Abstract
Here we show that plasma kallikrein (PKal) mediates a plasminogen (Plg) cascade in adipocyte differentiation. Ecotin, an inhibitor of serine proteases, inhibits cell-shape change, adipocyte-specific gene expression, and lipid accumulation during adipogenesis in culture. Deficiency of Plg, but not of urokinase or tissue-type plasminogen activator, suppresses adipogenesis during differentiation of 3T3-L1 cells and mammary-gland involution. PKal, which is inhibited by ecotin, is required for adipose conversion, Plg activation and 3T3-L1 differentiation. Human plasma lacking PKal does not support differentiation of 3T3-L1 cells. PKal is therefore a physiological regulator that acts in the Plg cascade during adipogenesis. We propose that the Plg cascade fosters adipocyte differentiation by degradation of the fibronectin-rich preadipocyte stromal matrix.
A dipogenesis is regulated by hormones such as leptin1 and by transcription factors such as peroxisome-proliferator-activated receptor-γ (PPARγ)2. However, it is not generally appreciated that, during adipocyte differentiation, the fibronectin-rich stromal matrix of preadipocytes is converted to the basement membrane of adipocytes3. Thus, although some of the intracellular events that occur during adipocyte differentiation are know understood4, the role of the extracellular matrix (ECM) is yet to be explored. Here we investigate the hypothesis that ECM-degrading proteases are required during adipogenesis. We focus on the Plg system of serine proteases, as plasmin directly cleaves various ECM molecules, including fibronectin5 and laminin6, and releases bound cytokines such as insulin-like growth factor I (IGF-I)7. Plg can be activated (converted to plasmin) by urokinase-type plasminogen activator (uPA) and by tissue-type plasminogen activator (tPA). Here we report the use of genetic and inhibitor-based approaches to evaluate the role of serine proteases during both adipogenic differentiation of preadipocytes in culture and repopulation of adipocytes during involution of the murine mammary gland in vivo. Our results demonstrate an important function of the Plg system of serine proteases during adipocyte differentiation.
Results
Inhibition of serine proteases reduces differentiation of 3T3-L1 cells
To identify the serine proteases in the plasminogen cascade that function during adipocyte differentiation, we used a new inhibitor-based approach to block their activity during differentiation of 3T3-L1 cells. Ecotin is a macromolecular inhibitor that inhibits a broad range of chymotrypsin-like serine proteases such as trypsin, chymotrypsin and elastase8. Wild-type (WT) ecotin is a poor inhibitor of uPA and plasmin; however, the ecotin mutant MM84,85RR (EcoRR) is a nanomolar inhibitor of uPA9. Addition of WT ecotin or EcoRR had no effect on preadipocytes, but reduced adipose conversion to <20% of control levels (Fig. 1a, b). Three lines of evidence indicate that the lack of lipid accumulation was a result of blockage of the differentiation pathway. First, most ecotin-treated cells failed to undergo morphologic differentiation (rounding up). Second, CCAAT/enhancer-binding protein-β (C/EBPβ; Fig. 1c) and PPARγ (Fig. 1d), both of which are required for adipogenic differentiation2,10, were only weakly expressed in ecotin-treated cells. Third, levels of glycerophosphate dehydrogenase (GPDH), an enzymatic marker for differentiated adipocytes11, were only ~20% of control levels in cells cultured with either form of ecotin (data not shown). These data support a functional role for serine proteases during differentiation of predipocytes in culture.
Figure 1. Inhibition of serine proteases during differentiation of 3T3-L1 cells reduces adipose conversion.
a , e–h, Staining of cells with Oil red O to visualize the extent of adipose conversion. b, i, Quantification of lipid accumulation; absorbance is designated as 1.0 for adipocytes. Data are means ± s.d. c, d, Western blotting of 3T3-L1 nuclear lysates to detect C/EBPβ (c) and PPARγ (d). Pre, 3T3-L1 preadipocytes; Adi, adipocytes, EcoWT, differentiating cells treated with WT ecotin; EcoRR, differentiating cells treated with EcoRR. Where indicated, adipocytes were treated with PAI-1 or α-2-antiplasmin (α2-AP).
To analyse further the functions of plasminogen and plasminogen activators, we treated 3T3-L1 cells with the serpin proteins α-2-antiplasmin (α2-AP, which inhibits plasmin12) and plasminogen-activator inhibitor-1 (PAI-1, which inhibits uPA and tPA13), and monitored their differentiation. Our data support the hypothesis that plasmin is required during differentiation of 3T3-L1 preadipocytes in culture. Treatment of cells with α2-AP during differentiation inhibited adipose conversion and lipid accumulation (Fig. 1h, i). On the other hand, uPA and tPA do not seem to be required for adipogenesis, as treatment with PAI-1 had no effect on 3T3-L1 differentiation (Fig. 1g, i).
Adipogenic differentiation of 3T3-L1 cells is Plg-dependent
We analysed the expression of uPA and tPA in both preadipocytes and differentiated adipocytes. We detected uPA and tPA in both the conditioned medium and cell lysates of 3T3-L1 preadipocytes (Fig. 2a). Two other caseinolytic serine proteases, with relative molecular masses of 80,000 (Mr 80K) and 120K (termed sp80 and sp120, respectively), were present in the conditioned media of both 3T3-L1 preadipocytes and differentiated adipocytes; these proteases were inhibited by phenylmethylsulfonyl fluoride (PMSF). An activity similar to sp80 was also present in fetal bovine serum (FBS). Expression of uPA and tPA decreased with adipogenic differentiation. These data raise the question of how Plg functions during adipogenesis. Preadipocytes grew normally in 10% FBS that was depleted of Plg (Fig. 2b). However, once cells were induced to differentiate, they exhibited <10% adipose conversion relative to cells cultured in complete FBS (Fig. 2c, d). Addition of exogenous Plg to Plg-depleted FBS rescued differentiation (Fig. 2e). Preadipocytes differentiated into adipocytes in normal human plasma, albeit to a lesser extent than in FBS (see later). However, no adipose conversion or lipid accumulation was seen in cells that were induced to undergo differentiation in Plg-deficient human plasma. Addition of physiological levels of exogenous human Plg restored adipose conversion to levels seen in normal human plasma (data not shown). These results show that Plg is required during adipocyte differentiation, when expression of ECM proteins, including fibronectin, is downregulated, and indicate that plasmin may be required to remodel the stromal ECM of preadipocytes.
