Abstract
Objectives
We assessed mortality associated with immunologic and virologic patterns of response at 6 months of highly active antiretroviral therapy (HAART) in HIV-infected individuals from resource-limited countries in Africa and South America.
Methods
Patients who initiated HAART between 1996 and 2007, were aged 16 years or older, and had at least one measurement (HIV-1 RNA plasma viral load or CD4 cell count) at 6 months of therapy (3 to 9 month window) were included. Therapy response was categorized as complete, discordant (virologic- or immunologic-only), and absent. Associations between 6-month response to therapy and all-cause mortality were assessed by Cox proportional hazards regression. Robust standard errors were calculated to account for intra-site correlation.
Results
A total of 7,160 patients, corresponding to 15,107 person-years, was analyzed. In multivariable analysis adjusted for age at HAART initiation, baseline clinical stage and CD4 cell count, year of HAART initiation, clinic, occurrence of an AIDS defining condition within the first 6 months of treatment, discordant and absent responses were associated with increased risk of death.
Conclusions
Similar to reports from high-income countries, discordant immunologic and virologic responses were associated with intermediate risk of death compared with complete and no response in this large cohort of HIV-1 patients from resource-limited countries. Our results support a recommendation for wider availability of plasma viral load testing to monitor antiretroviral therapy in these settings.
Keywords: Antiretroviral Therapy, Highly Active, Low-Income Population, CD4 Lymphocyte Count, Viral Load, Treatment Outcome, Cohort, Mortality
INTRODUCTION
The initiation of highly active antiretroviral therapy (HAART) generally leads to a rapid reduction in plasma HIV-1 RNA levels and to an increase in peripheral CD4+ cell counts [1–3]. However, some patients experience a discordant response, whereby the HIV-1 RNA plasma level is below the limit of detection but the CD4+ cell count increase is blunted. Conversely, some patients exhibit a sustained CD4+ cell count increase despite persistent viremia. Published data, mostly from high-income countries, have indicated that discordant responses are associated with an intermediate risk of death or clinical progression relative to complete response[4–7].
No published study has assessed the impact of discordant responses on mortality in low-income countries. Because most HIV treatment programmes rely only on clinical assessment and CD4 cell counts to monitor response to treatment, the potential association between early virological response and long term survival remains largely unexplored in these settings. Additionally, limited data are available on the prognostic value of discordant responses in naïve patients who start therapy with the non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens most commonly used in developing countries [8, 9]. We previously reported that the incidence of discordant responses in resource-constrained countries was similar to that observed in resource-rich settings [10]. In the present analysis, we assessed whether the risk of death was increased in patients starting HAART in resource-constrained settings who experienced discordant responses at 6 months.
METHODS
Study population
The Antiretroviral in Lower Income Countries collaboration of the International Databases to Evaluate AIDS (ART-LINC of IeDEA) is a large collaborative network of HIV/AIDS treatment programmes in low- and middle-income countries in Africa, South America and Asia [11, 12]. The collaboration was established in 2003 to characterize the prognosis of HIV-infected patients treated with HAART in resource-limited settings, to compare the experience between different settings, delivery modes and types of monitoring and to compare outcomes with those observed in industrialized nations. The data collected at participating sites are regularly transferred to data management and statistical teams at the University of Bern, Switzerland, and the University of Bordeaux, France, where data are cleaned and merged. The present analysis includes all data available up to 29 June 2007. All previously drug-naïve participants who initiated HAART between 1996 and 2007, were aged 16 years or older at treatment initiation, and had a known date of therapy initiation, a documented baseline CD4, and at least 1 measurement (CD4 cell count or plasma viral load (PVL)) or sufficient follow-up time (6 months after HAART initiation) were eligible for analysis. Sites that did not routinely measure HIV PVL or had >80% of PVL measurements missing at six months of treatment were excluded from the analysis. At all sites, local ethics committees or institutional review boards approved the study.
The following variables were assessed: age at therapy initiation (years); gender; clinic; stage of disease at HAART initiation, classified as less (Centers for Disease Control and Prevention [CDC] stages A or B, WHO stages I or II) or more advanced (CDC stage C, WHO stages III or IV); incidence of an opportunistic infection during the first 6 months of treatment; and baseline (−6 months to +1 week) CD4 cell count (cells/μL). The baseline date was the date of antiretroviral therapy initiation. Ascertainment of opportunistic conditions was made by the local medical staff. All reported conditions that met CDC stage C or WHO stage IV criteria were considered to be AIDS defining events. The exposure of interest was 6 month response to therapy (3 to 9 month window), categorized according to virological (HIV PVL < 500 copies/mL) and immunological (increase of at least 50 CD4 cells/μL) responses, considered jointly as complete (VR+IR+), virologic-only (VR+IR−), immunologic-only (VR−IR+), and absent (VR−IR−). First-line HAART regimens were categorized as NNRTI-based (one NNRTI plus two nucleoside reverse transcriptase inhibitors [NRTI]); protease inhibitor (PI)-based (two NRTIs plus one ritonavir-boosted or unboosted PI); or other (including triple NRTI regimens and any other regimen containing a minimum of 3 drugs).
