Abstract
Bradykinin causes vasodilation, stimulates tissue-type plasminogen activator (t-PA) release and, in rodents, increases muscle glucose uptake. Although bradykinin causes vasodilation partly by activating nitric-oxide synthase (NOS), the role of nitric oxide in regulating bradykinin-stimulated t-PA release is uncertain. This study examined the effect of high-dose NOS inhibition on bradykinin-stimulated t-PA release and glucose uptake in humans. We studied 24 healthy (12 women and 12 men), overweight and obese (body mass index >25 kg/m2), normotensive, nondiabetic subjects with normal cholesterol. We measured the effect of intra-arterial Nω-monomethyl-l-arginine (l-NMMA, 12 μmol/min) on forearm blood flow (FBF), net t-PA release, and glucose uptake at baseline and in response to intra-arterial bradykinin (50–200 ng/min) in subjects pretreated with the cyclooxygenase inhibitor aspirin. Measurements were repeated after isosorbide dinitrate (ISDN; 5 mg) or sildenafil (50 mg). l-NMMA decreased baseline FBF (P < 0.001), increased baseline forearm vascular resistance (P < 0.001), and increased the t-PA arterial-venous gradient (P = 0.04) without affecting baseline net t-PA release or glucose uptake. During l-NMMA, ISDN tended to decrease baseline net t-PA release (P = 0.06). l-NMMA blunted bradykinin-stimulated vasodilation (P < 0.001 for FBF and FVR). Bradykinin increased net glucose extraction (from −80 ± 23 to −320 ± 97 μg/min/100 ml at 200 ng/min bradykinin, P = 0.02), and l-NMMA (−143 ± 50 μg/min/100 ml at 200 ng/min, P = 0.045) attenuated this effect. In contrast, l-NMMA enhanced bradykinin-stimulated t-PA release (39.9 ± 7.0 ng/min/100 ml versus 30.0 ± 4.2 ng/min/100 ml at 200 ng/min, P = 0.04 for l-NMMA). In gender-stratified analyses, l-NMMA significantly increased bradykinin-stimulated t-PA release in women (F = 6.7, P = 0.02) but not in men. Endogenous NO contributes to bradykinin-stimulated vasodilation and glucose uptake but attenuates the fibrinolytic response to exogenous bradykinin.
Endothelial dysfunction, which is characterized by an impaired vasodilatory response to nitric-oxide synthase (NOS)-dependent agonists or by a decreased capacity of the endothelium to release tissue-type plasminogen activator (t-PA), predicts the development of cardiovascular events in patients at risk for coronary artery disease (Newby et al., 2001; Robinson et al., 2007; Van Guilder et al., 2008). Endothelial t-PA release occurs through both constitutive and regulated pathways. In constitutive release, newly synthesized t-PA is transported directly from the Golgi apparatus to the cell membrane and secreted, even in the absence of an extracellular stimulus, whereas in regulated secretion, stored t-PA is released from endothelial granules in response to activation of membrane receptors (van den Eijnden-Schrauwen et al., 1995; Emeis et al., 1996; Parmer and Miles, 1998).
The mechanisms governing the regulated secretion of t-PA are not completely understood. In vitro, in human microvascular endothelial cells, thrombin stimulates t-PA release via Gαq-, phospholipase Cβ-, inositol triphosphate-, and 5,6-epoxyeicosatrienoic acid and is independent of cyclooxygenase, NOS, and potassium channel stimulation (Muldowney et al., 2007). Inhibition of cyclooxygenase does not affect bradykinin-stimulated t-PA release from the human vasculature (Brown et al., 2000); however, the role of NO in the regulation of endothelial t-PA release in vivo in humans remains unclear. Sodium nitroprusside, a NO donor, does not stimulate t-PA release from the human forearm in most studies (Labinjoh et al., 2001; Van Guilder et al., 2005). Studies using the NOS inhibitor Nω-monomethyl-l-arginine (l-NMMA) to dissect out the effect of endogenous NO have provided conflicting results. Whereas Giannarelli et al. (2007) proposed that l-NMMA decreased t-PA release, we found no effect of l-NMMA given at a dose of 4 μmol/min on bradykinin-stimulated t-PA release (Brown et al., 2000). To the contrary, Smith et al. (2003) have reported that high dose l-NMMA (20 μmol/min) increases bradykinin-stimulated t-PA release. These data, if confirmed, suggest that complete inhibition of NOS unmasks an inhibitory effect of endogenous NO on endothelial t-PA release.
In addition to stimulating endothelial t-PA release, bradykinin may promote skeletal muscle glucose uptake. In rodents, bradykinin B2-receptor antagonism attenuates glucose uptake in skeletal muscle (Shiuchi et al., 2001), and ACE inhibition improves skeletal muscle glucose uptake in part through enhancement of the bradykinin-NO system (Shiuchi et al., 2002). In humans, two prior studies found no effect of intrafemoral artery infusion of bradykinin on insulin-simulated muscle glucose uptake (Nuutila et al., 1996; Laine et al., 1998).
