Abstract
Recent studies indicate that T-type calcium channels (T-channels) in the thalamus are cellular targets for general anesthetics. Here, we recorded T-currents and underlying low-threshold calcium spikes from neurons of nucleus reticularis thalami (nRT) in brain slices from young rats and investigated the mechanisms of their modulation by an anesthetic alcohol, 1-octanol. We found that 1-octanol inhibited native T-currents at subanesthetic concentrations with an IC50 of approximately 4 μM. In contrast, 1-octanol was up to 30-fold less potent in inhibiting recombinant CaV3.3 T-channels heterologously expressed in human embryonic kidney cells. Inhibition of both native and recombinant T-currents was accompanied by a hyperpolarizing shift in steady-state inactivation, indicating that 1-octanol stabilized inactive states of the channel. To explore the mechanisms underlying higher 1-octanol potency in inhibiting native nRT T-currents, we tested the effect of the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and PKC inhibitors. We found that PMA caused a modest increase of T-current, whereas the inactive PMA analog 4α-PMA failed to affect T-current in nRT neurons. In contrast, 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Go 6976), an inhibitor of calcium-dependent PKC, decreased baseline T-current amplitude in nRT cells and abolished the effects of subsequently applied 1-octanol. The effects of 1-octanol were also abolished by chelation of intracellular calcium ions with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. Taken together, these results suggest that inhibition of calcium-dependent PKC signaling is a possible molecular substrate for modulation of T-channels in nRT neurons by 1-octanol.
Multiple subtypes of neuronal voltage-gated Ca2+ channels have a key function in controlling cellular excitability and transmitter release. The proposed functions of low-voltage-activated or transient (T-type) Ca2+ channels (T-channels) include promotion of Ca2+-dependent burst firing, generation of low-amplitude intrinsic neuronal oscillations, elevation of Ca2+ entry, and boosting of dendritic signals. These functions of T-currents in thalamic circuitry may contribute to pacemaker activity, wakefulness and sleep cycles, and seizure susceptibility (Llinás, 1988; Perez-Reyes, 2003). We have shown previously that volatile general anesthetics at clinically relevant concentrations block recombinant and native T-current variants in peripheral and central neurons (Todorovic and Lingle 1998; Todorovic et al., 2000; Joksovic et al., 2005a,b). T-channels are abundantly expressed in thalamic nuclei, where they are crucial in control of the functional states of those neurons (McCormick and Bal, 1997; Llinás et al., 1998). Furthermore, inhibition of thalamic signaling has been implicated as a possibly common mechanism that contributes to such clinical effects of anesthetics as loss of consciousness and sedation (Alkire et al., 2000; Rudolph and Antkowiak, 2004). Therefore, the potential function of T-channels in the thalamus as targets for the action of general anesthetics remains an important issue in our understanding of the cellular mechanisms of anesthetic action.
Many normal alcohols have anesthetic capacity and are widely used to probe for molecular targets of anesthesia. Of particular interest for our studies is 1-octanol, which is a general anesthetic with estimated aqueous concentration EC50 values ranging from 57 to 130 μM for loss of righting reflex in tadpoles (Alifimoff et al., 1989). Within the same concentration range, 1-octanol may potentiate GABAA-receptor-gated currents, one of the major cellular targets of general anesthesia (Franks and Lieb, 1994). However, 1-octanol within the same concentration range also inhibits T-current in native systems, including adult dorsal root ganglion cells (Todorovic and Lingle, 1998), GH3 cell lines (Herrington and Lingle, 1992), hippocampal neurons (Takahashi et al., 1989), and thalamic relay neurons (Llinás et al., 2007). Moreover, we have demonstrated that 1-octanol completely blocks recombinant CaV3.1 (α1G) and CaV3.2 (α1H) T-channels, with estimated IC50 values of 160 and 220 μM, respectively (Todorovic et al., 2000). Given the proposed importance of the thalamus in sensory processing and anesthetic states, we examined the effect of 1-octanol, a representative anesthetic alcohol, on T-current and underlying low-threshold Ca2+ spikes (LTS) in the reticular thalamic nucleus (nRT), the main inhibitory structure in the thalamus.
Materials and Methods
In Vitro Tissue Slice Preparation.
We did most of these experiments on transverse rat brain slices cut through the middle anterior portion of the nRT (Paxinos and Watson, 1982) at a thickness of 250 to 300 μm, as we have done previously (Joksovic et al., 2005a,b, 2006, 2007). Gravid Sprague-Dawley rats were housed in a local animal facility in accordance with protocols approved by the University of Virginia Animal Use and Care Committee. We adhered to the guidelines in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
At the age of 7 to 14 days, rats of either sex were anesthetized with isoflurane and decapitated. The brains were rapidly removed and placed in chilled (4°C) cutting solution consisting of 2 mM CaCl2, 260 mM sucrose, 26 mM NaHCO3, 10 mM glucose, 3 mM KCl, 1.25 mM NaH2PO4, and 2 mM MgCl2 equilibrated with a mixture of 95% O2 and 5% CO2. We glued a block of tissue containing the thalamus to the chuck of a motorized vibroslice (WPI, Saratoga, FL) and cut slices in a transverse plane. We incubated the slices in 36°C oxygenated saline for 1 h, then placed them in a recording chamber that was superfused at a rate of 1.5 ml/min with 124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM MgCl2, 10 mM glucose, and 2 mM CaCl2 equilibrated with a mixture of 95% O2 and 5% CO2. Slices were maintained in the recording chamber at room temperature on room air, where they remained viable for at least 1 h.
Recording Procedures.
The extracellular saline solution typically used for recording in voltage-clamp and current-clamp experiments consisted of 2 mM CaCl2, 130 mM NaCl, 2.5 mM MgCl2, 10 mM glucose, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 0.001 mM tetrodotoxin (TTX). This was equilibrated with a mixture of 95% O2 and 5% CO2 for at least 30 min with final pH of 7.35 to 7.45. In some current-clamp experiments, TTX was omitted to study spike firing of nRT neurons. For recording T-currents in brain slices, we used an internal solution of 135 to 140 mM tetramethylammonium hydroxide (TMA-OH), 10 mM EGTA, 40 mM HEPES, and 2 mM MgCl2 titrated to pH 7.15 to 7.25 with hydrofluoric acid (HF) (Todorovic and Lingle 1998). In some experiments, we altered this internal solution by substituting BAPTA for EGTA. Recording electrodes for current-clamp studies contained 130 mM KCl, 5 mM MgCl2, 1 mM EGTA, 40 mM Na-HEPES, 2 mM MgATP, and 0.1 mM Na3-GTP at pH 7.2. For the data presented, membrane potential values were not corrected for the measured liquid junction potential of −10 mV in voltage-clamp experiments or for the potential of −5 mV in current-clamp experiments.
