Abstract
Since its widespread introduction, the hepatitis B vaccine has become an essential part of infant immunization programmes globally. The vaccine has been particularly important for countries where the incidence of hepatitis B virus-related hepatocellular carcinoma is high. Effective treatment options for individuals with chronic hepatitis B infection were limited until 1998 when lamivudine, the first nucleoside analogue drug, was introduced. As a single treatment agent, however, lamivudine has a significant drawback: it induces lamivudine-resistant hepatitis B virus strains that may pose a risk to the global hepatitis B immunization programme. Mutations associated with drug treatment can cause changes to the surface antigen protein, the precise part of the virus that the hepatitis B vaccine mimics. However, the emergence of antiviral drug-associated potential vaccine escape mutants (ADAP-VEMs) in treated patients does not necessarily pose a significant, imminent threat to the global hepatitis B immunization programme. Nonetheless, there is already evidence that current treatment regimens have resulted in the selection of stable ADAP-VEMs. Treatment is currently intended to prevent the long-term complications of hepatitis B virus infection, with little consideration given to potential adverse public health impacts. To address individual and public health concerns, trials are urgently needed to find the optimal combination of existing drugs that are effective but do not induce the emergence of ADAP-VEMs. This paper examines the mechanism of antiviral drug-selected changes in the portion of the viral genome that also affects the surface antigen, and explores their potential impact on current hepatitis B immunization programmes.
Résumé
Depuis son introduction à grande échelle, le vaccin contre l’hépatite B est devenu une composante essentielle des programmes de vaccination infantile partout dans le monde. Ce vaccin est particulièrement important pour les pays où l’incidence des carcinomes hépatocellulaires liés à une infection par le virus de l’hépatite B (VHB) est élevée. Jusqu’en 1998, date à laquelle est apparue la lamivudine, premier analogue nucléosidique, les options thérapeutiques efficaces pour traiter les individus atteints d’hépatite B chronique étaient limitées. En tant qu’agent thérapeutique unique, la lamivudine présente cependant un inconvénient notable : elle induit l’apparition de souches de VHB résistantes à la lamivudine qui risqueraient de compromettre les programmes de vaccination contre l’hépatite B à l’échelle mondiale. Les mutations associées au traitement par ce médicament peuvent entraîner des modifications au niveau des protéines antigéniques de surface, c’est-à-dire précisément dans la partie du virus qu’imite le vaccin contre l’hépatite B. Cependant, l’apparition de «mutants d’échappement au vaccin» potentiellement associés au traitement antiviral (ADAP-VEM), chez les malades traités ne représente pas nécessairement une menace importante et imminente pour le programme mondial de vaccination contre l’hépatite B. Néanmoins, il existe déjà des preuves de la sélection par les schémas thérapeutiques actuellement appliqués de mutants ADAP-VEM stables. On administre aujourd’hui le traitement pour prévenir les complications à long terme d’une infection par le VHB, en accordant peu d’attention à ses impacts négatifs éventuels sur la santé publique. Pour répondre aux préoccupations individuelles et de santé publique, il est urgent de procéder à des essais pour sélectionner une combinaison optimale de médicaments existants, qui soit efficace sans induire l’émergence de mutants ADAP-VEM. Le présent article étudie le mécanisme régissant les modifications sélectionnées par les antiviraux dans la partie du génome viral qui influe aussi sur l’antigène de surface ainsi que l’impact potentiel de ces modifications sur les programmes actuels de vaccination contre l’hépatite B.
Resumen
Desde su introducción generalizada, la vacuna contra la hepatitis B se ha convertido en un elemento esencial de los programas de inmunización de los lactantes en todo el mundo. La vacuna ha sido además especialmente útil en los países con alta incidencia de carcinoma hepatocelular relacionado con el virus de la hepatitis B. Las opciones terapéuticas eficaces para las personas con hepatitis B crónica eran limitadas hasta 1998, año en que se introdujo la lamivudina, el primer análogo nucleosídico. Como tratamiento único, sin embargo, la lamivudina tiene un inconveniente importante, y es que induce la aparición de cepas de virus de la hepatitis B resistentes a ese medicamento, fenómeno que puede hacer peligrar el programa mundial de inmunización contra la hepatitis B. Las mutaciones asociadas a la farmacoterapia pueden alterar la proteína del antígeno de superficie, precisamente la parte del virus mimetizada por la vacuna contra esa hepatitis. Sin embargo, la aparición de posibles mutantes de escape de la vacuna asociados al uso de antivirales (MEV-AUAV) en los pacientes tratados no supone necesariamente una amenaza relevante e inminente para el programa mundial de inmunización contra la hepatitis B. Así y todo, existen ya datos que demuestran que los actuales regímenes terapéuticos han provocado la aparición de MEV-AUAV. El tratamiento se orienta actualmente a prevenir las complicaciones a largo plazo de la infección que causa el virus de la hepatitis B, sin considerar apenas el impacto potencial en la salud pública. A fin de responder a la inquietud que ello ha suscitado en relación con la salud individual y la salud pública, es preciso emprender urgentemente ensayos que permitan hallar la combinación óptima de medicamentos ya existentes que sean eficaces pero no induzcan la aparición de MEV-AUAV. En este artículo se estudia el mecanismo de los cambios provocados por los antivirales en la parte del genoma viral que afecta también al antígeno de superficie, y se analiza el impacto potencial en los actuales programas de inmunización contra la hepatitis B.
