR-190 expression [1.4 ± 0.2 nM (n = 4)] is similar to that of fentanyl to regulate ERK phosphorylation (Fig. 1B).
Fentanyl Decreases miR-190 Expression via OPRM1.
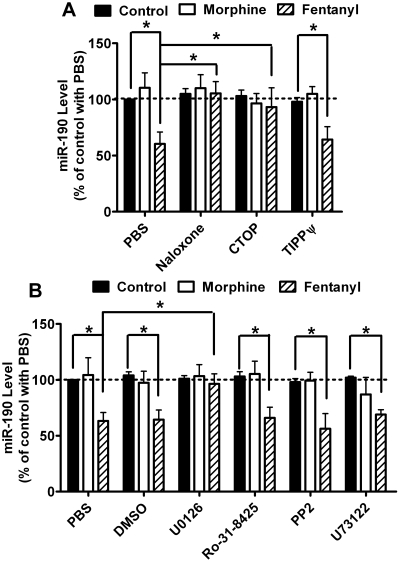
To understand the upstream signaling events controlling miR-190 expression, rat primary cultures were treated with agonists after incubation with the general opioid receptor antagonist naloxone or the OPRM1-specific antagonist Cys2-Tyr3-Orn5-Pen7-amide (CTOP). In the absence of antagonists, fentanyl decreased miR-190 expression to 60 ± 11% (n = 4, t = 5.695, p < 0.001) (Fig. 3A). In the presence of either antagonist, fentanyl did not decrease miR-190 expression: naloxone, 95 ± 11% (n = 4, t = 0.712 p > 0.05); CTOP, 93 ± 17% (n = 4, t = 0.997, p > 0.05) (Fig. 3A). The influence of naloxone (t = 6.315, p < 0.001) and CTOP (t = 4.643, p < 0.001) on fentanyl-induced miR-190 down-regulation was significant. However, when δ-opioid receptor-specific inhibitor H-Tyr-Ticψ[CH2NH]-Phe-Phe-OH (TIPPψ) was used, fentanyl induced a decrease in miR-190 expression (64 ± 11%, n = 4, t = 7.837, p < 0.001). Therefore, although δ-opioid receptor is detected in the hippocampus, fentanyl decreases the expression of miR-190 in primary cultures of rat hippocampal neurons via the activation of OPRM1.
Fig. 3.
ERK phosphorylation is required for the down-regulation of miR-190l. A, rat primary cultures were treated with PBS, 10 μM naloxone, 10 μM CTOP, or 10 μM TIPPψ for 3 h. Then the cultures were treated with 1 μM morphine or 10 nM fentanyl for an additional 3 days. The expression of miR-190 was determined by real-time PCR. The results of miR-190 were normalized against those of β-actin and further normalized against the result obtained from untreated cultures (control) in the PBS group. B, rat primary cultures were treated with PBS, 0.2% DMSO, 2 μM U0126, 4 μM Ro-31-8425, 10 μM PP2, or 10 μM U73122 for 3 h. Then the cultures were treated with 1 μM morphine or 10 nM fentanyl for an additional 3 days. The expression of miR-190 was determined as in A. Experiments were repeated four times. Data were analyzed by two-way ANOVA with post hoc Bonferroni test for comparisons. Error bars, S.D.; *, significant changes.
Fentanyl Decreases miR-190 Expression via ERK Phosphorylation.
To explore the mechanism of fentanyl to control the expression miR-190, inhibitors of mitogen-activated protein kinase kinase (U0126), PKC (Ro-31-8425), Src kinase (PP2), and phospholipase C (U73122) were used. The inhibitors were used at effective doses depending on previous reports (Muid et al., 1991; Jin et al., 1994; Favata et al., 1998; Nam et al., 2002). Because these inhibitors were dissolved in DMSO to generate stock solutions that are 500-fold of the final working concentrations, the ability of fentanyl to decrease miR-190 expression was determined in the rat primary cultures pretreated with 0.2% DMSO in medium. As indicated in Fig. 3B, fentanyl decreased the expression of miR-190 to 64 ± 9% (n = 4, t = 5.854, p < 0.001) of basal level in DMSO-treated cultures, which was not significantly different from that in PBS-treated cultures (t = 0.167, p > 0.05).