All recordings were obtained from thalamic neurons visualized with an infrared differential interference contrast camera (C2400; Hammamatsu, Hammamatsu City, Japan) on the Zeiss 2 FS Axioscope (Jena, Germany) with a 40× lens and patch-clamp pipette using a micromanipulator (MP-285; Sutter Instrument, Novato, CA).
Recombinant Human Embryonic Kidney Cells.
HEK 293 cells were stably transfected with rat α1Ib (CaV3.3.b) constructs as described previously (Murbartián et al., 2002; Joksovic et al., 2005b). Cells were typically used 1 to 2 days after plating. The standard extracellular saline for recording Ca2+ current contained 160 mM tetraethylammonium-Cl, 10 mM HEPES, and 2 mM CaCl2, adjusted to pH 7.4 with tetraethylammonium-OH. The internal solution consisted of 110 mM Cs-methane sulfonate, 14 mM phosphocreatine, 10 mM HEPES, 1 or 9 mM EGTA, 5 mM Mg-ATP, and 0.3 mM Tris-GTP; the pH was adjusted to 7.15 to 7.20 with CsOH (standard osmolarity, 300 mOsM). All recordings of currents from HEK 293 cells were done using an inverted Zeiss 200 Axiovert microscope (Jena, Germany) with a 40× lens.
Electrophysiological Recordings.
Recordings were made with standard whole-cell voltage-clamp technique (Hamill et al., 1981). Electrodes were fabricated from thin-walled microcapillary tubes (Drummond Scientific, Broomall, PA) and had final resistances of 3 to 6 MΩ. We recorded membrane currents with an Axoclamp 200B patch-clamp amplifier (Molecular Devices, Foster City, CA). Voltage commands and the digitization of membrane currents were done with Clampex 8.2 of the pClamp software package (Molecular Devices, Foster City, CA). Neurons were typically held (Vh) at −90 mV and depolarized to test potential (Vt) of −50 mV every 10 to 20 s to evoke inward Ca2+ currents. Data were analyzed using Clampfit (Molecular Devices) and Origin 7.0 (OriginLab Corp, Northampton, MA). We filtered currents at 5 to 10 kHz and typically compensated for 50 to 80% of series resistance (Rs). In most experiments, a P/5 protocol was used for on-line leakage subtraction.
Analysis of Current Inhibition.
The percentage reduction in peak T-current at a given 1-octanol concentration was used to generate concentration-response curves. For each such curve, all points are averages of multiple determinations obtained from at least five different cells. In most of the recombinant cells, we tested at least two different concentrations of 1-octanol, whereas in intact brain slices, only one concentration was used per cell in a given slice. On all plots, vertical bars indicate standard errors of the means (S.E.M). Mean values on concentration-response curves were fit to the following function: PB([1-octanol]) = PBmax/(1 + (IC50/[1-octanol])nH), where PBmax is the maximal percentage inhibition of peak T-current, IC50 is the concentration that produces 50% of maximal inhibition, and nH is the apparent Hill coefficient for inhibition. Fitted values are typically reported with 95% linear confidence limits.
Analysis of Current.
The voltage dependence of steady-state activation was described with a single Boltzmann distribution: G(V) = Gmax/(1 + exp[−(V − V50)/k]), where Gmax is the maximal estimated conductance, V50 is the half-maximal voltage, and k (millivolts) represents the voltage dependence of the distribution. The voltage-dependence of steady-state inactivation was described with a single Boltzmann distribution: I(V) = Imax/(1 + exp[(V − V50)/k]), where Imax is the maximal current, V50 is the half-maximal voltage, and k (millivolts) represents the voltage dependence of the distribution. The time course of current inactivation was fitted using a single exponential equation, f(t) = A1exp(−t/τ1), yielding one-time constants (τ1) and their amplitude (A1).
For all current-voltage (I-V) curves and steady-state inactivation curves, fitted values typically were reported with 95% linear confidence limits. Input resistance (Rin) was determined from the slope of the peak voltage versus the current plot that resulted from injecting 80- to 160-ms current ranging from 100 to 1000 pA. Statistical analysis was performed with either paired Student t test or unpaired Student's t test where appropriate, with statistical significance at p < 0.05.
Methodological Considerations Relevant to Intact Brain Slices.
Recordings from intact brain slices offer great advantages for studying neurons in an intact setting in vitro. However, because voltage control is compromised in whole-cell recordings from slices because of the presence of extensive cell processes, we paid close attention to the signs of good voltage control. Specifically, there was no extensive delay in the onset of current; also, the onset and offset kinetics depended on voltage, not on the amplitude of current. In whole-cell experiments, because intact nRT neurons have long processes, rapid components of recorded current, such as fast-activation kinetics or tail currents, are not likely to reflect the true amplitude and time course of Ca2+ current behavior. All our measurements of amplitudes from holding, peak, and steady-state currents were made at time points sufficient to ensure reasonably well clamped current conditions. Furthermore, using brain slices from young animals, in which dendrites are not fully developed, alleviated the space-clamp problem.
To facilitate application of drugs, we applied first 10 ml of external solution containing drug with bath flow of approximately 5 ml/min, and then continued with perfusion of 1 to 2 ml/min. All drugs were applied until an apparent steady-state effect on peak T-current was achieved. To avoid the accumulation of highly lipophilic substance in the tissue, we used only a single application of any given concentration of 1-octanol per slice.
Drugs and Chemicals.
TTX was obtained from Alomone Lab (Jerusalem, Israel). All other salts and chemicals were obtained from Sigma Chemical (St. Louis, MO). Stock solutions (100 μM) of phorbol 12-myristate 13-acetate (PMA) and its inactive stereoisomer (4αPMA), as well as Go 6976 at 10 mM, were made in dimethyl sulfoxide, while a 100 μM stock solution of TTX was prepared in distilled water. The maximal final concentration of dimethyl sulfoxide used in our experiments was 0.1%, which does not significantly affect native thalamic Ca2+ currents (Joksovic et al., 2005a,b).
Solutions.
