Abstract
Crofelemer, a purified proanthocyanidin oligomer extracted from the bark latex of Croton lechleri, is in clinical trials for secretory diarrheas of various etiologies. We investigated the antisecretory mechanism of crofelemer by determining its effect on the major apical membrane transport and signaling processes involved in intestinal fluid transport. Using cell lines and measurement procedures to isolate the effects on individual membrane transport proteins, crofelemer at 50 μM had little or no effect on the activity of epithelial Na+ or K+ channels or on cAMP or calcium signaling. Crofelemer inhibited the cystic fibrosis transmembrane regulator (CFTR) Cl− channel with maximum inhibition of ∼60% and an IC50 ∼7 μM. Crofelemer action at an extracellular site on CFTR produced voltage-independent block with stabilization of the channel closed state. Crofelemer did not affect the potency of glycine hydrazide or thiazolidinone CFTR inhibitors. Crofelemer action resisted washout, with <50% reversal of CFTR inhibition after 4 h. Crofelemer was also found to strongly inhibit the intestinal calcium-activated Cl− channel TMEM16A by a voltage-independent inhibition mechanism with maximum inhibition >90% and IC50 ∼6.5 μM. The dual inhibitory action of crofelemer on two structurally unrelated prosecretory intestinal Cl− channels may account for its intestinal antisecretory activity.
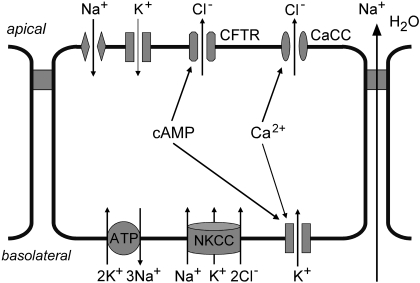
Secretory diarrhea remains a global health challenge in developing and developed countries. Intestinal fluid secretion involves Cl− influx into enterocytes through an Na+/K+/2Cl− symporter on the basolateral membrane and Cl− efflux through apical (lumen-facing) Cl− channels (Barrett and Keely, 2000; Field, 2003; Thiagarajah and Verkman, 2005) (Fig. 1). K+ channels and a 3Na+/2K+ pump establish the electrochemical driving force for Cl− secretion. Na+ and water secretion follow passively in response to active Cl− secretion. Bacterial enterotoxins produced by Vibrio cholerae and Escherichia coli elevate cyclic nucleotide concentrations in enterocytes, resulting in Cl− channel activation and fluid secretion. Na+ absorption through apical membrane Na+ channels and electrogenic Na+-coupled symporters oppose net fluid secretion. The rate of net intestinal fluid secretion, and hence the severity of secretory diarrhea, is expected to be sensitive to modulators of these transporting systems and to upstream cyclic nucleotide or calcium signaling pathways.
Fig. 1.
Cellular mechanisms of intestinal fluid secretion by enterocytes, showing chloride secretion through apical membrane chloride channels. See the Introduction for a further explanation.
Treatment of secretory diarrheas in developing countries is primarily supportive, involving replacement of intestinal fluid losses using oral rehydration salt solution. Although oral rehydration salt has greatly improved clinical outcome in cholera and other diarrheas, there remains significant mortality from infectious diarrheas, with recurrent major outbreaks. Potential targets for diarrhea therapy to further reduce morbidity and mortality include the bacteria itself (vaccines, antimicrobials), elaborated enterotoxins and their cellular uptake, cellular cyclic nucleotide signaling, and the prosecretory membrane transporters mentioned above (Field, 2003; Thiagarajah and Verkman, 2005). Our laboratory has focused on targeted inhibitors of the two principal apical membrane Cl− channels in enterocytes: the cystic fibrosis transmembrane regulator conductance (CFTR), a cAMP-stimulated Cl− channel; and calcium-activated Cl− channels (CaCCs). By high-throughput screening and follow-up chemistry, we identified inhibitors of these Cl− channels, including nanomolar-potency thiazolidinone (Ma et al., 2002), glycine hydrazide (Muanprasat et al., 2004) and pyrimido-pyrrolo-quinoxalinedione (Tradtrantip et al., 2009a) CFTR inhibitors, and 3-acyl-2-aminothiophene CaCC inhibitors (De La Fuente et al., 2008). We have also identified thiophene carboxylate activators of phosphodiesterases that reduce cyclic nucleotide concentrations and toxin-induced intestinal fluid secretion (Tradtrantip et al., 2009b).
Here, we investigate the antisecretory mechanism of crofelemer, a purified proanthocyanidin oligomer extracted from the blood-red bark latex of the South American medicinal plant Croton lechleri (dragon's blood). The sap of C. lechleri has been used in South American countries like Ecuador and Peru for many years to treat diarrheas, including dysentery and cholera, and various lung, stomach, and other conditions (Ubillas et al., 1994; Jones, 2003; Risco et al., 2003; Rossi et al., 2003). Additional pharmacological studies have shown that crofelemer reduced fluid secretion in cell culture and mouse models (Gabriel et al., 1999). Crofelemer is currently in clinical trials for the treatment of secretory diarrheas associated with acute infections including cholera, chronic diarrhea associated with HIV/AIDS, and diarrhea-predominant irritable bowel syndrome (Holodniy et al., 1999; DiCesare et al., 2002; Mangel and Chaturvedi, 2008). Here, we report that the antisecretory mechanism-of-action of crofelemer involves inhibition of both CFTR and CaCC Cl− channels at the luminal membrane of enterocytes.
Materials and Methods
Chemicals.