ملخص
منذ أن أدخل لقاح التهاب الكبد بي على نطاقٍ واسع، أصبح جزءاً أساسياً من برامج تمنيع الرضّع في العالم. وللقاح أهمية خاصة في البلدان التي تعاني من معدلات انتشار مرتفعة لسرطانة الخلية الكبدية المرتبطة بالتهاب الكبد بي. وقد كانت اختيارات المعالجة الفعّالة للمصابين بالتهاب الكبد بي المزمن محدودة حتى عام 1998 حين أدخل اللاميفودين، وأهو أول دواء مضاهئ للنكليوزيد. إلا أن للاميفودين، باعتباره دواء منفرد للمعالجة، عوائق هامة؛ منها أنه يحرّض ذراري فيروس التهاب الكبد بي المقاومة للاميفودين، وهو ما يعرض البرنامج العالمي للتمنيع ضد التهاب الكبد بي للخطر. فالطفرات التي رافقت المعالجة بالدواء يمكن أن تغيّر البروتينات على سطح المستضد وهو الجزء الدقيق من الفيروس الذي يحاكيه أو يشابهه لقاح التهاب الكبد بي. ومع ذلك فإن بزوغ الطفرات الهروبية المحتملة الحدوث للقاح والتي تترافق مع المعالجة الدوائية المضادة للفيروسات لدى المرضى الذين عولجوا بتلك الأدوية لا يعني بالضرورة تهديداً هاماً ووشيكاً للبرامج العالمية للتمنيع ضد التهاب الكبد بي. ولكن هناك بيِّنات متوافرة حالياً على أن النُظُم العلاجية الراهنة قد أدت إلى انتقاء الطفرات الهروبية المحتملة الحدوث في اللقاح والتي تترافق مع المعالجة الدوائية للفيروسات. وتهدف المعالجة في الوقت الحالي للوقاية من المضاعفات الطويلة الأمد للعدوى بفيروس التهاب الكبد بي، مع إيلاء القليل من الاهتمام للتأثيرات الضائرة المحتملة على الصحة العمومية. وللتصدي لجوانب القلق لدى الأفراد وعلى صعيد الصحة العمومية، تمس الحاجة للتعرُّف على التوليفة المثلى التي تضم الأدوية الموجودة حالياً والتي تتمتع بالفعالية دون أن تحرِّض على بزوغ الطفرات الهروبية المحتملة الحدوث مع اللقاح والتي تترافق مع المعالجة الدوائية المضادة للفيروسات. ويدرس الباحثون في هذه الورقة آلية التغيرات المنتقاة في الأدوية المضادة للفيروسات والتي تطرأ على جزء من جينوم الفيروس والذي تؤثر على سطح المستضد، كما تستكشف الدراسة التأثيرات المحتملة لها على برامج التمنيع ضد التهاب الكبد بي في الوقت الحالي.
Introduction
Since its widespread introduction in 1983, the hepatitis B vaccine has become an essential part of infant immunization programmes globally, and is the key component of the global hepatitis B control programme for the World Health Organization (WHO).1 Infection with hepatitis B virus (HBV) can cause acute liver disease, as well as chronic infection that may lead to liver failure or hepatocellular carcinoma. The vaccine has been particularly important for countries where the incidence of HBV-related hepatocellular carcinoma is high. In effect, the hepatitis B vaccine was the world’s first anticancer vaccine.
Effective treatment options for individuals with chronic hepatitis B infection were limited until 1998 when lamivudine, the first nucleoside analogue drug, was approved for treatment. Newer agents have been developed, but lamivudine remains the mainstay therapy in many countries with high HBV prevalence because of its safety, efficacy and low cost. As a single treatment agent, however, lamivudine has a significant drawback: monotherapy has resulted in the appearance of lamivudine-resistant HBV strains, a phenomenon that has been observed with single-drug regimens used to treat other infections such as tuberculosis and HIV infection.2 The emergence of these drug-resistant strains limits therapeutic options for individuals chronically infected with HBV; moreover, the spread of these strains may pose a risk to the global hepatitis B immunization programme. Mutations associated with drug treatment can cause changes to the surface antigen protein, the precise portion of the virus that the hepatitis B vaccine mimics. This article examines the mechanism of antiviral drug-selected changes in the part of the viral genome that also affects the surface antigen, and explores their potential impact on current hepatitis B vaccine programmes (Box 1).
Box 1. Glossary of terms related to hepatitis B viral replication.
ADAP-VEM
Antiviral drug-associated potential vaccine escape mutant.