Multiple independently controlled glass syringes attached to the common PVC tubing served as reservoirs for a gravity-driven perfusion system. Manually controlled valves were used to switch solutions. All experiments were done at room temperature (20–24°C). All drugs were prepared as stock solutions kept in sealed glass vials and freshly diluted to appropriate concentrations as needed. 1-Octanol was prepared from 2 mM stock solution obtained by mixing and stirring for several hours of 7.87 μl of 1-octanol with 25 ml of external solution in a sealed glass container. Aliquots of 1-octanol stock were then diluted to final concentrations and used fresh for each application. Determination of actual concentrations of 1-octanol in external solution was not performed. However, previous measurements with gas chromatography have indicated that the rate of volatilization of 1-octanol is less than 30% during first 2 h of stock preparation (C. J. Lingle and S. M. Todorovic, unpublished observations). During each experiment, solution was removed from the end of the chamber opposite the tubing by constant suction. Changes in Ca2+ current amplitude in response to applied drugs or ionic changes typically were complete in up to 3 min. Switch of flow between separate perfusion syringes, each containing control saline, resulted in no significant changes in the amplitude and kinetics of T-current.
Results
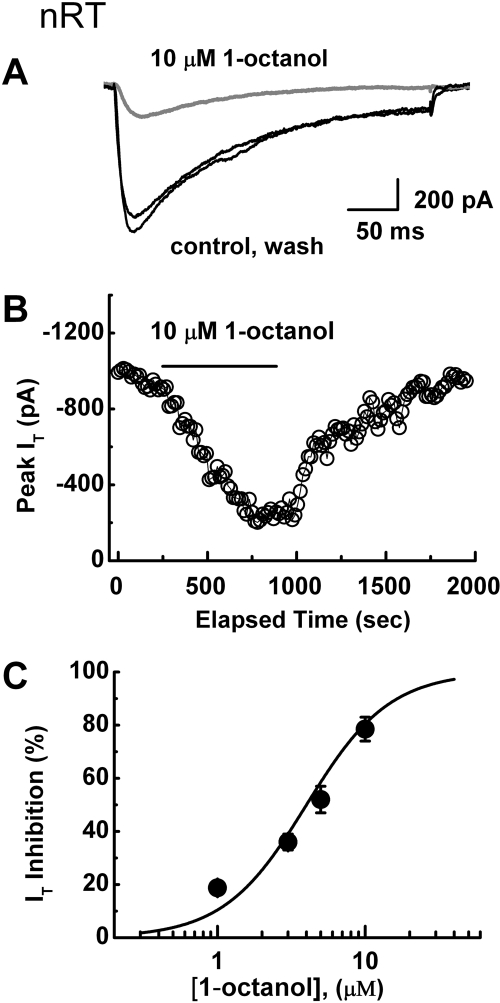
Bath application of 1-octanol potently inhibited T-current in nRT cells in brain slices that display characteristic slowly inactivating kinetics (Joksovic et al., 2005a,b). Figure 1A shows traces and Fig. 1B depicts the time course from the same voltage-clamp experiment in which 10 μM 1-octanol reversibly inhibited approximately 80% of the peak T-current without an apparent effect on macroscopic current inactivation kinetics. Figure 1C summarizes average data points with 1, 3, 5, and 10 μM 1-octanol demonstrating concentration-dependent, potent, and almost complete inhibition of T-current. The solid line represents a Hill plot giving an estimated IC50 of approximately 4 μM.
Fig. 1.
Potent inhibition of native slowly inactivating T-currents in nRT neurons by 1-octanol. A, traces represent T-current elicited from Vh −90 mV to Vt −50 mV in a representative nRT neuron before and after (black lines) as well as during (gray line) the application of 10 μM 1-octanol. This resulted in depression of the current amplitude but had no apparent effect on the macroscopic kinetics of the current. B, symbols represent the time course of the effect on peak T-current before, during, and after the application of 1-octanol to the same cell as that in A. Depolarizing pulse was applied every 10 s; the horizontal bar indicates time of the application. Inhibition of the peak current is gradual and partially reversible. C, concentration-response curve for T-current inhibition by 1-octanol. The solid symbols indicate the average of multiple determinations (n = 5 or more); vertical lines are ± S.E.M. (visible only if bigger than symbols). The solid line represents the best fit of the Hill equation. The fitted value for the curve is IC50 of 4 ± 1 μM, nH = 1.6 ± 0.5; maximal fitted effect was constrained to 100% inhibition.
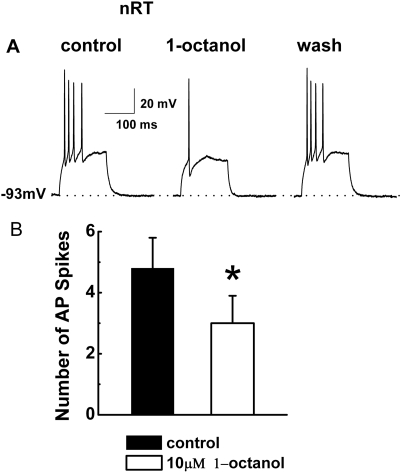
We hypothesized that 1-octanol may abolish LTS of nRT neurons that critically depend on T-channels. Thus, we used current-clamp recordings from these cells and assessed spike firing when the neuronal membrane was depolarized with current injections through the recording electrode from holding negative membrane potentials (e.g., −90 mV), which maximize the availability of T-channels. Figure 2A presents traces from a representative nRT cell before, during, and after the application of 10 μM 1-octanol. 1-Octanol reversibly depressed the number of action potentials that crowned LTS from four to one. The histograms in Fig. 2B indicate that in similar current-clamp experiments, 10 μM 1-octanol significantly decreased the membrane firing that accompanied LTS from approximately five to three action potentials (n = 6 cells, p < 0.05). Even though in most of the cells applications of 1-octanol in current-clamp experiments caused changes in baseline membrane potential, overall average changes did not significantly differ between baseline (−54.3 ± 3.2 mV) and 1-octanol (−51.0 ± 5.3 mV, n = 6, p > 0.05, data not shown).
Fig. 2.
1-Octanol inhibits burst firing and LTS in nRT cells. A, representative traces recorded in current-clamp mode from an nRT neuron at a holding potential of −93 mV, indicated by the horizontal dashed line. LTS was evoked on injection of a small depolarizing pulse (duration, 160 ms; amplitude, 60 pA) and elicited a burst of 4 action potentials (APs). This burst was greatly diminished in the presence of 10 μM 1-octanol (one AP), but was completely recovered on washout of 1-octanol (four APs). Bars indicate calibration. Holding potential was maintained by a constant injection of current via the recording electrode. B, the histogram compares the number of AP spikes in control (4.8 ± 1.0) and in the presence of 1-octanol (3.0 ± 1.0). *, p < 0.05 (n = 6 cells, paired Student's t test). Vertical bars represent S.E.