Outcomes
The endpoint was all cause mortality documented after the 6 (3 – 9) month measurement of response to therapy. Time was calculated from the 6-month measurement and ended at the earliest of the date of death or last follow-up visit. A patient was considered lost to follow-up if the last visit was recorded during the first year after starting HAART and the patient had at least 1 year of additional potential follow-up before the closing date of the database. This definition was chosen for the sake of comparability with other studies and was previously employed in a previous publication by this group [12]. The closing date was defined for each cohort as the date of the most recent follow-up recorded in the database. Death was ascertained by local medical staff.
Statistical analysis
Between-group comparisons were made by using the Chi Square test for categorical variables and the Kruskall-Wallis test for continuous variables. Kaplan Meier curves were used to show survival from 6-month response to HAART to death, and log-rank tests were used to test the hypothesis of equality between survival functions. Associations between independent variables and mortality were assessed using Cox proportional hazards regression with complete responders serving as the reference group. Wald tests were used to test for differences between the two types of immunologic and virologic discordant responses, using linear contrasts of the coefficients. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported. Huber-White robust standard errors were calculated to account for intra-site correlation. The proportional hazards assumption was assessed graphically by plotting scaled Schoenfeld residuals against survival time for each factor separately, and by log-log survival plots for categories of immunologic and virologic response, adjusted for other covariates.
Missing information on 6 month PVL or CD4 cell counts was imputed for those who had only one of these measurements, based on mortality status, loss to follow-up status, clinic, baseline CD4 cell count, sex, age, type of HAART regime, and year of HAART initiation. The ice command in Stata was used to multiply impute missing values [13]. In these imputations, values of the missing data were randomly sampled from their predicted distributions conditional on covariates and survival time. Analyses were run on each of 10 datasets that included the imputed values, and the results combined with Rubin’s rules [14]. Analyses were performed using Stata version 9.0 (Stata Corp., College Station, TX).
RESULTS
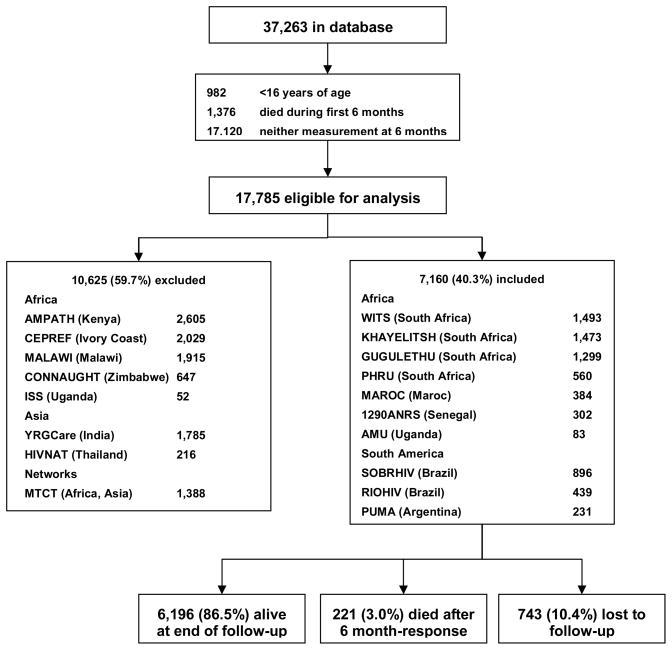
Of 37,263 records in the ART-LINC database, a total of 17,785 antiretroviral-naïve patients aged 16 years or older from 18 clinics, with at least one laboratory measurement at 6 months were potentially eligible for this analysis. Data from 8 clinics (5 from Africa, 2 from Asia and 1 Africa-Asia network) on 10,625 (59.7%) patients were excluded due to high levels of missing laboratory measurements at 6 months (Figure 1). Excluded and included patients were similar with regards to age (median 34 vs. 35 years, respectively, p=.86); sex (60.7% vs. 62.1% female, respectively, p=.08), and survival rates (1.49 vs. 1.48 per 100 person-years; p=.85). However excluded patients were more likely to start treatment on a non-standard regimen (9.1 % v. 3.7%, respectively; p<.001) and less likely to start on a PI-based regimen (1.9% vs. 15.7%, respectively; p<.001) than NNRTI-based regimens; had a higher baseline CD4 cell count (median 130 vs. 100 cells/μL; p<.001), a shorter follow-up (median 1.34 vs. 1.48 years; p<.001), and were less likely to be lost to follow-up (5.7% vs. 10.4%; p<.001).
