Abstract
Oncolytic viral therapy is a promising biological therapy for the treatment of cancer. Recent advances in genetic engineering have facilitated the construction of custom-built oncolytic viruses that can be exquisitely targeted to tumors by exploiting each cancer’s unique biology and their efficacy can be further enhanced by “arming” them with additional therapeutic genes. Such an approach allows the virus to unload its “therapeutic cargo” at the tumor site, thereby enhancing its anti-neoplastic properties. While several clever strategies have been recently described using genes that can induce cellular apoptosis/suicide and/or facilitate tumor/virus imaging, viruses armed with genes that also affect the tumor microenvironment present an exciting and promising approach to therapy. In this review we discuss recently developed oncolytic viruses armed with genes encoding for angiostatic factors, inflammatory cytokines, or proteases that modulate the extracellular matrix to regulate tumor vascularization, anti-tumor immune responses and viral spread throughout the solid tumor.
Keywords: Oncolytic viruses, Tumor microenvironment, angiogenesis, extracellular matrix, cytokines
INTRODUCTION
Oncolytic viral (OV) therapy is a promising biological treatment modality that exploits the tumor-specific replication propensity of some viruses. The underlying hypothesis is that viral replication leads to intratumoral amplification of the therapeutic virus, leading to the ultimate destruction of infected cancer cells with minimal damage to non neoplastic tissue. Several clever strategies have been employed to mutagenize and/or select for tumor-specific replicating viruses, though the basis for such targeted replication will not be discussed in this review (interested readers are directed to recent reviews [1–3]). Such “first generation” viruses typically are mutants with gene(s) deleted that are critical for virus replication in normal cells but dispensable in cancer cells. While the recent approval by the Chinese State Food and Drug Administration for H101 (Oncorine, an OV functionally identical to ONYX-015) resulted in the marketing of the world’s first oncolytic virus, the regulatory approval of OVs in US and Europe for standard clinical use has been lagging. Several Phase I/II clinical trials using oncolytic agents for cancer therapy have been completed and have underscored the safety of this approach. However, large randomized Phase-III studies need to be conducted within the US and Europe prior to their marketing in the western world [2].
As the first generation oncolytic viruses head for testing in human patients for efficacy, a variety of innovative approaches are being explored to enhance their therapeutic effects [4]. Rational combinations of viruses with various pharmacological drugs to enhance their efficacy are currently being tested in several preclinical and clinical studies [5, 6]. Recent advances in molecular biology techniques have facilitated the generation of recombinant viruses with increased potency and tumor specificity. One approach by which this is accomplished is to reinsert viral genes that were originally deleted in the first generation OV under the governance of tumor-specific promoters to enhance replication and lytic potency of viruses in a tumor-specific fashion [7]. Tumor tropism of viruses has also been genetically modified to create OVs whose binding to cells are exquisitely retargeted towards cancer/tissue specific epitopes [8]. So-called “arming” of oncolytic viruses refers to viruses that, apart from their lytic potential, are also harnessed as gene transfer vehicles. The variety of genes utilized to arm OVs include: 1) apoptosis inducing genes to enhance their tumor cell killing ability; 2) pro-drug activating enzymes which can convert nontoxic drug precursors to extremely toxic chemotherapeutic agents, which results in localized killing of both infected and uninfected bystander cells; 3) reporter genes, which facilitate in vivo monitoring of OV persistence so as to be able to monitor the pharmacodynamic and pharmacokinetics of the administered virus; and 4) genes that can modulate the tumor microenvironment. This last strategy is very significant as it employs a two-pronged approach that targets both the neoplastic cell and its microenvironment.
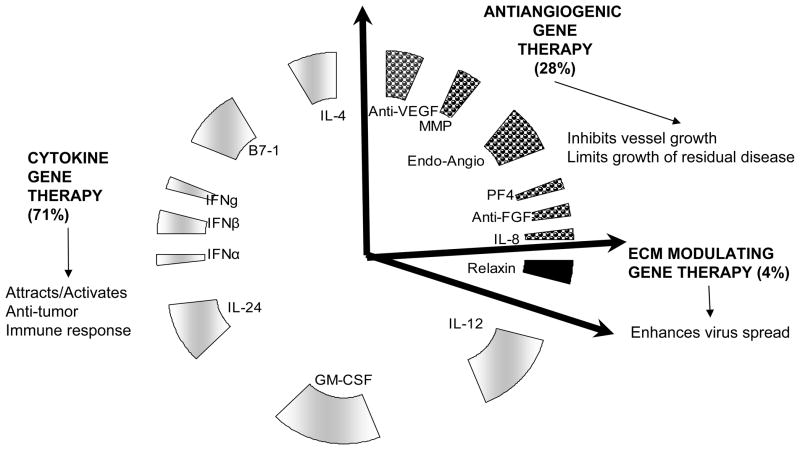
The tumor microenvironment consists of host fibroblasts, immune cells, endothelial cells, and pericyte cells. Together with cancer cells they secrete a complex extracellular matrix (ECM), which orchestrates a complex interplay of signals impacting tumor growth, progression, neovascularization, invasion, and metastasis. While ECM proteins provide structural support to cancer and stroma cells, their increased secretion also presents a barrier for efficient diffusion of therapeutics. Secreted cytokines and chemokines in the ECM also affect the recruitment of immune cells into the tumors, which plays an important role in tumor biology and impacts the efficacy of immunotherapy [9]. Recent studies have made it quite apparent that an effective anticancer agent needs to target both the neoplastic cells and the “cancer field” in which they grow to maximize their therapeutic efficacy. In this review we discuss the rationale, development and efficacy of oncolytic viruses armed with ECM-modulating therapeutic transgenes that can destroy tumor blood vessels, activate anti-tumor immune responses and enhance viral dissemination (Figure 1).
Figure 1. Oncolytic viruses encoding for genes that affect tumor microenvironment.
A large majority (71%) of the tumor microenvironment modulating oncolytic viruses express cytokines which can regulate antitumor immune response and also affect tumor stroma and angiogenesis. Twenty-five percent of oncolytic viruses are armed with the ability to modulate tumor angiogenesis by either secreting antiangiogenic genes or by reducing expression/bioavailability of angiogenic factors. A very small fraction (4%) of oncolytic viruses have been generated with the ability to digest the tumor extracellular matrix to enhance virus spread through the tumor interstitium.
ANGIOGENESIS TARGETING STRATEGIES
Angiogenesis is the development of a functional network of vasculature to perfuse dividing tumor cells with oxygen and nutrients and is considered an essential prerequisite for the growth of solid tumors [10]. This process is exquisitely controlled by the balanced secretion of both angiogenic and antiangiogenic growth factors into the tumor microenvironment. Normal homeostasis of these factors orchestrates a finely tuned mechanism which signals for the initial proliferation of endothelium (mature or progenitors) followed by a process of maturation to form functional blood vessels. In the tumor microenvironment, increased secretion of angiogenic factors along with a concurrent loss of antiangiogenic factors creates unchecked vascular proliferation. The resulting vasculature born out of this uncontrolled process is aberrant, and results in reduced tumor perfusion and increased hypoxia despite the increased microvessel density (Figure 2). Increased hypoxia in tumor tissue is associated with aggressive tumor growth, invasion, and resistance to therapy [11]. Destruction of tumoral vasculature using endogenous or synthetic antiangiogenic agents is emerging as a promising therapeutic modality for the treatment of solid tumors. While gene therapy of tumors with antiangiogenic genes is theoretically promising, major barriers to its implementation include limited viral gene transduction and low expression levels in tumors. Use of OVs as gene therapy vehicles provides for dosage amplification of the antiangiogenic therapeutic gene in a cancer cell specific manner.
Figure 2. Difference between normal and tumor vasculature.
Athymic nude mice with intracranial U87ΔEGFR glioma cell tumors were sacrificed when the mice showed evidence of tumor burden. The harvested tissue including tumor and normal brain was fixed and stained for CD31 endothelial marker (brown) and counterstained with hematoxylin. A: Highlights the regular thickness of vasculature in the normal, non-tumor bearing hemisphere. Note the uniform distribution, and regular thickness of the vasculature. B: Tumor vasculature resulting from the uncontrolled secretion of angiogenic factors is highly aberrant. Note the large extended lumens, irregular thickness, tortuous structure and very high blood vessel density.
Apart from the oncolytic destruction of malignant cancer cells, the impact of OV treatment on the tumor microenvironment has been the subject of several studies. Treatment of tumors with oncolytic adenovirus such as the ONYX-015 (with E1A gene expression) has been associated with reduced VEGF production and tumor-associated angiogenesis [12, 13]. Similarly, oncolytic HSV-1 has also been shown to be capable of infecting and lysing proliferating tumor endothelial cells resulting in reduced micro vessel density in treated tumors [14–16]. While these studies have demonstrated the reduction of tumor vasculature as a direct effect of viral infection, two recent studies reported increased vessel density in tumors that regrow after oncolysis [17, 18]. Consistent with this finding is the increased angiogenic response associated with infection of certain wild type viruses [19–21]. More significantly, combination of OV therapy with angiogenesis inhibitors has shown to increase viral spread [22], reduce antiviral inflammation and enhance viral propagation [23], leading to increased antitumor efficacy [24]. Additionally, increased replication of oncolytic HSV in hypoxic cells has been noted [25], suggesting the hypoxia that ensues after antiangiogenic treatment may also contribute towards the observed synergy between oncolytic HSV and antiangiogenic treatments in vivo. Thus, the combination of oncolytic viral therapy with antiangiogenic gene delivery is an attractive means to enhance oncolysis. In this section we review some of the antiangiogenic gene therapy strategies used in conjunction with oncolytic viral therapy.