Figure 2. The Plg system is regulated and required during 3T3-L1 cell differentiation.
a–e, Staining with Oil red O to detect adipocytes. a, Casein/Plg zymograms. FBS, fetal bovine serum; CM, conditioned medium; Pre, 3T3-L1 preadipocytes; Adi, adipocytes. b, Preadipocytes grown in Plg-depleted serum. c, Cells differentiated in normal serum. d, Cells differentiated in Plg-depleted serum. e, Cells differentiated in Plg-depleted serum with exogenous Plg added. f, Quantification of adipose conversion; absorbance is designated as 1.0 for adipocytes. Data are means ± s.d.
Serine-protease inhibitors reduce adipogenesis during mammary-gland involution
We sought evidence that serine proteases function during adipogenesis in vivo. For this analysis we used the mammary gland, which has an adipose stroma. After lactation and weaning in the mouse, the mammary gland undergoes a programme of remodelling during involution to replace the secretory epithelial tissue involved in lactation with adipose tissue. Adipogenesis during involution occurs on a short timescale. Infiltration of adipocytes can be detected by day 2 of involution14, and both uPA and tPA are upregulated during this process15. To evaluate serine-protease function in vivo during mammary adipogenesis, we treated female CF1 mice with WT ecotin or EcoRR over days 1–4 of involution, when the mammary gland is normally repopulated with adipocytes. On day 5, the density of adipose tissue of involution was greatly reduced in mammary glands treated with either form of ecotin (Fig. 3a–c). These data indicate that serine proteases have an important role in adipogenesis in vivo. Concomitant with the reduction in adipogenesis, deposition of stromal ECM, as shown by staining for collagen, increased significantly in mice treated with WT ecotin or EcoRR (Fig. 3e, f). We also observed increased deposition of the stromal proteins type I collagen (Fig. 3g–i) and fibronectin (Fig. 3j–l) in animals treated with either from of ecotin. WT ecotin, which does not inhibit uPA or plasmin, was as effective as EcoRR in blocking adipogenesis, and neither WT ecotin nor EcoRR inhibited tPA (Fig. 4). Surprisingly, when we examined epithelial apoptosis and involution14, mice treated with EcoRR showed delayed epithelial involution, whereas those treated with WT ecotin did not (data not shown). These data indicate that neither uPA nor tPA is required for Plg activation during adipogenesis in vivo. Moreover, the lack of effect of WT ecotin on epithelial remodelling shows that differentiation of epithelia and adipocytes are not necessarily linked, but rather may be regulated independently.
Figure 3. Adipogenesis is reduced in female mice treated with ecotin during mammary-gland involution.
Animals were treated with carrier (control), WT ecotin (EcoWT) or EcoRR and examined after 5 days. a–c, Staining with Oil red O to detect adipocytes. d–f, Staining with Masson’s Trichrome to detect collagen fibrils (blue). g–i, Immunohistochemistry to detect type I collagen (blue). j–l, Immunohistochemistry to detect fibronectin (blue). Scale bar represents 100 μm.
Figure 4. Adipocyte differentiation is impaired during involution in Plg-deficient, but not uPA-deficient, mice.
a–d, Staining with Oil red O to detect adipocytes. e–h, Staining with Masson’s Trichrome to detect collagen fibrils (blue). Scale bar represents 100 μm.
Adipogenesis is impaired in Plg-deficient, but not uPA-deficient, mice
As Plg is required for 3T3-L1 adipogenesis, is it also critical in vivo? Plg-deficient mice16 exhibited impaired adipogenesis (Fig. 4a, b) and increased collagen deposition in mammary glands (Fig. 4e, f), as was the case in mice treated with WT ecotin or EcoRR. This indicates that Plg is required during involution for normal adipocyte differentiation. Interestingly, healthy adult Plg−/− mice of the same skeletal size, as confirmed using X-rays, were leaner and lighter than wild-type controls (at 23 weeks of age, Plg−/−, 18.8 ± 2.1 g, n = 4; control, 22.8 ± 1.0 g, n = 5; P < 0.005). However, uPA did not seem to contribute significantly to adipocyte differentiation in the mammary gland. Mice lacking either uPA (Fig. 4c, d, g, h) or both uPA and tPA17 (data not shown) showed no significant alteration in adipogenesis or collagen accumulation during involution. These data show that neither uPA nor tPA is required for adipogenesis in the mammary gland and therefore indicate the the possible presence of an alternative plasminogen activator during adipogenesis.
Plasma kallikrein is required during adipogenesis
We sought to identify another serine protease that is inhibited by both WT ecotin and EcoRR and can activate Plg. Lysates of involuting mammary gland at day 5 from either wild-type or Plg−/− mice, assayed by zymography on casein–Plg gels, showed the presence of a serine protease of Mr 80K, which was similar to the sp80 protease that was detected in medium conditioned by 3T3-L1 cells (Fig. 5a). sp80 was also present in normal mouse and human plasma, in FBS, and in the conditioned medium of 3T3-L1 cells grown in the presence, but not absence, of FBS. These data indicate that sp80 may be derived from plasma or serum, rather than from adipogenic cells.