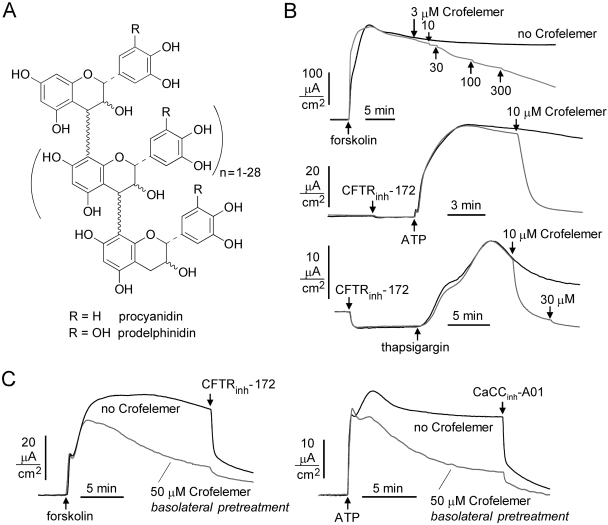
Forskolin, apigenin, and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma-Aldrich (St. Louis, MO). 8-(4-Chlorophenylthio)-cAMP (CPT-cAMP) was purchased from Calbiochem (San Diego, CA). The small-molecule CFTR inhibitors CFTRinh-172 and GlyH-101, and the CaCC inhibitor CaCCinh-01, were synthesized as reported previously (Ma et al., 2002; Muanprasat et al., 2004; De La Fuente et al., 2008). Crofelemer was provided by Napo Pharmaceuticals Inc. (South San Francisco, CA). Crofelemer was prepared by extraction from the bark latex of C. lechleri. After chilling the bark latex to induce a phase separation, the solid residues were discarded, and the supernatant was extracted with butanol. The crofelemer-containing aqueous phase was filtered by tangential flow and subjected to low-pressure liquid chromatography on an ion-exchange column. The crofelemer-enriched fraction was purified on a Sephadex column, and crofelemer was eluted using a mobile phase of aqueous acetone. Crofelemer was then dried under vacuum. Crofelemer is a proanthocyanidin oligomer with an average molecular mass of 2100 Da, in agreement with a previously reported average molecular mass of 2300 Da (Ubillas et al., 1994). Figure 2A shows the putative structure of crofelemer. The material used for the studies here is the same as that used in clinical trials, in which it is formulated for oral dosing as modified-release tablets (125 or 250 mg of crofelemer per tablet).
Fig. 2.
Crofelemer reduces Cl− secretion in T84 human intestinal cells in response to cAMP and calcium-elevating agonists. A, chemical structure of crofelemer (see Materials and Methods for explanation) (Ubillas et al., 1994). B, short-circuit current in T84 cells after activation of Cl− secretion by forskolin (10 μM), ATP (100 μM), or thapsigargin (1 μM). Indicated concentrations of crofelemer were added to the luminal bathing solution. Where indicated, cells were pretreated with 20 μM CFTRinh-172 to inhibit CFTR Cl− current. C, short-circuit current measurements showing CFTR (left)- and CaCC (right)-dependent Cl− current in the presence of crofelemer (50 μM) added to the basolateral bathing solution 10 min before measurements. CFTR was inhibited by pretreatment with CFTRinh-172 (20 μM) for measurement of ATP-induced CaCC activation.
Cell Culture.
Fisher rat thyroid (FRT) cells expressing human CFTR were generated as described previously (Ma et al., 2002). FRT cells expressing human TMEM16A (cDNA provided by Dr. Luis Galietta, Gaslini Institute, Genoa, Italy) were generated similarly. FRT cells were cultured in F-12 Modified Coon's medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 350 μg/ml hygromycin, and 500 μg/ml G418 (Geneticin). Primary cultures of human bronchial epithelial cells were maintained at an air-liquid interface as described previously (Yamaya et al., 1992). T84 cells were cultured in DMEM/Ham's F-12 (1:1) medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were grown on Snapwell porous filters (Corning Life Sciences, Lowell, MA) at 37°C in 5% CO2/95% air.
Short-Circuit Current Measurements.
FRT cells (stably expressing CFTR or TMEM16A) were cultured on Snapwell filters until confluence (transepithelial resistance >500 Ω · cm). Short-circuit current was measured in Ussing chambers (Vertical diffusion chamber; Corning Life Sciences) with Ringer's solution bathing the basolateral surface and half-Ringer's bathing the apical surface. Ringer's solution contained 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM sodium-HEPES, and 10 mM glucose, pH 7.3. Half-Ringer's solution was the same, except that 65 mM NaCl was replaced with sodium gluconate, and CaCl2 was increased to 2 mM. The basolateral membrane was permeabilized with 250 μg/ml amphotericin B, as described previously (Ma et al., 2002). Chambers were bubbled continuously with air. For T84 cells and bronchial epithelial cells, cells were bathed in symmetrical HCO3−-buffered solution containing 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM d-glucose, 5 mM HEPES, and 25 mM NaHCO3, pH 7.4, and aerated with 5% CO2 at 37°C. For the measurement of apical K+ conductance in T84 cells, NaHCO3 and NaCl were replaced with sodium gluconate, and sodium gluconate in basolateral solution was replaced with potassium gluconate and bubbled with air. The basolateral membrane was permeabilized with 20 μM amphotericin B. Short-circuit current was measured using a DVC-1000 voltage-clamp apparatus (World Precision Instruments, Inc., Sarasota, FL).
Cyclic Nucleotide Assays.
T84 cells were grown in 24-well plates, treated for 45 min with crofelemer and then for 10 min with 0 or 20 μM forskolin, lysed by sonication, and centrifuged to remove cell debris, and the supernatant was assayed for cAMP according to manufacturer's instructions (Parameter cAMP immunoassay kit; R&D Systems, Minneapolis, MN).
Patch-Clamp Analysis of CFTR and TMEM16A Cl− Channel Function.