Oncolytic strategies targeting vascular endothelial growth factor (VEGF)
Vascular endothelial growth factor is comprised of a family of growth factors which includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and Placenta-like growth factor [26–28]. These are homodimeric heparin binding secreted proteins which function as a potent stimulators of growth and migration of vascular endothelial cells derived from arteries, veins and even lymphatics (reviewed in [29]). VEGF expression in human cancers correlates with increased tumor vascularity and in many instances worse prognosis, and its over-expression in human tumors promotes neovascularization and malignant growth. VEGF binds to its tyrosine kinase receptors: VEGF receptor-1 (VEGFR-1/Flt-1) and VEGF receptor 2 (VEGFR-2/KDR) on endothelial cells activate a signaling cascade resulting in increased endothelial cell proliferation, migration and differentiation (reviewed in [11]). Several strategies targeting VEGF secretion and/or VEGF receptor activation in conjunction with oncolytic virus treatment have been tested (Table 1). VEGF blockade via expression of soluble VEGF receptors conjugated to antibody Fc domains have been shown to function as potent inhibitors of angiogenesis and led to significant inhibition of tumor growth of both murine (Lewis lung carcinoma, T241 fibrosarcoma) and human (BxPC3 pancreatic carcinoma) tumors in mice [30].
Table 1.
List of Oncolytic viruses delivering angiostatic gene therapy discussed in this paper
| OV NAME | THERAPEUTIC GENE | VIRUS TYPE | OV MUTATION | IN VIVO CANCER MODEL USED (EFFICACY +/−) | REF. |
|---|---|---|---|---|---|
| VEGF Targeting OV: | |||||
| ZD55-sflt-1 | sFlt-1 | Ad | E1B-55kDa deleted | Colorectal (+) | [31] |
| Ad-ΔB7-shVEGF | shRNA-VEGF | Ad | E1A mutated and ΔE1B | Glioma (+) | [33] |
| AdΔB7-KOX | F435-KOX | Ad | E1A mutated and ΔE1B | Glioma (+) | [34] |
| MMP Targeting OV: | |||||
| AdΔ24TIMP3 | TIMP3 | Ad | 24 b.p. deletion of E1A gene. | Glioma (−) | [45] |
| rQT3 | TIMP3 | HSV-1 | ΔICP34.5, and ΔICP6 | MPNST | [46] |
| Endostatin-Angiostatin Targeting OV: | |||||
| HSV-Endo | Endostatin | HSV-1 | ΔICP6 | Colon Carcinoma (+) | |
| AE618 | Endostatin- Angiostatin fusion Pr. | HSV-1 | ΔICP34.5, and ΔICP6 | Lung Cancer (+) | [58] |
| CNHK200-mE | Endostatin | Ad | E1B55kDa deleted | Hepatocellular carcinoma (+) | [60] |
| CNHK500-mE | Mouse Endostatin | Ad | hTERT controlled E1A, and Hypoxia regulated E1B. | Nasopharyngeal tumor (+) | [61] |
| Platelet Growth Factor Targeting OV: | |||||
| bG47Δ-PF4 | Platelet Factor-4 | HSV-1 | ΔICP34.5, ΔICP6, and ΔICP47 | Glioma (+) Malignant Peripheral Nerve Sheath Tumors (+) |
[65] |
| Fibroblast Growth Factor Targeting OV: | |||||
| bG47Δ-dnFGFR | dn Fibroblast growth factor | HSV-1 | ΔICP34.5−/−, ΔICP6, and ΔICP47 | Glioma (+) Malignant Peripheral Nerve Sheath Tumors (+) |
[72] |
| Interleukin 8 Targeting OV: | |||||
| Ad-ΔB7-U6shIL8 | shRNA-IL-8 | Ad | E1A mutated and ΔE1B | Liver Cancer (+) and Non small cell lung cancer (+) Pulmonary metastasis of breast (+) |
[76] |
Abbreviations: VEGF: Vascular Endothelial growth factor; sflt-1: soluble VEGF receptor flt-1; Ad: Adenovirus; sh-VEGF: small hairpin targeting VEGF; TIMP3: Tissue inhibitor of metalloproteinases; HSV-1: Herpes Simplex virus, dnFGFR: dominant negative Fibroblast growth factor receptor; shIL8: small hairpin targeting IL8.
Conditionally replicating adenovirus (CRAd) mediated delivery of soluble VEGFR-1 (sFlt-1) was recently tested for efficacy against human subcutaneous colon cancer (SW620) xenografts in mice [31]. In this study, the gene encoding sFlt-1 was inserted into the backbone of an E1B-55-kDa deleted CRAd, creating ZD55-sflt-1. This construct facilitated expression and secretion of sFlt-1 in cells infected with ZD55-sflt-1, compared to cells infected with the ZD55 control CRAd. Treatment of tumor bearing mice with ZD55-sflt-1 resulted in marked reduction in microvessel density and tumor growth [31]. In a similar approach, administration of oncolytic adenovirus in conjunction with a non-replicating adenovirus expressing soluble VEGFR-2 (sflk-1) was tested for anti-tumor efficacy [32]. Co-infection of Ad Flk1-Fc with CRAd dl922/947 permitted for replication and packaging of the replication-deficient virus and facilitated increased sFlk1-Fc gene expression and increased therapeutic efficacy in vivo [32]. Direct inhibition of VEGF gene expression in conjunction with OV has also been tested for antitumor efficacy [33]. An oncolytic adenovirus (Ad-ΔB7-shVEGF) expressing short hairpin RNA (shRNA) against VEGF was created. Ad-ΔB7-shVEGF infected cells showed reduced secretion of VEGF, reduced angiogenesis and reduced tumor growth compared to control Ad-ΔB7 infected cells [33]. A more novel approach to target VEGF expression recently exploited gene expression of a VEGF-targeted zinc finger protein (ZFP) transcriptional repressor (F435-KOX). F435-KOX is a recombinant fusion protein created by fusing F435 ZFP (VEGF promoter specific binding ZFP) with Kruppel associated box repression domain derived from human protein KOX1. This recombinant protein functions as a VEGF-specific transcriptional repressor and reduced the amount of secreted VEGF [34]. Expression of F435-KOX within a CRAd resulted in enhanced antitumor efficacy and reduced micro vessel density in treated tumors in vivo [34].
Tissue Inhibitor of Metalloproteinase
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases involved in degrading and remodeling the ECM. Increased expression and/or activity of MMPs have been associated with increased tumor angiogenesis, invasion, spread and metastasis [35]. Apart from direct ECM remodeling, MMP activity can also release growth factors from the matrix increasing their bioavailability, resulting in increased activation of their receptors [36]. The tissue inhibitors of metaloproteinases (TIMPs) are a family of proteins that control extracellular matrix remodeling by regulating MMP function [37]. Among the four known TIMP proteins (TIMP1, TIMP2, TIMP3 and TIMP4), TIMP3 has the widest spectrum of activity. Mutations in TIMP3 have been identified in familial cases of Sorsby’s fundus dystrophy, a disease characterized by excessive sub-retinal neovascularization and atrophy of retinal pigment epithelium [38]. Reduced TIMP3 gene expression and increased MMP activity have been observed in multiple different human tumors and are considered to be linked to an increased invasive, proteolytic and angiogenic phenotype in multiple human cancers [39, 40]. Overexpression of TIMP3 significantly suppressed growth of various tumor types in vitro and in vivo [41–43]. TIMP3 gene delivery by non replicating vectors has been associated with both autocrine and parcrine antitumor effects [44]. Based on these studies, TIMP3 expressing adenovirus and HSV-1 derived oncolytic viruses have been created and tested for therapeutic efficacy in mice (Table 1). Lamfers et al tested the antitumor efficacy of TIMP3 gene delivery in conjunction with replication competent adenoviruses [45]. A conditionally replication competent Ad expressing TIMP3 (AdΔ24TIMP-3) was created by inserting TIMP3 cDNA under the control of a CMV promoter into the E3 region of an adenovirus containing a 24 bp deletion in its E1A region. Despite reduction in MMP activity, in vivo treatment of intracranial gliomas with AdΔ24TIMP-3 did not result in increased anti-tumor efficacy [45]. The effect of TIMP3 gene delivery in the context of an oncolytic HSV-1 virus against human neuroblastoma tumors in vivo was tested by Mahller et al in 2008 [46]. In this study the anti-tumor efficacy of rQT3, an oncolytic HSV-1 expressing TIMP3 under the control of an immediate early viral promoter, was compared to a control virus expressing luciferase [46]. This construction facilitated early and robust expression of the therapeutic transgene, and reduced MMP activity in vitro. In vivo, rQT3 treated tumors had reduced microvessel density, increased intratumoral virus replication and reduced tumor growth [46]. While these studies investigated the effects of TIMP3 gene delivery in the context of different oncolytic virus backbones and tumor types, the opposing results in these two studies could be a reflection of higher levels of gene expression from an immediate early viral gene in HSV-1 relative to a CMV promoter used in the adenovirus construct [47]. Nevertheless, these studies underscore the importance of testing oncolytic viral gene therapy in multiple different viral backbones and tumor types. It should be noted that while the use of TIMP3 gene therapy to inhibit MMP activity is being exploited for its antiangiogenic and anti-invasive effects, treatment of tumors with proteases such as MMP has also been shown to enhance virus dissemination through the tumors. The use of proteases to modulate tumor extracellular matrix to enhance virus mobilization is discussed later in this review (see section on proteases and glycosidases).