Fentanyl did not affect the expression of miR-190 (96 ± 9%, n = 4, t = 0.588, p > 0.05) in U0126-treated rat primary cultures. U0126 blocks the phosphorylation of ERK by inhibiting the activities of MEK1 and MEK2 (Favata et al., 1998). Hence, blocking ERK activation attenuated fentanyl-induced miR-190 down-regulation (t = 5.513, p < 0.001) (Fig. 3B). In the rat primary cultures pretreated with other inhibitors, fentanyl decreased miR-190 expression similarly to that in PBS-pretreated cultures (Fig. 3B). Therefore, fentanyl decreases miR-190 expression via ERK phosphorylation.
To further confirm this hypothesis, the agonist-induced ERK phosphorylation was determined. As indicated in Table 2, morphine and fentanyl increased the amount of phosphorylated ERK to 193 ± 8% (n = 4, t = 19.79, p < 0.001) and 202 ± 13% (n = 4, t = 21.75, p < 0.001), respectively, in rat primary cultures. Pretreatment with DMSO, TIPPψ, PP2, and U73122 did not affect the ERK phosphorylation, whereas U0126, naloxone, and CTOP attenuated the ERK phosphorylation induced by the agonists (Table 2). Consistent with a previous report (Zheng et al., 2008b), the PKC inhibitor Ro-31-8425 attenuated morphine-induced but not fentanyl-induced ERK phosphorylation, suggesting that β-arrestin2-mediated (fentanyl-induced) but not PKC-mediated (morphine-induced) ERK phosphorylation is critical for miR-190 down-regulation. Therefore, the agonist-selective regulation on miR-190 expression is related to agonist-selective ERK phosphorylation.
TABLE 2.
ERK phosphorylation induced by morphine and fentanyl
Rat primary cultures were pretreated as in Fig. 3, and mouse primary cultures were pretreated as in Fig. 4. Then, 1 μM morphine or 10 nM fentanyl was used to treat the cultures for 5 min. The phosphorylation of ERK was determined by normalizing the immunoreactivities of phosphorylated ERK against those of total ERK. The percentages were calculated by further normalizing the results against those in the two PBS samples. The basal level of ERK phosphorylation was not affected by the treatment. Experiments were repeated four times. Data were analyzed by two-way ANOVA with post hoc Bonferroni test for comparisons (rat primary cultures as one experiment, mouse primary cultures as another experiment). Values presented are percentages of control values.
| Pretreatment | ERK Phosphorylation |
||
|---|---|---|---|
| Control | Morphine | Fentanyl | |
| % | % | % | |
| Rat primary cultures | |||
| PBS | 100 | 193 ± 8* | 202 ± 13* |
| Naloxone | 104 ± 4 | 106 ± 5 | 113 ± 11 |
| CTOP | 104 ± 9 | 107 ± 9 | 106 ± 3 |
| TIPPψ | 97 ± 8 | 107 ± 9 | 106 ± 3 |
| DMSO | 101 ± 7 | 181 ± 11* | 185 ± 8* |
| U0126 | 97 ± 11 | 107 ± 10 | 96 ± 6 |
| Ro-31-8425 | 103 ± 3 | 113 ± 13 | 176 ± 5* |
| PP2 | 106 ± 8 | 181 ± 12* | 196 ± 3* |
| U73122 | 94 ± 8 | 194 ± 6* | 176 ± 12* |
| Primary cultures from wild-type mice | |||
| PBS | 100 | 173 ± 7* | 186 ± 12* |
| DMSO | 105 ± 3 | 181 ± 13* | 166 ± 5* |
| U0126 | 107 ± 6 | 89 ± 11 | 104 ± 13 |
| Ro-31-8425 | 98 ± 8 | 109 ± 15 | 171 ± 16 |
| Primary cultures from β-arrestin2 mice | |||
| PBS | 99 ± 11 | 175 ± 8* | 106 ± 11 |
P < 0.05.