Whole-cell recordings were made on FRT cells stably expressing CFTR or TMEM16A. The pipette solution for CFTR contained 140 mM N-methyl-d-glucamine chloride (NMDG-Cl), 5 mM EGTA, 1 mM MgCl2, 1 mM Tris-ATP, and 10 mM HEPES, pH 7.2. The pipette solution for TMEM16A contained 130 mM CsCl, 0.5 mM EGTA, 1 mM MgCl2, 1 mM Tris-ATP, and 10 mM HEPES, pH 7.2. The bath solution contained 140 mM NMDG-Cl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4. All measurements were done at room temperature (22–25°C). Pipettes were pulled from borosilicate glass and had resistances of 3 to 5 MΩ after fire-polishing. Seal resistances were between 3 and 10 GΩ. After establishing the whole-cell configuration, CFTR was activated by forskolin and IBMX, and TMEM16A was activated by ATP. Whole-cell currents were elicited by applying hyperpolarizing and depolarizing voltage pulses from a holding potential of 0 mV to potentials between −100 and +100 mV in steps of 20 mV. The current output was filtered at 5 kHz. Currents were digitized and analyzed using an AxoScope 10.0 system and a Digidata 1440A AC/DC converter (Molecular Devices, Sunnyvale, CA).
Single-channel analysis of CFTR was done in the cell-attached configuration using fire-polished pipettes with a resistance of 6 to 10 MΩ. The pipette solution contained 140 mM NMDG-Cl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES, pH 7.4, and the KCl bath solution contained 140 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES, pH 7.4. Recordings were performed at room temperature using an Axopatch-200B (Molecular Devices). Voltage and current data were low-pass-filtered at 1 kHz and stored for later analysis. Single-channel data were digitally filtered at 25 Hz and analyzed using Clampfit 10.0 software (Molecular Devices).
Calcium Signaling Measurements.
Measurements of [Ca2+]i in confluent monolayers of T84 cells were done by loading cells with fura-2 by 30-min incubation at 37°C with 2 μM fura-2-AM (Invitrogen, Carlsbad, CA). Fura-2-loaded T84 cells were mounted in a perfusion chamber on the stage of an inverted fluorescence microscope. The cells were superfused with 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM d-glucose, and 10 mM HEPES, pH 7.4. Fura-2 fluorescence was recorded at excitation wavelengths of 340 and 380 nm, and the results were expressed as a 340/380 fluorescence ratio. After obtaining baseline measurements, 100 μM ATP was added in the perfusate. Measurements were made in the absence and presence of 50 μM crofelemer.
Results
Crofelemer Inhibits Cl− Secretion by T84 Human Intestinal Epithelial Cells.
To test whether crofelemer reduces intestinal cell Cl− secretion, short-circuit current was measured in T84 cells in symmetrical physiological solutions (without plasma membrane permeabilization). Figure 2B shows crofelemer concentration-dependent inhibition of the increase in short-circuit current produced by the cAMP agonist forskolin (top) and the calcium agonists ATP (middle) and thapsigargin (bottom). Measurements with the calcium agonists were done in the presence of CFTRinh-172 to inhibit CFTR. Whereas crofelemer inhibition of forskolin-induced current, which is mainly CFTR-dependent, was slow, weak, and partial, inhibition of ATP and thapsigargin-induced current was nearly complete at 10 μM crofelemer. This inhibition of current induced by calcium agonists suggests that crofelemer inhibits both CFTR and CaCC channels, with apparently much stronger inhibition of the latter. Further measurements were done using transfected cell systems to study crofelemer effects on CFTR and CaCCs in isolation.
Although crofelemer is membrane-impermeable because of its size and polarity, and thus should access only the luminal surface of intestinal epithelial cells, we investigated possible effects of basolateral application of crofelemer on apical membrane CFTR and CaCC. Studies were done in T84 cells in which 50 μM crofelemer was added to the basolateral bathing solution for 10 min before measurement of short-circuit current. It is interesting that, although crofelemer did not affect the initial currents induced by the CFTR and CaCC agonists forskolin and ATP, respectively, crofelemer slowly reduced Cl− secretion over many minutes (Fig. 2C). Although probably not relevant to its antidiarrheal mechanisms, crofelemer may act at the basolateral surface of T84 cells to inhibit one or more transporters involved in transepithelial Cl− secretion.
Crofelemer Is a Partial Antagonist of CFTR Cl− Conductance.
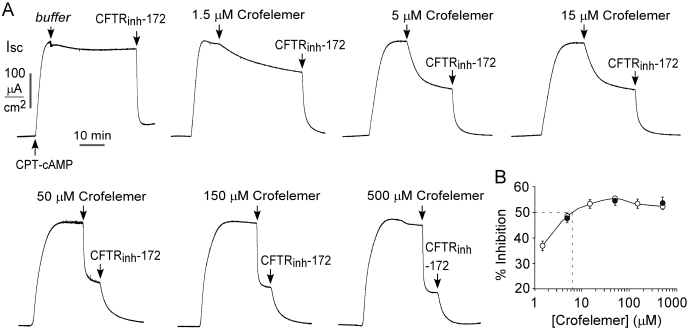
CFTR Cl− current was measured in CFTR-expressing FRT cells, in which the basolateral membrane was permeabilized by amphotericin B, and a transepithelial Cl− gradient was applied. FRT cells were used here because they lack intrinsic chloride channel activity, form tight junctions, grow quickly, and are readily transfected with chloride channel cDNAs and reporter indicators (Verkman and Galietta, 2009). Under these conditions, the measured current provides a direct quantitative measure of CFTR Cl− conductance. Figure 3A shows apical membrane current measurements in which CFTR Cl− conductance was stimulated by CPT-cAMP, which was followed by the addition of different concentrations of crofelemer in the apical bathing solution. Increasing concentrations of crofelemer produced notably more rapid, although partial, inhibition of CFTR Cl− current. The addition of crofelemer to the basolateral bathing solution did not inhibit current (data not shown). As summarized in Fig. 3B (○) the apparent IC50 value (giving 50% inhibition of Cl− current) for crofelemer was ∼7 μM, and the maximal inhibition was ∼60%. Similar results were obtained when the apical and basolateral bathing solutions were switched (high Cl− in apical solution) (Fig. 3B, ●), indicating that crofelemer inhibition of CFTR does not depend on Cl− concentration. In contrast to the partial inhibition by crofelemer, maximal CFTR inhibition by CFTRinh-172 or GlyH-101 is approximately 100% (see below).