Figure 1.
Patients’ disposition and main outcomes.
Of the 7,160 patients included in the analysis, 4,347 (60.7%) were female, with a median age of 34 years (interquartile range [IQR] 30 – 41) and a median baseline CD4 cell count of 100 (IQR 42 – 173) cells/μL. Most patients (82.2%) were at an advanced stage of disease and were prescribed a NNRTI-based regimen as first line therapy (80.6%). Of the 7,160 patients, 5,663 (79.1%) had both PVL and CD4 cell count measured at 6 months of therapy, and 1,497 (20.9%) had either CD4 cell count (1,181) or PVL (316). The rate of missing response was similar in Africa and South America (21.1 and 20.2%, respectively). Data on 6-month measurements were imputed for these patients, and the following results are based on imputed data.
At 6 (3 – 9) months, 4,974 patients (69.5%) showed complete response; 1,260 (17.6%) had virologic-only response; 540 (7.5%) had immunologic-only response; and 386 (5.4%) showed no response. Baseline CD4 cell count was lowest for immunologic-only responders, followed by complete responders, non-responders and virologic-only responders (p<.001) (Table 1).
Table 1.
Patient baseline characteristics and outcomes according to immunologic and virologic responses at 6 months of therapy.
| Variable | Total | VR+IR+ | VR+IR− | VR−IR+ | VR−IR− |
|---|---|---|---|---|---|
| N = 7,160 | N = 4,974 | N = 1,260 | N = 540 | N = 386 | |
| Baseline characteristics | |||||
| Female gender n (%) | 4,347 (60.7) | 3,147 (63.3) | 724 (57.5) | 288 (53.3) | 188 (48.7) |
| Age years (IQR)* | 34 (30 – 41) | 34 (30 – 41) | 36 (31 – 43) | 33 (29 – 39) | 34 (30 – 40) |
| Baseline CD4 count cells/μL (IQR)* | 100 (42 – 173) | 93(40 – 162) | 141 (73 – 217) | 76 (27 – 148) | 117 (43 – 200) |
| Clinical stage n (%) | |||||
| Less advanced | 753 (10.5) | 532 (10.7) | 164 (13.0) | 30 (5.6) | 27 (7.0) |
| Advanced | 5,886 (82.2) | 4,095 (82.3) | 1,012 (80.3) | 462 (85.5) | 317 (82.1) |
| Unknown | 521 (7.3) | 347 (7.0) | 84 (6.7) | 48 (8.9) | 42 (10.9) |
| Regimen type n (%) | |||||
| NNRTI based | 5,769 (80.6) | 4,212 (84.7) | 1,038 (82.4) | 309 (57.2) | 210 (54.4) |
| PI based | 1,125 (15.7) | 607 (12.2) | 164 (13.0) | 200 (37.0) | 154 (39.9) |
| Other ** | 266 (3.7) | 155 (3.1) | 58 (4.6) | 31 (5.7) | 22 (5.7) |
| Geographic region | |||||
| Africa | 5,595 (78.1) | 3,929 (70.2) | 962 (17.2) | 435 (7.7) | 268 (4.8) |
| South America | 1,566 (21.9) | 892 (57.0) | 257 (16.4) | 233 (14.9) | 184 (11.7) |
| Outcomes | |||||
| Follow-up time in years (IQR)* | 1.01 (0.54 – 1.72) | 0.99 (0.53 – 1.59) | 0.97 (0.47 – 1.57) | 1.37 (0.76 – 1.37) | 1.23 (0.62 – 3.94) |
| Mortality rate per 100PYs (95% CI) | 1.46 (1.28 – 1.67) | 1.03 (0.85 – 1.25) | 1.83 (1.36 – 2.45) | 1.88 (1.32 – 2.68) | 3.72 (2.77 – 4.99) |
| Loss to follow-up n (%) | 743 (10.4) | 422 (8.5) | 129 (10.2) | 116 (21.5) | 76 (19.7) |
Abreviations: VR+IR+: Complete response; VR+IR−: Virologic-only response; VR−IR+: Immunologic-only response; VR−IR−: Non-response; NNRTI: Non-nucleoside reverse transcriptase inhibitor; PI: Protease inhibitor
Median (Interquartile range)
Other: any other non-standard combination of 3 or more drugs.
The median follow-up time was 1.01 year (IQR 0.54 – 1.72; Table 1), and varied significantly across clinics (data not shown). The two Brazilian clinics had the longest follow-up times, with medians of 4.02 (IQR 1.13 – 6.21); and 4.23 years (IQR 1.82 – 6.43). The overall rate of loss-to-follow-up was 10.4%, and was highest among virologic non-responders (Table 1).