Antiangiogenic peptides (Angiostatin and Endostatin)
Angiostatin is a small peptide fragment containing the first 3 or 4 Kringle domains of plasminogen. It is generated by sequential digestion of plasminogen by urokinase plasminogen activator (uPA), serine proteases and MMP to generate angiostatin (reviewed in [48]). The antiangiogenic effects of angiostatin were first identified to be responsible for the suppression of neovascularization and growth of metastasis in a Lewis lung carcinoma model in mice [49]. Angiostatin binds and antagonizes endothelial cell the surface receptors integrin αvβ3, ATP synthase, and angiomotin and interferes with pro-angiogenic signaling through these receptors [50–52]. Consistent with these observations, recombinant angiostatin delivered to established intracranial gliomas was shown to suppress tumor growth and angiogenesis in athymic nude mice bearing human U87 glioma [53]. Endostatin is another small peptide with potent antiangiogenic effects. It is a 20kDa fragment generated by proteolytic processing of collagen type XVIII [54]. Endostatin mediates its antiangiogenic effects by binding with heparin sulfate proteoglycans (HSPG), glypican or integrins α5β1 on endothelial cells (reviewed in [48]). Antiangiogenic and anti-tumorigenic effects of endostatin have been reported in multiple tumor models in vivo [55, 56]. The observed antiangiogenic effects of endostatin and angiostatin led to the concept of enhancing oncolytic viral therapy efficacy with angiostatin/endostatin gene delivery (Table 1).
To test the utility of expressing these angiogenic proteins by OVs, a replication conditional HSV-1 virus was created in which the murine endostatin gene (HSV-endo) was inserted into the HSV-1 genome [57]. Cells infected with HSV-Endo secreted biologically active endostatin, which did not interfere with viral replication. Treatment of subcutaneous tumors in mice with HSV-endo revealed efficient tumor destruction accompanied by reduced vessel density [57]. Later, Yang et al created an oncolytic HSV (AE618) within the doubly attenuated G207 background expressing a recombinant endostatin-angiostatin fusion protein [58]. AE618 significantly suppressed the growth of human endothelial cells in vitro and also suppressed growth of xenograft tumors in vivo [58].
The effect of antiangiogenic gene delivery in the context of a CRAd has been tested by several groups using different strategies. Concurrent delivery of oncolytic E1B55-kDa deleted virus expressing human TRAIL with a similar construct expressing Kringle 5 domains of plasminogen showed enhanced tumor killing ability in vivo [59]. An E1B55-kDa deleted adenovirus expressing the mouse endostatin gene (CNHK200-mE) showed higher endostatin gene expression compared to a replication defective version and exhibited potent antitumor efficacy against mouse xenografts in vivo [60]. The same group has recently shown that a human telomerase and hypoxia driven CRAd armed with the mouse endostatin gene (CNHK500-mE) [61] had efficient production of endostatin and increased efficacy against subcutaneous xenografts in mice [61]. Collectively these studies indicate the potential of combining oncolytic viral therapy with endostatin/angiostatin gene delivery.
Platelet factor-4 (Chemokine, CXC Motif, Ligand 4; CXCL4)
Platelet Factor 4 is a 70 amino acid secreted protein released from the alpha-granules of activated platelets and is known to promote antiangiogenic and antitumorigenic effects (reviewed in [62]). PF-4 binds to HSPG and CXCR3 on endothelial cells to mediate its antiangiogenic effects [63]. Apart from direct binding to these receptors, PF-4 can also directly bind to VEGF and bFGF, sequestering them from binding to their receptors on endothelial cells and thus antagonizing their proangiogenic effects (reviewed in [48]). Overexpression of PF-4 has been shown to suppress angiogenesis and tumor growth in mice [64]. Liu et al inserted the gene encoding for PF-4 into the oncolytic HSV-1 G47Δ viral backbone to create bG47Δ-PF4, an oncolytic HSV expressing PF-4. Treatment of human U87 glioma and mouse malignant peripheral nerve sheath tumors (MPNSTs) with bG47Δ-PF4 revealed a significant increase in anti-tumor efficacy compared to control bG47Δ-treated tumors (Table 1) [65].
Fibroblast growth factor (FGF)
Fibroblast Growth Factor (FGF) is comprised of a family of secreted growth factors that bind to four different receptor tyrosine kinases (FGFR1-4). Binding of FGF to its receptors leads to receptor dimerization and autophosphorylation, and initiates a cascade of signal transduction events which regulate diverse processes such as apoptosis, proliferation, migration, and angiogenesis (reviewed in [66]). Normally, FGFR signaling is highly regulated; dysregulation of this pathway via both overexpression and activation mutations has been noted in multiple cancers including breast, prostate, thyroid, melanoma and MPNSTs [67–69]. Blockade of FGF signaling in tumors as an antiangiogenic and anti-cancer therapeutic approach has shown promise in several animal models [70, 71]. The strategy of arming oncolytic HSV with a dominant negative FGFR (dn FGFR) was tested in MPNSTs grown as xenografts in mice (Table 1) [72]. The virus showed enhanced antiangiogenic and anti-tumor efficacy against two different tumor models [72]. These studies indicated that dn FGFR is an excellent therapeutic transgene to “arm” oncolytic viruses for future clinical translational studies.
Interleukin 8
Interleukin 8 (IL-8) is a proinflammatory cytokine that functions as a potent angiogenic factor by stimulating VEGF expression and increased autocrine activation of VEGF receptor [73]. Apart from its increased angiogenesis, it activates a signaling cascade that regulates diverse cell functions including proliferation, migration and invasion [74]. A positive correlation between IL-8 expression and aggressive tumor growth and metastasis has been noted in various studies [74]. IL-8 expression has been found in multiple tumor types including melanoma, lung, prostate, gastric, ovarian, and bladder. Blockade of IL-8 signaling by humanized anti-IL-8 monoclonal antibody has shown potential as an anticancer agent [75].
The potential of targeting oncolytic viral therapy in conjunction with blockade of IL-8 signaling was tested by Yoo et al with a CRAd expressing a short hairpin targeting IL-8 gene expression (Table 1). The CRAd reduced secretion of IL-8 in infected cells and exerted significant anti-tumor and anti-angiogenic effects against tumors grown in mice [76]. This study underscores the potential of using shRNA to reduce intratumoral concentrations of tumorigenic cytokines as a therapeutic strategy.
IMMUNOMODULATORY CHEMOKINE/CYTOKINE AUGMENTED ONCOLYTIC THERAPY
Chemokines and cytokines secreted in the tumor microenvironment can both support and control tumor growth. While some cytokines can regulate the antitumor immune response and exert a potent anti-tumor immune response, other cytokines can also modulate the response of tumor stroma and affect tumor angiogenesis [77]. Given the pleiotropic effects of cytokines on tumor biology, several strategies aimed at delivering or interfering with cytokine expression via oncolytic viral therapy have been utilized to enhance anti-tumor efficacy. In this section we discuss some of the approaches used to modulate cytokine gene expression in conjunction with oncolytic viral therapy.
Interleukin-12
Interleukin-12 (IL-12) is a secreted hetero-dimeric chemokine composed of IL-12α and IL-12β proteins, and is a very promising antitumor agent. It leads to the activation of natural killer (NK) cells, cytotoxic T cells, as well as the development of TH-1 type immune responses, and is involved in initiating anti-immune responses to various pathogens [78]. Apart from its immunomodulatory role, it was found to inhibit bFGF-induced corneal neovascularization [79]. Suppression of tumor-associated angiogenesis was dependant on its ability to induce IFN-γ expression and the presence of intact IFN-γ signaling receptors in neoplastic cells [79, 80]. IL-12 treatment has also been shown to reduce the production of VEGF, MMPs, and lead to NK cell mediated cytolytic destruction of endothelium [81, 82]. Consistent with its antiangiogenic effect, increased IL-12 expression has been associated with antitumor effects in several preclinical models of cancer.
Based on the potent antiangiogenic and antitumorigenic effects of IL-12, its expression in conjunction with oncolytic viruses has been tested in several preclinical models (Tables 2 and 4). A conditionally replication competent γ34.5-deleted HSV-1 expressing IL-12α and IL-12β under the control of the murine early growth response promoter (HSV M002) was created to test therapeutic efficacy of this strategy. Treatment of mice bearing intracerebral neuroblastoma cells with HSV M002 led to a significant increase in the observed anti-tumor effect. Virus infection was accompanied by a pronounced influx of CD4+ and CD8+ T cells and macrophages, indicating the activation of antitumor immune responses by IL-12 expression [83]. Consistent with these results, Wong et al studied the effect of combining IL-12 gene therapy within the NV1023 backbone of an oncolytic HSV. NV1042 is based on the NV1023 and harbors a deletion of viral α47 gene with an insertion of murine IL-12α and IL-12β genes under the governance of the viral α4TK promoter [84]. NV1042 treated murine squamous cell carcinoma tumors showed a striking reduction in tumor volume accompanied by a reduction in tumor take upon rechallenge [84]. The antitumor effects of this virus were subsequently also observed in several different preclinical model of cancer [85, 86]. Similarly, Varghese et al reported increased therapeutic efficacy of NV1042 relative to multiple different oncolytic HSVs (G207, G47Δ, NV1023, NV1042) against poorly immunogenic mouse prostrate cancer tumors [87]. Apart from its immunomodulatory role, a significant reduction in angiogenesis was also observed in NV1042-treated tumors compared to NV1023-treated tumors, indicating that antiangiogenic effects of IL-12 contributed to the observed anti-tumor efficacy [88].