Agonist-Selective Regulation on miR-190 Results from Agonist-Selective ERK Phosphorylation.
Morphine induces ERK phosphorylation via PKC pathway, whereas fentanyl functions in a β-arrestin2-dependent manner (Zheng et al., 2008b). Primary cultures of hippocampal neurons from wild-type and β-arrestin2(−/−) mice were used to determine the role played by β-arrestin2. In addition, the PKC inhibitor was used to determine the contribution of PKC.
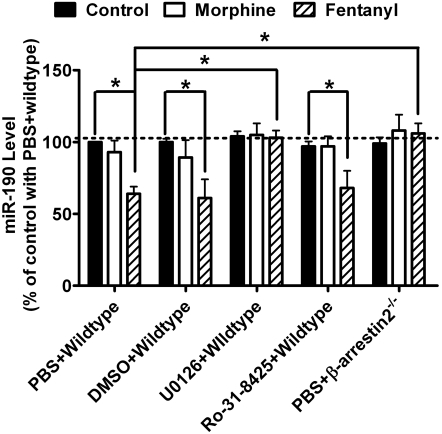
Fentanyl induced the decrease in miR-190 expression in the primary cultures from wild-type mice (61 ± 13%, n = 4, t = 5.912, p < 0.001) (Fig. 4). In addition, both morphine (173 ± 7%, n = 4, t = 11.28, p < 0.001) and fentanyl (186 ± 12%, n = 4, t = 13.29, p < 0.001) induced ERK phosphorylation in these primary cultures (Table 2). When U0126 was used to treat the primary cultures before agonists, neither ERK phosphorylation (morphine, 89 ± 11%, n = 4, t = 1.700, p > 0.05; fentanyl, 104 ± 13%, n = 4, t = 0.618, p > 0.05) nor miR-190 down-regulation (103 ± 5%, n = 4, t = 0.493, p > 0.05) was observed. Therefore, U0126 attenuates (t = 5.314, p < 0.001) fentanyl-induced miR-190 down-regulation by blocking ERK phosphorylation.
Fig. 4.
β-arretsin2-mediated ERK phosphorylation is required for the down-regulation of miR-190. Primary cultures from wildtype C57/BL6 mice were treated with PBS, 0.2% DMSO, 2 μM U0126 or 4 μM Ro-31-8425 for 3 h. Primary cultures from β-arretsin2−/− C57/BL6 mice were treated with PBS for 3 h. Then the cultures were treated with 1 μM morphine or 10 nM fentanyl for additional three days. The expression of miR-190 was determined by real-time PCR. The results of miR-190 were normalized against those of β-actin, and further normalized against the result obtained from untreated cultures (control) in “PBS+wild type” group. Experiments were repeated for four times. Data were analyzed by two-way ANOVA with post hoc Bonferroni test for comparisons. Error bars, S.D.; *, significant changes.
However, when the PKC inhibitor Ro-31-8425 was used, morphine-induced ERK phosphorylation was attenuated (109 ± 15%, n = 4, t = 1.391, p > 0.05), whereas fentanyl still induced ERK phosphorylation (171 ± 16%, n = 4, t = 10.97, p < 0.001). Likewise, fentanyl also induced the down-regulation of miR-190 (68 ± 12%, n = 4, t = 5.255, p < 0.001) in Ro-31-8425–treated rat primary cultures, suggesting that PKC activation does not mediate this down-regulation (Fig. 4).
When primary cultures from β-arrestin2−/− mice were used, fentanyl did not induce ERK phosphorylation (106 ± 11%, n = 4, t = 0.927, p > 0.05), whereas morphine did (175 ± 8%, n = 4, t = 11.59, p < 0.001) (Table 2). In addition, the down-regulation of miR-190 induced by fentanyl was not observed (98 ± 17%, n = 4, t = 0.985, p > 0.05) (Fig. 4). When the ability of fentanyl to decrease miR-190 expression in the primary cultures from β-arrestin2−/− mice was compared with that in the primary cultures from wild-type mice, a significant difference was identified (t = 0.538, p < 0.001). Therefore, the decrease in miR-190 expression requires fentanyl-induced β-arrestin2-mediated ERK phosphorylation.