“a” determinant
A specific region of the hepatitis B virus surface antigen. Neutralizing antibodies that recognize this antigen must form for the vaccine to provide protection against the disease.
Code
The verb “to code for” denotes the process of providing the genetic template for a specific protein.
Codon
Groups of three amino acids (nucleotides) that together form a unit of genetic code in a DNA or RNA molecule.
Envelope gene
A gene which codes for the surface antigen (envelope) of the hepatitis B virus.
Genome
The complete set of genes or genetic material (DNA and RNA) present in a cell or organism.
Genotype
The genetic constitution of an organism.
Monotherapy
Treatment with a single therapeutic agent.
Polymerase
An enzyme that acts in the polymerization of new DNA or RNA during the processes of replication and transcription.
Reading frame
A non-overlapping set of three-nucleotide sequence codons in DNA or RNA. There are three possible ways to read a nucleotide sequence, depending upon whether reading starts with the first, second or third base in the sequence. For example, with the nucleotide sequence TGCTGCTGC, the three possible reading frames are TGC TGC TGC, GCT GCT GCT and CTG CTG CTG.
Surface antigen
The outer envelope protein of the hepatitis B virus.
Transcriptase
An enzyme that catalyzes the formation of RNA from a DNA template during transcription.
Reverse transcription
Genetic copying in the reverse direction in a small number of species (e.g. human immunodeficiency and hepatitis B viruses) compared with transcription in other species.
Vaccine escape mutant
A viral strain that has undergone changes in antigenicity that make the vaccine-generated antibody response ineffective in vivo.
Features of the virus
HBV is an enveloped, partly double-stranded DNA virus containing a compact, circular genome of overlapping reading frames (Fig. 1). Human HBV causes both acute and long-term infections, including chronic and neoplastic liver disease. The virus exists as eight genotypes labelled A to H, with varied geographical distributions. Differences between genotypes involve approximately 8% of the genomic sequence.3–5 HBV uses an encoded enzyme reverse transcriptase to replicate its viral genome. Reverse transcription is an error-prone process that generates a large number of nucleotide changes within the viral genome. This process results in new, closely-related viral species; as a result, at any given time in a particular host the viral population consists of a swarm of similar but discrete viruses.6,7
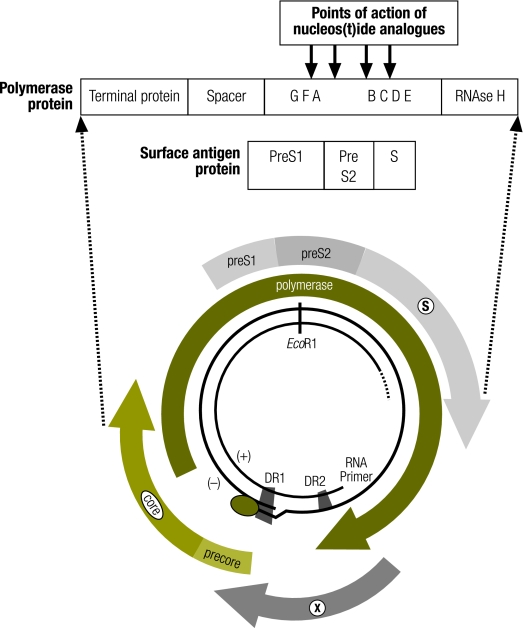
Fig. 1.
Genome structure of the hepatitis B virus showing overlapping reading frames of the polymerase and surface antigen
DR1, direct repeat sequence 1; DR2, direct repeat sequence 2; EcoR1, the cut site of the restriction endonuclease EcoR1 derived from E. coli; X, X gene encoding the HBV X protein; PreS1 and PreS2, large envelope proteins; S, the small envelope protein.
Vaccine escape mutants
The hepatitis B vaccine is an effective means of preventing HBV infection, producing protective levels of antibodies in up to 95% of recipients.8 The envelope gene of HBV produces proteins of three different lengths: two larger proteins, preS1 and preS2, as well as the smaller S protein. The commercially available hepatitis B vaccine used in most programmes is a yeast-derived recombinant surface antigen of the small S protein alone. Antibodies elicited by the hepatitis B vaccine specifically target the “a” determinant of the surface antigen (Fig. 1).