In another set of experiments, we included TTX in the external solution and applied a 100-ms preceding hyperpolarizing pulse, which resulted in rebound LTS, because sufficient hyperpolarization of the neuronal membrane caused deinactivation of T-channels. This protocol allowed us to measure the effects of 1-octanol on isolated LTS and input resistance in current-clamp experiments (Joksovic et al., 2006). On average, 10 μM 1-octanol decreased the amplitude of isolated LTS in nRT neurons for 35 ± 5% (n = 5, p < 0.01, data not shown). In contrast, average input resistance (Rin) was not significantly affected in these cells (control, 118 ± 2 MΩ; 1-octanol, 162 ± 60 MΩ; n = 6, p > 0.05, data not shown). Somewhat greater 1-octanol-induced inhibition of isolated T-currents than LTS and associated burst firing may be related to better separation of T-currents from other ionic conductances in voltage-clamp experiments.
Next we considered the possibility that potent inhibition of native T-current in nRT neurons by 1-octanol may be due to its direct interaction with a specific molecular isoform of T-channels that is expressed in this thalamic nucleus. Previous molecular studies using in situ hybridization demonstrated that nRT has abundant transcripts for CaV3.2 (α1H) and CaV3.3 (α1I), but sparse CaV3.1 (α1G) isoforms of the pore-forming α1 subunit (Talley et al., 1999). Thus, we tested the sensitivity of recombinant rat CaV3.3 channels, which characteristically display slow inactivation kinetics similar to those of T-currents of native nRT cells (Joksovic et al., 2005b).
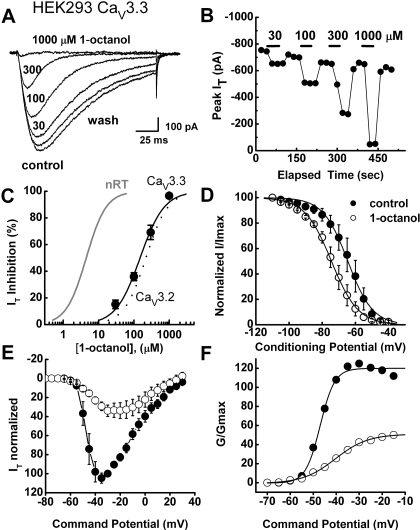
Figure 3A shows traces of inhibition of recombinant CaV3.3 current in HEK 293 cells with escalating concentrations of 1-octanol (30, 100, 300, and 1000 μM). Figure 3B shows the time course from the same cell. Here, 1-octanol rapidly induced a reversible, almost complete concentration-dependent inhibition of CaV3.3 current. However, the black solid line representing the Hill plot of the average concentration-response curve in Fig. 3C shows that this agent was more than 30-fold less potent in inhibiting recombinant CaV3.3 (IC50, 150 μM) than native nRT T-currents (solid gray line; IC50, 4 μM). The dashed black line in Fig. 3C represents the Hill plot of the 1-octanol block of recombinant human CaV3.2 channels with an IC50 of 219 μM (from Todorovic et al., 2000). Most of our recordings with recombinant cells were done with intracellular solution containing 10 mM EGTA. Hence, we also examined the ability of 100, 300, and 1000 μM 1-octanol to inhibit recombinant CaV3.3 currents with 1 mM intracellular EGTA to provide more physiological concentrations of intracellular Ca2+ (Harney et al., 2006). However, in these experiments with low concentrations of EGTA we obtained similar potency of 1-octanol in inhibiting peak CaV3.3 current (fitted IC50, 176 ± 1.0 μM; nH 1.4 ± 0.1, n = 6 cells; data not shown). Thus, it seems that inhibitory effect of 1-octanol on recombinant CaV3.3 currents is not critically dependent on intracellular Ca2+ concentrations.
Fig. 3.
1-Octanol inhibits recombinant T-currents less potently than do native nRT T-currents. A, traces depict the inhibitory effect of escalating concentrations of 1-octanol on the amplitude of CaV3.3 current in a representative HEK 293 cell (Vh −90 mV, Vt −30 mV). A progressive increase in concentrations of 1-octanol leads to almost complete inhibition of T-current amplitude and apparent speeding of current inactivation kinetics. B, time course of T-current inhibition with 30, 100, 300, and 1000 μM 1-octanol from the same cell as that in A. Horizontal bars indicate the duration of drug application. Note rapid onset and almost complete washout of the effect after each application. A depolarizing test pulse was applied every 20 s. C, symbols indicate the concentration-response curve for CaV3.3 T-type current inhibition by 1-octanol. The solid symbols indicate the average of multiple determinations (n = 5 or more); vertical lines are ± S.E.M. (visible only if bigger that symbols). The solid black line represents the best fit of the Hill equation for CaV3.3. The fitted value for the curve is IC50 of 149.4 ± 35.6 μM, nH = 1.3 ± 0.4; maximal fitted effect was constrained to 100% inhibition. The solid gray line is the same as that in Fig. 1C, indicating that native T-current in nRT cells is 30-fold more potently inhibited by 1-octanol than recombinant CaV3.3 current. The dashed line represents the best fit of the Hill equation for CaV3.2 current (from Todorovic et al., 2000) yielding an IC50 of 219 μM, nH = 1.4; curve fit was constrained to 100% inhibition. D, the steady-state inactivation kinetics of T-currents in CaV3.3-transfected HEK cells were examined by paired-pulse protocols at different conditioning potentials. Double-pulse protocols to −30 mV were used to elicit T-currents, separated by 3.5-s prepulse to potentials ranging from −110 to −40 mV before (filled symbols) and during (open symbols) the application of 300 μM 1-octanol. The data were best fitted with the Boltzmann equation, yielding V50 of −64.5 ± 0.3 mV (k = 7.4 ± 0.3 mV) in control and −74.4 ± 0.4 mV (k = 8.0 ± 0.3 mV) in the presence of 1-octanol (n = 5 cells). E, the average I-V relationships before (●) and during (○) the application of 300 μM 1-octanol. Currents were elicited by progressing from −80 to 30 mV in 5-mV increments from a holding potential of −90 mV (n = 6 cells). Note the depression of the current amplitude at most potentials and the slight shift of the I-V curve to the right in the presence of 1-octanol. F, normalized conductance in whole-cell recordings from current-voltage experiments depicted in E of this figure. The average half-conductance (V50) was in control −47.2 ± 0.4 mV (k = 3.2 ± 0.3 mV) and in the presence of 1-octanol was −40.4 ± 0.4 mV (k = 7.1 ± 0.4 mV). Fits were done using the Boltzmann equation. The estimated reversal potential was taken to be 50 mV.