Among those who had a 6-month measurement, a total of 221 deaths were reported during 15,107 PYs, corresponding to a mortality rate of 1.46 (95% CI 1.28 – 1.67) per 100 PYs. Mortality rates varied by clinic, and were generally higher in the African clinics (1.83 per 100 PYs; 95% CI 1.57 – 2.14) than in the South American clinics (0.96 per 100 PYs; 95% CI 0.75 – 1.23).
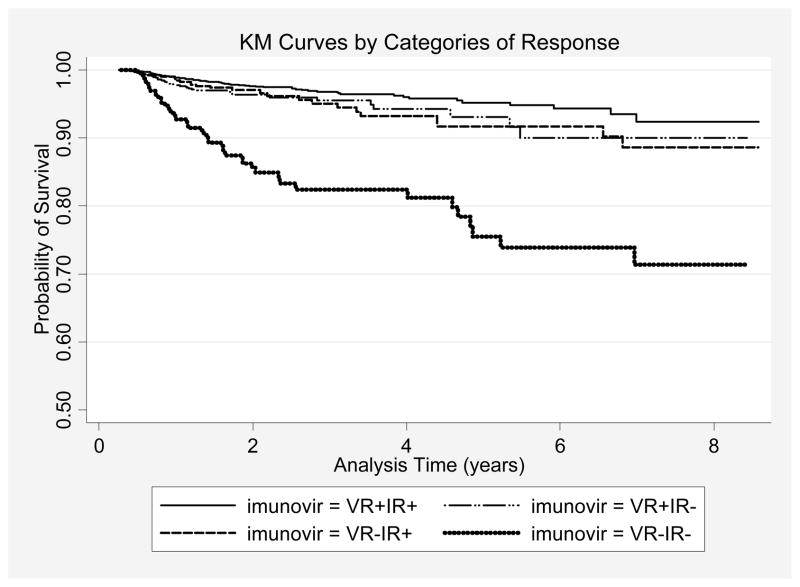
The mortality rate was highest among non-responders, followed by immunologic-only, virologic-only, and complete responders (3.72 [95%CI 2.77 – 4.99], 1.88 [95%CI 1.32 – 2.68], 1.83 [95%CI 1.36 – 2.45], and 1.03 [95% CI 0.85 – 1.25], respectively; Table 1). The log-rank test for equality of survival functions showed significant differences between these groups (p<.001), with the complete responders having the best survival and the non-responders having the worst survival (Figure 2).
Figure 2.
Kaplan-Meier curves of probability of survival according to categories of immunologic and virologic responses at 6 months in 7,160 patients in ART-LINC of IeDEA Collaboration.
In multivariable analysis adjusted for age at HAART initiation, clinical stage and CD4 cell count at HAART initiation, occurrence of an AIDS defining condition during the first 6 months of therapy, clinic, and year of HAART initiation, discordant immunologic and virologic responses as well as non-responses were associated with an increased risk of death (Table 2). Compared with complete responders, virologic-only responders had a HR of 1.90 (95% CI 1.38 – 2.63), immunologic-only responders had a HR of 2.00 (95% CI 1.30 – 3.30), and non-responders had a HR of 5.48 (95% CI 2.87 – 10.46). The two of discordant responses were not significantly different from each other (Wald test p = .46). A higher baseline CD4 cell count was independently associated with improved survival (HR = 0.81 for an increase in 100 cells/μL; 95% CI 0.68 – 0.96). Having an AIDS-defining condition during the initial 6 months of therapy was associated with a HR of 1.89 (95% CI 1.18 – 3.02). Plots of scaled Schoenfeld residuals for each variable included in the final model did not show violation of the proportional hazards assumption.
Table 2.