This study tested the hypothesis that high-dose NOS inhibition enhances bradykinin-stimulated t-PA release and attenuates bradykinin-stimulated glucose uptake in overweight and obese humans. We conducted the study in this population because endothelium-dependent vasodilation and t-PA release are diminished in obesity (Van Guilder et al., 2005, 2008).
Materials and Methods
Subjects.
Twenty-four (12 women and 12 men) healthy subjects with a body mass index (BMI) >25 kg/m2 participated in the study (ClinicalTrials.gov Identifier: NCT00685945). Two women were receiving injectable hormonal contraceptive therapy, whereas the other women were between 12 and 22 days after their last menstrual cycle (luteal phase). After written informed consent was obtained, all subjects underwent a complete history and physical examination, and an electrocardiogram and routine laboratory were obtained. Subjects with renal, pulmonary, endocrine, hematological, or cardiovascular disease (including hypertension defined as an untreated seated systolic/diastolic blood pressure greater than 140/90) were excluded. Subjects with fasting cholesterol greater than 5.7 mM (220 mg/dl) and smokers were excluded.
Experimental Protocol.
The study protocol was approved by the Vanderbilt University Institutional Review Board and conducted according to the Declaration of Helsinki. Studies were performed in the morning in a temperature-controlled room. Subjects were studied in the supine position after an overnight fast. All subjects received 325 mg of acetylsalicylic acid (nonselective COX inhibitor) before placement of the catheters. Acetylsalicylic acid was administered to exclude a contribution of prostacyclin to bradykinin-mediated vasodilation. We have previously demonstrated bradykinin-stimulated t-PA release to be COX-independent (Brown et al., 2000). To ensure that COX inhibition was achieved, serum thromboxane B2 was assayed by gas chromatography/electron capture ionization/mass spectrometry as previously described in seven subjects (Daniel et al., 1994). The mean serum thromboxane B2 concentration was 0.61 ± 0.12 ng/ml (reference range 40.2–415 ng/ml), indicating complete COX inhibition.
An intravenous catheter was placed in the antecubital vein in both arms. After subdermal administration of 1% lidocaine, a 20-gauge polyurethane catheter (Cook Inc., Bloomington, IN) was inserted into the brachial artery of the nondominant arm allowing direct intra-arterial administration of drugs. Before the infusion of vasoactive drugs, arterial catheter patency was maintained by infusion of 0.9% sodium chloride at a rate of 1 ml/min. After placement of catheters, subjects were allowed to rest 30 min before baseline measurements were made. Forearm blood flow (FBF) was measured by silastic-in-mercury strain-gauge plethysmography. After measurement of baseline FBF and blood sampling, graded doses of bradykinin (Clinalfa AG, Läufelfingen, Switzerland) were infused at 50, 100, and 200 ng/min. Each dose was infused for 5 min, and FBF was measured during the last 2 min of infusion. Thirty minutes after administration of bradykinin, a continuous intra-arterial infusion of l-NMMA (a NO synthase inhibitor; Bachem, Torrance, CA) at 12 μmol/min was started. While continuing the infusion of l-NMMA, baseline measurements and infusion of bradykinin were repeated. After the second bradykinin infusion, 12 subjects received 5 mg of isosorbide dinitrate (ISDN, an exogenous NO donor; Major Pharmaceuticals Inc., Livonia MI), and 12 subjects received 50 mg of sildenafil [phosphodiesterase type 5 (PDE5)] inhibitor to increase cGMP without increasing NO (Pfizer, New York, NY). This dose of ISDN is approximately 20-fold higher than the Emax for vasodilation (Leeson et al., 1997). The dose of sildenafil yields a plasma concentration approximately 95-fold higher than the IC50 for PDE5 inhibition (Boolell et al., 1996; Nichols et al., 2002). Sixty minutes after the administration of either ISDN or sildenafil, the continuous intra-arterial infusion of l-NMMA at 12 μmol/min was restarted, and baseline measurements and bradykinin infusion were repeated. Because safety requirements precluded prolonged instrumentation of the brachial artery and because ISDN and sildenafil have relatively long half-lives, it was not feasible to randomize the order of treatment with l-NMMA versus l-NMMA plus oral study medication. One subject did not complete the study because l-NMMA was erroneously administered before the first bradykinin infusion.
Forearm Perfusion Measurements.
FBF was measured by silastic-in-mercury strain-gauge plethysmography (Hokanson et al., 1975). The wrist was supported in a sling to raise the forearm to above the level of the atrium, and the strain gauge was placed at the widest part of the forearm. The strain gauge was connected to a plethysmograph (model EX-5; D. E. Hokanson, Inc., Bellevue, WA), which was calibrated to measure the percentage change in volume and connected to a chart recorder. For each measurement, a cuff placed around the upper arm was inflated to 40 mm Hg, with a rapid cuff inflator (model E-10; D. E. Hokanson, Inc., Bellevue, WA) to occlude venous outflow from the extremity. The hand was excluded from the measurement of blood flow by inflation of a pediatric sphygmomanometer cuff around the wrist to 200 mm Hg before and during measurements of FBF. Flow measurements were recorded for ∼7 of 15 s, and the slope was derived from the first three or four pulses; five to seven such readings were obtained for each mean value.