In contrast to the effect of 1-octanol on native T-current in nRT cells, traces on Fig. 3A with 100 and 300 μM 1-octanol suggest that 1-octanol enhances the speed of macroscopic current inactivation. Thus, we fitted the decaying portion of the current with a single exponential function, finding that 1-octanol caused a significant increase in the CaV3.3 current inactivation rate as shown by a 3-fold decrease in inactivation time constant (τ) from 120 ± 20 to 41 ± 4 ms (n = 8, p < 0.005) for 100 μM 1-octanol (data not shown). In addition, 300 μM 1-octanol shifted the steady-state inactivation of CaV3.3 channels to hyperpolarized potentials by approximately 10 mV (Fig. 3D). In average, control steady-state inactivation was significantly shifted from −63.4 ± 3.4 to −72.7 ± 2.8 mV in the presence of 1-octanol (n = 6 cells, p < 0.01). We also examined the effects of 300 μM 1-octanol on I-V (Fig. 3E) and activation-voltage (Fig. 3F) relationships. The application of 1-octanol inhibited currents over the wide range of potentials, but there was no significant effect on midpoint of activation (control V50, −47.2 ± 1.7 mV, and −45.7 ± 3.1 mV in the presence of 1-octanol; n = 6 cells, p > 0.05).
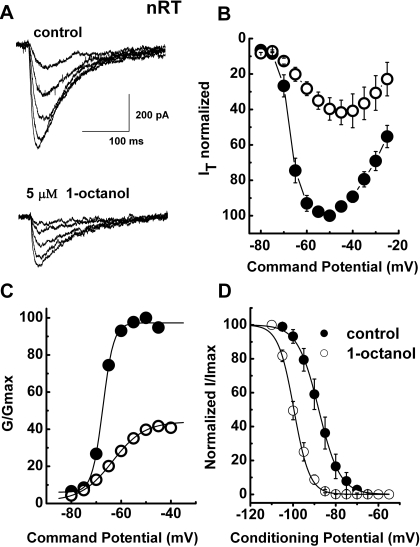
We did biophysical studies to discern possible differences in the mechanisms of modulation of nRT and CaV3.3 T-type currents that could account for the higher potency of the inhibition of native currents by 1-octanol. Figure 4A shows a family of inward currents evoked by progressive depolarizing steps from −70 to −50 mV from a holding potential (Vh) of −90 mV in a representative nRT cell before (top traces) and during (bottom traces) the application of 5 μM 1-octanol. Figure 4B depicts the average I-V curves from 5 nRT cells in experiments similar to those shown in Fig. 4A. It is evident that 5 μM 1-octanol depressed peak current at all test potentials (Vt) more positive than −70 mV. From these experiments, we calculated the apparent voltage dependence of activation (Fig. 4C). The effect of 1-octanol on the midpoint of activation (V50) in nRT neurons was shifted in depolarized potentials by approximately 4 mV (control, −67.4 ± 1.0 mV; 1-octanol, −62.5 ± 2.0 mV; n = 5, p > 0.05). It is noteworthy that 1-octanol had a significant voltage-dependent effect on steady-state inactivation, shifting midpoint of inactivation V50 to hyperpolarized potentials by approximately 12 mV (Fig. 4D). In average, in control conditions V50 occurred at –87.8 ± 2.0 mV, and in the presence of 1-octanol in the same cells V50 was shifted to −100 ± 1.0 mV (n = 10, p < 0.001). This was much like its effect in recombinant CaV3.3 channels.
Fig. 4.
The effects of 1-octanol on the kinetic properties of T-currents in nRT neurons. A, a family of raw T-current traces in a representative nRT cell elicited with test potentials from −70 to −50 mV in 5-mV increments from a holding potential of −90 mV in control conditions (top traces) and in the presence of 5 μM 1-octanol in external solution (bottom traces). Bars indicate calibration. B, the average I-V relationships before (●) and during (○) the application of 5 μM 1-octanol. Currents were elicited by progressing from −80 to −20 mV in 5-mV increments from a holding potential of −90 mV (n = 5 cells). Note the depression of the current amplitude at most potentials and the slight shift of the I-V curve to the right in the presence of 1-octanol. C, normalized conductance in whole-cell recordings from current-voltage experiments depicted in B. 1-Octanol shifted half-conductance (V50) to the right from −67.2 ± 0.4 mV (k = 2.4 ± 0.4 mV) in control to −63.8 ± 2.5 mV (k = 5.7 ± 2.6 mV). Fits were done using the Boltzmann equation. D, the steady-state inactivation kinetics of T-currents in nRT neurons were examined by paired-pulse protocols at different conditioning potentials. Double-pulse protocols to −50 mV were used to elicit T-currents separated by 3.5-s prepulse to potentials ranging from −110 to −50 mV in 5-mV increments before (filled symbols) and during (open symbols) the application of 1-octanol. The data were best fitted with the Boltzmann equation, yielding V50 of −88.0 ± 0.2 mV (k = 5.0 ± 0.1 mV) in control and −99.6 ± 0.2 mV (k = 3.7 ± 0.2 mV) in the presence of 1-octanol (n = 10 cells).
Based on our results, we conclude that potent inhibition of native nRT currents by 1-octanol cannot be entirely explained either by a direct interaction with a particular known isoform of T-channels or specific biophysical features of channel block. Thus, we considered the possibility that 1-octanol interacts with second messengers in intact nRT neurons. Protein kinase C (PKC), an important signaling molecule, has been proposed as the putative target of general anesthesia (Hemmings 1998; reviewed in Rebecchi and Pentyala, 2002). Previous data from other systems indicate that 1-octanol and related normal alcohols may either stimulate (Shen et al., 1999) or inhibit (Slater et al., 1993) the activity of PKC. Thus, we tested the ability of PKC activators and inhibitors to alter the baseline T-currents in nRT cells and to consequently affect the ability of 1-octanol to inhibit T-currents.