It has been recognized that the administration of hepatitis B vaccine can increase the mutation rate of the virus. In high-prevalence countries such as China, Thailand as well as Province of Taiwan, China, monitoring for more than a decade has shown that hepatitis B immunization programmes have increased the incidence of HBV variants with mutations in the surface antigen protein9,10 even as they reduce the overall burden of chronic hepatitis B infection.11 Mutations in and around the “a” determinant may lead to an alteration in the antigenicity of the surface antigen protein so that antibodies directed against the surface antigen protein may fail to neutralize the virus.12–20 Infection of immunized individuals with a vaccine escape mutant (VEM)20 is therefore possible. VEMs are typically characterized by the presence of single-amino-acid changes in the S protein.12,21 Some of these variants are associated with high levels of viraemia and have persisted in the host for more than 10 years, suggesting they are stable and transmissible variants. In addition, the antibody responses against the surface antigen protein elicited by the recombinant yeast-derived vaccine now in use are weaker and more specific than those achieved with the previous plasma-derived surface antigen vaccine.22–24 In Province of Taiwan, China, up to 28% of children with chronic hepatitis B infection also harbour hepatitis B surface antigen mutants. So VEMs capable of causing infection in fully immunized individuals are not uncommon in countries with high rates of endemic HBV infection and universal hepatitis B infant immunization programmes.12,20 However, to date the emergence of VEMs has not had a known negative impact on any country’s immunization programme.25
Treatment of infection
Five oral antiviral agents have been approved by the United States Food and Drug Administration for the treatment of chronic hepatitis B infection: lamivudine, adefovir, entecavir, telbivudine and tenofovir.26,27 The same agents are licensed in the United Kingdom and Europe. Each of these nucleoside analogue drugs has an excellent safety and efficacy profile. Effective long-term suppression of HBV replication by these agents is associated with histological and clinical improvement.
Before the approval of these direct antiviral agents, interferon, an immune potentiator, had been used to treat chronic hepatitis B infection.28 However, interferon was not consistently successful when used as monotherapy.26,27 More recently, standard interferon has been replaced by interferon conjugated with polyethylene glycol (PEG-IFN). This combination administered for 48 weeks achieves a sustained response rate in about 30% of patients.29,30 The combination of PEG-IFN plus lamivudine29,30 does not improve the virological response compared with PEG-IFN alone. However, compared with lamivudine monotherapy, this combination was more effective in preventing the emergence of lamivudine-resistant HBV.31 The combination of PEG-IFN with more potent nucleoside and nucleotide analogues such as entecavir or tenofovir needs to be tested.
Problems with drug therapy
The HBV genome is circular and organized into four open reading frames that overlap with each other. As a consequence of this arrangement, the part of the polymerase gene that codes for the reverse transcriptase enzyme – the target of antiviral therapy – overlaps the neutralization domain of the surface gene that codes for the S protein – the target of antibodies elicited by the hepatitis B vaccine (Fig. 1). Treatment of HBV-infected individuals with nucleoside analogues results in mutations in the polymerase gene, many of which are associated with alterations in the “a” determinant of the surface antigen.32–34 Resistance to lamivudine is, in fact, common in patients on monotherapy, rising from 15% after the first year of treatment to 67% when used continuously for 4 years.35 The genomic changes can be stable, and in at least one case a drug-resistant strain has been transmitted to another individual.36 Consequently, in populations where lamivudine has been widely used to treat patients continuously for periods of several years, viruses with alterations in the surface antigen are likely to occur relatively frequently, and some will be antiviral drug-associated potential vaccine escape mutants (ADAP-VEMs). Although drug-driven changes in the S protein have been well described, the effect of these changes on the antigenicity of the surface antigen has been little studied.37,38
Public health risk of escape mutants
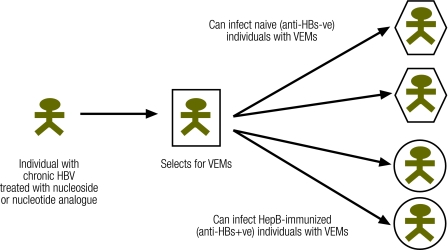
The use of nucleoside and nucleotide analogues in the treatment of chronic hepatitis B infection results in mutations in the HBV genome that can have an impact on public health (Fig. 2). However, the emergence of such ADAP-VEMs in treated patients does not necessarily pose a significant, imminent threat to the global hepatitis B immunization programme. For instance, some ADAP-VEMs selected by lamivudine have been shown to be less fit virologically; that is, they are rapidly replaced when the drug is removed and are unlikely to be a dominant or co-dominant viral population in terms of virus transmission.29 For a new viral species to pose a threat to an immunization programme, we propose that it must display the following characteristics: (i) it must be a stable mutant, (ii) the changes in antigenicity must be sufficient to prevent the antibody generated by the vaccine from neutralizing it (i.e. it must be a true ADA-VEM), (iii) it must be transmissible, cause infection in immunized individuals and have opportunities to spread, and (iv) it must cause acute or chronic disease in infected individuals.
Fig. 2.
Potential impact of hepatitis B VEMs and ADAP-VEMs on public health
ADAP-VEM, antiviral drug-associated potential vaccine escape mutants; Anti-HBs−ve, anti-hepatitis B surface antigen-negative; Anti-HBs+ve, anti-hepatitis B surface antigen-positive; HBV, hepatitis B virus; VEMs, vaccine escape mutants
Of these four characteristics, to date we have evidence that three have been fulfilled, although we do not know if ADAP-VEMs have the same propensity to cause disease as do strains of HBV that are already circulating. However, given the high rate of HBV drug resistance and the discovery of ADAP-VEMs in individuals who have received lamivudine therapy, we suggest at least two features of HBV treatment programmes may increase the likelihood that an ADAP-VEM of public health importance will emerge.