Blood Sampling and Biochemical Assays.
After measurement of FBF, simultaneous arterial and venous samples were obtained from the infused arm before and after each dose of bradykinin. Blood samples were collected on ice and centrifuged immediately, and plasma was stored at −70°C until the time of assay.
Blood for measurement of t-PA was collected in tubes containing 0.105 M acidified sodium citrate, and antigen levels were determined using a two-site enzyme-linked immunosorbent assay (Biopool, Trinity Biotech USA, Berkeley Heights, NJ) as described previously (Ridker et al., 1992). Glucose concentrations were determined colorimetrically with the Vitros 250 Chemistry System (GMI, Inc., Ramsey, MN). Insulin concentrations were determined by radioimmunoassay. Venous blood for measurement of bradykinin was drawn into ice-cold anhydrous ethanol and centrifuged after 1 h; the supernatant was saved at −70°C until the time of assay. Bradykinin concentrations were determined using a commercially available enzyme immunoassay (Peninsula Laboratories, Inc., San Carlos, CA).
Ateriovenous concentration gradients were calculated by subtracting the plasma level measured in simultaneously collected venous and arterial blood. Forearm plasma flow was calculated from the FBF and arterial hematocrit corrected for 1% trapped plasma. Thus, individual net release or uptake rates at each time point were calculated by the following formula: net release = (Cv − CA) × [FBF × (101 − hematocrit/100)], where Cv and CA represent the concentration of t-PA or glucose in the brachial vein and artery, respectively. Net t-PA release is expressed as nanogram per minute per 100 ml, and net glucose uptake is expressed as microgram per minute per 100 ml.
Statistical Analysis.
Data are presented as means ± S.E.M. Baseline characteristics between gender groups were compared using independent Student's t test. The effect of bradykinin on hemodynamic, fibrinolytic, and glucose uptake variables were determined using general linear model-repeated measures ANOVA in which the between-subject variable was gender, and the within-subjects variables were drug (control, +l-NMMA, +l-NMMA plus ISDN or +l-NMMA plus sildenafil) and dose of bradykinin. A Bonferroni correction was done for multiple comparisons. Bradykinin data were log-transformed before analysis. A two-tailed P value less than 0.05 was considered statistically significant. Statistical analyses were performed with the statistical package SPSS for Windows (Version 17.0; SPSS, Chicago, IL).
Results
Baseline Subject Characteristics.
Table 1 provides the baseline clinical characteristics of the subjects. Twenty four (12 women and 12 men) overweight and obese (BMI >25 kg/m2), normotensive, nondiabetic subjects with normal serum cholesterol were studied. There was no significant difference between women and men in BMI (P = 0.29), age (34.1 ± 1.3 versus 29.3 ± 2.1 years, P = 0.07), heart rate (P = 0.98), systolic blood pressure (P = 0.24), diastolic blood pressure (P = 0.89), mean arterial pressure (P = 0.61), serum cholesterol (160.8 ± 6.0 versus 176.5 ± 5.8 mg/dl, P = 0.07), or insulin (P = 0.80). Fasting blood glucose was significantly lower in women compared with men (76.6 ± 2.4 versus 83.1 ± 1.8 mg/dl, P = 0.04).
Table 1.
Baseline subject characteristics
| Characteristic | |
|---|---|
| Gender (F/M) | 12/12 |
| Ethnicity (Caucasian/African American/Indian) | 17/6/1 |
| Age (years) | 31.7 ± 1.3 |
| BMI (kg/m2) | 31.9 ± 1.2 |
| Heart rate (bpm) | 67.0 ± 1.7 |
| Systolic blood pressure (mm Hg) | 118.2 ± 1.9 |
| Diastolic blood pressure (mm Hg) | 70.0 ± 1.1 |
| Mean arterial pressure (mm Hg) | 86.0 ± 1.1 |
| Cholesterol (mg/dl) | 168.7 ± 4.4 |
| Hematocrit (%) | 41.5 ± 0.7 |
| Fasting blood glucose (mg/dl) | 79.8 ± 1.6 |
| Fasting venous insulin (μU/ml) | 7.8 ± 0.8 |
Effect of l-NMMA, ISDN, and Sildenafil on Baseline MAP, FBF, FVR, Net t-PA Release and Fasting Blood Glucose Uptake.