Table 2.
List of OV delivering single Cytokine gene therapy discussed in this review
| OV NAME | THERAPEUTIC GENE | VIRUS TYPE | OV MUTATION | CANCER MODEL TESTED (EFFICACY) | REF. |
|---|---|---|---|---|---|
| Interleukin 12 Targeting OV: | |||||
| HSVM002 | IL-12α/IL-12β | HSV-1 | ΔICP34.5 | Neuroblastoma | [83] |
| NV1042 | IL-12α/IL-12β | HSV-1 | 15Kb deletion of UL56-ICP4 promotor region. | Squamous cell carcinoma (+). Hepatic (+). Prostate (+) |
[84, 88] [85] [87] |
| rVSV-IL12 | IL-12α/IL-12β | VSV | na | Squamous cell carcinoma | [90] |
| vMyxIL-12 | IL-12α/IL-12β | Myxoma | na | Not tested | [89] |
| Ad-DHscIL12 | IL-12α/IL-12β | Ad | Hypoxia controlled E1A (deleted for 24 b.p), and E2F-1 controlled E4. E1A gene, and Δ6.7K/gp19K E3 genes. | Pancreatic cancer | [92] |
| YKL-IL12 | IL-12α/IL-12β | Ad | E1B55kDa deleted | Melanoma (+) | [91] |
| Granulocyte Macrophage Targeting OV: | |||||
| NV1034 | GM-CSF | HSV-1 | 15Kb deletion of UL56-ICP4 promotor region. | Squamous cell Carcinoma (−) Prostate (−) Liver (+) Colorectal and Hepatoma. |
[84] [87] [96] [95] [95] |
| JS1/ICP34.5−/47− | GM-CSF | HSV-1 | ICP34.5−/−, and ICP47− | Lymphoma (+)* | [97] |
| E2F/GM/ΔE3 | GM-CSF | Ad | E2F regulated E1A and ΔE3 | Non small cell lung carcinoma (+) | E2F/G M/ΔE3 |
| CG0070 | GM-CSF | Ad | E2F re000gulated E1A. | Bladder transitional cell carcinoma (+) | [102] |
| TOA2 | GM-CSF | Ad | E2F and hTERT driven E1A, and ΔE3 | Lung cancer (+) | [104] |
| JX-594 | GM-CSF | VV | ΔTK | Liver cancer (+)** | [109] |
| MV GM-CSF | GM-CSF | MV | na | Lymphoid tumors (+) | [111] |
| Interleukin 24 Targeting OV: | |||||
| ZD55-IL-24 | IL-24 | Ad | E1B55kDa deleted | Colorectal carcinoma (+) Leukemia |
[112] [114] |
| Ad.PEG-E1A- mda-7 | IL-24 | Ad | PEG driven E1A | Prostate cancer (+) | [115] |
| AdCN103 | IL-24 | Ad | hTERT driven E1A, and Δ E3 | Hepatocellular carcinoma (+) | [116] |
| Ad.sp- E1A(Delta24)- IL-24 | IL-24 | Ad | Survivin driven E1A (with 24 bp deletion), and ΔE3 | Lung Cancer (+) | [117] |
| CRAdRGDflt- IL24 | IL-24 | Ad | Flt-1 driven E1A RGP peptide inserted knob domain. | Glioma(+) | [118] |
| B-Lymphocyte Activation Antigen B7-1: | |||||
| vHsv-B7.1-Ig | B7-1 | HSV-1 | ΔICP34.5−/−, ΔICP6 | Neuroblastoma (−) | [122] |
| G47ΔB7-1-Ig | Il-18 and B7-1 | HSV-1 | ΔICP34.5−/−, ΔICP6, and ICP47 | Prostate cancer (−) | [123] |
| Interleukin 4 Targeting OV: | |||||
| R8306 | IL-4 | HSV-1 | ΔICP34.5 | Glioma (+) | [127] |
| rHSVQ1-mIL4 | IL-4 | HSV-1 | ΔICP34.5, and ΔICP6 | Glioma (+) | [47] |
| HYPR-Ad-IL4 | IL-4 | Ad | Hypoxia regulated E1A, and ΔE3 | Glioma (+) | [128] |
| VSV-IL-4 | IL-4 | VSV | na | Breast and Melanoma | [129] |
| Interferon targeting OV: | |||||
| KD3-IFN | IFNα | Ad | Overexpression of ADP, and mutant E1A | Hepatocellular carcinoma | [135] |
| ZD-55- IFNβ | IFNβ | Ad | E1B55kDa deleted | Cervical Cancer model (+) | [137] |
| JX-795 | IFNβ | VV | ΔTK, ΔB18R | Murine tumor cell lines | [132] |
| CNHK300- mIFNγ | IFNγ | Ad | hTERT driven E1A | Liver Cancer models | [138] |
Abbreviations: IL-12: Interleukin 12; HSV-1: Herpes simplex virus 1; VSV: Vesicular Stomatitis virus; Ad: Adenovirus; MV: Measles virus; IL-24; Interleukin 24; B7-1: B-Lymphocyte Activation Antigen; IL-18: Interleukin 18; IL-4: Interleukin 4; IFN: Interferon; VV: Vacinia virus; MV: Measles virus;
Is currently being evaluated in human patients for efficacy [98].
Is currently being evaluated in human patients for efficacy [110]. IL-12: Interleukin 12; GM-CSF: Granulocyte macrophage stimulating factor.
Table 4.
List of doubly armed OV discussed in this review
| OV NAME | THERAPEUTIC GENE | VIRUS TYPE | OV MUTATION | CANCER MODEL TESTED (EFFICACY) | REF. |
|---|---|---|---|---|---|
| YKL-IL12/B7 | IL-12, and B7-1 | Ad | E1B55kDa deleted | Melanoma (+) | [91] |
| YKL-GB | GM-CSF and B7-1 | Ad | E1B55kDa deleted | Murine melanoma | [103] |
| G47ΔIL-18/B7 | Il-18 and B7-1 | HSV-1 | ΔICP34.5−/−, ΔICP6, and ICP47 | Prostate cancer (+) | [123] |
| OV-RLX-5T35H | Relaxin and GMCSF | Ad | E2F regulated E1A, and Ad5 shafy and Ad 35 knob | Prostate Cancer (+) | OV-RLX-5T35H |
Myxoma virus is a pox virus that exhibits preferential infection and killing of cancer cells. Stanford et al created a Myxoma virus expressing IL-12 and showed it produce IL-12 in infected cells without interfering with viral replication or showing signs of increased pathology [89]. Future studies will highlight the antitumor efficacy of this recombinant virus in vivo.
In addition to DNA viruses, IL-12 gene expression mediated by oncolytic Vesicular Stomatitis Virus (rVSV-IL12), a tumor-specific replicating negative strand RNA virus, also showed increased anti-tumor efficacy against a squamous cell carcinoma model in mice [90].
The antitumor effects of IL-12 in conjunction with CRAd were tested by the creation of an E1B-55kDa deleted virus expressing IL-12 (YKL-IL12) [91]. This virus was able to significantly suppress the growth of murine B16 melanoma relative to control YKL-1-treated tumors [91]. More recently, the immuno-stimulatory properties of IL-12 were exploited by incorporating its gene into a hypoxia-dependant CRAd [92]. The antitumor efficacy of this CRAd was tested in a novel permissive model of pancreatic cancer developed in Syrian hamsters. A single intratumoral injection of the virus expressing IL-12 (Ad-DHscIL12) achieved H2T-specific immune responses and had significant antitumor effects compared to animals treated with a control CRAd expressing luciferase [92]. Based on these results, a CRAd expressing IL-12 and B-lymphocyte activation antigen, B7-1, was created and tested for efficacy. This virus as discussed below showed significant improvement in antitumor efficacy [91].