Unadjusted and adjusted Cox proportional hazards and 95% confidence intervals of all-cause mortality for 7,160 patients*
| Variable | Unadjusted HR | P | Adjusted HR | P |
|---|---|---|---|---|
| 6-month response | ||||
| Complete (ref) | 1.00 | 1.00 | ||
| Virologic-only | 1.85 (1.35 – 2.52) | <.001 | 2.11 (1.55 – 2.87) | <.001 |
| Immunologic-only | 1.76 (1.09 – 2.86) | .002 | 1.88 (1.16 – 3.04) | .002 |
| Absent | 4.68 (2.57 – 8.53) | <.001 | 5.73 (3.30 – 9.95) | <.001 |
| Baseline CD4 cell count (100cells increase/μL) | 0.79 (0.68 – 0.91) | .002 | 0.81 (0.68 – 0.98) | .039 |
| Age in years | ||||
| 16 – 29 (ref) | 1.00 | 1.00 | ||
| 30 – 39 | 1.00 (0.72 – 1.39) | .989 | 1.04 (0.69 – 1.52) | .847 |
| 40 – 49 | 1.12 (0.93 – 1.36) | .226 | 1.18 (0.88 – 1.57) | .262 |
| 50+ | 1.43 (0.93 – 2.20) | .103 | 1.58 (0.95 – 2.64) | .078 |
| Clinical stage | ||||
| Less advanced (ref) | 1.00 | |||
| Advanced | 1.06 (0.58 – 1.94) | .838 | ||
| Unknown | 1.10 (0.56 – 2.15) | .773 | ||
| AIDS defining event | 1.66 (1.07 – 2.56) | .022 | 1.88 (1.17 – 3.02) | .009 |
| HAART Regimen | ||||
| NNRTI based (ref) | 1.00 | 1.00 | ||
| PI-based | 1.31 (0.91 – 1.89) | .147 | 1.50 (0.70 – 3.18) | .292 |
| Other | 0.58 (0.36 – 0.94) | .026 | 0.45 (0.25 – 0.80) | .007 |
Adjusted for year of therapy initiation and clinic as fixed effects
The Cox model fitted to the complete case data is shown in Table 3. Although the HTs are qualitatively similar to the corresponding estimates in Table 2, several estimates were no longer statistically significant in the smaller sample, i.e. immunologic-only response, baseline CD4 cell count, AIDS defining event, and other HAART regimen, while the HR for age 50+ became statistically significant in the smaller sample.
Table 3.
Unadjusted and adjusted Cox proportional hazards and 95% confidence intervals of all-cause mortality for 4,944 patients with complete data*
| Variable | Unadjusted HR | P | Adjusted HR | P |
|---|---|---|---|---|
| 6-month response | ||||
| Complete (ref) | 1.00 | 1.00 | ||
| Virologic-only | 1.46 (1.05 – 2.04) | .024 | 1.50 (1.08 – 2.09) | .015 |
| Immunologic-only | 1.51 (0.78 – 2.92) | .218 | 1.53 (0.90 – 2.58) | .111 |
| Absent | 4.56 (2.01 – 10.36) | <.001 | 5.43 (2.20 – 13.42) | <.001 |
| Baseline CD4 cell count (100cells increase/μL) | 0.85 (0.76 – 0.95) | .007 | 0.90 (0.71 – 1.13) | .373 |
| Age in years | ||||
| 16 – 29 (ref) | 1.00 | 1.00 | ||
| 30 – 39 | 0.99 (0.64 – 1.55) | .990 | 1.06 (0.65 – 1.72) | .820 |
| 40 – 49 | 1.18 (0.94 – 1.48) | .154 | 1.24 (0.85 – 1.82) | .266 |
| 50+ | 1.58 (1.02 – 2.48) | .041 | 2.00 (1.22 – 3.28) | .006 |
| Clinical stage | ||||
| Less advanced (ref) | 1.00 | |||
| Advanced | 1.04 (0.58 – 1.85) | .891 | ||
| Unknown | 1.04 (0.55 – 1.98) | .900 | ||
| AIDS defining event | 1.45 (0.91 – 2.30) | .115 | ||
| HAART Regimen | ||||
| NNRTI based (ref) | 1.00 | 1.00 | ||
| PI-based | 1.21 (0.87 – 1.67) | .243 | 1.57 (0.65 – 3.75) | .311 |
| Other | 0.71 (0.48 – 1.05) | .094 | 0.51 (0.22 – 1.20) | .123 |
Adjusted for year of therapy initiation and clinic as fixed effects
In a subanalysis including only patients taking either NNRTI- or PI-based regimens (N = 6,882), there was a significant interaction between type of regimen and response (likelihood ratio Chi square p-value = .006). Among non-responders, the hazard of death was increased if the initial regimen was NNRTI-based.
Sensitivity analyses
We assessed the extent to which different loss to follow-up rates may have affected our results by excluding from the multivariable model clinics with loss to follow-up rates greater than 15%. The HRs for the 3 responses remained similar to those in the full model (HR for VR+IR− 1.68, 95%CI 1.08 – 2.62; HR for VR−IR+ 1.71, 95%CI 0.69 – 4.24; and HR for VR−IR− 7.61, 95%CI 5.69 – 10.17). Exclusion of the Brazilian clinics also did not substantially change the estimated HRs, suggesting no bias due to different lengths of follow-up in our analysis (HR for VR+IR− 1.75, 95%CI 1.22 – 2.51; HR for VR−IR+ 1.35, 95%CI 0.50 – 3.63; and HR for VR−IR− 7.84, 95%CI 6.11 – 10.05). The HRs of discordant responses were also similar in African (HR for VR+IR− 1.79, 95%CI 1.18 – 2.40; HR for VR−IR+ 1.35, 95%CI 0.50 – 3.67; and HR for VR−IR− 7.92, 95%CI 6.16 – 10.17) and South American regions (HR for VR+IR− 1.91, 95%CI 0.71 – 5.16; HR for VR−IR+ 1.54, 95%CI 1.16 – 2.05; and HR for VR−IR− 4.07, 95%CI 2.23 – 7.44). Overall, the results of these sensitivity analyses showed consistency of HRs for discordant responses.