The first feature of such programmes that can increase the risk is the type of antiviral agent. Lamivudine, although it is relatively inexpensive, has been shown to rapidly result in the selection of primary antiviral drug-resistant polymerase variants. These variants in turn also select for compensatory mutations, some of which may have altered surface antigens.33,34,39 In contrast, the likelihood that mutants will be generated seems to be much lower for newer agents such as entecavir, and for combination therapies including PEG-IFN, although the risk is not zero.30,35,38,40 All of these newer medications, however, are more expensive and their long-term efficacy is still being established. Consequently, for the foreseeable future many countries, especially in the Asia-Pacific region, will probably continue to treat chronic hepatitis B infection with lamivudine even though it is no longer considered the first-line treatment, and despite the fact that drug resistance may rapidly emerge.41 The proliferation of medications and substandard drugs, as has occurred with treatments for malaria (for instance), is an additional risk as treatment programmes expand in developing countries.
The second potentially problematic feature of current treatment programmes is the way patients are selected. Antiviral drugs are the only long-term treatment option for chronic hepatitis B infection,40 and many investigators have argued that all patients who are viraemic should be treated. Because of the strong correlation between HBV viral load and the likelihood of developing cirrhosis and hepatocellular carcinoma, reducing viral replication as early as possible after infection is likely to be beneficial. In addition to being costly, this approach may generate antiviral drug resistance since current agents never lead to eradication of the virus, but only to control of replication. This effect, in turn, can complicate second-line therapies and may favour the appearance of ADAP-VEMs. Treatment in industrialized countries is therefore usually reserved for patients likely to respond to therapy and those who have advanced disease.41 However, there is no consensus globally regarding which patients to treat, and WHO has not yet produced international recommendations to guide therapy (Daniel Lavanchy, personal communication, 2008). Inappropriate inclusion criteria, improper application of these criteria or lack of compliance with treatment could greatly influence the emergence of both drug resistance and ADAP-VEMs.
The public health risk of treatment programmes in different populations may also depend upon features of the circulating viral genotype. There is evidence that the genotype of the virus influences the speed and frequency of development of resistance to treatment, which may in turn influence the likelihood that ADAP-VEMs will emerge. For example, genotype A-1 (common in north-western Europe and North America) more frequently develops surface protein changes with lamivudine therapy than genotype D-1 (concentrated in Mediterranean countries but distributed globally).42 The viral genotype also influences how frequently infected individuals develop antibodies to the hepatitis B “e” antigen (HBeAg). During the natural history of chronic hepatitis B, persons infected with genotype D-1 are more likely to become HBeAg-negative than those infected with genotype A-1.43 In general, when HBeAg is detectable in the blood, the infection is more transmissible. Among children born to mothers who are HBeAg-positive, 90% will become infected whereas only 10% of children born to HBeAg-negative mothers will become infected. The disease process can also be more active and more rapidly progressive in HBeAg-positive individuals,43 although disease progression may also be influenced more by the underlying HBV genotype than by HBeAg status alone.44,45 The proportion of infected individuals who are HBeAg-positive could therefore influence the total numbers of persons treated with antiviral medications, the risk of generating ADAP-VEMs and the subsequent risk of secondary transmission of these mutants.
Given these features, the likelihood that escape mutants will emerge as a result of HBV treatment programmes is probably greatest in settings of high HBV prevalence, where treatment is widely available and where regimens are inappropriate or adherence is uneven. Escape mutants may also appear in settings where HIV/HBV co-infection is prevalent since HIV is known to increase HBV replication,46 but it has not been confirmed that co-infection alters the rate of developing resistance. Within these settings there may be subgroups of particular interest. For example, the efficiency of transmission from an HBeAg-positive mother to her child during the perinatal period creates a very high risk for chronic hepatitis B infection. This risk makes it important to monitor for ADAP-VEMs among treated women of childbearing age, especially if lamivudine is used as monotherapy, since neither hepatitis B immunoglobulin nor active immunization would prevent infection with an ADAP-VEM transmitted from mother to child. In addition, the child may then spread the ADAP-VEM to other children, immunized or not. Subsequent treatment of chronic hepatitis B in all persons infected when young is also complicated if they have been infected with a drug-resistant strain. On the other hand, it has been estimated that the proportion of all HBV infections acquired among children more than 5 years of age ranges from 10% where prevalence of hepatitis B infection is high to as much as 90% where prevalence is low.47 These numbers suggest that monitoring for ADAP-VEMs may be needed in other populations in addition to HBV-infected HBeAg-positive women of childbearing age.
Geographical sites of high risk for the emergence of ADAP-VEMs of public health importance should be mapped. To this end, official and unofficial treatment programmes that already record the type of antiviral treatment, treatment criteria and the extent of substandard medications could be combined with descriptive epidemiology (e.g. high burden of disease, viral genotype, proportion of HBV-infected individuals with surface antigen positivity) to identify high-risk settings.