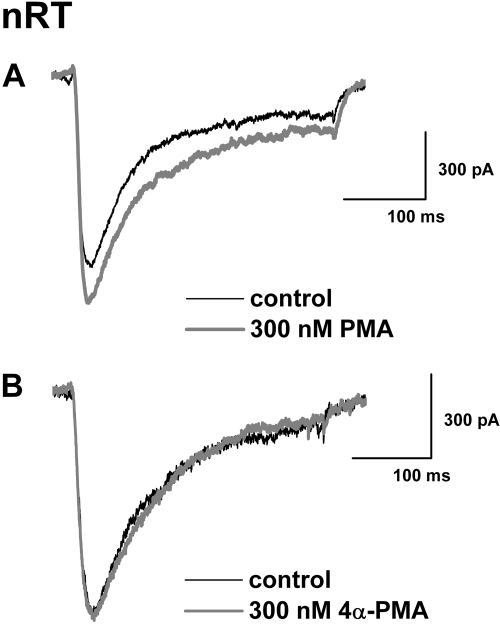
In these set of experiments, we first applied PKC activator, 300 nM PMA to nRT neurons. Figure 5A shows traces from a representative neuron. PMA caused a small (approximately 17%) increase in baseline peak T-current without obvious changes in current kinetics. In a control experiment, 4α-PMA, an inactive form of PMA, had a minimal effect on baseline T-current at the same concentrations (Fig. 5B). Overall, we found that 300 nM PMA increased baseline T-current in nRT cells by 16 ± 3% (p < 0.001, n = 7), whereas 300 nM 4αPMA had no significant effect on baseline T-current in these cells (6 ± 5% increase, p > 0.05, n = 5).
Fig. 5.
PMA enhances the amplitude of nRT T-current. A, representative traces of peak T-type current amplitudes (Vh −90 mV, Vt −50 mV) before (control, black trace) and during applications of 300 nM PMA (gray trace). PMA application increased T-current amplitude approximately 17%. B, representative traces of peak T-current amplitudes (Vh −90 mV, Vt −50 mV) before (control, black trace) and during applications of 300 nM 4αPMA (gray trace). Application of 4αPMA had very little effect on T-current amplitude.
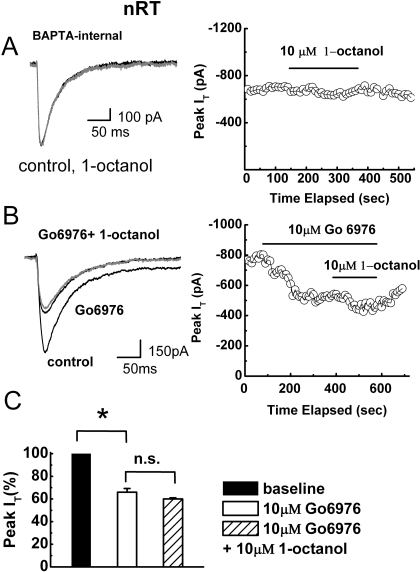
Our data are consistent with a modest stimulation of baseline T-current in nRT cells by PKC activation. However, because 1-octanol consistently inhibited T-currents in these cells, it is unlikely that its effects are mediated by PKC stimulation. We reasoned that if 1-octanol inhibits PKC in nRT cells, pharmacological or physiological inhibitors of PKC would also inhibit baseline T-current in nRT and interfere with the activity of 1-octanol to modulate this current. Molecular studies with in situ hybridization indicate that both calcium-dependent and Ca2+-independent isoforms of PKC have been expressed in the thalamus (Minami et al., 2000). To probe for the importance of Ca2+ ions in mediating 1-octanol inhibition of T-current in nRT cells, we used BAPTA, a high-affinity chelator of Ca2+ ions, that at physiological pH buffers Ca2+ approximately 100-fold faster than EGTA and can diminish cytosolic Ca2+ fast enough to suppress a biological response in the brain slices (Harney et al., 2006). Hence, we dialyzed BAPTA into cells via recording pipette solution at a high concentration of 10 mM. We used only nRT cells that had been dialyzed for at least 10 min to assure diffusion of BAPTA into dendritic regions. In the cells that still had significant T-currents under these recording conditions, we found that BAPTA completely abolished the ability of 10 μM 1-octanol to inhibit T-currents (2 ± 1% block, p > 0.05, n = 5). Figure 6A shows traces (left) and the time course of T-current (right) from a representative nRT neuron in the presence of BAPTA in internal solution. Furthermore, Fig. 6 B shows that short-term application of 10 μM Go 6976, a specific Ca2+-dependent PKC inhibitor, decreased baseline T-current amplitude approximately 40%, whereas subsequently coapplied 10 μM 1-octanol inhibited less than 10% of T-current amplitude. The histograms in Fig. 6C summarize average data obtained when Go 6976 and 1-octanol were coapplied to the same cells. Overall, 1-octanol had no significant effect on baseline T-current in nRT cells treated with Go 6976 (n = 5, p > 0.05). In contrast, when we applied 10 μM Go 6976 on recombinant HEK 293 cells expressing CaV3.3 channels, there was no statistically significant effect on peak T-current amplitude (1 ± 6% change, n = 3, p > 0.05; data not shown). Thus, it seems that baseline native and recombinant T-currents are differently regulated and that the effects of 1-octanol on nRT currents are at least partly mediated by the inhibition of Ca2+-dependent PKC pathway.
Fig. 6.
Calcium-dependent PKC is the possible cellular target of 1-octanol inhibition of T-current in nRT cells. A, traces (left) and time course (right) from a representative nRT cell dialyzed with 10 mM BAPTA. Note that 10 μM 1-octanol had little effect on T-current amplitude or current kinetics. B, traces (left) and time course (right) from another nRT cell. Note that 10 μM 1-octanol was applied to this cell after it had been exposed to 10 μM Go 6976, a specific Ca2+-dependent PKC inhibitor. By itself, Go 6976 inhibited approximately 40% of baseline T-current but, with 1-octanol, the two together inhibited approximately 48% of the current. C, the average data from experiments similar to those presented in B indicate that Go 6976 reduced peak T-current to 66 ± 3% of the baseline level (p < 0.001, n = 5) but that coapplication of 10 μM 1-octanol to the same cells did not significantly affect the new baseline (60 ± 1%, p > 0.05). n.s., not significant (p > 0.05).