Granulocyte macrophage colony stimulating Factor
Granulocyte macrophage stimulating factor (GM-CSF) is a cytokine that engenders protective immunity primarily by stimulating the recruitment, maturation and function of dendritic cells [93]. These antigen-presenting cells phagocytose tumor cell debris, and then prime CD4+ and CD8+ T cells, NK cells, and antibody producing B cells. The activated effector T cells manifest tumor-specific cytotoxicity and produce a broad range of cytokines resulting in the recruitment of granulocytes, eosinophils, and macrophages, which together elicit a potent anti-tumor immune response (reviewed in [94]). Due to its powerful ability to activate systemic rejection of cancer cells, GM-CSF has been one of the most well studied cytokines for immunotherapy, and several oncolytic viruses expressing GM-CSF have been created and tested for efficacy (Tables 2 and 4). Wong et al describe the creation and testing of the first oncolytic virus expressing GM-CSF was created in 2001 [84]. The conditionally replication-competent HSV vector NV1034 was created by inserting the gene encoding GM-CSF within the attenuated NV1023 backbone. This virus produced murine GM-CSF in infected cells, but did not improve anti-tumor efficacy against squamous cell carcinoma and two different mouse prostate cancer models in mice [84, 87]. The relatively low level of cytokine production by NV1034 may contribute to the observed absence of improved efficacy over the control. In contrast to these reports, two subsequent studies showed significant efficacy of NV1034 relative to NV1023 in two different mouse models [95, 96]. The improved efficacy was found to depend on the presence of CD4+ and CD8+ T lymphocytes in mice [95]. In a subsequent study, GM-CSF was incorporated in the context of an ICP34.5-deleted and ICP47-deleted oncolytic HSV-1 derived from strain JS1 [97]. This strain showed enhanced tumor cell killing compared to other HSV-1 strains, and deletion of ICP47 enhanced oncolytic potential of an ICP34.5-deleted mutant, due to the earlier expression of the Us11 gene product during the virus life-cycle, which compensates in part for the lack of ICP34.5. As predicted from its role in suppressing class I antigen processing in Golgi, deletion of ICP47 also improved immuno-stimulating properties of the virus [97]. GM-CSF expressed in the context of this virus showed significant tumor regression, compared to mice treated with control JS1/ICP34.5-/47- virus. This increased anti-tumor efficacy correlated with an increased anti-tumor immune response and also subsequent rejection of tumor cells in surviving mice [97]. This virus, renamed OncoVEXGM-CSF, was evaluated in a Phase I trial for safety in patients with cutaneous or subcutaneous tumors of breast, head and neck, gastrointestinal cancers, and malignant melanoma who had previously failed therapy [98]. OncoVEXGM-CSF was well tolerated and is currently being evaluated in a large, multinational, randomized phase III trial for efficacy in melanoma patients [99].
Murine GM-CSF expressed in the context of a CRAd (E2f-GM: an E3 deleted CRAd, with E1a under the control of E2F promoter) also showed potent antitumor responses in xenograft tumor models, which could be attributed to both oncolytic activity and GM-CSF induced inflammation and innate immunity [100]. The investigators later created another CRAd in which the E3 gene region was maintained [101]. This virus also showed increased anti-tumor efficacy against human hepatocellular carcinoma and retained replication potential and murine GM-CSF expression in cells expressing high levels of E2F [101]. Subsequently, a nearly identical CRAd expressing the human GM-CSF (CG0070) was shown to be efficacious against bladder transitional cell carcinoma in mice [102]. Expression of GM-CSF and B7-1 within an E1B-55kDa deleted CRAd (YKL-GB) showed enhanced antitumor activity compared to control CRAd in immune competent mice bearing murine melanoma B16-F10 tumors [103]. Recently, the GM-CSF gene was incorporated within the genome of a telomerase-driven CRAd to created TOA2 [104]. This virus showed strong tumor cell selectivity and efficient anti-tumor efficacy in mouse models of human head/neck and hepatocellular cancer, underscoring the therapeutic benefit of this approach [104].
While oncolytic HSV and adenovirus show efficient anti-tumor efficacy following intratumoral injection, their rapid neutralization in serum limits their usage for eradication of systemic disease [105, 106]. In contrast, vaccinia virus (VV) has evolved for bloodstream dissemination and systemic delivery of oncolytic VV to tumors has been described [107, 108]. JX-594, an oncolytic VV expressing GM-CSF that is deleted for viral thymidine kinase and expresses human GM-CSF was engineered. The anti-tumor efficacy of JX-594 was tested in two different immune competent tumor models: a rabbit liver tumor model with reproducible lung metastasis, and a carcinogen induced rat liver cancer model [109]. Significant anti-tumor efficacy was observed against both of these tumor models with concomitant induction of tumor specific CTLs [109]. This virus was evaluated in a Phase I clinical trial for safety in human patients with hepatocellular carcinoma [110]. Ongoing clinical trials will unveil its efficacy in patients.
Negative strand RNA viruses are also being evaluated as oncolytic agents for cancer therapy. Attenuated replication competent measles virus (MV) mutants have shown efficacy in multiple tumor models. Grote et al created a MV expressing GM-CSF (MV GM-CSF) and evaluated tumor regression in mice engineered to express CD46 the MV receptor [111]. MV GM-CSF had enhanced anti-tumor efficacy, which correlated with increased neutrophil accumulation in tumors, indicating the development of an anti-tumor immune response [111].
Interleukin-24
Melanoma differentiation associated gene-7/Interleukin-24 (MDA-7/IL-24) is a member of the Il-10 family of genes, and has been shown to selectively induce apoptosis in a wide variety of malignant cells and to spare normal cells. The mechanisms of IL-24-mediated tumor cell killing are manifold and vary according to tumor type. Apart from direct tumor cell killing, Il-24 has also been shown to inhibit tumor angiogenesis, metastasis and invasion. Thus, IL-24 may present as an ideal candidate transgene for gene therapy, and has been used to enhance antitumor efficacy of several oncolytic viruses (Table 2). The first oncolytic vector incorporating IL-24 gene expression was made in an E1B55kDa deleted CRAd backbone [112]. The resulting recombinant CRAd (ZD-55-IL-24) expressed IL-24 in infected cells and, despite the induction of apoptosis, did not interfere with viral replication. Treatment of established tumors showed potent antitumor efficacy alone and in combination with a CRAd expressing apoptosis-inducing TRAIL compared to control CRAds [112–114]. Incorporation of the IL-24 gene in transcriptionally targeted adenoviruses wherein E1A is driven by a cancer-specific promoter (elevated gene-3, hTERT, or survivin) also revealed potent antiangiogenic and antitumor effects at not only the injected tumor but also distant tumors established in the contralateral flank of mice [115–117]. Recently, a double armed strategy incorporating IL-24 within a CRAd driven by the human VEGFR-1/Flt-1 promoter, in conjunction with an RGD peptide-modified fiber to enhance its infectivity, exhibited enhanced anti-tumor efficacy against intracranial gliomas in mice [118].
B-Lymphocyte Activation Antigen B7-1
Efficient anti-tumor immunity requires the recognition and binding of T cells to processed peptide antigens complexed with molecules of the major histocompatibility complex (MHC), and a second costimulatory signal provided by the interaction of the CD28 molecule on the T cell surface with its ligand, B-lymphocyte activation antigen (B7-1), on the surface of an antigen-presenting cell (APC) [119]. B7-1 binding provides regulatory signals leading to T cell activation, resulting in the induction of tumor specific cytotoxic T lymphocyte (CTL) responses. Exogenous expression of the costimulatory ligand B7-1 on melanoma cells led to the rejection of murine melanoma tumors, indicating that B7 expression renders tumor cells capable of effective antigen presentation, leading to their eradication in vivo [120]. Because activation of T cells by B7-1 co-stimulatory molecule depends on the ability of B7-1 to cross link T cell surface receptors (CD28 and CTLA-4), Todo et al hypothesized that a secreted dimeric soluble B7-1 molecule would facilitate efficient activation of a tumor specific CTL response. They designed a soluble B7-1 wherein two molecules of B7-1 extracellular domains were linked together by the Fc portion of IgG1. The antineoplastic efficacy of this approach was demonstrated in immune competent mice bearing murine neuroblastoma. In this study, co-delivery of a replication-deficient amplicon with oncolytic HSV-1 virus (G207) resulted in increased antitumor efficacy that was dependant on the presence of intact CD8+ T cells [121]. Based on these results, several soluble B7-1 expressing oncolytic viruses derived from HSV-1 or adenoviruses have been created and tested for efficacy (Tables 2 and 4). Based on these results, Dr. Todo (University of Tokyo) created an oncolytic HSV-1 virus armed with soluble B7-1 gene expression and tested it for anti-tumor efficacy in mice bearing subcutaneous neuroblastoma tumors. Expression of B7-1 alone in an oncolytic HSV-1 did not demonstrate a significant increase in therapeutic efficacy relative to tumors treated with parent oncolytic HSV [122]. However, combination of three viruses armed with soluble B7-1, IL-18, or IL-12 resulted in increased anti-tumor efficacy [122]. These results indicated that oncolytic viruses armed with more than one immuno-stimulatory gene would have increased therapeutic efficacy. Dr. Todo subsequently created an oncolytic HSV-1 armed with an expression cassette encoding soluble B7-1 and murine IL-18 genes connected by an internal ribosome entry site (IRES) in an HSV-1 backbone deleted for the γ34.5, and α47 genes and with a disrupted ICP6 viral genes [123]. Treatment of subcutaneous tumors in mice demonstrated enhanced antitumor efficacy of this doubly armed oncolytic virus compared to parent or singly armed oncolytic viruses [123]. Similarly, oncolytic adenoviruses doubly armed with genes encoding B7-1 and GM-CSF or IL-12 were engineered in an E1B 55kDa deleted virus [91, 103]. Therapeutic efficacy of YKL-GBAd (E1B deleted adenovirus expressing B7-1 and GM-CSF) was evaluated in immune competent mice bearing murine melanoma tumors. Significant inhibition of tumor growth was observed in mice treated with YKL-GB compared to control YKL-1 treated mice [103]. Therapeutic testing of YKL-IL12/B7 (E1B deleted adenovirus expressing B7-1 and IL-12) in murine melanomas revealed a higher incidence of complete tumor regression compared to the control YKL-1 virus [91]. Together these results underscore the significance of combining immuostimulatory genes with viral oncolysis to enhance antitumor efficacy in vivo.