Table 2 presents the effect of drug treatment on baseline MAP, FBF, FVR, and net t-PA release and fasting blood glucose uptake. l-NMMA increased MAP slightly but significantly (F = 7.1, P = 0.01). Administration of ISDN decreased MAP during administration of l-NMMA (P = 0.02), whereas sildenafil did not affect MAP during l-NMMA. l-NMMA significantly decreased baseline FBF (F = 35.1, P < 0.001), even when ISDN (P = 0.003) or sildenafil (P = 0.01) was given concurrently. Likewise, l-NMMA significantly increased baseline FVR (F = 38.2, P < 0.001), whether or not ISDN (P = 0.005) or sildenafil (P = 0.006) was administered. l-NMMA increased the baseline arterial-venous t-PA concentration gradient (Table 3) but did not affect net t-PA release. ISDN tended to decrease baseline t-PA release during l-NMMA (P = 0.06 after controlling for gender). There was no effect of sildenafil on baseline t-PA release and no effect of any treatment on baseline glucose uptake.
Table 2.
Effect of drug treatment on baseline measurements
| Control | +l-NMMA | +l-NMMA + ISDN | +l-NMMA + Sildenafil | |
|---|---|---|---|---|
| Mean arterial pressure (mm Hg) | 85.7 ± 0.8 | 87.2 ± 0.8* | 83.3 ± 1.8† | 85.2 ± 1.6 |
| Forearm blood flow (ml/min/100 ml) | 4.0 ± 0.2 | 2.4 ± 0.2*** | 2.2 ± 0.4** | 2.8 ± 0.4* |
| Forearm vascular resistance (mm Hg/ml/min/100 ml) | 23.4 ± 1.6 | 41.4 ± 2.9*** | 46.8 ± 6.7** | 35.5 ± 3.7** |
| Net t-PA release (ng/min/100 ml) | 0.2 ± 0.4 | 0.6 ± 0.2 | −0.4 ± 0.5†† | 0.3 ± 0.7 |
| Net fasting glucose uptake (μg/min/100 ml) | −80 ± 23 | −74 ± 7 | −71 ± 28 | −67 ± 13 |
P < 0.05,
P < 0.01,
P < 0.001 vs. control.
P < 0.05 versus l-NMMA alone;
P, 0.06 versus l-NMMA alone after controlling for gender.
Table 3.
Effect of drug treatment on arterial-venous (AV) gradient
| Control | +l-NMMA | +l-NMMA + ISDN | +l-NMMA + Sildenafil | |
|---|---|---|---|---|
| Venous-arterial glucose difference (mg/dl) | ||||
| Baseline | −3.6 ± 0.9 | −5.9 ± 0.7 | −5.2 ± 1.9 | −4.2 ± 0.8 |
| Bradykinin (200 ng/min) | −3.1 ± 1.0 | −1.7 ± 1.0* | −0.2 ± 2.4* | −1.8 ± 0.7* |
| Venous-arterial t-PA difference (ng/ml) | ||||
| Baseline | 0.1 ± 0.2 | 0.5 ± 0.2† | −0.4 ± 0.5 | 0.4 ± 0.4 |
| Bradykinin (200 ng/min) | 3.0 ± 0.5*** | 6.3 ± 1.0***††† | 7.6 ± 1.4***†† | 5.3 ± 1.7** |
P < 0.05,
P < 0.01,
P < 0.001 versus baseline.
P < 0.05,
P < 0.01,
P < 0.001 versus control.
Effect of l-NMMA, ISDN, and Sildenafil on FBF, FVR, and t-PA Response to Exogenous Bradykinin.
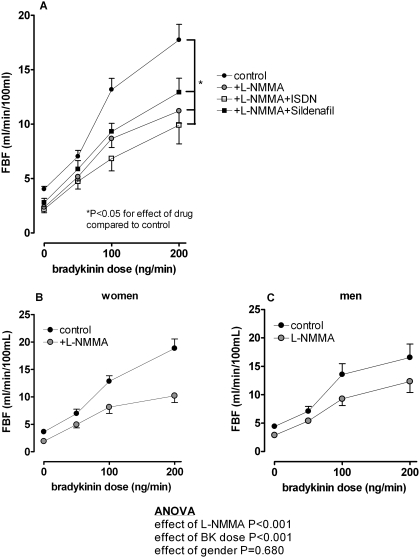
Bradykinin caused a dose-dependent increase in FBF in all subjects (from 4.0 ± 0.2 to 17.7 ± 1.4 ml/min/100 ml, P < 0.001; Fig. 1A). There was no significant effect of gender on bradykinin-stimulated FBF (P = 0.91). l-NMMA significantly blunted bradykinin-stimulated FBF in all subjects (from 2.4 ± 0.2 to 11.2 ± 1.1 ml/min/100 ml, P < 0.001 for effect of drug), and this response was not significantly different between women and men (Fig. 1, B and C). Neither ISDN nor sildenafil altered the FBF response to bradykinin during l-NMMA.
Fig. 1.
A, effect of l-NMMA, ISDN, and sildenafil on FBF response to exogenous bradykinin in all subjects. Neither ISDN nor sildenafil altered the FBF response to bradykinin during l-NMMA. B and C, effect of l-NMMA on FBF response to exogenous bradykinin in women (B) and men (C). ANOVA results are for the comparison between women and men.