Discussion
The major finding of our study is that the anesthetic alcohol 1-octanol induced a potent concentration-dependent inhibition (IC50, 4 μM) of native slowly inactivating T-currents and underlying burst firing in nRT neurons in intact brain slices. These effects could be important for the sedative and hypnotic action of anesthetic alcohols, because the in vitro effect of 1-octanol in nRT neurons was well within the concentrations necessary to induce loss of righting reflex in the tadpole anesthesia assay (Alifimoff et al., 1989). That assay is frequently used to estimate hypnotic properties of various drugs, because its results generally correlate well with measures of anesthetic potency in mammals (Franks and Lieb, 1994). The inhibitory effect of 1-octanol on T-channel-dependent LTS and neuronal burst firing in nRT neurons (this study) and thalamo-cortical relay neurons (Soltesz et al., 1991; Llinás et al., 2007) could at least contribute to the inhibitory effect of anesthetics on neuronal firing in the thalamo-cortical sensory pathway. Such inhibitory effect on thalamo-cortical circuitry is observed during in vivo anesthesia with other general anesthetics (Angel, 1991; Detsch et al., 2002). The effect of 1-octanol on LTS and burst firing of thalamic neurons in brain slices is similar to the effects of other classes of clinically used conventional volatile anesthetics such as enflurane (Joksovic et al., 2005a) and isoflurane (Ries and Puil, 1999). Hence, different classes of general anesthetics may have a common inhibitory effect on thalamocortical information transfer, which critically depends on the activity of T-channels. Our data further support the potential importance of T-channels as cellular targets for general anesthesia.
In at least two reports, potent inhibition of native T-currents by 1-octanol has been demonstrated. This was based on the findings that 1 μM 1-octanal blocked 75% of T-current in neonatal dorsal root ganglion cells (Scott et al., 1990), and 20 μM 1-octanol completely blocked T-currents in inferior olivary neurons (Llinás 1988). However, full-concentration response curves were not generated, and the underlying mechanism of this potent inhibition of T-currents by 1-octanol was not further investigated in these studies.
Our study with recombinant T-channel isoforms expressed in HEK 293 cells indicates that these isoforms are inhibited by 1-octanol at concentrations up to 30-fold less potent than T-currents in native nRT cells. Binding of some drugs to inactivated states of the channel is an important property because it can provide pharmacological selectivity to their action. In particular, this mechanism can stabilize channel in inactive states and thus decrease availability of channel at physiological membrane potentials. We explore this hypothesis and found that 1-octanol significantly decreased availability of channel as evidenced by a hyperpolarizing shift of approximately 10 mV in steady-state inactivation curves of both recombinant CaV3.3 (Fig. 3) and native nRT T-currents (Fig. 4). Although this is an important mechanism contributing to the preferential reduction of peak T-current at depolarized membrane potentials, it is unlikely that this could account for a large difference in potency in native versus recombinant T-currents.
One possible reason for discrepancy in potency of T-current inhibition by 1-octanol between the native and recombinant cells could be different contents of membrane lipids. For example, it has been shown that membrane composition of lipid bilayers can drastically affect ability of anesthetics such as halothane and propofol (Hemmings and Adamo, 1994), as well as 1-octanol (Shen et al., 1999), to interact with lipid-bound regulatory domains of PKC. Furthermore, in our study, the potent inhibitory effect of 1-octanol on T-current in nRT cells was critically dependent on constitutive Ca2+-dependent PKC, as shown by the fact that it was abolished with agents to inhibit it, as do the Ca2+ ion chelator BAPTA and Go 6976, a specific inhibitor of Ca2+dependent isoforms of PKC. We also found that PMA induced a small but significant up-regulation of baseline T-current in nRT cells. Furthermore, Go 6976 blocked baseline T-current, strongly suggesting that endogenous PKC in nRT cells modulates T-current. In contrast, Go 6976 at the same concentration did not significantly affect recombinant CaV3.3 currents. In addition, changing intracellular Ca2+ buffering capacity with low concentrations of EGTA did not increase potency of 1-octanol in inhibiting recombinant CaV3.3 currents in HEK 293 cells. Thus, baseline T-current activity seems to be differently regulated in native and recombinant cells. This is important, indicating a novel form of T-current modulation in nRT neurons by PKC. Earlier, we reported that nRT T-currents, LTS, and burst firing can be directly modulated by other endogenous agents, such as reducing amino acid l-cysteine (Joksovic al., 2006) and S-nitrosothiols (Joksovic et al., 2007). Thus, different regulatory mechanisms regulate the expression of T-current in the finely tuned neuronal excitability of these cells.
The molecular basis for PKC modulation of T-current in nRT cells remains unknown. Several isoforms of PKC in neurons can be Ca2+-dependent (e.g., α, βI, βII, γ), or Ca2+-independent (e.g., δ, ε, η, θ, μ) (Rebecchi and Pentyala, 2002). In situ hybridization studies have shown that PKC isoforms can be differentially expressed in the central nervous system, including the thalamus (Minami et al., 2000). Our results strongly suggest that Ca2+-dependent PKC isoforms are tonically active in the up-regulation of T-current in nRT neurons and can be potently inhibited by 1-octanol. Recent studies with recombinant T-channel isoforms expressed in Xenopus laevis oocytes (Park et al., 2006) and mammalian cells (Chemin et al., 2007) indicate that the amplitude of currents of all three known isoforms, CaV3.2, CaV3.2, and CaV3.3, may be enhanced up to 3- to 4-fold by PKC activation with prolonged incubation with PMA. Park et al. (2006) further showed that critical sites located in the I-II loop of the channel are responsible for the interaction of PMA and CaV3.1. However, the exact amino acid residues targeted by PKC action, presumably phosphorylation, have not been identified yet. It is interesting that the stimulatory effects of PKC upon T-current amplitude were strictly temperature-dependent in study using mammalian cells (Chemin et al., 2007), whereas similar effects were observed in oocytes at room temperature (Park et al., 2006). It is noteworthy that different T-current isoforms in two adjacent nuclei of the thalamus seem to be differentially regulated by PKC. For example, PMA inhibits T-current in thalamocortical cells of ventrobasal nucleus that express predominantly CaV3.1 channels (Cheong et al., 2008) but enhances T-current in nRT cells that express CaV3.2 and CaV3.3 channels (this study). Extensive molecular studies in both recombinant and native cells are needed to decipher the precise mechanisms of the molecular interaction between T-channels, PKC, and anesthetic alcohols.