DISCUSSION
We report on survival associated with immunologic and virologic discordant responses at 6 months after HAART initiation in a large collaboration of cohorts from lower-income countries. Our analysis shows that both types of discordant responses are associated with increased risk of death relative to complete responders, which is in agreement with previous reports from developed countries [4–6, 15].
Understanding the relationship between early responses to HAART and mortality has critical implications for guiding treatment modifications, particularly for those patients who show discordant responses, because partial response to therapy has been associated with increased risk of disease progression and death in several studies. In a study conducted in France, virologic-only and non-responders had a higher probability of clinical progression, whereas immunologic-only and complete responders had similar risks [16]. In contrast, in other studies immunologic-only response was also associated with a higher risk of clinical progression. In one cohort of antiretroviral experienced patients with advanced HIV disease starting protease inhibitor based HAART that was followed for over 30 months, discordant responders at 12 months experienced significantly more AIDS-defining events than complete responders, with immunologic-only responders having a slightly higher probability of being event-free compared to virologic-only responders [4]. In another study involving over 2,100 antiretroviral experienced and naïve patients followed for a median of 44 months, immunologic- and virologic-only responders had significantly lower risk of clinical progression than non-responders, but had a 2.3 and 1.9-fold greater risks of death or of experiencing a new AIDS-defining event than complete responders, respectively [5]. Similar findings were recently reported by Tan et al. among 404 patients from an urban clinic in the US [15].
Few studies have assessed the prognostic value of discordant responses in previously naïve patients and in recipients of NNRTI-base regimens. Our results are similar to those reported by Moore et al.[6], who assessed the independent association of discordant responses with mortality in 2,217 antiretroviral-naïve individuals initiating HAART in British Columbia. These authors also reported that discordant responses were associated with increased risk of death when compared with complete responders. Likewise, in a study involving previously HAART naïve injection drug users, although discordant responders experienced increased mortality in comparison to complete responders, progression rates did not differ whether early response was immunologic only or virologic only [17]. Another study of 850 virologically suppressed patients showed that poor CD4 cell recovery was associated with higher risk of death or an AIDS defining event, and that consequences of poor response were greater at lower baseline CD4 values[18].
We found a significant interaction between use of NNRTI-based regimen and non-response, suggesting that a poor response to this class of drug has more deleterious effect on long-term survival than a PI-based regimen. Although our findings should be interpreted with caution due to inherent limitations of this type of analysis, in particular selection-by-indication bias, data suggesting that failure of PI-based regimens is associated with less rapid progression than NNRTI regimens are accumulating [19, 20]. One concern that arises from the wide use of NNRTI-based regimens is that qualitative differences in immunologic-only response could exist between PI and NNRTI recipients due to different genetic barriers to resistance as well as potential intrinsic properties of individual drugs or classes of drugs [21, 22]. A study of 1,138 previously HAART-naive HIV-infected individuals from British Columbia showed that patients who developed resistance to NNRTI had a risk 3.02 times higher of progressing to death than those who had no resistance [23]. In a study assessing the impact of time from virologic failure to treatment switch, Petersen et al. showed that delayed modification after failure of a NNRTI-based regimen was associated with increased risk of immunologic failure and mortality [24]. Further studies are thus needed to clarify the prognostic importance of different patterns of immunologic and virologic response in recipients of PI and non-PI regimens.
Our study has several limitations. First, data on adherence were not available. Several studies have shown the independent association between poor adherence and increased mortality [6, 25, 26]. Second, other possible confounders could not be assessed in our study. For example, studies on the pathogenesis of immunologic-only response have suggested possible roles of factors associated with viral subtype [27] [28], phenotype [29], and/or fitness [22], as well as T-cell activation [30, 31] and host-related determinants such as genetic polymorphisms [32]. Poor CD4 cell recovery has been associated with regimens containing didanosine plus tenofovir [33], and polymorphisms of interleukin-6 and central major histocompatibility complex genes [34]. Third, the definition of immunologic response used in the present and in other studies (an increase in CD4 cell count of at least 50 cells) may be subject to a regression towards the mean effect, by which those with the highest baseline values would be less likely to respond than those with low baseline values [35]. Fourth, the exclusion of nearly 60% of patients due to lack of information on the 6-month response could have introduced a bias into our results. Most ART-LINC participating sites do not perform routine PVL for treatment monitoring; therefore the analysis was limited to a sample that may not be representative of countries where ARV programmes have been rolled out. Although excluded and included patients were similar in baseline characteristics and survival rates, the relatively short follow-up time precludes further extrapolations as to whether such selection might affect long-term outcomes. Therefore, our results may not be applicable to patients from lower income countries who do not have a 6-month laboratory assessment. Finally, we also noticed a generally higher rate of loss to follow-up among non-responders. That these patients also were more likely to die indicates a potential ascertainment bias that could have led to underestimating the hazard for this group.