Fig. 3.
Crofelemer inhibition of CFTR Cl− conductance. A, apical membrane current in CFTR-expressing FRT cells after permeabilization with amphotericin B and in the presence of a transepithelial Cl− gradient (apical [Cl−], 75 mM; basolateral [Cl−], 150 mM). CFTR Cl− conductance was activated by 100 μM CPT-cAMP followed by the addition of indicated concentrations of crofelemer to the luminal solution. B, crofelemer concentration inhibition of CFTR Cl− current measured at 20 min after crofelemer application (S.E., n = 3–5). Data shown for experiments as in A (○) and with reversed Cl− gradient (apical [Cl−], 150 mM; basolateral [Cl−], 75 mM) (●).
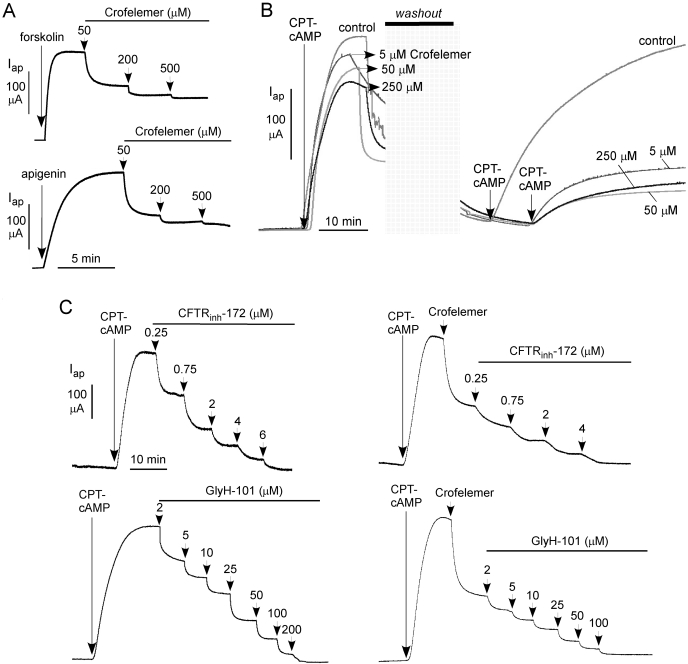
Measurements were done to investigate whether the crofelemer inhibition potency depends on the CFTR activation mechanism. Figure 4A shows similar responses to 50, 200, and 500 μM crofelemer using agonists that activate CFTR directly (apigenin) or through cAMP-dependent CFTR phosphorylation (forskolin). The reversibility of crofelemer inhibition of CFTR was investigated, because washout during secretory diarrhea is a concern with the use of a nonabsorbable antisecretory agent. Figure 4B shows apical current measurements, in which CFTR Cl− current was stimulated by CPT-cAMP and then inhibited by different concentrations of crofelemer. After extensive washing, residual CFTR inhibition was determined from the current after restimulation by CPT-cAMP. In control studies in the absence of crofelemer, washout (of CPT-cAMP) followed by restimulation produced a current similar to that seen in the initial stimulation. However, after inhibition with different concentrations of crofelemer, washout studies showed partial (25–35%) reversal of CFTR inhibition over 30 min. Extended time studies showed <50% reversal of crofelemer inhibition at 4 h (data not shown).
Fig. 4.
Characterization of crofelemer inhibition of CFTR Cl− conductance. A, crofelemer inhibition of CFTR after different agonists including forskolin (20 μM) and apigenin (100 μM). B, slow reversibility of crofelemer inhibition of CFTR. Where indicated, crofelemer was added, the apical solution was washed extensively, and CPT-cAMP was readded. C, investigation of possible synergy/competition of crofelemer with small-molecule CFTR inhibitors. Left, apical membrane current after CFTR activation by CPT-cAMP and inhibition by CFTRinh-172 or GlyH-101. Right, crofelemer (50 μM) was added to inhibit CFTR Cl− current by ∼50 to 60%, followed by indicated concentrations of CFTRinh-172 or GlyH-101.
To evaluate the possibility that the site of action of crofelemer on CFTR might overlap with that of the small-molecule thiazolidinone and glycine hydrazide CFTR inhibitors, we compared CFTR inhibition in the absence and presence of preadded crofelemer. Figure 4C (left) shows concentration-inhibition studies of CFTR inhibition by CFTRinh-172 and GlyH-101. Maximal inhibition was ∼100%, with IC50 values of ∼1 and ∼8 μM, respectively. Figure 4C (right) shows similar concentration-inhibition measurements, in which 50 μM crofelemer was added initially to inhibit CFTR Cl− current by ∼50%. Despite the partial antagonist mechanism of crofelemer, CFTRinh-172 and GlyH-101 were able to inhibit CFTR by nearly 100%. The similar IC50 values for CFTRinh-172 and GlyH-101 in the absence and presence of crofelemer suggests nonoverlapping CFTR inhibition sites for crofelemer and CFTRinh-172 or GlyH-101.