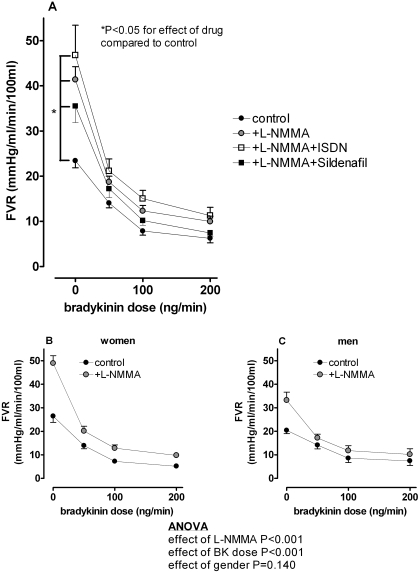
Bradykinin significantly decreased FVR in all subjects (from 23.4 ± 1.6 to 6.2 ± 1.0 mm Hg/ml/min/100 ml, P < 0.001; Fig. 2A), with no significant effect of gender (P = 0.75). l-NMMA blunted the decrease in FVR in response to bradykinin in all subjects (P < 0.001 for effect of l-NMMA). This FVR response was not significantly different between women and men (Fig. 2, B and C). Likewise, neither ISDN nor sildenafil altered the FVR response to bradykinin during l-NMMA.
Fig. 2.
A, effect of l-NMMA, ISDN, and sildenafil on FVR response to exogenous bradykinin in all subjects. Neither ISDN nor sildenafil altered the FVR response to bradykinin during l-NMMA. Effect of l-NMMA on FVR response to exogenous bradykinin in women (B) and men (C). ANOVA results are for the comparison between women and men.
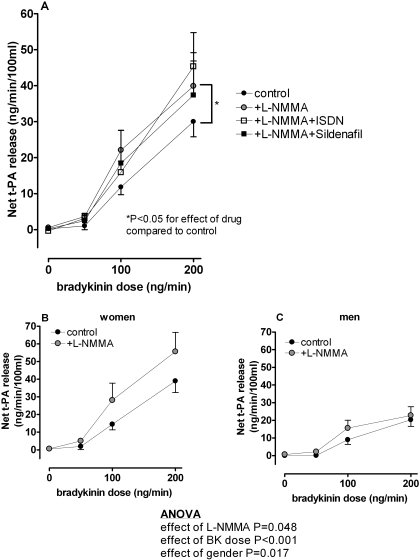
Bradykinin caused a 150-fold increase in net t-PA release (from 0.2 ± 0.4 to 30.0 ± 4.2 ng/min/100 ml, P < 0.001; Fig. 3A), and women had a significantly greater response compared with men (39.0 ± 6.5 versus 20.3 ± 3.7 ng/min/100 ml, P = 0.02). l-NMMA significantly enhanced bradykinin-stimulated t-PA release in all subjects (from 0.6 ± 0.2 to 39.9 ± 7.0 ng/min/100 ml, P = 0.04 for effect of l-NMMA). l-NMMA significantly increased bradykinin-stimulated t-PA release in women (F = 6.7, P = 0.02; Fig. 3B) but not in men (Fig. 3C). Neither ISDN nor sildenafil significantly altered bradykinin-stimulated net t-PA release during l-NMMA. To exclude the possibility that the effect of l-NMMA on bradykinin-stimulated t-PA release was a result of an increase in local concentration of agonist due to decreased flow, we measured venous bradykinin concentrations during the 200 ng/min bradykinin infusion. l-NMMA did not significantly affect venous bradykinin concentration (3.7 ± 0.5 versus 3.1 ± 0.4 fmol/ml in the absence of l-NMMA, P = 0.14). There was no significant effect of gender on bradykinin concentrations (P = 0.16).
Fig. 3.
A, effect of l-NMMA, ISDN, and sildenafil on net t-PA response to exogenous bradykinin in all subjects. Effect of l-NMMA on t-PA response to exogenous bradykinin in women (B) and men (C). The increase in bradykinin-stimulated t-PA release in the presence of l-NMMA was significantly greater in women compared with men (F = 6.7, P = 0.02).
Effect of l-NMMA, ISDN, and Sildenafil on Fasting Blood Glucose Uptake to Exogenous Bradykinin.
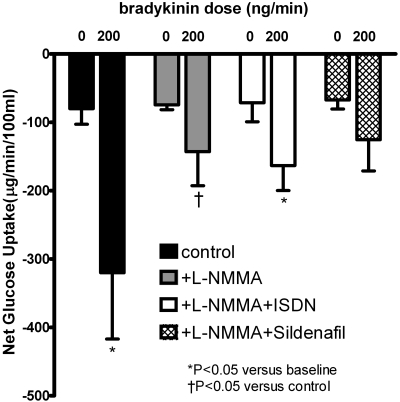
Bradykinin significantly increased net glucose uptake (from −80 ± 23 to −320 ± 97 μg/min/100 ml, P = 0.02; Fig. 4). This resulted from an increase in flow without a dilutional decrease in the arterial-venous glucose gradient (Table 3). The effect of bradykinin on glucose uptake was not significant in the presence of l-NMMA or l-NMMA plus sildenafil. Bradykinin decreased the arterial-venous glucose gradient in the presence of l-NMMA, whether or not ISDN or sildenafil was given concurrently. These responses were similar in women and men.