Discussion
In this article we raise the possibility of a threat to the global hepatitis B immunization programme because of the use of lamivudine and other nucleoside or nucleotide analogue therapeutic agents to treat individuals with chronic hepatitis B infection. Although the threat is theoretical, there is already evidence that current treatment regimens have resulted in the selection of stable ADAP-VEMs. Even though the transmission of ADAP-VEMs to individuals immunized with HBV vaccine has been observed in only one case,36 VEMs generated by hepatitis B vaccine have spread more widely and caused infection in previously immunized individuals.
Knowing this, what should our response be now? At the very least, we must learn more about ADAP-VEMs, their transmissibility and their potential to cause infection and disease in immunized individuals. This will require virological surveillance and clinical follow-up of infected individuals and those undergoing treatment, and also, possibly, surveillance of their close contacts. The initial focus of these activities should be high-risk settings until the level of risk is defined and understood better. Incident cases of HBV in these situations could also be examined for VEMs, especially if a new case is epidemiologically linked to an individual undergoing treatment for chronic hepatitis B infection. Follow-up of such cases of HBV, however, would depend on the availability of testing, and currently no suitable commercial tests are available.
At present, treatment aims to prevent the long-term complications of HBV infection, with little consideration given to potential adverse public health impacts. The number of potent antiviral agents is limited, their development by manufacturers is episodic and trials that have evaluated combination therapies are lacking. Because of these factors, monotherapy remains the usual practice in most settings. As with other infections, more potent combination therapies for HBV would reduce the chance of drug-resistance and lead to early and longer-lasting control of HBV replication. Such therapies would not only benefit the individual but would also simultaneously reduce the likelihood that ADAP-VEMs of global public health significance will emerge. Trials are urgently needed to identify the optimal combination of existing drugs that can address both individual and public health needs. International therapeutic guidelines for chronic hepatitis B such as those issued by the Asian-Pacific Association for the Study of the Liver,48 the European Association for the Study of the Liver International Consensus Conference49 and the American Association for the Study of Liver Disease50 should ideally consider both of these elements, and will need to be refined as more is learned about ADAP-VEMs. More effective novel agents are clearly needed that target other parts of the virus.
It is still essential to prevent the spread of wild, vaccine-sensitive strains of HBV. Well-tested measures such as safe sex and avoiding the risks associated with injection drug use will also help to reduce horizontal transmission of both the wild virus and VEMs. Hepatitis B immunization for infants of mothers with HBV will reduce perinatal transmission of the wild virus but may not prevent transmission of VEMs. The global hepatitis B immunization programme will continue to reduce new incident infections of hepatitis B and the burden of chronic HBV disease globally, although it is simultaneously generating VEMs. One simulation has predicted that the spread of VEMs selected out by immunization would result in relatively few new infections for the foreseeable future.51 However, it is not yet clear whether the emergence of ADAP-VEMs in a population will be speeded up by the simultaneous use of both the vaccine and treatment. Of course, hepatitis B vaccines that incorporate HBV proteins not altered by immunization or drug therapy are the ultimate solution to prevent the appearance of viral escape mutants generated by vaccines or antiviral drugs. ■
Acknowledgements
We thank Paul Desmond, the director of the Gastroenterology Department of St Vincent’s Hospital, Melbourne, Australia, for advice on clinical and pharmacological aspects that affect treatment of individuals with chronic hepatitis B infection.
Footnotes
Competing interests: None declared.
References
- 1.Kane M. Global status of hepatitis B immunization. Lancet. 1997;350:1102. doi: 10.1016/S0140-6736(05)70464-5. [DOI] [PubMed] [Google Scholar]
- 2.Hanson DL, Adjé-Touré C, Talla-Nzussouo N, Eby P, Borget MY, Ya Kouadio L, et al. HIV Type 1 drug resistance in adults receiving highly active antiretroviral therapy in Abidjan, Côte d’Ivoire. AIDS Res Hum Retroviruses. 2009;25:489–95. doi: 10.1089/aid.2008.0273. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, et al. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575–83. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 4.Lindh M, Andersson AS, Gusdal A. Genotypes, nt 1858 variants and geographic origin of hepatitis B virus large-scale analysis using a new genotyping method. J Infect Dis. 1997;175:1285–93. doi: 10.1086/516458. [DOI] [PubMed] [Google Scholar]