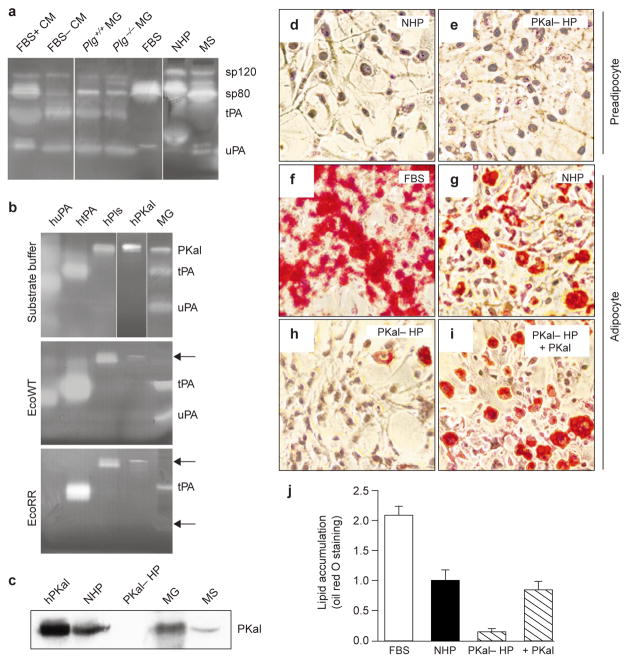
Figure 5. PKal is present during adipogenesis and is required for differentiation of 3T3-L1 cells.
a, b, Casein/Plg zymograms. a, Incubation in substrate buffer. FBS, fetal bovine serum; CM, conditioned medium; MG, mammary-gland lysate; NHP, normal human plasma; MS, mouse serum. b, Incubation in substrate buffer alone or in buffer containing WT ecotin (EcoWT) or EcoRR. Inhibited bands are indicated by arrowheads. Human enzymes are given the prefix ‘h’. Pls, plasmin. c, Western blotting to detect PKal. PKal– HS, prekallikrein-deficient human plasma. d, e, Staining with Oil red O of preadipocytes grown in normal (d) or prekallikrein-deficient (e) human plasma. f–i, Staining with Oil red O of adipocytes differentiated in FBS (f), normal human plasma (g), prekallikrein-deficient human plasma (h), or prekallikrein-deficient human plasma with exogenous human PKal added (i). j, Quantification of adipose conversion; absorbance is designated as 1.0 for cells grown in NHP. Data are means ± s.d.
Of the mammalian serine proteases that are present in databases, a compelling candidate is plasma kallikrein (PKal), which has a similar size and is inhibited by subnanomolar concentrations of WT ecotin18. Mouse sp80 and human PKal were inhibited both by WT ecotin and by EcoRR (Fig. 5b), whereas uPA was inhibited only by EcoRR. We identified sp80 as PKal by western blotting of mammary lysates and mouse serum with a polyclonal antibody against human prekallikrein (Fig. 5c). PKal has been shown to activate both pro-uPA19,20 and Plg21,22. To investigate the function of PKal during adipocyte differentiation, we took advantage of Fletcher trait, a rare form of human plasma prekallikrein deficiency23. Both normal and prekallikrein-deficient human plasma supported growth of 3T3-L1 preadipocytes (Fig. 5d, e). Preadipocytes differentiated into adipocytes in normal human plasma, albeit to a lesser extent than in FBS (Fig. 5f, g). However, very little adipose conversion and only 14% lipid accumulation was observed in cells that were induced to undergo differentiation in prekallikrein-deficient human plasma (Fig. 5h, j). Addition of physiological levels of exogenous human PKal restored adipose conversion to 85% of that seen in normal human plasma (Fig. 5i, j). These data indicate that PKal is required for adipogenesis.
PKal and Plg promote adipogenesis under serum-free differentiation conditions
We sought to determine whether proteolysis by serine proteases promotes differentiation of 3T3-L1 cells. To this end, we induced the differentiation of 3T3-L1 preadipocytes in the absence of FBS. Under these conditions, cells underwent adipose conversion to a much lesser extent than cells differentiated in the presence of FBS (Fig. 6a, b, g). Addition of exogenous Plg did not enhance the extent of adipose conversion (Fig. 6c, g). However, addition of both Plg and PKal promoted adipose conversion to about 50% of that observed in FBS (Fig. 6a, d, g). PKal alone did not significantly increase adipose conversion (data not shown). The PKal zymogen, prekallikrein, was ineffective in promoting adipogenesis in both the presence and the absence of Plg (data not shown), indicating that activation of prekallikrein may also be required. Interestingly, Factor XII, the principal activator of prekallikrein, is also inhibited by nanomolar concentrations of ecotin18. The increase in adipose conversion observed in the presence of Plg and PKal was not significantly affected by addition of PAI-1 (Fig. 6e, g), but was reduced in the presence of EcoRR (Fig. 6f, g). These data indicate that although 3T3-L1 preadipocytes express both uPA and tPA (Fig. 2a), PKal is required during adipogenesis to activate Plg and promote adipose conversion.
Figure 6. Plg and PKal promote adipose conversion in the absence of FBS.
a–f, Staining with Oil red O of cells differentiated in the presence of FBS (a). in the absence of FBS (b). in the absence of FBS with the addition of exogenous Plg (c), in the absence of FBS with the addition of exogenous Plg and PKal (d), in the absence of FBS with the addition of exogenous Plg, PKal and PAI-1 (e), or in the absence of FBS with the addition of exogenous Plg, PKal and ecotin RR (f). g, Quantification of adipose conversion; absorbance is designated as 1.0 for cells grown in the absence of FBS (b) Data are means ± s.d.