Fig. 4.
Effect of l-NMMA, ISDN, and sildenafil on net glucose uptake response to exogenous bradykinin. Bradykinin caused a significant increase in net glucose uptake (P = 0.02). *, P < 0.05 versus baseline; †, P < 0.05 versus control.
Discussion
This study examined the effect of high-dose NOS inhibition on endothelium-dependent vasodilation, t-PA release, and glucose uptake in overweight and obese subjects. High-dose NOS inhibition enhanced t-PA release while attenuating endothelium-dependent vasodilation, confirming that endogenous NO attenuates vascular t-PA release in overweight and obese subjects. This study is unique in demonstrating a gender-specific effect of NOS inhibition in this group. In addition, this study provides the first evidence that bradykinin increases muscle glucose uptake in humans through a NOS-dependent pathway.
Studies regarding the contribution of NO to the regulation of stimulated t-PA release have provided conflicting data. The majority of studies using the NO donor sodium nitroprusside indicate that NO does not stimulate t-PA release (Hrafnkelsdóttir et al., 1998; Labinjoh et al., 2001; Van Guilder et al., 2005). We previously reported no effect of a submaximal dose (4 μmol/min) of the NOS inhibitor l-NMMA on bradykinin-stimulated t-PA release in healthy volunteers (Brown et al., 2000). In the current study, we found that a 3-fold higher dose of l-NMMA significantly enhances endothelial t-PA release in response to bradykinin in overweight and obese subjects, as Smith et al. (2003) had reported previously in normal-weight males. Thus, administration of high-dose l-NMMA appears to unmask an inhibitory effect of NO on t-PA release. These data may also explain the finding of Newby and co-workers that intra-arterial administration of tumor necrosis factor-α enhances bradykinin-stimulated t-PA release while inhibiting NO-dependent vasodilation (Chia et al., 2003).
Giannarelli et al. (2007) reported that NOS inhibition decreases bradykinin-stimulated t-PA release. This apparently contradictory finding may result from the specific methodology applied by the investigators. That is, the investigators infused the NOS inhibitor l-NMMA using an “NO clamp” technique; in this technique, sodium nitroprusside is infused concurrently during l-NMMA at a dose of 0.3 to 0.4 μg/100 ml/min to maintain normal FBF. Thus, it is not possible to determine whether administration of the NOS inhibitor or the NO donor affected bradykinin-stimulated t-PA release.
The mechanisms whereby NO could inhibit t-PA release have been explored in vitro and in animal studies. In the rat isolated hind limb, the NO donors sodium nitroprusside and atrial natriuretic factor inhibit bradykinin-stimulated t-PA release, whereas the cGMP analog 8-bromo-cGMP does not reproduce the inhibitory effect, suggesting that NO inhibits t-PA release through a cGMP-independent pathway (Tranquille and Emeis, 1993). Matsushita et al. (2003) have reported that NO inhibits exocytosis of Weibel-Palade bodies (a possible storage site of t-PA) via a cGMP-independent pathway by nitrosylating cysteine residues of N-ethylmaleimide-sensitive factor (NSF), thereby inhibiting NSF disassembly of soluble NSF attachment protein receptor. t-PA concentrations are increased in endothelial NOS-deficient mice (Iafrati et al., 2005). Consistent with an NO-dependent, cGMP-independent mechanism, in the present study the NO donor ISDN tended to decrease basal t-PA release when NOS was inhibited, whereas the PDE5 inhibitor sildenafil did not.
We used oral ISDN and sildenafil to determine whether giving exogenous NO or preventing the degradation of cGMP would reverse the effect of l-NMMA. A limitation of this approach is that systemic administration of these drugs can lower systemic MAP, as we observed for ISDN. Because changes in FVR alone do not stimulate t-PA release (Brown et al., 1999), the systemic effect of ISDN and sildenafil would not confound the primary outcome. An alternative approach would have been to use the NO “clamp” as described previously (Ueda et al., 2004). Although this approach may be more elegant, the use of oral ISDN and sildenafil is more applicable to the clinical situation.