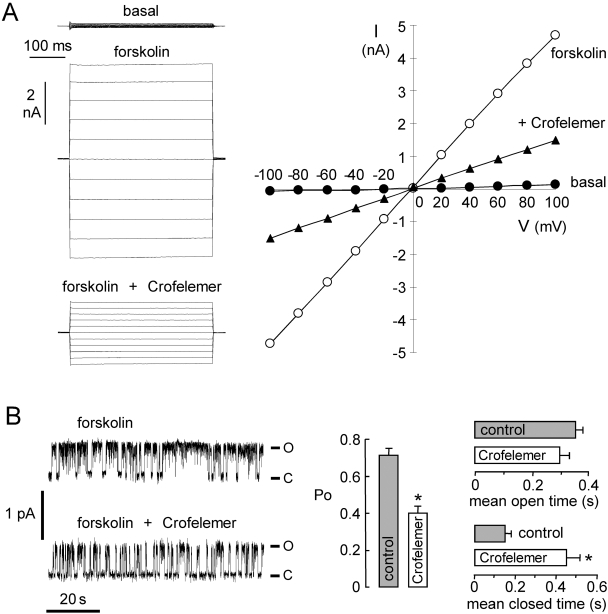
Patch-clamp was done to investigate the molecular mechanism of CFTR inhibition by crofelemer. Whole-cell membrane current was measured in CFTR-expressing FRT cells (Fig. 5A, left). Stimulation by 10 μM forskolin produced a membrane current of 179 ± 18 pA/pF (n = 3) at +100 mV (total membrane capacitance, 15.8 ± 4 pF). Crofelemer at 50 μM gave ∼60% inhibition of CFTR Cl− current. Figure 5A (right) shows an approximately linear current-voltage relationship for CFTR, as expected for CFTR. The CFTR current-voltage relationship remained linear after crofelemer addition, indicating a voltage-independent block mechanism, as expected for an uncharged inhibitor.
Fig. 5.
Patch-clamp analysis of crofelemer inhibition of CFTR. A, left, whole-cell CFTR current recorded at a holding potential at 0 mV and pulsing to voltages between ±100 mV in steps of 20 mV in the absence and presence of 50 μM crofelemer. CFTR was stimulated by forskolin. Right, I-V plot of mean currents at the middle of each voltage pulse from experiments as in A (S.E., n = 3). Fitted IC50 = 6.5 μM. B, left, single-channel recordings were done in the cell-attached configuration. CFTR was activated by 10 μM forskolin and 100 μM IBMX. Pipette potential was +80 mV. Right, summary of crofelemer effect on CFTR channel Po, mean open time, and mean closed time (S.E., n = 3–4; ∗, P < 0.05). o, open channel state; c, closed channel state.
To assess single-channel CFTR properties, cell-attached patch recordings were done on CFTR-expressing FRT cells in the absence or presence of crofelemer in the pipette solution (Fig. 5B). The addition of 10 μM forskolin and 100 μM IBMX to the bath resulted in CFTR channel opening. CFTR unitary conductance was 7.2 ± 0.1 pS (n = 4), which was not affected by crofelemer. Crofelemer reduced channel activity remarkably, as seen by the less frequent channel openings (Fig. 5B, left). Analysis of single-channel recordings indicated that crofelemer significantly reduced open-channel probability (Po) from 0.71 ± 0.04 (n = 4) to 0.40 ± 0.04 (n = 3). Mean channel open time was not significantly changed, but mean channel closed time was significantly increased (Fig. 5B, bottom), indicating that crofelemer stabilizes the channel closed state. These results suggest that crofelemer inhibits CFTR by an altered channel-gating mechanism.
Crofelemer Is a Strong Inhibitor of the CaCC TMEM16A.
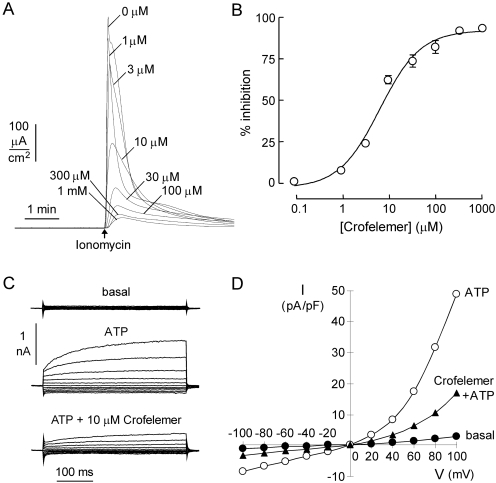
The data in Fig. 2B suggest that crofelemer strongly inhibits CaCC(s) in T84 cells. Recent work has implicated the protein TMEM16A as a CaCC in multiple epithelial cells, including intestinal epithelia, as well as in smooth muscle, nerve, and other cell types (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). To test whether TMEM16A is the CaCC target of crofelemer, FRT epithelial cells stably expressing TMEM16A were pretreated with different concentrations of crofelemer, followed by the addition of 1 μM ionomycin to stimulate TMEM16A Cl− current. Measurements were made in the presence of a transepithelial Cl− gradient, so that current is a direct, quantitative measure of TMEM16A Cl− conductance. Figure 6A shows crofelemer concentration-dependent inhibition of TMEM16A Cl− current, which was nearly complete at high concentrations of crofelemer. Figure 6B shows an IC50 for crofelemer inhibition of TMEM16A of ∼6.5 μM.
Fig. 6.
Crofelemer inhibition of calcium-activated Cl− channels. A, apical membrane current in TMEM16A-expressing FRT cells in the presence of a transepithelial Cl− gradient (apical [Cl−], 70 mM; basolateral [Cl−], 140 mM). B, crofelemer concentration-dependence of TMEM16A Cl− current inhibition. C, whole-cell TMEM16A current recorded at a holding potential of 0 mV and pulsing to voltages between ± 100 mV in steps of 20 mV in the absence and presence of 10 μM crofelemer. TMEM16A was stimulated by 100 μM ATP. D, I-V plot of mean currents (at the middle of each voltage pulse). Mean currents were normalized as current densities (measured in picoamperes per picofarads).