A PKal-mediated Plg cascade promotes fibronectin cleavage during adipocyte differentiation
To evaluate our hypothesis that PKal activates Plg during adipocyte differentiation, we analysed the extent of Plg activation by 3T3-L1 cells by western blotting of the plasmin protease domain in the presence and absence of PKal. We first confirmed that PKal activates Plg at physiologically relevant concentrations, albeit to a lesser extent than that observed with uPA or tPA (Fig. 7a). However, PKal is present in plasma at relatively high concentrations (30–50 μg ml−1; ref. 24) compared with uPA (3–5 ng ml−1) or tPA (5–10 ng ml−1; ref. 13). We next monitored activation of Plg during adipogenesis, and found that 3T3-L1 cells differentiated in the absence of FBS did not effectively activate Plg, and that addition of PKal significantly enhanced Plg activation (Fig. 7b). These data indicate that although 3T3-L1 cells express uPA and tPA (Fig. 2a), PKal may be required to activate Plg during 3T3-L1 differentiation.
Figure 7. PKal-mediated Plg activation promotes fibronectin degradation during adipocyte differentiation.
a, PKal activates Plg at physiologically relevant concentrations. Western blot showing generation of the Mr 25K protease domain (PD) of plasmin at 5, 10 and 15 min. b, Activation of Plg in the conditioned medium of 3T3-L1 cells is enhanced in the presence of PKal. Western blot of conditioned media from 3T3-L1 cells differentiated in the absence of FBS but with exogenous plasminogen (Plg), plasma kallikrein (PKal) or PAI-1, showing generation of plasmin (Pls) and the protease domain. c, Western blot (against human fibronectin) showing fibronectin (FN) cleavage products after incubation with serine proteases. d, Western blot (against rat fibronectin) of whole-cell lysates from preadipocytes (Pre), differentiated adipocytes (Adi) and cells differentiated in the presence of WT ecotin (EcoWT), showing that fibronectin is present in lysates from 3T3-L1 preadipocytes but is downregulated in adipocytes. e, Western blot (against endogenous mouse fibronectin) of conditioned media from preadipocytes, differentiated adipocytes, and cells differentiated in the presence of WT ecotin (EcoWT) and EcoRR, showing that fibronectin is cleaved during adipocyte differentiation. f, Western blot showing generation of the protease domain of plasmin at 5, 10 and 15 min in the absence (Control) and presence of fibrin and fibronectin. g–l, Staining with Oil red O of preadipocytes (g), of adipocytes (h) and of 3T3-L1 cells differentiated in the presence of fibronectin (i), fibronectin matrix and cytochalasin D (j), WT ecotin (k), or WT ecotin and cytochalasin D (l).
We next sought to identify the molecular target of the Plg cascade. As increased deposition of fibronectin was observed in the mammary glands of ecotin-treated mice (Fig. 3j–l), we tested the hypothesis that the Plg cascade mediates fibronectin cleavage during adipocyte differentiation. We assayed uPA, tPA, plasmin and PKal for their ability to cleave fibronectin and found that only plasmin cleaves fibronectin into several fragments, including one of Mr 20K (Fig. 7c). Fibronectin was associated with 3T3-L1 preadipocytes and was downregulated in differentiated adipocytes, but not in cells treated with ecotin during differentiation (Fig. 7d) or in the absence of Plg (data not shown). When we analysed conditioned media for cleavage of endogenous mouse fibronectin during differentiation, we detected a cleaved fragment of Mr 20K in the conditioned medium of differentiated adipocytes, but not in that of preadipocytes or of cells cultured during differentiation with WT ecotin or EcoRR (Fig. 7e). Moreover, mammary lysates also generated fibronectin cleavage products that were suppressed by ecotin treatment (data not shown). These findings indicate that the Plg cascade may promote adipocyte differentiation by degrading the fibronectin-rich stromal ECM of preadipocytes.
PKal-mediated activation of the Plg cascade may be selective for plasmin generation in the region of the stromal ECM. We found that PKal-mediated, but not uPA-mediated activation of Plg is enhanced in the presence of fibronectin (Fig. 7f). tPA-mediated activation of Plg was enhanced by fibrin, but not by fibronectin (data not shown). These data indicate that PKal may be an important activator of Plg in fibrin-independent processes.
In support of a mechanism in which fibronectin degradation is required during adipogenesis, we found that addition of an exogenous fibronectin matrix suppressed differentiation of 3T3-L1 cells (Fig. 7i). This suppression was overcome by addition of cytochalasin D, which disrupts the actin cytoskeleton and fibronectin at the cell surface (Fig. 7j). Similarly, the suppressive effects of ecotin were overcome by concomitant addition of cytochalasin D (Fig. 7k, l). These data indicate that remodelling of the stromal matrix of preadipocytes may be an important early step that promotes cell-shape change and expression of adipocyte-specific genes during differentiation.
Discussion
We have shown that extracellular proteolysis is a key mechanism for regulating adipocyte differentiation, and have demonstrated for the first time that PKal functions in the physiological Plg-activation cascade during adipogenesis. Although our in vivo study concentrated on the rapid and easily measurable adipogenesis in the mammary fat pad during mammary involution, our preliminary data clearly show the reduction of fat deposits at other sites, such as the epidydymal fat pad in male Plg−/− mice (data not shown). However, because adipogenesis does eventually occur in the mammary gland and at other sites in Plg−/− mice, further proteolytic pathways may also contribute to adipocyte differentiation. We propose that the mammary fat pad during involution constitutes a rapid and sensitive system for evaluating the factors that regulate adipogenesis.