We have reported previously that bradykinin stimulates t-PA release to a greater extent in the forearm vasculature of women compared with in men. This gender effect is estrogen-independent as, during ACE inhibition, bradykinin-stimulated t-PA release is increased in postmenopausal as well as premenopausal women compared with age-matched men and 17β-estradiol treatment does not alter t-PA release in postmenopausal women (Pretorius et al., 2005, 2008). We again observed a gender difference in the t-PA response to bradykinin in the current study. In addition, we found that l-NMMA enhanced bradykinin-stimulated t-PA release in the women studied but not in men. Whether this represents a true gender difference in the contribution of NO to the regulation of t-PA release or reflects differences in NO bioavailability in the overweight and obese men and women studied is not possible to ascertain. Smith et al. (2003) reported that high-dose l-NMMA increased t-PA release in men but studied healthy men rather than obese subjects in whom endothelial function is diminished. The males in our study had significantly higher fasting glucose concentrations, and this may have affected endothelial fibrinolytic function.
In addition to causing vasodilation and endothelial t-PA release, bradykinin has been reported to increase muscle glucose uptake through a NO-dependent pathway in rodents (Shiuchi et al., 2001). By use of [18F]fluoro-deoxyglucose to measure muscle glucose uptake, Nuutila and co-workers reported that infusion of bradykinin in the femoral artery increases blood flow without increasing either basal or insulin-stimulated muscle glucose uptake in normal or obese subjects (Nuutila et al., 1996; Laine et al., 1998). Compared with this earlier work, we infused a 4-fold higher dose of bradykinin after normalizing for forearm or leg muscle volume and achieved a significantly greater increase in FBF. We administered bradykinin for 15 min versus 100 min and may have avoided tachyphylaxis. Using direct measurement of arterial and venous glucose concentrations, we found that bradykinin increased muscle glucose uptake and that this effect was diminished by the NOS inhibitor l-NMMA. It is noteworthy that in the present study, l-NMMA decreased glucose extraction (the arterial-venous gradient) as well as flow, suggesting a direct effect on bradykinin-stimulated muscle glucose uptake.
Our study results have relevance to the use of ACE inhibitors. ACE inhibitors exert their pharmacological effects by decreasing angiotensin II formation and bradykinin degradation (Brown and Vaughan, 1998). We have previously reported that ACE inhibition increases constitutive t-PA release through endogenous bradykinin (Pretorius et al., 2003). In addition, our study also provides new information regarding the beneficial effect of bradykinin on glucose uptake in humans. The present study suggests this effect may be enhanced in individuals with endothelial dysfunction.
Acknowledgments
We thank Delia Woods for nursing assistance and Jeff Petro for technical assistance.
This work was supported in part by the National Institutes of Health [Grants HL085740, HL060906, HL065193]; and the National Institutes of Health National Center for Research Resources [Grant 1UL1-RR024975] (Vanderbilt Clinical and Translational Science Award).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160168
- NOS
- nitric-oxide synthase
- ACE
- angiotensin-converting enzyme
- BMI
- body mass index
- FBF
- forearm blood flow
- FVR
- forearm vascular resistance
- ISDN
- isosorbide dinitrate
- MAP
- mean arterial pressure
- l-NMMA
- Nω-monomethyl-l-arginine
- NSF
- N-ethylmaleimide-sensitive factor
- t-PA
- tissue-type plasminogen activator
- COX
- cyclooxygenase
- PDE5
- phosphodiesterase type 5
- ANOVA
- analysis of variance.
References
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. (1996) Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 8:47–52 [PubMed] [Google Scholar]
- Brown NJ, Gainer JV, Murphey LJ, Vaughan DE. (2000) Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B2 receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation 102:2190–2196 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Gainer JV, Stein CM, Vaughan DE. (1999) Bradykinin stimulates tissue plasminogen activator release in human vasculature. Hypertension 33:1431–1435 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Vaughan DE. (1998) Angiotensin-converting enzyme inhibitors. Circulation 97:1411–1420 [DOI] [PubMed] [Google Scholar]
- Chia S, Qadan M, Newton R, Ludlam CA, Fox KA, Newby DE. (2003) Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol 23:695–701 [DOI] [PubMed] [Google Scholar]
- Daniel VC, Minton TA, Brown NJ, Nadeau JH, Morrow JD. (1994) Simplified assay for the quantification of 2,3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl 653:117–122 [DOI] [PubMed] [Google Scholar]
- Emeis JJ, van den Eijnden-Schrauwen Y, Kooistra T. (1996) Tissue-type plasminogen activator and the vessel wall: Synthesis, storage and secretion., in Vascular Control of Hemostasis (van Hinsbergh VWM. ed) pp 187–206, Harwood Academic Publishers [Google Scholar]
- Giannarelli C, De Negri F, Virdis A, Ghiadoni L, Cipriano A, Magagna A, Taddei S, Salvetti A. (2007) Nitric oxide modulates tissue plasminogen activator release in normotensive subjects and hypertensive patients. Hypertension 49:878–884 [DOI] [PubMed] [Google Scholar]