Whole-cell membrane current was measured in TMEM16A- expressing FRT cells (Fig. 6C). Stimulation by 100 μM ATP produced a membrane current of 56 ± 13 pA/pF (n = 3) at +100 mV. Pretreatment with 10 μM crofelemer inhibited ATP-induced TMEM16A Cl− current by 58% (24 ± 6 pA/pF, n = 3). Figure 6D shows an outward-rectifying current-voltage relationship for TMEM16A. The TMEM16A current-voltage relationship remained outward-rectifying after crofelemer addition, as expected for an uncharged inhibitor. The percentage inhibition of TMEM16A by crofelemer was voltage-independent (data not shown), as seen from the similar shapes of the current-voltage relationships without versus with crofelemer, indicating a voltage-independent block mechanism. These results define a second, distinct luminal membrane Cl− channel target of crofelemer.
Crofelemer Has Little Effect on Apical Cation Channels and cAMP/Calcium Signaling.
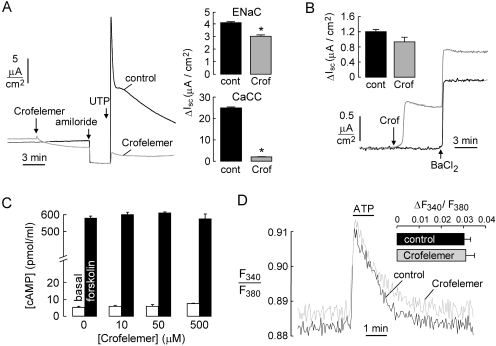
The apical membrane of enterocytes also contains Na+ and K+ channels, which are potential additional targets of crofelemer. To investigate whether crofelemer alters the activity of the epithelial cell Na+ channel ENaC, short-circuit current was measured in primary cultures of human bronchial epithelial cells, which robustly express ENaC and in which the change in short-circuit current after amiloride provides a quantitative measure of ENaC activity (Yamaya et al., 1992). Figure 7A shows that pretreatment of the cell culture with 50 μM crofelemer produced a small, ∼20% inhibition of ENaC activity. Human bronchial epithelial cells also express TMEM16A and have robust CaCC activity. Crofelemer pretreatment produced a >90% reduction in short-circuit current after the addition of calcium-elevating agonist UTP, in agreement with the conclusions from T84 cells and TMEM16A-transfected FRT cells, above.
Fig. 7.
Little or no effect of crofelemer on apical membrane cation channels and intracellular cAMP and calcium signaling. A, left, short-circuit current in primary cultures of CFTR-deficient human bronchial epithelial cells without versus with pretreatment with 50 μM crofelemer in the luminal solution. Where indicated, amiloride (10 μM) and UTP (100 μM) were added. Right, summary of differences in short-circuit current after amiloride and UTP additions (S.E., n = 3; ∗, P < 0.05). B, apical membrane K+ current in human bronchial epithelial cells after basolateral membrane permeabilization with 20 μM amphotericin B and in the presence of a K+ gradient (apical [K+], 5 mM; basolateral [K+], 150 mM). C, cAMP levels in T84 cell homogenates under basal conditions and at 10 min after treatment with 20 μM forskolin. Differences ± crofelemer were not significant. D, calcium signaling measured by fura-2 fluorescence in T84 cells under basal conditions and after ATP addition (100 μM). Where indicated, cells were pretreated with 50 μM crofelemer. Inset, peak ATP increase in fura-2 fluorescence ratio (S.E., n = 4). Difference was not significant.
Possible inhibition of apical K+ channels by crofelemer was tested in human bronchial epithelial cells, in which the basolateral membrane was permeabilized with amphotericin B in the presence of a transepithelial K+ gradient. Under these conditions, the small measured current is an apical membrane K+ current. Apical K+ current was measured after the addition of BaCl2, a nonspecific inhibitor of K+ channels. Figure 7B shows that pretreatment with 50 μM crofelemer produced a small, ∼22% inhibition of apical membrane K+ current.
Last, we tested the possibility that crofelemer action on apical membrane receptor(s) might affect major intracellular signaling pathways, which might secondarily modulate the activities of basolateral membrane transporters to inhibit transcellular Cl− secretion indirectly. Figure 7C shows that crofelemer at 50 μM had no significant effect on basal or forskolin-stimulated cAMP concentrations in T84 cells. Figure 7D shows that crofelemer did not alter basal cytoplasmic calcium concentration or affect the elevation in calcium concentration after ATP treatment in T84 cells.
Discussion
Crofelemer is a polyphenolic molecule isolated from the latex of the plant species C. lechleri of the family Euphorbiaceae. Crofelemer is an amorphous, dark red-brown powder consisting of an oligomeric proanthocyanidin of varying chain lengths with an average molecular mass of 2100 Da. Crofelemer has been characterized by 1H NMR, 13C NMR, and mass spectrometry, producing the putative structure shown in Fig. 2A (Ubillas et al., 1994). The polymer chains mostly range from 3 to 30 monomer units linked together in a random sequence through either C-4→C-6 and/or C-4→C-8. The monomeric components are (+)-catechin, (−)-epicatechin, (+)-gallocatechin, and (−)-epigallocatechin. The relative and absolute configuration of the monomer units was established by optical rotation, circular dichroism, and 13C NMR, and from analysis of monomers after depolymerization. Recently completed clinical studies in adults with acute infectious diarrhea, one with predominantly E. coli diarrhea and the other with V. cholera, showed significant clinical benefit of crofelemer in the resolution of the diarrheas (Sharma et al., 2008; Bardhan et al., 2009).
The cellular antisecretory targets of crofelemer were investigated, focusing on the principal luminal membrane determinants of intestinal fluid secretion, including ion channels and signaling pathways. We found that crofelemer inhibited apical membrane cAMP-stimulated (CFTR) and calcium-stimulated (CaCC) Cl− channels, with little effect on cation channels or cAMP/calcium signaling. It is noteworthy that crofelemer inhibited two distinct Cl− channels, which are unrelated in their sequences and putative structures. The dual cellular actions of crofelemer, together with its slow washout, may account for its broad antisecretory activity in diarrheas caused by bacterial enterotoxins, viruses, and other effectors. Inhibition of both CFTR and CaCCs is of particular interest because of cAMP/calcium cross-talk in enterocytes and thus the potential involvement of both types of Cl− channels in some diarrheas. Crofelemer action at the basolateral surface of T84 cells also reduced net Cl− secretion, perhaps by inhibition of one or more transporters involved in transcellular Cl− secretion such as the basolateral Na+/K+ pump, NKCC symporter, or K+ channel(s). Because crofelemer has minimal oral absorption, it is not expected to access the basolateral surface of enterocytes in the intestine, and its action on basolateral transporter(s) is probably not important in its antidiarrheal mechanisms.