Surprisingly, uPA and tPA have relatively minor roles in adipogenesis both in vivo and in culture. uPA has been considered to be the primary enzyme involved in cell-mediated Plg activation by virtue of its high affinity for Plg and of the presence of a specific receptor, uPAR. However, mice lacking either the gene for uPA or the genes for both uPA and tPA do not have a marked phenotype with regard to adipogenesis, indicating the possible presence of other Plg activators. Other enzymes that have been implicated as Plg activators include PKal21,22 and Factor XII25. PKal is a compelling candidate because of its high concentration, ubiquitous presence and ability to localize to the cell surface. It is also a potent activator of pro-uPA26,27. The requirement for PKal and Plg during adipogenesis indicates that PKal is a physiological component of the Plg-activation cascade. Although adipogenesis is attenuated in human plasma that lacks PKal or Plg, it remains to be determined whether this cascade has the same function in humans and mice as it does in culture. Our findings imply that PKal may have a significant role in other physiological and pathological processes that involve Plg activation in vivo.
It is clear from our results that proteolysis selectively affects terminally differentiating adipocytes rather than proliferating preadipocytes, as cleavage of cell-associated fibronectin, which is a substrate for plasmin, was regulated during adipocyte differentiation. How is the PKal–Plg system regulated during adipogenesis? During adipocyte differentiation, PKal may function as an activator of pro-uPA or as a direct activator of Plg. PKal-mediated activation of pro-uPA can occur on the surface of platelets26 and of endothelial cells27. Although expression of uPA is regulated during 3T3-L1 differentiation, uPA-deficient mice do not exhibit an adipogenic phenotype, which leads us to favour the hypothesis that PKal directly activates Plg during adipogenesis. Our data indicate that PKal can activate Plg at physiologically relevant concentrations.
This raises the question of what the nature of the process that is regulated during adipogenesis. The functions of the PKal–Plg cascade may be regulated during differentiation by endogenous cell-surface receptors or inhibitors. One possibility is that binding to the cell surface is regulated. uPAR may serve as an acquired receptor for PKal. uPAR binds to pro-uPA at a site within uPAR domain 1 (ref. 28). The cleaved, two-chain form of high-molecular-mass kininogen, which is a substrate for PKal and contains a high-affinity binding site for prekallikrein29, can also bind to uPAR through interactions within uPAR domains 2 and 3 (ref. 30). Thus, uPAR may function to promote PKal activity at the cell surface. However, uPAR is virtually undetectable in the involuting mammary gland15, indicating that this is not the principal mechanism at this site. PKal activation is known to occur through Factor XII (ref. 20), which in turn requires activation by a cell-surface-dependent mechanism that may itself be regulated. Another model involves PAI-1, a serpin that inhibits both uPA and tPA31, but not PKal (data not shown). Increased levels of PAI-1 are associated with obesity in mice and humans32,33, and this protein is expressed by mature adipocytes in culture and in vivo34. PAI-1 may also function as a regulator of adhesion to the ECM35. However, the precise function of PAI-1 in development of adipose tissue is unclear. PAI-1 is barely detectable in the mammary gland during involution with its associated adipogene-sis15 and, moreover, our findings indicate that PKal, which is not inhibited by PAI-1, may function to promote adipocyte differentiation despite the presence of high PAI-1 levels observed in obesity.
How does extracellular proteolysis of the preadipocyte microenvironment facilitate adipogenesis? Our data support a model in which the Plg cascade is required for remodelling of the fibronectin-rich ECM of preadipocytes. This remodelling then gives rise to alterations in cell–ECM adhesion and cytoarchitecture, and promotes transcription of adipocyte-specific genes. Our finding that C/EBPβ and PPARγ, both of which are crucial to adipocyte differentiation, are only weakly induced in ecotin-treated cells, indicates that serine proteases may function at critical steps in adipocyte differentiation. Fibronectin is a component of the preadipocyte ECM — addition of exogenous fibronectin during differentiation of preadipocytes in culture inhibits cell-shape change and prevents adipogenesis — and is a substrate of plasmin5 (Fig. 7). As differentiation of 3T3-L1 cells proceeds, synthesis of stromal ECM components such as fibronectin and fibrillar is reduced36,37 and cleavage of fibronectin increases (Fig. 7). Thus, Plg activation results in rapid removal of fibronectin from the microenvironment of committed cells.
How does fibronectin function to suppress adipogenesis? One possibility is that cytoskeletal organization and signalling downstream of adhesion receptors for fibronectin foster a fibroblastic phenotype, whereas receptors for basement-membrane proteins foster an adipogenic phenotype. This hypothesis, which remains untested, is supported by the observation that cytochalasin D, which disrupts adhesion38 and cytoskeletal structure, overcomes the suppressive effects of fibronectin and ecotin (Fig. 7j, l). A related, and also untested, hypothesis is that cleavage of fibronectin results in loss of syndecan, an integral membrane heparan sulphate proteoglycan that binds to fibronectin39. Loss of syndecan may downregulate Wnt signalling and thereby promote adipogenesis40,41.
Alternatively, the Plg system may activate and release differentiation-promoting growth factors from sequestration in the stromal ECM. The bio-availability of IGF-I, a physiologically relevant regulator of adipocyte differentiation42, is modulated by specific, high-affinity IGF-binding proteins (IGFBPs). Transgenic mice with increased IGFBP concentration exhibit impaired adipogenesis in vivo43. Plasmin and other serine proteases can cleave IGFBPs and release active IGF44,45. Thus, extracellular proteolysis is an important regulatory mechanism for the function of ECM molecules, as well as for cytokines, during adipogenesis. Whether the impact of proteolysis on fibronectin or IGFBPs is rate-limiting in vivo remains to be determined. Interestingly, matrix metalloproteinases also regulate adipogenesis both in vivo and in culture but, unlike the Plg system, their absence stimulates adipogenesis46, indicating that the two classes of enzymes have distinct targets.