In conclusion, we found that in developing countries both types of immunologic and virologic discordant responses were associated with higher mortality rates when compared with complete responses. No published study has assessed the impact of discordant responses on mortality in low-income countries, where most patients initiate HAART with NNRTI-based regimens. Most studies published so far have been conducted in developed countries and have included patients mostly using PI-based regimens. Our results suggest that, as in resource-rich settings, both immunologic and virologic assessments are important for predicting mortality in patients receiving HAART in resource-constrained countries, and provide a strong argument for recommending the wider availability of plasma viral load testing to monitor antiretroviral therapy in these settings.
Acknowledgments
The ART-LINC collaboration of the International epidemiological Databases to Evaluate AIDS (IeDEA) is funded by the US National Institutes of Health (Office of AIDS Research and National Institute of Allergy and Infectious Diseases) and the French Agence Nationale de Recherches sur le Sida et les hepatitis virales (ANRS). This study was partially supported by the Fogarty International Center, NIH (grant 3 D43 TW01038). We are grateful to Joyce Snyder and Claire Graber for technical support and to Eric Balestre for data management.
Collaborating centres
Centre de Prise en Charge de Recherches et de Formation (CEPREF)/Agence Nationale de Recherches sur le Sida et les hepatitis virales (ANRS) 1203 COTRAME Cohort (Abidjan, Côte d’Ivoire); Senegalese Antiretroviral Access Initiative (ISAARV), ANRS 1290 (Dakar, Senegal); Academic Model for the Prevention and Treatment of HIV/AIDS (AMPATH), Moi University College of Health Sciences/University of Indiana (Eldoret, Kenya); Adherence Monitoring Uganda (AMU) cohort, Makerere-University of California in San Francisco (UCSF; Kampala, Uganda); Kamuzu Central Hospital/Lighthouse Trust (Lilongwe, Malawi); Connaught Clinic (Harare, Zimbabwe); Gugulethu ART Programme, (Cape Town, South Africa); Khayelitsha ART Programme, (Cape Town, South Africa); Operational Research on ART (OPERA), Perinatal HIV Research Unit (Soweto, South Africa); Morocco Antiretroviral Treatment 14 Cohort, Centre Hospitalier Universitaire (Casablanca, Morocco); MTCT-Plus Initiative, International Center for AIDS Care and Treatment Programs, Mailman School of Public Health, Columbia University, New York, USA; Prospective Evaluation in the Use and Monitoring of Antiretrovirals in Argentina (PUMA), Buenos Aires, Argentina; South Brazil HIV Cohort (SOBRHIV), Hospital de Clinicas (Porto Alegre, Brazil); Rio de Janeiro HIV Cohort, Hospital Universitario Clementino Fraga Filho (Rio de Janeiro, Brazil); Y R Gaitonde Centre for AIDS Research and Education (YRG) Care Cohort (Chennai, India); HIV-NAT, Thai Red Cross AIDS Research Centre (Bangkok, Thailand).
Writing committee
Suely Hiromi Tuboi, Antonio Guilherme Pacheco, Lee H. Harrison, Roslyn A. Stone, Margaret May, Martin W. G. Brinkhof, François Dabis, Matthias Egger, Denis Nash, David Bangsberg, Paula Braitstein, Constantin T. Yiannoutsos, Robin Wood, Eduardo Sprinz, and Mauro Schechter.
The ART-LINC of IeDEA Central Coordinating Team
Eric Balestre, Martin Brinkhof, François Dabis (principal investigator), Matthias Egger (principal investigator), Claire Graber, Beatrice Fatzer, Olivia Keiser, Charlotte Lewden, Mar Pujades, Mauro Schechter (principal investigator).