Interleukin 4
Interleukin 4 (IL-4) is a secreted cytokine that binds to its type I (IL-4 R/γc) or its type II receptor (IL-4 R/IL-13Rα1) (reviewed in [124]). It plays a key role in the growth and maturation of T helper cells and assists in the development of a humoral response. Its increased levels have been associated with activation of anti-tumor immune response and subsequent cancer cell rejection by the induction of CD4+ T helper cells. Over expression of IL-4 by constitutive cloning or by viral vectors has been shown to suppress tumor growth in multiple different tumor models [125, 126]. The activation of in vivo localized anti-tumor immune response by Il-4 delivery has been tested in the context of multiple oncolytic viruses (Table 2). Andreansky et al created an oncolytic HSV expressing Il-4 under the control of Egr-1 promoter (R8306) in the backbone of an HSV deleted for both the copies of the γ34.5 gene and expressing IL-4 within the deleted γ34.5 gene locus [127], which resulted in efficient expression of IL-4 in infected cells. Treatment of intracranial gliomas in a syngeneic mouse model showed increased survival of mice [127]. Subsequently, Terada et al constructed an oncolytic HSV expressing murine IL-4 in the context of an oncolytic HSV deleted for both the copies of γ34.5, and with a disrupted viral ICP6 gene [47]. OV expressing IL-4 was able to slow down tumor growth of syngeneic primary and metastatic brain tumor models [47].
IL-4 gene expression within the backbone of a hypoxia-dependent replication-competent adenovirus was tested for antitumor efficacy against three different established models of glioma in mice [128]. Treatment of established tumors with such a CRAd resulted in efficient suppression of tumor growth, and increased leukocyte infiltration of tumors in mice [128]. Apart from DNA viruses expressing IL-4 in the context of Vesicular Stomatitis Virus, a negative strand RNA virus that preferentially replicates in neoplastic cells, also showed evidence of increased anti-tumor efficacy against mammary adenocarcinoma in mice [129].
Interferon (IFN)
Interferons constitute a large family of secreted cytokines divided into three classes: type I, II, and III. They are pleiotropic cytokines which have antiproliferative, cytotoxic, antiangiogenic, and immuno-modulatory functions. Recombinant IFNα isoforms are used clinically to treat various kinds of cancer [130]. Interestingly, they also initiate an extremely powerful antiviral response that can attenuate replication of most viruses [131]. The sensitivity of most viruses to IFN interferes with efficient viral replication in normal cells; however, most cancer cells are resistant to these antiviral effects. Thus, deletion of viral genes that can counter the effects of IFN imposes a tumor selective replication potential phenotype. Such a strategy has been used in the engineering of several viruses including VSV and HSV-1. While the pleiotropic antitumor effects of IFN are lucrative to harness, the accompanying antiviral effects have limited the enthusiasm for use in the context of OV. However a few clever approaches have used this strategy to create tumor-selective viruses, and also harness the antitumor effects of IFN signaling (Table 2).
For example, VV contains a gene (B18R) encoding a secreted protein that binds and neutralizes IFNβ [132]. The ability of this virus to neutralize secreted IFN renders it extremely insensitive to nature’s most powerful antiviral cytokine. Conversely, deletion of B18R viral gene renders the virus exquisitely sensitive to IFN signaling. Kirn et al evaluated the feasibility of using IFNβ expression in a B18r deleted VV backbone to enhance its safety and antitumor potency. Removal of the IFN binding protein B18R made the virus selective for replication in cancer cells that are unresponsive to IFN. This B18R-deleted VV backbone was then engineered to express secreted active IFNβ to increase its anticancer effects and also facilitate expression and secretion of the cytokine [132]. The recombinant VV (JX-795) showed complete tumor responses with immune-mediated protection against tumor formation upon subsequent challenge with cancer cells in vivo [132].
The natural ability of adenoviruses to evade IFN’s effects [131] has also been harnessed to deliver IFN cytokine gene therapy. For example, adenoviruses counteract IFNα antiviral effects by inhibiting IFN induction and also by a small viral RNA (VA-RNAI) that interferes with activation of the cellular PKR, which constitutes the major antiviral signaling pathway activated in infected cells [133, 134]. CRAds expressing IFNα were first generated in an Ad backbone that over-expressed adenovirus death protein (ADP) and also had a dl1101/1107 mutation in the E1A region that facilitated efficient and tumor selective replication of the virus [135]. This virus showed increased antineoplastic efficacy in athymic mice bearing human hepatocellular cancer xenografts and in immunocompetent Syrian hamsters bearing hamster kidney cancer tumors [135]. The efficacy was further enhanced when TRAIL was administered systemically to the animals [136]. ZD55-IFNβ was engineered by incorporating IFNβ gene within the backbone of an E1B-55-kDa deleted virus. Efficient production of IFNβ in cells infected with ZD-55-IFNβ did not enhance virus-mediated cancer cell killing in vitro and in vivo [137]. IFNγ-expressing CRAd (CNHK300-mIFNγ) was created in the backbone of a human TERT regulated CRAd. This virus induced regression of xenografts in liver cancer models in both immunodefficient and immunocompetent mice, by both direct cell killing, antiangiogenesis and activation of immune responses [138].
PROTEASES AND GLYCOSIDASES
One of the major barriers for effective drug delivery within the tumor parenchyma is the ubiquitous extracellular matrix (ECM) secreted by cancer cells. Despite the increased cellularity of most solid tumors, there is also an increased extracellular space (ECS) within the tumor. Diffusion studies have further revealed that the newly enlarged ECS is also filled with an ECM that is more complex than that found in the corresponding normal tissue [139]. This matrix forms a complex scaffold that modulates tumor cell proliferation, cell adhesion, and motility [140, 141]. While there are distinct similarities between the extracellular matrix of tumors and non neoplastic tissue, the ECM of most tumors also includes mesenchymal proteins that are absent in normal tissue [141] and render the matrix of these tumors very distinct from normal tissue [140, 142]. Increased expression and extracellular accumulation of tumor ECM also reduces the ECS and increases the internal pressure within the tumor. These changes lead to an increase in the fractional volume and tortuosity of the extracellular space, which can present as the major biophysical factors that limit passive molecular diffusion in the tumor tissue and can be limiting for spread of therapeutics [142]. This ECM-mediated impediment is corroborated by the long standing observations noting inadequate diffusion of therapeutic molecules through the tumor parenchyma [139]. Consistent with these diffusion studies, mathematical modeling of HSV-1 distribution in solid tumors also highlighted the role of tumor ECM in impeding viral spread [143]. Inefficient dispersal of OV through the solid tumor has also been previously noted and is considered to be one of the major limitations of this therapy [144]. Nevertheless, strategies to overcome this barrier have showed success: treatment with hyaluronidase improved intratumoral biodistribution and anticancer efficacy of therapeutic agents [145, 146]. Similarly, Kuriyama et al found that treatment of solid tumors with mixtures of collagenase/dispase or trypsin enhanced oncolytic adenoviral spread through the tumor, indicating that digestion of ECM could be a useful strategy to enhance OV spread [147].
Based on these observations several innovative strategies to arm oncolytic viruses with matrix modulating enzymes to enhance OV spread have been investigated in recent years (Tables 3 and 4). In this section we discuss some of oncolytic viruses armed with matrix-dissolving enzymes to enhance their dissemination throughout the solid tumor.
Table 3.
List of OV singly armed with matrix modulating enzymes discussed in this review
| OV NAME | THERAPEUTIC GENE | VIRUS TYPE | OV MUTATION | CANCER MODEL TESTED (EFFICACY) | REF. |
|---|---|---|---|---|---|
| Collagen Targeting OV: | |||||
| Ad-ΔE1B-RLX | RLX | Ad | ΔE1B-55kDa | Hepatocellular carcinoma, and lung metastasis (+) | [150] |
Abbreviations: RLX: Relaxin; Ad Adenovirus;
Collagen is a major constituent of tumor ECM for most tumors and its role in impeding spread of OV was investigated in a very elegant study from Dr. Jain’s laboratory at Harvard medical school in Boston [148]. In this study, they used second harmonic generation imaging of fibrillar collagen to visualize spread of labeled viral particles in subcutaneous tumors. In vivo imaging revealed that viral particles distributed primarily in collagen-free areas and had very limited penetration in collagen-rich areas. They further showed that treatment of tumors with collagenase improved viral distribution and gene expression [148], and that tumors over expressing matrix metalloproteinases had a better response to oncolytic viral therapy [149]. Given the significance of collagen in interfering with viral spread, Kim et al tested the efficacy of a CRAd expressing relaxin [150]. Relaxin is a small peptide hormone that can decrease the production of interstitial collagen and also induce the production of metalloproteinases and procollagenase [151]. A CRAd expressing relaxin showed greater viral persistence, spread and survival compared to a control CRAd [150]. In a subsequent study, expression of relaxin in the context of a fiber-modified CRAd also showed increased viral spread and anti-tumor efficacy in two highly metastatic tumor models in mice [152]. In a very elegant study performed in Dr. Hay’s laboratory at the New York School of Medicine, collagen-1 was found to impede virus spread in a transwell chamber assay [153]. Based on this result they then created a non-replicating adenovirus expressing MMP8, a metalloproteinase that can break down Collagen I. Intratumoral injection of AdMMP8 along with a replication-competent CRAd reduced tumor cell growth and reduced expression of collagen in virus infected areas. Collectively, these studies highlight the power of arming viruses with proteases to increase spread and anti-tumor efficacy.