Plasmin acts in many fibrin-independent physiological and pathological processes, including neuronal cell death, cancer progression, and apoptosis of epithelial cells during mammary-gland involution. In the mammary gland, lack of Plg promotes survival of differentiated secretory epithelium47, but inhibits adipocyte differentiation. Although there may be a reciprocal relationship between epithelial and mesenchymal cells to maintain tissue volume, it is also clear from ecotin studies that these processes are regulated by activation of the Plg cascade by distinct components. The identities of the plasminogen activators that regulate other Plg-dependent processes remain to be determined. Our results have opened further avenues of research by which to elucidate the physiological functions of PKal, its activators, its target plasmin and their substrates.
Methods
Preparation of ecotin
Ecotins were prepared and purified as described9. Purified samples were tested and found to be free of endotoxin. Samples used for animal injections were diluted in PBS, pH 7.4.
Differentiation of 3T3-L1 cells
3T3-L1 cells (American Type Culture Collection) were grown to confluence in DMEM containing 4.5 g l−1 glucose supplemented with 10% FBS (growth medium). Differentiation was induced by culturing cells in growth medium containing differentiation cocktail (0.22 μM insulin, 0.6 μM dexamethasone and 0.5 mM methylisobutylxanthine) as described48. Concurrently, 500 nM of WT ecotin, 500 nM EcoRR, 500 nM human PAI-1 (mutant human recombinant PAI-1; Calbiochem) or 2 μM human α2-AP (Calbiochem) was added to cells. Fresh growth medium was added to cells after 2 days; adipose conversion was analysed 6 days after induction.
To assess the effects of Plg depletion, FBS was passed over a lysine–Sepharose column several times and the flow-through fraction was collected49. Depletion of Plg from the flow-through fraction was confirmed by western blotting using a rabbit polyclonal antibody against human Plg (Dako, Carpinteria, California). 3T3-L1 cells were induced and maintained up to day 4 in medium containing 10% Plg-depleted FBS. For reconstitution experiments, 50 μg ml−1 human glu-type Plg (American Diagnostica Inc., Greenwich, Conneticut) was added to the medium. To assess the effects of Plg deficiency, 3T3-L1 cells were induced to differentiate without FBS in 5% pooled normal human plasma (George King Bio-Medical Inc., Overland Park, Kansas), Plg-deficient human plasma (American Diagnostica Inc.) or Plg-deficient human plasma reconstituted with 100 μg ml−1 human glu-type Plg for 2 days. Cells were maintained in fresh growth medium for a further 4 days.
To assess the effects of PKal deficiency, 3T3-L1 cells were induced to differentiate without FBS in 5% pooled normal human plasma, prekallikrein-deficient human plasma (George King Bio-Medical Inc.) or prekallikrein-deficient human plasma reconstituted with 50 μg ml−1 human PKal (Enzyme Research Laboratories, South Bend, Indiana) for 2 days. Cells were maintained in fresh growth medium for a further 4 days.
To assess the ability of serine proteases to promote differentiation under serum-free conditions, 3T3-L1 cells were induced to differentiate as described above but without FBS. Concurrently, 1 ∝M human glu-type Plg, 200 nM PKal, 500 nM PAI-1 and 500 nM EcoRR were added. Cells were maintained under these serum-free conditions until day 2 and were then switched to serum-containing growth medium for a further 4 days.
To assess the effects of fibronectin on 3T3-L1 differentiation, 6-well plates were coated with 50 μg ml−1 human fibronectin (Roche) at 4 °C for 12 h. Cells were grown and differentiated as described above. Cytochalasin D (0.5 μg ml−1; Sigma) was added together with the differentiation cocktail. After 2 days cells were switched to growth medium.
In vivo adipocyte differentiation
For ecotin treatment, female CF1 mice crossed with CD1 males were allowed to undergo normal pregnancy. The number of pups was normalized to 8 for each experiment and they were weaned after 7–10 days of lactation (day 0 of involution). Mice were injected intraperitoneally with 100 μg of WT ecotin, EcoRR or PBS twice a day on days 1–4 of involution and were killed on day 5 of involution. Each cohort contained four mice per treatment and experiments were repeated three times. Mammary glands from uPA-deficient C57BL/6 mice (n = 6), Plg-deficient C57BL/6 mice (n = 7), and wild-type littermate controls (n = 6 each) were collected on day 5 of involution. Plg−/− and wild-type littermates were weighed weekly at the ages of 4–25 weeks; weights were compared by ANOVA to calculate the P value. Mice lacking the genes for both uPA and tPA were generated as described17. To prepare frozen sections, tissue samples were snap-frozen in liquid nitrogen for biochemical analyses, placed in OCT and frozen; for paraffin sections they were fixed in 4% paraformaldehyde.
Oil red O staining, Masson Trichrome staining and immunohistochemistry
3T3-L1 cells were stained with Oil red O as described50. Oil red O dye was extracted into isopropanol and absorbance was measured at 510 nm. Frozen sections of mammary gland (10 μm) were fixed in 50% ethanol and stained in a 0.2% Oil red O solution; they were then counterstained with Meyer’s hematoxylin. Paraffin-embedded (CF1 mice) and frozen (uPA- and Plg-deficient mice and controls) tissue sections were stained using an Accustain™ Masson Trichrome Stain kit (Sigma). To detect fibronectin, paraffin sections were stained using a rabbit polyclonal antibody against rat fibronectin (Calbiochem). To detect type I collagen, paraffin sections were stained using a rabbit polyclonal type I antibody against mouse collagen (Calbiochem).