Crofelemer acted as a partial antagonist of CFTR Cl− conductance, with a concentration-dependent rate of inhibition over several minutes. Washout of the crofelemer was slow, occurring over hours. It is unknown why, unlike thiazolidinone and glycine hydrazide CFTR inhibitors, crofelemer inhibition of CFTR Cl− conductance is partial even at very high concentrations. Some possibilities for such partial inhibition include partial external CFTR pore blockade by the large crofelemer molecule and an intrinsically inefficient allosteric inhibition mechanism. Patch-clamp analysis indicated that crofelemer action on the extracellular-facing CFTR surface produces voltage-independent channel inhibition with stabilization of the channel closed state and without rapid channel flicker. Therefore, crofelemer inhibition of CFTR is unlikely to involve direct pore occlusion. In contrast, CFTR inhibitors of the glycine hydrazide class produced a voltage-dependent block, with inward rectification of residual CFTR Cl− current, and direct pore occlusion with rapid flicker in membrane current (Muanprasat et al., 2004; Sonawane et al., 2006, 2007, 2008). The independence of crofelemer and GlyH-101 action seen in Fig. 4C is consistent with crofelemer action at a site different from that of GlyH-101, which occludes the CFTR pore. The larger molecular size of crofelemer compared with GlyH-101 is consistent with crofelemer action at a site outside of the CFTR pore. Prior studies (Gabriel et al., 1999) suggested evidence for CFTR inhibition by crofelemer in T84 cells in the presence of a large Cl− gradient. However, in the previous studies, inhibition of CaCCs was not recognized, and measurements were not done in a defined system in which CFTR could be studied in isolation from CaCCs and other ion channels.
We discovered that crofelemer strongly inhibited CaCC(s). There is evidence, albeit indirect, suggesting that CaCCs in intestinal epithelial cells provide an important route for Cl− and fluid secretion in secretory diarrheas caused by certain drugs, including some antiretrovirals and chemotherapeutics, and some viruses (Morris et al., 1999; Barrett and Keely, 2000; Kidd and Thorn, 2000; Takahashi et al., 2000; Gyömörey et al., 2001; Rufo et al., 2004; Schultheiss et al., 2005, 2006; Thiagarajah and Verkman, 2005; Lorrot and Vasseur, 2007). In addition to their expression in intestinal epithelial cells, CaCCs are broadly expressed in many cell types, in which they are involved in different functions, including transepithelial fluid secretion, olfactory and sensory signal transduction, smooth muscle contraction, and cardiac excitation (Hartzell et al., 2005; Verkman and Galietta, 2009). The molecular identity of CaCCs was unclear until recently, when three independent laboratories reported TMEM16A (anoctamin-1) as a CaCC (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). Several lines of evidence supported the conclusion that TMEM16A is a CaCC, including the demonstration that CaCC Cl− currents in TMEM16A-transfected cells are similar in electrophysiological characteristics with native CaCCs and reduction in CaCC Cl− current after RNAi knockdown of TMEM16A. TMEM16A is expressed broadly in epithelial and other cell types in multiple organs, including intestinal epithelium. It is not known at this time whether intestinal epithelial cells express other CaCCs as well. We found here that crofelemer strongly inhibited human TMEM16A, which probably accounts, at least in part, for its inhibition of Cl− current in T84 cells after addition of calcium-elevating agonists. The precise role of TMEM16A in various secretory diarrheas remains to be elucidated.
In conclusion, the cellular antisecretory action of crofelemer seems to involve two distinct Cl− channel targets on the luminal membrane of epithelial cells lining the intestine. We did not investigate the possibility that crofelemer metabolites formed in the intestine might have additional cellular effects on enterocytes. The dual inhibition of CFTR and CaCC Cl− channels by crofelemer provides insights into the understanding of its therapeutic effects in the treatment of secretory diarrheal disorders of various etiologies (Holodniy et al., 1999; DiCesare et al., 2002; Mangel and Chaturvedi, 2008), which share the common feature of excessive Cl− secretion.
Acknowledgments
We acknowledge the indigenous people of the Northwest Amazon for their expertise on the use of C. lechleri latex that led to the crofelemer work.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK72517, DK35124, DK86125]; the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL73856]; the National Institutes of Health National Eye Institute [Grant EY13574]; the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering [Grant EB00415]; the Cystic Fibrosis Foundation [Grant R613]; and an unrestricted gift from Napo Pharmaceuticals.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061051
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CaCC
- calcium-activated chloride channel
- CFTRinh-172
- thiazolidinone cystic fibrosis transmembrane conductance regulator inhibitor
- CPT-cAMP
- chlorophenylthio-cAMP
- FRT
- Fisher rat thyroid
- GlyH-101
- glycine hydrazide cystic fibrosis transmembrane conductance regulator inhibitor
- IBMX
- 3-isobutyl-1-methylxanthine
- NMDG-Cl
- N-methyl-d-glucamine chloride.