NeuroD Expression Changes Consistently with miR-190 Expression.
One of the mechanisms by which miRNAs exert their functions is inhibition of the expression of their target mRNAs. To investigate the functional relevance of reduced miR-190, the targets of miR-190 conserved in human, mouse, and rat were predicted by searching the database [http://genome.ewha.ac.kr/miRGator/miRGator.html (Nam et al., 2008)]. The mRNAs of the presumptive targets were quantified by real-time PCR in rat primary cultures after agonist treatment, because miRNAs frequently destabilize their target mRNAs (Bartel, 2004).
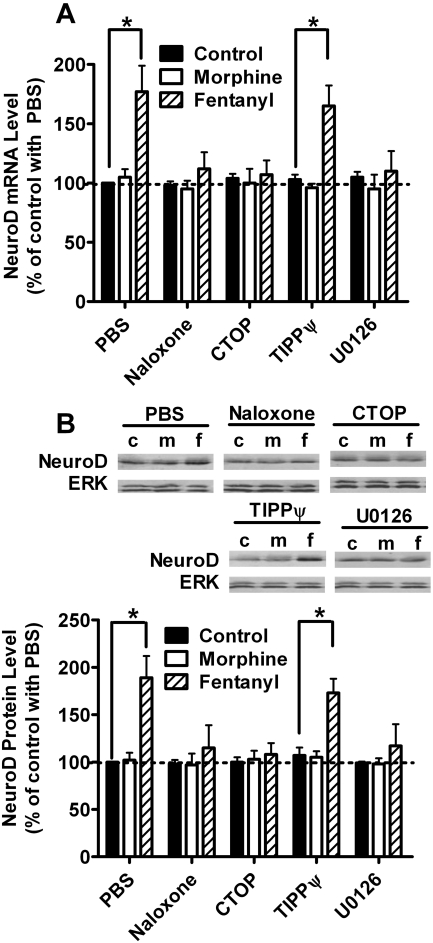
The mRNA levels of one selected target, neurogenic differentiation 1 (NeuroD), increased after treatment with fentanyl (177 ± 22%, n = 4, t = 9.755, p < 0.001) but not morphine (105 ± 7%, n = 4, t = 0.633, p > 0. 05) (Fig. 5A). Such fentanyl-specific increase was also observed when the rat primary cultures were pretreated with TIPPψ (165 ± 17%, n = 4, t = 8.630, p < 0.001) but was not observed with naloxone (112 ± 14%, n = 4, t = 1.520, p > 0.05), CTOP (107 ± 12%, n = 4, t = 0.887, p > 0.05), or U0126 (110 ± 17%, n = 4, t = 1.267, p > 0.05), suggesting that NeuroD is a possible target of miR-190.
Fig. 5.
NeuroD expression was consistent with miR-190 expression. Rat primary cultures were treated with PBS, 10 μM naloxone, 10 μM CTOP, 10 μM TIPPψ, or 2 μM U0126 for 3 h. Then the cultures were treated with 1 μM morphine or 10 nM fentanyl for an additional 3 days. The mRNA levels of NeuroD were determined by real-time PCR (A). The PCR results of NeuroD were normalized against those of β-actin and further normalized against the result obtained from untreated cultures (control) in the PBS group. The protein levels of NeuroD (B) were determined by immunoblotting after nuclear extraction as described under Materials and Methods. The immunoreactivities of NeuroD were normalized against those of total ERK and further normalized against the result obtained from untreated cultures (control) in the PBS group. Experiments were repeated for four times. Data were analyzed by two-way ANOVA with post hoc Bonferroni test for comparisons. Error bars, S.D.; *, significant changes.