In conclusion, we have demonstrated that 1-octanol potently inhibits native T-currents in nRT neurons, probably by modulating Ca2+-dependent PKC signaling. Moreover, T-channels in nRT neurons have a key function in thalamocortical oscillations, which in turn regulate physiological processes, such as level of arousal and sleep, awareness, and sensory information transfer, as well as pathological processes such as absence seizures (Steriade, 2005). Thus, PKC modulation in nRT cells by anesthetic alcohols may contribute to the sedative, hypnotic, and anticonvulsant effects of general anesthesia.
Acknowledgments
We thank Dr. Edward Perez-Reyes for contribution of CaV3.3 HEK cells and Dr. Julie Sando for helpful discussion and critical comments.
This work was supported by the grants from the National Institutes of Health National Institute of General Medical Sciences [Grant GM070726] and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant F31-NS059190].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059931.
- T-channel
- transient (T-type) Ca2+ channel
- LTS
- low-threshold Ca2+ spikes
- nRT
- reticular thalamic nucleus
- HEK
- human embryonic kidney
- I-V
- current-voltage
- TTX
- tetrodotoxin
- PMA
- phorbol 12-myristate 13-acetate
- Go 6976
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- PKC
- protein kinase C.
References
- Alifimoff JK, Firestone LL, Miller KW. (1989) Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol 96: 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH. (2000) Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn 9: 370–386 [DOI] [PubMed] [Google Scholar]
- Angel A. (1991) The G. L. Brown lecture. Adventures in anaesthesia. Exp Physiol 76: 1–38 [DOI] [PubMed] [Google Scholar]
- Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P. (2007) Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem 282: 32710–32718 [DOI] [PubMed] [Google Scholar]
- Cheong E, Lee S, Choi BJ, Sun M, Lee CJ, Shin HS. (2008) Tuning thalamic firing modes via simultaneous modulation of T- and L-type Ca2+ channels controls pain sensory gating in the thalamus. J Neurosci 28: 13331–13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C. (2002) Differential effects of isoflurane on excitatory and inhibitory synaptic inputs to thalamic neurones in vivo. Br J Anaesth 89: 294–300 [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. (1994) Molecular and cellular mechanisms of general anaesthesia. Nature 367: 607–614 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R. (2006) Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci 26: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Lingle CJ. (1992) Kinetic and pharmacological properties of low-voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells. J Neurophysiol 68: 213–232 [DOI] [PubMed] [Google Scholar]
- Hemmings HC., Jr (1998) General anesthetic effects on protein kinase C. Toxicol Lett 100–101: 89–95 [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Adamo AI. (1994) Effects of halothane and propofol on purified brain protein kinase C activation. Anesthesiology 81: 147–155 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources ( 1996) Guide for the Care and Use of Laboratory Animals, 7th ed.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Joksovic PM, Bayliss DA, Todorovic SM. (2005a) Different kinetic properties of two T-type Ca2+ currents of rat reticular thalamic neurones and their modulation by enflurane. J Physiol 566: 125–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksovic PM, Brimelow BC, Murbartián J, Perez-Reyes E, Todorovic SM. (2005b) Contrasting anesthetic sensitivities of slow T-type calcium channels of reticular thalamic neurons and recombinant Cav3.3 channels. Br J Pharmacol 144: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksovic PM, Doctor A, Gaston B, Todorovic SM. (2007) Functional regulation of T-type calcium channels by s-nitrosothiols in the rat thalamus. J Neurophysiol 97: 2712–2721 [DOI] [PubMed] [Google Scholar]
- Joksovic PM, Nelson MT, Jevtovic-Todorovic V, Patel MK, Perez-Reyes E, Campbell KP, Chen CC, Todorovic SM. (2006) CaV3.2 is the major molecular substrate for redox regulation of T-type Ca2+ channels in the rat and mouse thalamus. J Physiol 574: 415–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR. (1988) The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664 [DOI] [PubMed] [Google Scholar]
- Llinás RR, Choi S, Urbano FJ, Shin HS.(2007)Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci U S A 104: 17819–17824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP.(1998)Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 96: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. (1997) Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215 [DOI] [PubMed] [Google Scholar]
- Minami H, Owada Y, Suzuki R, Handa Y, Kondo H. (2000) Localization of mRNAs for novel, atypical as well as conventional protein kinase C (PKC) isoforms in the brain of developing and mature rats. J Mol Neurosci 15: 121–135 [DOI] [PubMed] [Google Scholar]
- Murbartián J, Arias JM, Lee JH, Gomora JC, Perez-Reyes E. (2002) Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1). FEBS Lett 528: 272–278 [DOI] [PubMed] [Google Scholar]
- Park JY, Kang HW, Moon HJ, Huh SU, Jeong SW, Soldatov NM, Lee JH. (2006) Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density. J Physiol 577: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1982) The Rat Brain in Stereotaxic Coordinates, Academic Press, Australia [Google Scholar]
- Perez-Reyes E. (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83: 117–161 [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. (2002) Anaesthetic actions on other targets: protein kinase C and guanine nucleotide-binding proteins. Br J Anaesth 89: 62–78 [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E. (1999) Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol 81: 1795–1801 [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. (2004) Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci 5: 709–720 [DOI] [PubMed] [Google Scholar]
- Scott RH, Wootton JF, Dolphin AC. (1990) Modulation of neuronal T-type calcium channel currents by photoactivation of intracellular guanosine 5′-O(3-thio) triphosphate. Neuroscience 38: 285–294 [DOI] [PubMed] [Google Scholar]
- Slater SJ, Cox KJ, Lombardi JV, Ho C, Kelly MB, Rubin E, Stubbs CD. (1993) Inhibition of protein kinase C by alcohols and anaesthetics. Nature 364: 82–84 [DOI] [PubMed] [Google Scholar]
- Shen YM, Chertihin OI, Biltonen RL, Sando JJ. (1999) Lipid-dependent activation of protein kinase C-alpha by normal alcohols. J Biol Chem 274: 34036–34044 [DOI] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. (1991) Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol 441: 175–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. (2005) Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurol Sci 28: 317–324 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wakamori M, Akaike N. (1989) Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett 104: 229–234 [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19: 1895–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. (1998) Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol 79: 240–252 [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Perez-Reyes E, Lingle CJ. (2000) Anticonvulsants but not general anesthetics have differential blocking effects on different T-type current variants. Mol Pharmacol 58: 98–108 [DOI] [PubMed] [Google Scholar]