References
- Bardhan PK, Khan WA, Salam A, Saha D, Golman D, Harris MS, Chaturvedi P. (2009) Safety and efficacy of a novel anti-secretory anti-diarrheal agent Crofelemer (NP-303), in treatment of adult infectious diarrhea and cholera, with or without the use of antibiotics. 13th International Conference on Emerging Infectious Diseases in the Pacific Rim; Kolkata, India pp 18–19 2009 Apr 6–9; [Google Scholar]
- Barrett KE, Keely SJ. (2000) Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572 [DOI] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594 [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Namkung W, Mills A, Verkman AS. (2008) Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol 73: 758–768 [DOI] [PubMed] [Google Scholar]
- DiCesare D, DuPont HL, Mathewson JJ, Ashley D, Martinez-Sandoval F, Pennington JE, Porter SB. (2002) A double blind, randomized, placebo-controlled study of SP-303 (Provir) in symptomatic treatment of acute diarrhea among travelers to Jamaica and Mexico. Am J Gastroenterol 97: 2585–2588 [DOI] [PubMed] [Google Scholar]
- Field M. (2003) Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Davenport SE, Steagall RJ, Vimal V, Carlson T, Rozhon EJ. (1999) A novel plant-derived inhibitor of cAMP-mediated fluid and chloride secretion. Am J Physiol 276: G58–G63 [DOI] [PubMed] [Google Scholar]
- Gyömörey K, Garami E, Galley K, Rommens JM, Bear CE. (2001) Non-CFTR chloride channels likely contribute to secretion in the murine small intestine. Pflugers Arch 443 (Suppl 1): S103–S106 [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. (2005) Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758 [DOI] [PubMed] [Google Scholar]
- Holodniy M, Koch J, Mistal M, Schmidt JM, Khandwala A, Pennington JE, Porter SB. (1999) A double blind, randomized, placebo-controlled phase II study to assess the safety and efficacy of orally administered SP-303 for the symptomatic treatment of diarrhea in patients with AIDS. Am J Gastroenterol 94: 3267–3273 [DOI] [PubMed] [Google Scholar]
- Jones K. (2003) Review of sangre de drago (Croton lechleri)–a South American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: traditional uses to clinical research. J Altern Complement Med 9: 877–896 [DOI] [PubMed] [Google Scholar]
- Kidd JF, Thorn P. (2000) Intracellular Ca2+ and Cl− channel activation in secretory cells. Annu Rev Physiol 62: 493–513 [DOI] [PubMed] [Google Scholar]
- Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31. doi: 10.1186/1743-422X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel AW, Chaturvedi P. (2008) Evaluation of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patients. Digestion 78: 180–186 [DOI] [PubMed] [Google Scholar]
- Morris AP, Scott JK, Ball JM, Zeng CQ, O'Neal WK, Estes MK. (1999) NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am J Physiol 277: G431–G544 [DOI] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. (2004) Discovery of glycine hydrazide pore occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco E, Ghia F, Vila R, Iglesias J, Alvarez E, Cañigueral S. (2003) Immunomodulatory activity and chemical characterisation of sangre de drago (dragon's blood) from Croton lechleri. Planta Med 69: 785–794 [DOI] [PubMed] [Google Scholar]
- Rossi D, Bruni R, Bianchi N, Chiarabelli C, Gambari R, Medici A, Lista A, Paganetto G. (2003) Evaluation of the mutagenic, antimutagenic and antiproliferative potential of Croton lechleri (Muell. Arg.) latex. Phytomedicine 10: 139–144 [DOI] [PubMed] [Google Scholar]
- Rufo PA, Lin PW, Andrade A, Jiang L, Rameh L, Flexner C, Alper SL, Lencer WI. (2004) Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl− secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am J Physiol Cell Physiol 286: C998–C1008 [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G, Hennig B, Schunack W, Prinz G, Diener M. (2006) Histamine-induced ion secretion across rat distal colon: involvement of histamine H1 and H2 receptors. Eur J Pharmacol 546: 161–170 [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Siefjediers A, Diener M. (2005) Muscarinic receptor stimulation activates a Ca2+-dependent Cl− conductance in rat distal colon. J Membr Biol 204: 117–127 [DOI] [PubMed] [Google Scholar]
- Sharma A, Bolmal C, Dinakaran N, Rajadhyaksha G, Ernst J. (2008) Crofelemer improves acute diarrhea symptoms, in Proceedings of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25–28; Washington, DC.American Society for Microbiology, Washington, DC [Google Scholar]
- Sonawane ND, Hu J, Muanprasat C, Verkman AS. (2006) Luminally-active, nonabsorbable CFTR inhibitors as potential therapy to reduce intestinal fluid losses in cholera. FASEB J 20: 130–132 [DOI] [PubMed] [Google Scholar]
- Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. (2007) Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology 132: 1234–1244 [DOI] [PubMed] [Google Scholar]
- Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. (2008) Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem Biol 15: 718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Sato Y, Shiomi Y, Cantarelli VV, Iida T, Lee M, Honda T. (2000) Mechanisms of chloride secretion induced by thermostable direct haemolysin of Vibrio parahaemolyticus in human colonic tissue and a human intestinal epithelial cell line. J Med Microbiol 49: 801–810 [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Verkman AS. (2005) New drug targets for cholera therapy. Trends Pharmacol Sci 26: 172–175 [DOI] [PubMed] [Google Scholar]
- Tradtrantip L, Sonawane N, Namkung W, Verkman AS.(2009a)Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem 52: 6447–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tradtrantip L, Yangthara B, Padmawar P, Morrison C, Verkman AS.(2009b)Thiophenecarboxylate suppressor of cyclic nucleotides discovered in a small-molecule screen blocks toxin-induced intestinal fluid secretion. Mol Pharmacol 75: 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubillas R, Jolad SD, Bruening RC, Kernan MR, King SR, King Sesin DF, Barrett M, Stoddart CA, Flaster T, Kuo J, et al. (1994) SP-303, an antiviral oligomeric proanthocyanidin from the latex of Croton lechleri (sangre de drago). Phytomedicine 1: 77–106 [DOI] [PubMed] [Google Scholar]
- Verkman AS, Galietta LJ. (2009) Chloride channels as drug targets. Nat Rev Drug Discov 8: 153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. (1992) Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol 262: L713–L724 [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215 [DOI] [PubMed] [Google Scholar]