NeuroD protein levels were monitored at the same time. The increase in NeuroD protein level was observed in the rat primary cultures treated with fentanyl (189 ± 9%, n = 4, t = 9.515, p < 0.001) but not morphine (102 ± 8%, n = 4, t = 0.214, p > 0.05) (Fig. 5B). Such increase was also observed when the cultures were pretreated with TIPPψ (173 ± 15%, n = 4, t = 9.421, p < 0.001) but was not observed with naloxone (115 ± 24%, n = 4, t = 1.604, p > 0.05), CTOP (108 ± 12%, n = 4, t = 885, p > 0.05), or U0126 (117 ± 23%, n = 4, t = 1.817, p > 0.05), which is consistent with the changes of NeuroD mRNA levels.
NeuroD Is One miR-190 Target.
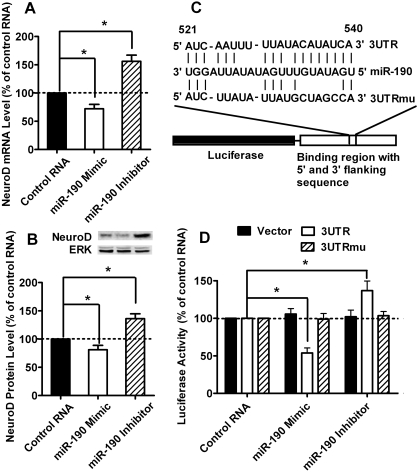
To confirm that NeuroD was one of the miR-190 targets, the miR-190 mimic and miR-190 inhibitor were purchased and used to transfected the rat primary cultures as described under Materials and Methods. As indicated in Fig. 6A, miR-190 mimic decreased the mRNA level of NeuroD (72 ± 8%, n = 4, q = 5.084, p < 0.01), whereas miR-190 inhibitor increased the mRNA level of NeuroD (156 ± 11%, q = 10.17, p < 0.001). The protein level of NeuroD displayed similar changes: the miR-190 mimic decreased it (81 ± 8%, n = 4, q = 3.981, p < 0.01), whereas the miR-190 inhibitor increased it (136 ± 9%, n = 4, q = 7.983, p < 0.001) (Fig. 6B).
Fig. 6.
NeuroD is one target of miR-190. A and B, rat primary cultures were transfected with control RNA, miR-190 mimic, or miR-190 inhibitor by using Lipofectamine 2000. Two days after transfection, the mRNA (A) and protein (B) levels of NeuroD were determined by real-time PCR and immunoblotting, respectively. The results were normalized against internal control (β-actin for mRNA and total ERK for protein) and further normalized against the results obtained from cultures transfected with control RNA. Data were analyzed by one-way ANOVA with Dunnett test as post hoc test to do comparisons. C, schematics of the 3UTR and 3UTRmu reporters. The first nucleotide after the stop codon of rat NeuroD mRNA is designated as number 1. D, HEK293 cells were transfected with one of the RNAs, one of the reporters, and the luciferase reporter system by using Lipofectamine 2000. RNAs included control RNA, miR-190 mimic, and miR-190 inhibitor. Reporters included vector, 3UTR, and 3UTRmu. The luciferase expression was determined as described under Materials and Methods. The results were normalized against internal control (R. reniformis luciferase) and further normalized against the results obtained from cultures transfected with control RNA in each group. Data were analyzed by two-way ANOVA with post hoc Bonferroni test for comparisons. Experiments (A–D) were repeated four times. Error bars, S.D.; *, significant changes.
miR-190 is predicted to bind to nucleotides 521 to 540 of the 3′-UTR of rat NeuroD mRNA, which is conserved in human, mouse, and rat. To assess the functional interaction between miR-190 and NeuroD mRNA, approximately 300 nucleotides of the 3′-UTR (with the predicted miR-190 targeting site in the middle) were inserted into the 3′-UTR of a firefly luciferase reporter mRNA (3UTR). As a control, another luciferase reporter was constructed with the miR-190 targeting site mutated (3UTRmu) (Fig. 6C). The reporters were then cotransfected with a miR-190 mimic or a miR-190 inhibitor into HEK293 cells. As expected, the miR-190 mimic suppressed luciferase expression from the 3UTR construct (54 ± 7%, n = 4, t = 9.780, p < 0.001), whereas the miR-190 inhibitor enhanced the luciferase expression (137 ± 13%, n = 4, t = 7.491, p < 0.001), presumably by blocking the inhibitory effects of endogenous miR-190 (Fig. 6D). In contrast, luciferase expression from the 3UTRmu construct was not affected by the miR-190 mimic (99 ± 8%, n = 4, t = 0.231, p > 0.05) or inhibitor (103 ± 6%, n = 4, t = 0.705, p > 0.05) (Fig. 6D). These results illustrate a specific interaction between miR-190 and NeuroD mRNA and support the hypothesis that increased NeuroD expression resulted directly from the reduction of miR-190 expression.