- 5.Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology. 1995;38:24–34. doi: 10.1159/000150411. [DOI] [PubMed] [Google Scholar]
- 6.Torresi J, Earnest-Silveira L, Civitico G, Walters T, Lewin SR, Fyfe J, et al. Restoration of replication phenotype of lamivudine resistant hepatitis B virus mutants by compensatory changes in the “fingers” sub-domain of the viral polymerase selected as a consequence of mutations in the overlapping S gene. Virology. 2002;299:88–99. doi: 10.1006/viro.2002.1448. [DOI] [PubMed] [Google Scholar]
- 7.Torresi J, Earnest-Silveira L, Deliyannis G, Edgtton K, Zhuang H, Locarnini SA, et al. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293:305–13. doi: 10.1006/viro.2001.1246. [DOI] [PubMed] [Google Scholar]
- 8.Hessel L, West DJ. Antibody responses to recombinant hepatitis B vaccines. Vaccine. 2002;20:2164–5. doi: 10.1016/S0264-410X(02)00117-2. [DOI] [PubMed] [Google Scholar]
- 9.Theamboonlers A, Chongsrisawat V, Jantaradsamee P, Poovorawan Y. Variants within the “a” determinant of HBs gene in children and adolescents with and without hepatitis B vaccination as part of Thailand’s Expanded Program on Immunization (EPI). Tohoku J Exp Med. 2001;193:197–205. doi: 10.1620/tjem.193.197. [DOI] [PubMed] [Google Scholar]
- 10.He C, Nomura F, Itoga S, Isobe K, Nakai T. Prevalence of vaccine-induced escape mutants of hepatitis B virus in the adult population in China: a prospective study in 176 restaurant employees. J Gastroenterol Hepatol. 2001;16:1373–7. doi: 10.1046/j.1440-1746.2001.02654.x. [DOI] [PubMed] [Google Scholar]
- 11.Su FH, Chen JD, Cheng SH, Lin CH, Liu YH, Chu FY. Seroprevalence of Hepatitis-B infection amongst Taiwanese university students 18 years following the commencement of a national Hepatitis-B vaccination program. J Med Virol. 2007;79:138–43. doi: 10.1002/jmv.20771. [DOI] [PubMed] [Google Scholar]
- 12.Carman WF. The clinical significance of surface antigen variants of hepatitis B virus. J Viral Hepat. 1997;4(Suppl 1):11–20. doi: 10.1111/j.1365-2893.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 13.Wands JR, Fujita YK, Isselbacher KJ, Degott C, Schellekens H, Dazza MC, et al. Identification and transmission of hepatitis B virus-related variants. Proc Natl Acad Sci USA. 1986;83:6608–12. doi: 10.1073/pnas.83.17.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karthigesu VD, Allison LM, Fortuin M, Mendy M, Whittle HC, Howard CR. A novel hepatitis B virus variant in the sera of immunized children. J Gen Virol. 1994;75:443–8. doi: 10.1099/0022-1317-75-2-443. [DOI] [PubMed] [Google Scholar]
- 15.Carman WF, Wallace L, Ward K, Hawkins A, Rice S, Tedder R. Fulminant reactivation of hepatitis B due to envelope protein mutant that escaped detection by monoclonal HBsAg ELISA. Lancet. 1995;345:1406–7. doi: 10.1016/S0140-6736(95)92599-6. [DOI] [PubMed] [Google Scholar]
- 16.Carman WF, Trautwein C, van Deursen FJ, Colman K, Dornan E, McIntyre G, et al. Hepatitis B virus envelope variation after transplantation with and without hepatitis B immune globulin prophylaxis. Hepatology. 1996;24:489–93. doi: 10.1002/hep.510240304. [DOI] [PubMed] [Google Scholar]
- 17.Carman WF. The clinical significance of surface antigen variants of hepatitis B virus. J Viral Hepat. 1997;4:11–20. doi: 10.1111/j.1365-2893.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 18.Waters JA, Kennedy M, Voet P, Hauser P, Petre J, Carman W, et al. Loss of the common “a” determinant of hepatitis B surface antigen by a vaccine-induced escape mutant. J Clin Invest. 1992;90:2543–7. doi: 10.1172/JCI116148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.19. Oon CJ, Lim GK, Ye Z, Goh KT, Tan KL, Yo SL, et al. Molecular epidemiology of hepatitis B virus vaccine variants in Singapore. Vaccine 1995;13:699-702. [DOI] [PubMed]
- 20.Hsu HY, Chang MH, Liaw SH, Ni YH, Chen HL. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology. 1999;30:1312–7. doi: 10.1002/hep.510300511. [DOI] [PubMed] [Google Scholar]
- 21.Karthigesu VD, Allison LM, Fortuin M, Mendy M, Whittle HC, Howard CR. A novel hepatitis B virus variant in the sera of immunized children. J Gen Virol. 1994;75:443–8. doi: 10.1099/0022-1317-75-2-443. [DOI] [PubMed] [Google Scholar]
- 22.Papaevangelou G, Dandolos E, Roumeliotou-Karayannis A, Richardson SC. Immunogenicity of recombinant hepatitis B vaccine. Lancet. 1985;1:455–6. doi: 10.1016/S0140-6736(85)91171-7. [DOI] [PubMed] [Google Scholar]
- 23.Stevens CE, Taylor PE, Tong MJ, Toy PT, Vyas GN, Nair PV, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987;257:2612–6. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]
- 24.Jilg W, Schmidt M, Deinhardt F. Prolonged immunity after late booster doses of hepatitis B vaccine. J Infect Dis. 1988;157:1267–9. doi: 10.1093/infdis/157.6.1267. [DOI] [PubMed] [Google Scholar]