References
- 1.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. Jama. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 4.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001;183:1328–1335. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 5.Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, Geraci A, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–160. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 6.Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, Montaner JS. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–293. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 7.Grabar S, Le Moing V, Goujard C, Egger M, Leport C, Kazatchkine MD, et al. Response to highly active antiretroviral therapy at 6 months and long-term disease progression in HIV-1 infection. J Acquir Immune Defic Syndr. 2005;39:284–292. doi: 10.1097/01.qai.0000160925.33935.72. [DOI] [PubMed] [Google Scholar]
- 8.Copackaged drug regimen approved for PEPFAR. AIDS Alert. 2005;20:46. [PubMed] [Google Scholar]
- 9.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: 2006. [PubMed] [Google Scholar]
- 10.Tuboi SH, Brinkhof MW, Egger M, Stone RA, Braitstein P, Nash D, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 11.Dabis F, Balestre E, Braitstein P, Miotti P, Brinkhof WG, Schneider M, et al. Cohort Profile: Antiretroviral Therapy in Lower Income Countries (ART-LINC): international collaboration of treatment cohorts. Int J Epidemiol. 2005;34:979–986. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- 12.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 13.Acock AC. Journal of Marriage and Family. 2005. Working with missing values; pp. 1012–1028. [Google Scholar]
- 14.Rubin D. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 15.Tan R, Westfall AO, Willig JH, Mugavero MJ, Saag MS, Kaslow RA, Kempf MC. Clinical Outcome of HIV-Infected Antiretroviral-Naive Patients With Discordant Immunologic and Virologic Responses to Highly Active Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2008;47:553–558. doi: 10.1097/QAI.0b013e31816856c5. [DOI] [PubMed] [Google Scholar]
- 16.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine MD, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Lucas G, Astemborski J, Kirk G, Vlahov D, Galai N. Discordant Responses to HAART and Clinical Outcomes among Injection Drug Users in Baltimore, Maryland. Thirteenth Conference on Retroviruses and Opportunistic Infections; Denver. Abstract 527 2006. [Google Scholar]
- 18.Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–546. doi: 10.1097/QAI.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG, Barbour JD, Grant RM, Martin JN. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. Aids. 2002;16:201–207. doi: 10.1097/00002030-200201250-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 21.Deeks SG, Grant RM. Sustained CD4 responses after virological failure of protease inhibitor-containing therapy. Antivir Ther. 1999;4 (Suppl 3):7–11. [PubMed] [Google Scholar]
- 22.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344:472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 23.Hogg RS, Bangsberg DR, Lima VD, Alexander C, Bonner S, Yip B, et al. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med. 2006;3:e356. doi: 10.1371/journal.pmed.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. Aids. 2008;22:2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O’Shaughnessy MV, Montaner JS. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. Aids. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 27.Santoro-Lopes G, Harrison LH, Tavares MD, Xexeo A, Dos Santos AC, Schechter M. HIV disease progression and V3 serotypes in Brazil: is B different from B-Br? AIDS Res Hum Retroviruses. 2000;16:953–958. doi: 10.1089/08892220050058362. [DOI] [PubMed] [Google Scholar]
- 28.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;358:1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sufka SA, Ferrari G, Gryszowka VE, Wrin T, Fiscus SA, Tomaras GD, et al. Prolonged CD4+ cell/virus load discordance during treatment with protease inhibitor-based highly active antiretroviral therapy: immune response and viral control. J Infect Dis. 2003;187:1027–1037. doi: 10.1086/368359. [DOI] [PubMed] [Google Scholar]
- 30.Hunt PW, Deeks SG, Bangsberg DR, Moss A, Sinclair E, Liegler T, et al. The independent effect of drug resistance on T cell activation in HIV infection. Aids. 2006;20:691–699. doi: 10.1097/01.aids.0000216369.30948.18. [DOI] [PubMed] [Google Scholar]
- 31.Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, Stevens RA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–133. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 32.Nasi M, Pinti M, Bugarini R, Troiano L, Lugli E, Bellodi C, et al. Genetic polymorphisms of Fas (CD95) and Fas ligand (CD178) influence the rise in CD4+ T cell count after antiretroviral therapy in drug-naive HIV-positive patients. Immunogenetics. 2005;57:628–635. doi: 10.1007/s00251-005-0031-z. [DOI] [PubMed] [Google Scholar]
- 33.Barreiro P, Soriano V, Casas E, Gonzalez-Lahoz J. Different degree of immune recovery using antiretroviral regimens with protease inhibitors or non-nucleosides. Aids. 2002;16:245–249. doi: 10.1097/00002030-200201250-00014. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez S, Rosenow AA, James IR, Roberts SG, Nolan RC, French MA, Price P. Recovery of CD4+ T Cells in HIV patients with a stable virologic response to antiretroviral therapy is associated with polymorphisms of interleukin-6 and central major histocompatibility complex genes. J Acquir Immune Defic Syndr. 2006;41:1–5. doi: 10.1097/01.qai.0000188990.57760.e3. [DOI] [PubMed] [Google Scholar]
- 35.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]