Discussion
miRNAs, as one important family of small RNAs, play important roles in a variety of biological processes. However, their relation with GPCRs, especially OPRM1, has not been reported extensively. In current study, the different abilities of OPRM1 agonists to modulate the expression of miRNAs were demonstrated. Some miRNAs were regulated by both morphine and fentanyl and some were regulated by only one of the two agonists. One of these miRNAs, miR-190, was under agonist-selective regulation. Fentanyl decreased the miR-190 expression through ß-arrestin2-mediated ERK phosphorylation. Because morphine uses the PKC pathway to induce ERK phosphorylation, it did not affect the expression of miR-190.
Although the mechanism used by fentanyl to modulate the expression of miR-190 has not been identified in the current study, the transcriptional regulation is a possible mechanism. miRNA is initially transcribed as part of a large primary transcript and then undergoes extensive processing for maturation (Cullen, 2004). Thus the initial transcription is a potential checkpoint in the expression of miRNA (Zeng, 2006). In addition, more than 50% of mammalian miRNAs are located within the introns of normal genes (Rodriguez et al., 2004). Therefore, the expression of miRNAs can be regulated by the promoters of their host genes. Because miR-190 locates in the locus of talin2 in human, mouse, and rat, the fentanyl may modulate the expression of miR-190 by controlling the promoter activity of talin2. The agonist-selective ERK phosphorylation also supports this hypothesis. There are a vast number of transcriptional factors under the regulation of ERK in the neurons (Grewal et al., 1999). In addition, the nucleus translocation of phosphorylated ERK after fentanyl treatment may easily affect the transcription of talin2 gene locus via transcriptional factors. The ability of agonist-selective ERK phosphorylation to activate different sets of transcriptional factors further supports the hypothesis (Narita et al., 2006; Zheng et al., 2008b).
However, we still could not eliminate other possibilities. For example, agonist-selective ERK phosphorylation may lead to different effects on the miR-190 stability or on the processing of the initial transcript of miR-190, which may also result in agonist-selective regulation on miR-190 expression. In addition, ERK and its downstream targets may not regulate transcription of the talin2 gene locus directly. β-Arrestin may be involved in this process, not only because fentanyl induces ERK phosphorylation via β-arrestin-pathway but also because β-arrestin could translocate into the nucleus and regulate gene transcription (Ma and Pei, 2007). Because β-arrestin functions as a scaffold protein for ERK phosphorylation (DeWire et al., 2007), it may translocate into the nucleus with phosphorylated ERK at the same time. Thus it is possible that ERK phosphorylation mediates the decrease in miR-190 expression by influencing the nucleus translocation of β-arrestin or the effects of β-arrestin on transcriptional regulation.
One of the miR-190 targets we identified is NeuroD. However, miR-190 may have other targets in addition to NeuroD. Hence the functions of miR-190 may not be limited in regulating the expression of NeuroD. Being a transcription factor involved in the differentiation and migration of neurons, NeuroD has been identified to regulate a large number of genes (Seo et al., 2007). In addition, NeuroD-null mice show severe defects in granule cells of the cerebellum and hippocampal dentate gyrus (Cho and Tsai, 2004). Thus, the agonist-selective regulation of miR-190 and NeuroD may contribute to the functions of the various opioid agonists.
Current results also have important implications with regard to agonist-selective signaling. The differential influence of agonists on the expression of miR-190 and NeuroD results from the agonist-selective ERK activation. This observation supports the hypothesis that differential changes in gene expression may result from the agonist-selective signaling (Zheng et al., 2008a,b). Such agonist-selective regulation on gene expression could have important implication in the long-term use of various agonists.