Substrate zymography and western blotting
Frozen mammary tissue was homogenized in RIPA buffer (50 mM Tris–Cl pH 8.0, 150 mM NaCl, 1% NP40, 0.5% deoxycholate and 0.1% SDS) and the supernatant was collected. Conditioned media were collected from 3T3-L1 cells grown under normal and serum-free conditions. Cell lysates were prepared by scraping cells into RIPA buffer. 3T3-L1 nuclear lysates were prepared as described51. Mammary lysates were normalized to wet tissue weight; 3T3-L1 conditioned media and lysates were normalized to cell number. PPARγ was detected by western blotting of nuclear lysates using a goat polyclonal antibody raised against a peptide that maps within an internal region of human PPARγ (Santa Cruz). C/EBPβ was detected by western blotting of nuclear lysates using a rabbit polyclonal antibody raised against a peptide that maps at the carboxy terminus of rat C/EBPβ (Santa Cruz). For substrate zymography, samples were loaded onto non-reducing SDS–polyacrylamide gels containing 1 mg ml−1 casein and 10 μg ml−1 Plg52. Human tPA, high-molecular-mass uPA, plasmin (American Diagnostica Inc.) and PKal were used as controls. Gels were incubated overnight at 37 °C in the presence or absence of 500 nM WT ecotin or EcoRR. The identity of PKal was confirmed by western blotting using a sheep polyclonal antibody against human prekallikrein (Enzyme Research Laboratories).
Plasminogen activation and fibronectin cleavage
To test PKal for its ability to activate Plg, 1 μM human glu-type Plg was incubated with 10 nM human high-molecular-mass uPA, 40 nM tPA or 40 nM PKal at 37 °C in activity buffer (50 mM Tris–Cl pH 7.5, 10 mM CaCl2 and 0.01% Tween-20) on a 96-well plate coated with PBS (control), 50 μg ml−1 human fibronectin (Roche) or 2 mg ml−1 human fibrinogen (Sigma). Fibrinogen was converted to fibrin using 500 nM human thrombin (Sigma). Aliquots were removed after 5, 10 and 15 min and were reduced. Plg was detected by western blotting using a rabbit polyclonal antibody against human Plg (Dako). Reduced samples of human glu-type Plg and human plasmin were used as controls. To assay for Plg activation, 3T3-L1 cells were induced to differentiate under serum-free conditions as described above. At the time of induction, 1 μM human glu-type Plg, 200 nM PKal and 500 nM PAI-1 were added to the medium. Conditioned media were collected after 24 h and were reduced. Plg was detected by western blotting using the rabbit polyclonal antibody against human Plg.
To assay for fibronectin cleavage, 500 nM human plasma fibronectin was incubated with 10 nM human tPA, high-molecular-mass uPA, PKal or plasmin in activity buffer for 30 min at 37 °C. Cleavage products were reduced and subjected to 10% SDS–PAGE. Fibronectin was detected by western blotting using a monoclonal mouse antibody against human fibronectin (Calbiochem). To detect endogenous fibronectin, conditioned media and cell lysates were prepared from 3T3-L1 cells grown and differentiated in the presence of FBS. Cell-associated fibronectin was detected by western blotting of reduced 3T3-L1 cell lysates using a rabbit polyclonal antibody against rat fibronectin (Calbiochem). Cleaved mouse fibronectin present in conditioned media was reduced and assayed by western blotting using a rabbit antibody against mouse fibronectin (Gibco BRL).
Acknowledgments
This work was supported by grants from the National Cancer Institute (to C.S.C. and Z.W.).
References
- 1.Hwang CS, Loftus TM, Mandrup S, Lane MD. Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol. 1997;13:231–259. doi: 10.1146/annurev.cellbio.13.1.231. [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB. PPARγ: An essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- 3.Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995;309:697–710. doi: 10.1042/bj3090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowherd RM, Lyle RE, McGehee RE. Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, et al. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–4636. [PubMed] [Google Scholar]
- 6.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 7.Booth BA, Boes M, Bar RS. IGFBP-3 proteolysis by plasmin, thrombin, serum: heparin binding, IGF binding, and structure of fragments. Am J Physiol. 1996;271:E465–E470. doi: 10.1152/ajpendo.1996.271.3.E465. [DOI] [PubMed] [Google Scholar]
- 8.Chung CH, Ives HE, Almeda S, Goldberg AL. Purification from Escherichia coli of a periplasmic protein that is a potent inhibitor of pancreatic proteases. J Biol Chem. 1983;258:11032–11038. [PubMed] [Google Scholar]
- 9.Wang CI, Yang Q, Craik CS. Isolation of a high affinity inhibitor of urokinase-type plasminogen activator by phage display of ecotin. J Biol Chem. 1995;270:12250–12256. doi: 10.1074/jbc.270.20.12250. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 11.Pairault J, Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci USA. 1979;76:5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwaan HC. The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev. 1992;11:291–311. doi: 10.1007/BF01307184. [DOI] [PubMed] [Google Scholar]
- 13.Rijken DC. Plasminogen activators and plasminogen activator inhibitors: biochemical aspects. Baillieres Clin Haematol. 1995;8:291–312. doi: 10.1016/s0950-3536(05)80269-0. [DOI] [PubMed] [Google Scholar]
- 14.Lascelles AK, Lee CS. In: Lactation: A Comprehensive Treatise. Larson BL, editor. Academic; New York: 1978. pp. 115–176. [Google Scholar]
- 15.Lund LR, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 18.Ulmer JS, Lindquist RN, Dennis MS, Lazarus RA. Ecotin is a potent inhibitor of the contact system proteases factor XIIa and plasma kallikrein. FEBS Lett. 1995;365:159–163. doi: 10.1016/0014-5793(95)00466-m. [DOI] [PubMed] [Google Scholar]
- 19.Ichinose A, Fujikawa K, Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J Biol Chem. 1986;261:3486–3489. [PubMed] [Google Scholar]