CONCLUSIONS
The ability of cancer cell-specific replication provides oncolytic viruses the exclusive ability to dose-amplify themselves and a carrier gene from a single treatment. Thus the combination of virus-mediated cancer cell lysis with therapeutic transgene expression in a single entity has tremendous promise in cancer therapy. In this review we have discussed viruses that apart from tumor-specific replication potential can also deliver genes capable of modulating the tumor microenvironment. This two-pronged approach of treating cancer has resulted in a battery of “armed OV” with tremendous therapeutic potential. As these viruses are being examined for their oncolytic and anti-tumor properties, it is also extremely important to elucidate the unique effects that each engineered OV has on the tumor microenvironment and host immune responses. A more clear understanding of the benefits and pitfalls of each approach can then lead to the design of strategies to derive maximal benefit from each approach.
Acknowledgments
The authors would like to thank the Ohio State University Comprehensive Cancer Center and the Dardinger Center for Neuro-Oncology and Neurosciences funds. This work was supported by funding from: NINDS/NIH 1R01NS064607; NINDS/NIH 1K01NS059575; NINDS/NIH 1R21NS056203 (to BK); NCI/NIH R21CA133663 and NCI/NIH R01CA114004 (to TPC) and NIH/NCI P01CA089248, NIH/NIMH R21MH082421, NIH/NINDS R21NS063290, NIH/NINDS U01NS061811 (to EAC). This material is based upon work supported under a grant by the Alliance for Cancer Gene Therapy.
References
- 1.Haseley A, Christopher A, Chaudhary A, Kaur B. Advances in Oncolytic virus therapy for glioma. Recent Patents on CNS Drug Discovery. 2009;4:1–13. doi: 10.2174/157488909787002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 4.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chemical Reviews. 2009 doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Gao L, Yeagy B, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- 7.Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr Cancer Drug Targets. 2007;7:181–189. doi: 10.2174/156800907780058880. [DOI] [PubMed] [Google Scholar]
- 8.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- 10.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 11.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito Y, Sunamura M, Motoi F, et al. Oncolytic replication-competent adenovirus suppresses tumor angiogenesis through preserved E1A region. Cancer Gene Ther. 2006;13:242–252. doi: 10.1038/sj.cgt.7700902. [DOI] [PubMed] [Google Scholar]
- 13.Rao XM, Zheng X, Waigel S, Zacharias W, McMasters KM, Zhou HS. Gene expression profiles of normal human lung cells affected by adenoviral E1B. Virology. 2006;350:418–428. doi: 10.1016/j.virol.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Mahller YY, Vaikunth SS, Currier MA, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15:279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 15.Cinatl J, Jr, Michaelis M, Driever PH, et al. Multimutated herpes simplex virus g207 is a potent inhibitor of angiogenesis. Neoplasia. 2004;6:725–735. doi: 10.1593/neo.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benencia F, Courreges MC, Conejo-Garcia JR, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16:765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 17.Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res. 2007;67:440–444. doi: 10.1158/0008-5472.CAN-06-3145. [DOI] [PubMed] [Google Scholar]
- 18.Kurozumi K, Hardcastle J, Thakur R, et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16:1382–1391. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–26979. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhary A, Hiscott P, Hart CA, Kaye SB, Batterbury M, Grierson I. Suppression of thrombospondin 1 and 2 production by herpes simplex virus 1 infection in cultured keratocytes. Mol Vis. 2005;11:163–168. [PubMed] [Google Scholar]
- 22.Libertini S, Iacuzzo I, Perruolo G, et al. Bevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947. Clin Cancer Res. 2008;14:6505–6514. doi: 10.1158/1078-0432.CCR-08-0200. [DOI] [PubMed] [Google Scholar]
- 23.Kurozumi K, Hardcastle J, Thakur R, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 24.Liu TC, Castelo-Branco P, Rabkin SD, Martuza RL. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol Ther. 2008;16:1041–1047. doi: 10.1038/mt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17:51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 27.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 28.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CJ, Farnebo F, Yu EY, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Zou W, Wang J, et al. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Thorne SH, Tam BY, Kirn DH, Contag CH, Kuo CJ. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol Ther. 2006;13:938–946. doi: 10.1016/j.ymthe.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15:295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 34.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008;16:1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 35.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 36.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 37.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber BH, Vogt G, Pruett RC, Stohr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- 39.Kotzsch M, Farthmann J, Meye A, et al. Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression levels in breast cancer. Eur J Cancer. 2005;41:2760–2768. doi: 10.1016/j.ejca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T, Kubota T, Kabuto M, et al. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994;81:69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- 41.Spurbeck WW, Ng CY, Vanin EF, Davidoff AM. Retroviral vector-producer cell-mediated in vivo gene transfer of TIMP-3 restricts angiogenesis and neuroblastoma growth in mice. Cancer Gene Ther. 2003;10:161–167. doi: 10.1038/sj.cgt.7700577. [DOI] [PubMed] [Google Scholar]
- 42.Ahonen M, Ala-Aho R, Baker AH, et al. Antitumor activity and bystander effect of adenovirally delivered tissue inhibitor of metalloproteinases-3. Mol Ther. 2002;5:705–715. doi: 10.1006/mthe.2002.0606. [DOI] [PubMed] [Google Scholar]
- 43.Tran PL, Vigneron JP, Pericat D, et al. Gene therapy for hepatocellular carcinoma using non-viral vectors composed of bis guanidinium-tren-cholesterol and plasmids encoding the tissue inhibitors of metalloproteinases TIMP-2 and TIMP-3. Cancer Gene Ther. 2003;10:435–444. doi: 10.1038/sj.cgt.7700592. [DOI] [PubMed] [Google Scholar]
- 44.Finan KM, Hodge G, Reynolds AM, et al. In vitro susceptibility to the pro-apoptotic effects of TIMP-3 gene delivery translates to greater in vivo efficacy versus gene delivery for TIMPs-1 or -2. Lung Cancer. 2006;53:273–284. doi: 10.1016/j.lungcan.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Lamfers ML, Gianni D, Tung CH, et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005;65:9398–9405. doi: 10.1158/0008-5472.CAN-04-4264. [DOI] [PubMed] [Google Scholar]
- 46.Mahller YY, Vaikunth SS, Ripberger MC, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68:1170–1179. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13:705–714. doi: 10.1038/sj.gt.3302717. [DOI] [PubMed] [Google Scholar]
- 48.Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: nature’s antiangiogenic therapy. Neuro-oncol. 2005;7:106–121. doi: 10.1215/S115285170400119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 50.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J Biol Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- 51.Moser TL, Kenan DJ, Ashley TA, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joe YA, Hong YK, Chung DS, et al. Inhibition of human malignant glioma growth in vivo by human recombinant plasminogen kringles 1–3. Int J Cancer. 1999;82:694–699. doi: 10.1002/(sici)1097-0215(19990827)82:5<694::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 54.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 55.Joki T, Machluf M, Atala A, et al. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat Biotechnol. 2001;19:35–39. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- 56.Read TA, Sorensen DR, Mahesparan R, et al. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 57.Mullen JT, Donahue JM, Chandrasekhar S, et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer. 2004;101:869–877. doi: 10.1002/cncr.20434. [DOI] [PubMed] [Google Scholar]
- 58.Yang CT, Lin YC, Lin CL, et al. Oncolytic herpesvirus with secretable angiostatic proteins in the treatment of human lung cancer cells. Anticancer Res. 2005;25:2049–2054. [PubMed] [Google Scholar]
- 59.Liu XY, Qiu SB, Zou WG, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol Ther. 2005;11:531–541. doi: 10.1016/j.ymthe.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Li G, Sham J, Yang J, et al. Potent antitumor efficacy of an E1B 55kDa-deficient adenovirus carrying murine endostatin in hepatocellular carcinoma. Int J Cancer. 2005;113:640–648. doi: 10.1002/ijc.20581. [DOI] [PubMed] [Google Scholar]
- 61.Su C, Na M, Chen J, et al. Gene-viral cancer therapy using dual-regulated oncolytic adenovirus with antiangiogenesis gene for increased efficacy. Mol Cancer Res. 2008;6:568–575. doi: 10.1158/1541-7786.MCR-07-0073. [DOI] [PubMed] [Google Scholar]
- 62.Bikfalvi A. Recent developments in the inhibition of angiogenesis: examples from studies on platelet factor-4 and the VEGF/VEGFR system. Biochem Pharmacol. 2004;68:1017–1021. doi: 10.1016/j.bcp.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 65.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV Armed with Platelet Factor 4, an Antiangiogenic Agent, Shows Enhanced Efficacy. Mol Ther. 2006;14:789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005;16:179–186. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60:7163–7169. [PubMed] [Google Scholar]
- 69.Bello L, Giussani C, Carrabba G, Pluderi M, Costa F, Bikfalvi A. Angiogenesis and invasion in gliomas. Cancer Treat Res. 2004;117:263–284. doi: 10.1007/978-1-4419-8871-3_16. [DOI] [PubMed] [Google Scholar]
- 70.Auguste P, Gursel DB, Lemiere S, et al. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001;61:1717–1726. [PubMed] [Google Scholar]
- 71.Aoki T, Kato S, Fox JC, et al. Inhibition of autocrine fibroblast growth factor signaling by the adenovirus-mediated expression of an antisense transgene or a dominant negative receptor in human glioma cells in vitro. Int J Oncol. 2002;21:629–636. [PubMed] [Google Scholar]