Acknowledgments
We thank Dr. Liao Dezhi (University of Minnesota, Minneapolis, MN) for advice on preparing primary cultures and Dr. Robert Lefkowitz (Duke University, Durham, NC) for β-arrestin2(−/−) mice.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA007339, DA016674, DA000564, DA011806, K05-DA70544, K05-DA00513].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.060848.
- GPCR
- G protein-coupled receptor
- ERK
- extracellular signal-regulated kinase
- PKC
- protein kinase C
- OPRM1
- μ-opioid receptor
- miRNA
- microRNA
- PCR
- polymerase chain reaction
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
- Ro-31-8425
- 3-(8-(aminomethyl)-6,7,8,9-tetrahydropyrido(1,2-a)indol-10-yl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine
- U73122
- 1-[6-[[17β-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- NeuroD
- neurogenic differentiation 1
- UTR
- untranslated region
- HEK
- human embryonic kidney
- CTOP
- Cys2-Tyr3-Orn5-Pen7-amide
- TIPPψ
- H-Tyr-Ticψ[CH2NH]-Phe-Phe-OH
- DMSO
- dimethyl sulfoxide
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance
- %MPE
- percentage of maximum possible effect.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. (1995) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15: 3328–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Borgland SL. (2001) Acute opioid receptor desensitization and tolerance: is there a link? Clin Exp Pharmacol Physiol 28: 147–154 [DOI] [PubMed] [Google Scholar]
- Cho JH, Tsai MJ. (2004) The role of BETA2/NeuroD1 in the development of the nervous system. Mol Neurobiol 30: 35–47 [DOI] [PubMed] [Google Scholar]
- Chu J, Zheng H, Loh HH, Law PY. (2008) Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell Signal 20: 1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. (2004) Transcription and processing of human microRNA precursors. Mol Cell 16: 861–865 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69: 483–510 [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82: 1226–1236 [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281: 10856–10864 [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. (1999) Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9: 544–553 [DOI] [PubMed] [Google Scholar]
- Jin W, Lo TM, Loh HH, Thayer SA. (1994) U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res 642: 237–243 [DOI] [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70: 676–685 [DOI] [PubMed] [Google Scholar]
- Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. (2008) Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 29: 2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. (1998) mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53: 377–384 [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol 67: 280–287 [DOI] [PubMed] [Google Scholar]
- Kosik KS. (2006) The neuronal microRNA system. Nat Rev Neurosci 7: 911–920 [DOI] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. (2007) Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35: 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Pei G. (2007) Beta-arrestin signaling and regulation of transcription. J Cell Sci 120: 213–218 [DOI] [PubMed] [Google Scholar]
- Muid RE, Dale MM, Davis PD, Elliott LH, Hill CH, Kumar H, Lawton G, Twomey BM, Wadsworth J, Wilkinson SE. (1991) A novel conformationally restricted protein kinase C inhibitor, Ro 31–8425, inhibits human neutrophil superoxide generation by soluble, particulate and post-receptor stimuli. FEBS Lett 293: 169–172 [DOI] [PubMed] [Google Scholar]
- Nam JS, Ino Y, Sakamoto M, Hirohashi S. (2002) Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res 8: 2430–2436 [PubMed] [Google Scholar]
- Nam S, Kim B, Shin S, Lee S. (2008) miRGator: an integrated system for functional annotation of microRNAs. Nucleic Acids Res 36: D159–D164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T. (2006) mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience 138: 609–619 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. (2004) Identification of mammalian microRNA host genes and transcription units. Genome Res 14: 1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439: 283–289 [DOI] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. (2007) Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J 26: 5093–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281: 1261–1273 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320: 1–13 [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422 [DOI] [PubMed] [Google Scholar]
- Zeng Y. (2006) Principles of micro-RNA production and maturation. Oncogene 25: 6156–6162 [DOI] [PubMed] [Google Scholar]
- Zheng H, Chu J, Qiu Y, Loh HH, Law PY. (2008a) Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A 105: 9421–9426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008b) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol 73: 178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]