- 25.Basuni AA, Butterworth L, Cooksley G, Locarnini S, Carman WF. Prevalence of HBsAg mutants and impact of hepatitis B infant immunisation in four Pacific Island countries. Vaccine. 2004;22:2791–9. doi: 10.1016/j.vaccine.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562–8. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 28.Thomas H, Foster G, Platis D. Mechanisms of action of interferon and nucleoside analogues. J Hepatol. 2003;39(Suppl 1):93–8. doi: 10.1016/S0168-8278(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 29.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–17. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 30.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 31.Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562–8. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh CT, Chien RN, Chu CM, Liaw YF. Clearance of the original hepatitis B virus YMDD-motif mutants with emergence of distinct lamivudine-resistant mutants during prolonged lamivudine therapy. Hepatology. 2000;31:1318–26. doi: 10.1053/jhep.2000.7296. [DOI] [PubMed] [Google Scholar]
- 33.Torresi J, Earnest-Silveira L, Civitico G, Walters T, Lewin SR, Fyfe J, et al. Restoration of replication phenotype of lamivudine resistant hepatitis B virus mutants by compensatory changes in the “fingers” sub-domain of the viral polymerase selected as a consequence of mutations in the overlapping S gene. Virology. 2002;299:88–99. doi: 10.1006/viro.2002.1448. [DOI] [PubMed] [Google Scholar]
- 34.Torresi J, Earnest-Silveira L, Deliyannis G, Edgtton K, Zhuang H, Locarnini SA, et al. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293:305–13. doi: 10.1006/viro.2001.1246. [DOI] [PubMed] [Google Scholar]
- 35.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok ASF. Management of Hepatitis B: Summary of a Clinical Research Workshop. Hepatology. 2007;45:1056–75. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 36.Thibault V, Aubron-Olivier C, Agut H, Katlama C. Primary infection with a lamivudine-resistant hepatitis B virus. AIDS. 2002;16:131–3. doi: 10.1097/00002030-200201040-00020. [DOI] [PubMed] [Google Scholar]
- 37.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–9. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 38.Locarnini S, Hatzakis A, Heathcote J, Keeffe EB, Liang TJ, Mutimer D, et al. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9:679–93. [PubMed] [Google Scholar]
- 39.Torresi J. The virological and clinical significance of mutations in the overlapping envelope and polymerase genes of hepatitis B virus. J Clin Virol. 2002;25:97–106. doi: 10.1016/S1386-6532(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 40.Sheldon J, Soriano V. Hepatitis B virus escape mutants induced by antiviral therapy. J Antimicrob Chemother. 2008;61:766–8. doi: 10.1093/jac/dkn014. [DOI] [PubMed] [Google Scholar]
- 41.Papatheodoridis GV, Hadziyannis SJ. Review article: current management of chronic hepatitis B. Aliment Pharmacol Ther. 2004;19:25–37. doi: 10.1046/j.1365-2036.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- 42.Sheldon J, Ramos B, Garcia-Samaniego J, Rios P, Bartholomeusz A, Romero M, et al. Selection of hepatitis B virus (HBV) vaccine escape mutants in HBV-infected and HBV/HIV co-infected patients failing antiretroviral drugs with anti-HBV activity. J Acquir Immune Defic Syndr. 2007;46:279–82. doi: 10.1097/QAI.0b013e318154bd89. [DOI] [PubMed] [Google Scholar]
- 43.Fung SK, Lok AS. Hepatitis B virus genotypes: do they play a role in the outcome of HBV infection? Hepatology. 2004;40:790–2. doi: 10.1002/hep.1840400407. [DOI] [PubMed] [Google Scholar]
- 44.Liu C-J, Kao J-H, Chen D-S. Therapeutic implications of hepatitis B virus genotypes. Liver Int. 2005;25:1097–107. doi: 10.1111/j.1478-3231.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 45.Toan NL, Song L, Kremsner PG, Duy D, Binh V, Koeberlein B, et al. Impact of the hepatitis B virus genotype and genotype-mixtures on the course of liver disease in Vietnam. Hepatology. 2006;43:1375–84. doi: 10.1002/hep.21188. [DOI] [PubMed] [Google Scholar]
- 46.Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863–70. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 47.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39(Suppl 1):64–9. doi: 10.1016/S0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 48.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement of the management of chronic hepatitis B: a 2008 update. Hepatology International 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proceedings of the European Association for the Study of the Liver (EASL) International Consensus Conference on Hepatitis B. 14-16 September, 2002. Geneva, Switzerland. J Hepatol. 2003;39(Suppl 1):S1–235. [PubMed] [Google Scholar]
- 50.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 51.Wilson JN, Nokes DJ, Carman WF. The predicted pattern of emergence of vaccine-resistant hepatitis B: a cause for concern? Vaccine. 1999;17:973–8. doi: 10.1016/S0264-410X(98)00313-2. [DOI] [PubMed] [Google Scholar]