- 72.Liu TC, Zhang T, Fukuhara H, et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res. 2006;12:6791–6799. doi: 10.1158/1078-0432.CCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 73.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 75.Mian BM, Dinney CP, Bermejo CE, et al. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9:3167–3175. [PubMed] [Google Scholar]
- 76.Yoo JY, Kim JH, Kim J, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15:635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 77.Lowenstein PR, Kroeger K, Castro MG. Immunology of neurological gene therapy: how T cells modulate viral vector-mediated therapeutic transgene expression through immunological synapses. Neurotherapeutics. 2007;4:715–724. doi: 10.1016/j.nurt.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 79.Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 80.Coughlin CM, Salhany KE, Gee MS, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 81.Cavallo F, Quaglino E, Cifaldi L, et al. Interleukin 12-activated lymphocytes influence tumor genetic programs. Cancer Res. 2001;61:3518–3523. [PubMed] [Google Scholar]
- 82.Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood. 1999;93:1612–1621. [PubMed] [Google Scholar]
- 83.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong RJ, Patel SG, Kim S, et al. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum Gene Ther. 2001;12:253–265. doi: 10.1089/10430340150218396. [DOI] [PubMed] [Google Scholar]
- 85.Jarnagin WR, Zager JS, Klimstra D, et al. Neoadjuvant treatment of hepatic malignancy: an oncolytic herpes simplex virus expressing IL-12 effectively treats the parent tumor and protects against recurrence-after resection. Cancer Gene Ther. 2003;10:215–223. doi: 10.1038/sj.cgt.7700558. [DOI] [PubMed] [Google Scholar]
- 86.Wong RJ, Chan MK, Yu Z, et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10:251–259. doi: 10.1158/1078-0432.CCR-0197-3. [DOI] [PubMed] [Google Scholar]
- 87.Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–265. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 88.Wong RJ, Chan MK, Yu Z, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10:4509–4516. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 89.Stanford MM, Barrett JW, Gilbert PA, Bankert R, McFadden G. Myxoma virus expressing human interleukin-12 does not induce myxomatosis in European rabbits. J Virol. 2007;81:12704–12708. doi: 10.1128/JVI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin EJ, Wanna GB, Choi B, et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope. 2007;117:210–214. doi: 10.1097/01.mlg.0000246194.66295.d8. [DOI] [PubMed] [Google Scholar]
- 91.Lee YS, Kim JH, Choi KJ, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res. 2006;12:5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 92.Bortolanza S, Bunuales M, Otano I, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther. 2009;17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 94.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–298. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 95.Malhotra S, Kim T, Zager J, et al. Use of an oncolytic virus secreting GM-CSF as combined oncolytic and immunotherapy for treatment of colorectal and hepatic adenocarcinomas. Surgery. 2007;141:520–529. doi: 10.1016/j.surg.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Derubertis BG, Stiles BM, Bhargava A, et al. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 2007;14:590–597. doi: 10.1038/sj.cgt.7701053. [DOI] [PubMed] [Google Scholar]
- 97.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 98.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 99.gov CT. In.
- 100.Bristol JA, Zhu M, Ji H, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7:755–764. doi: 10.1016/s1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 101.Zhu M, Bristol JA, Xie Y, et al. Linked tumor-selective virus replication and transgene expression from E3-containing oncolytic adenoviruses. J Virol. 2005;79:5455–5465. doi: 10.1128/JVI.79.9.5455-5465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12:305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 103.Choi KJ, Kim JH, Lee YS, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 104.Lei N, Shen FB, Chang JH, et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 105.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Law M, Hollinshead R, Smith GL. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J Gen Virol. 2002;83:209–222. doi: 10.1099/0022-1317-83-1-209. [DOI] [PubMed] [Google Scholar]
- 108.Vanderplasschen A, Mathew E, Hollinshead M, Sim RB, Smith GL. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci U S A. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 111.Grote D, Cattaneo R, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- 112.Zhao L, Gu J, Dong A, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845–858. doi: 10.1089/hum.2005.16.845. [DOI] [PubMed] [Google Scholar]
- 113.Zhao L, Dong A, Gu J, et al. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 114.Qian W, Liu J, Tong Y, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- 115.Sarkar D, Lebedeva IV, Su ZZ, et al. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- 116.Luo J, Xia Q, Zhang R, et al. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin Cancer Res. 2008;14:2450–2457. doi: 10.1158/1078-0432.CCR-07-4596. [DOI] [PubMed] [Google Scholar]
- 117.Zhang KJ, Wang YG, Cao X, et al. Potent Antitumor Effect of Interleukin-24 Gene in the Survivin Promoter and Retinoblastoma Double-Regulated Oncolytic Adenovirus. Hum Gene Ther. 2009 doi: 10.1089/hum.2008.205. [DOI] [PubMed] [Google Scholar]
- 118.Kaliberova LN, Krendelchtchikova V, Harmon DK, et al. CRAdRGDflt-IL24 virotherapy in combination with chemotherapy of experimental glioma. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 120.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 121.Todo T, Martuza RL, Dallman MJ, Rabkin SD. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 2001;61:153–161. [PubMed] [Google Scholar]
- 122.Ino Y, Saeki Y, Fukuhara H, Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res. 2006;12:643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 123.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 124.Gilmour J, Lavender P. Control of IL-4 expression in T helper 1 and 2 cells. Immunology. 2008;124:437–444. doi: 10.1111/j.1365-2567.2008.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 126.Giezeman-Smits KM, Okada H, Brissette-Storkus CS, et al. Cytokine gene therapy of gliomas: induction of reactive CD4+ T cells by interleukin-4-transfected 9L gliosarcoma is essential for protective immunity. Cancer Res. 2000;60:2449–2457. [PubMed] [Google Scholar]
- 127.Andreansky S, He B, van Cott J, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 128.Post DE, Sandberg EM, Kyle MM, et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67:6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 131.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 132.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anderson KP, Fennie EH. Adenovirus early region 1A modulation of interferon antiviral activity. J Virol. 1987;61:787–795. doi: 10.1128/jvi.61.3.787-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kitajewski J, Schneider RJ, Safer B, et al. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45:195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- 135.Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 136.Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He LF, Gu JF, Tang WH, et al. Significant antitumor activity of oncolytic adenovirus expressing human interferon-beta for hepatocellular carcinoma. J Gene Med. 2008;10:983–992. doi: 10.1002/jgm.1231. [DOI] [PubMed] [Google Scholar]
- 138.Su C, Peng L, Sham J, et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 139.Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol (Berl) 2005;110:435–442. doi: 10.1007/s00401-005-1078-5. [DOI] [PubMed] [Google Scholar]
- 140.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 141.Delpech B, Maingonnat C, Girard N, et al. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur J Cancer. 1993;29A:1012–1017. doi: 10.1016/s0959-8049(05)80214-x. [DOI] [PubMed] [Google Scholar]
- 142.Vargova L, Homola A, Zamecnik J, Tichy M, Benes V, Sykova E. Diffusion parameters of the extracellular space in human gliomas. Glia. 2003;42:77–88. doi: 10.1002/glia.10204. [DOI] [PubMed] [Google Scholar]
- 143.Mok W, Stylianopoulos T, Boucher Y, Jain RK. Mathematical modeling of herpes simplex virus distribution in solid tumors: implications for cancer gene therapy. Clin Cancer Res. 2009;15:2352–2360. doi: 10.1158/1078-0432.CCR-08-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Currier MA, Adams LC, Mahller YY, Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12:407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- 145.Haselsberger K, Radner H, Pendl G. Boron neutron capture therapy for glioblastoma: improvement of boron biodistribution by hyaluronidase. Cancer Lett. 1998;131:109–111. doi: 10.1016/s0304-3835(98)00206-7. [DOI] [PubMed] [Google Scholar]
- 146.Baumgartner G, Gomar-Hoss C, Sakr L, Ulsperger E, Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors--experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:85–99. [PubMed] [Google Scholar]
- 147.Kuriyama N, Kuriyama H, Julin CM, Lamborn K, Israel MA. Pretreatment with protease is a useful experimental strategy for enhancing adenovirus-mediated cancer gene therapy. Hum Gene Ther. 2000;11:2219–2230. doi: 10.1089/104303400750035744. [DOI] [PubMed] [Google Scholar]
- 148.McKee TD, Grandi P, Mok W, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 149.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67:10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 150.Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 151.Unemori EN, Pickford LB, Salles AL, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98:2739–2745. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ganesh S, Gonzalez Edick M, Idamakanti N, et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67:4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [DOI] [PubMed] [Google Scholar]
- 153.Cheng J, Sauthoff H, Huang Y, et al. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol Ther. 2007;15:1982–1990. doi: 10.1038/sj.mt.6300264. [DOI] [PubMed] [Google Scholar]