- Hokanson DE, Sumner DS, Strandness DE., Jr (1975) An electrically calibrated plethysmograph for direct measurement of limb blood flow). IEEE Trans Biomed Eng 22:25–29 [DOI] [PubMed] [Google Scholar]
- Hrafnkelsdóttir T, Wall U, Jern C, Jern S. (1998) Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet 352:1597–1598 [DOI] [PubMed] [Google Scholar]
- Iafrati MD, Vitseva O, Tanriverdi K, Blair P, Rex S, Chakrabarti S, Varghese S, Freedman JE. (2005) Compensatory mechanisms influence hemostasis in setting of eNOS deficiency. Am J Physiol Heart Circ Physiol 288:H1627–H1632 [DOI] [PubMed] [Google Scholar]
- Labinjoh C, Newby DE, Pellegrini MP, Johnston NR, Boon NA, Webb DJ. (2001) Potentiation of bradykinin-induced tissue plasminogen activator release by angiotensin-converting enzyme inhibition. J Am Coll Cardiol 38:1402–1408 [DOI] [PubMed] [Google Scholar]
- Laine H, Yki-Jarvinen H, Kirvela O, Tolvanen T, Raitakari M, Solin O, Haaparanta M, Knuuti J, Nuutila P. (1998) Insulin resistance of glucose uptake in skeletal muscle cannot be ameliorated by enhancing endothelium-dependent blood flow in obesity. J Clin Invest 101:1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J. (1997) Non-invasive measurement of endothelial function: effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart 78:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, et al. (2003) Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldowney JA, 3rd, Painter CA, Sanders-Bush E, Brown NJ, Vaughan DE. (2007) Acute tissue-type plasminogen activator release in human microvascular endothelial cells: the roles of Galphaq, PLC-beta, IP3 and 5,6-epoxyeicosatrienoic acid. Thromb Haemost 97:263–271 [PubMed] [Google Scholar]
- Newby DE, McLeod AL, Uren NG, Flint L, Ludlam CA, Webb DJ, Fox KA, Boon NA. (2001) Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation 103:1936–1941 [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Muirhead GJ, Harness JA. (2002). Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 53Suppl 1: 5S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutila P, Raitakari M, Laine H, Kirvelä O, Takala T, Utriainen T, Mäkimattila S, Pitkänen OP, Ruotsalainen U, Iida H, et al. (1996) Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest 97:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer RJ, Miles LA. (1998) Targeting of tissue plasminogen activator to the regulated pathway of secretion. Trends Cardiovasc Med 8:306–312 [DOI] [PubMed] [Google Scholar]
- Pretorius M, Luther JM, Murphey LJ, Vaughan DE, Brown NJ. (2005) Angiotensin-converting enzyme inhibition increases basal vascular tissue plasminogen activator release in women but not in men. Arterioscler Thromb Vasc Biol 25:2435–2440 [DOI] [PubMed] [Google Scholar]
- Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. (2003) Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation 107:579–585 [DOI] [PubMed] [Google Scholar]
- Pretorius M, van Guilder GP, Guzman RJ, Luther JM, Brown NJ. (2008) 17Beta-estradiol increases basal but not bradykinin-stimulated release of active t-PA in young postmenopausal women. Hypertension 51:1190–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Vaughan DE, Stampfer MJ, Manson JE, Shen C, Newcomer LM, Goldhaber SZ, Hennekens CH. (1992) Baseline fibrinolytic state and the risk of future venous thrombosis. A prospective study of endogenous tissue-type plasminogen activator and plasminogen activator inhibitor. Circulation 85:1822–1827 [DOI] [PubMed] [Google Scholar]
- Robinson SD, Ludlam CA, Boon NA, Newby DE. (2007) Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 27:1651–1656 [DOI] [PubMed] [Google Scholar]
- Shiuchi T, Cui TX, Wu L, Nakagami H, Takeda-Matsubara Y, Iwai M, Horiuchi M. (2002) ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension 40:329–334 [DOI] [PubMed] [Google Scholar]
- Shiuchi T, Nakagami H, Iwai M, Takeda Y, Cui T, Chen R, Minokoshi Y, Horiuchi M. (2001) Involvement of bradykinin and nitric oxide in leptin-mediated glucose uptake in skeletal muscle. Endocrinology 142:608–612 [DOI] [PubMed] [Google Scholar]
- Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. (2003) Endothelial release of tissue-type plasminogen activator in the human forearm: role of nitric oxide. J Cardiovasc Pharmacol 42:311–314 [DOI] [PubMed] [Google Scholar]
- Tranquille N, Emeis JJ. (1993) The role of cyclic nucleotides in the release of tissue-type plasminogen activator and von Willebrand factor. Thromb Haemost 69:259–261 [PubMed] [Google Scholar]
- Ueda S, Wada A, Umemura S. (2004) Methodological validity and feasibility of the nitric oxide clamp technique for nitric oxide research in human resistant vessels. Hypertens Res 27:351–357 [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ. (1995) Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood 85:3510–3517 [PubMed] [Google Scholar]
- Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, DeSouza CA. (2005) Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab 289:E807–E813 [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Stauffer BL, Greiner JJ, DeSouza CA. (2008) Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 294:H1685–H1692 [DOI] [PMC free article] [PubMed] [Google Scholar]