- 20.Hauert J, Nicoloso G, Schleuning WD, Bachmann F, Schapira M. Plasminogen activators in dextran sulfate-activated euglobulin fractions: a molecular analysis of factor XII- and prekallikrein-dependent fibrinolysis. Blood. 1989;73:994–999. [PubMed] [Google Scholar]
- 21.Colman RW. Activation of plasminogen by human plasma kallikrein. Biochem Biophys Res Commun. 1969;35:273–279. doi: 10.1016/0006-291x(69)90278-2. [DOI] [PubMed] [Google Scholar]
- 22.Miles LA, Greengard JS, Griffin JH. A comparison of the abilities of plasma kallikrein, beta-Factor XIIa, Factor XIa and urokinase to activate plasminogen. Thromb Res. 1983;29:407–417. doi: 10.1016/0049-3848(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 23.Saito H, et al. Heterogeneity of human prekallikrein deficiency (Fletcher trait): evidence that five of 18 cases are positive for cross-reacting material. N Engl J Med. 1981;305:910–914. doi: 10.1056/NEJM198110153051602. [DOI] [PubMed] [Google Scholar]
- 24.Raspi G. Kallikrein and kallikrein-like proteinases: purification and determination by chromatographic and electrophoretic methods. J Chromatogr B. 1996;684:265–287. doi: 10.1016/0378-4347(96)00144-2. [DOI] [PubMed] [Google Scholar]
- 25.Schousboe I, Feddersen K, Rojkjaer R. Factor XIIa is a kinetically favorable plasminogen activator. Thromb Haemost. 1999;82:1041–1046. [PubMed] [Google Scholar]
- 26.Loza JP, Gurewich V, Johnstone M, Pannell R. Platelet-bound prekallikrein promotes pro-urokinase-induced clot lysis: a mechanism for targeting the factor XII dependent intrinsic pathway of fibrinolysis. Thromb Haemost. 1994;71:347–352. [PubMed] [Google Scholar]
- 27.Lin Y, et al. High molecular weight kininogen peptides inhibit the formation of kallikrein on endothelial cell surfaces and subsequent urokinase- dependent plasmin formation. Blood. 1997;90:690–697. [PubMed] [Google Scholar]
- 28.Behrendt N, et al. The ligand-binding domain of the cell surface receptor for urokinase- type plasminogen activator. J Biol Chem. 1991;266:7842–7847. [PubMed] [Google Scholar]
- 29.Tait JF, Fujikawa K. Identification of the binding site for plasma prekallikrein in human high molecular weight kininogen. A region from residues 185 to 224 of the kininogen light chain retains full binding activity. J Biol Chem. 1986;261:15396–15401. [PubMed] [Google Scholar]
- 30.Colman RW, et al. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–1487. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dano K, et al. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 32.Samad F, Loskutoff DJ. Tissue distribution and regulation of plasminogen activator inhibitor-1 in obese mice. Mol Med. 1996;2:568–582. [PMC free article] [PubMed] [Google Scholar]
- 33.Juhan-Vague I, Alessi MC. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thromb Haemost. 1997;78:656–660. [PubMed] [Google Scholar]
- 34.Samad F, Yamamoto K, Loskutoff DJ. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-alpha and lipopolysaccharide. J Clin Invest. 1996;97:37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loskutoff DJ, Curriden SA, Hu G, Deng G. Regulation of cell adhesion by PAI-1. Apmis. 1999;107:54–61. doi: 10.1111/j.1699-0463.1999.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiner FR, Shah A, Smith PJ, Rubin CS, Zern MA. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry. 1989;28:4094–4099. doi: 10.1021/bi00435a070. [DOI] [PubMed] [Google Scholar]
- 37.Bortell R, Owen TA, Ignotz R, Stein GS, Stein JL. TGF beta 1 prevents the down-regulation of type I procollagen, fibronectin, and TGF beta 1 gene expression associated with 3T3-L1 pre-adipocyte differentiation. J Cell Biochem. 1994;54:256–263. doi: 10.1002/jcb.240540214. [DOI] [PubMed] [Google Scholar]
- 38.Ali IU, Hynes RO. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977;471:16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- 39.Tumova S, Woods A, Couchman JR. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J Biol Chem. 2000;275:9410–9417. doi: 10.1074/jbc.275.13.9410. [DOI] [PubMed] [Google Scholar]
- 40.Alexander CM, et al. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nature Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 41.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 42.Smith PJ, Wise LS, Berkowitz R, Wan C, Rubin CS. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 43.Rajkumar K, Modric T, Murphy LJ. Impaired adipogenesis in insulin-like growth factor binding protein-1 transgenic mice. J Endocrinol. 1999;162:457–465. doi: 10.1677/joe.0.1620457. [DOI] [PubMed] [Google Scholar]
- 44.Campbell PG, Andress DL. Plasmin degradation of insulin-like growth factor-binding protein-5 (IGFBP-5): regulation by IGFBP-5-(201–218) Am J Physiol. 1997;273:E996–E1004. doi: 10.1152/ajpendo.1997.273.5.E996. [DOI] [PubMed] [Google Scholar]
- 45.Zheng B, Clarke JB, Busby WH, Duan C, Clemmons DR. Insulin-like growth factor-binding protein-5 is cleaved by physiological concentrations of thrombin. Endocrinology. 1998;139:1708–1714. doi: 10.1210/endo.139.4.5945. [DOI] [PubMed] [Google Scholar]
- 46.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. doi: 10.1083/jcb.152.4.693. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lund LR, et al. Lactational development and involution of the mammary gland requires plasminogen. Development. 2000;127:4481–4492. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- 48.Bernlohr DA, Angus CW, Lane MD, Bolanowski MA, Kelly TJ. Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci USA. 1984;81:5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deutsch DG, Mertz ET. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 51.Finbloom DS, et al. Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol Cell Biol. 1994;14:2113–2118. doi: 10.1128/mcb.14.3.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]