Abstract
Abuse-liability-related effects of subtype-selective GABAA modulators were explored relative to the prototypic benzodiazepine lorazepam. 7-Cyclobutyl-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-3-phenyl-1,2,4-triazolo[4,3-b]pyridazine (TPA123) has weak partial agonist efficacy at α1-, α2-, α3-, and α5-containing GABAA receptors, whereas 7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine (TPA023) has weaker partial agonist efficacy at α2 and α3 and none at α1 and α5 subtypes. For both compounds, preclinical data suggested efficacy as nonsedating anxiolytics. Self-injection of TPA123 (0.0032–0.1 mg/kg) and TPA023 (0.0032–0.32 mg/kg) was compared with lorazepam (0.01–0.32 mg/kg) in baboons. TPA123 and lorazepam maintained self-injection higher than vehicle at two or more doses in each baboon; peak rate of self-injection of lorazepam was higher than TPA123. Self-injected lorazepam and TPA123 also increased rates of concurrently occurring food-maintained behavior. After the availability of self-administered TPA123 doses ended, an effect consistent with a mild benzodiazepine-like withdrawal syndrome occurred. In contrast with lorazepam and TPA123, TPA023 did not maintain self-administration. Positron emission tomography studies showed that TPA023 produced a dose-dependent inhibition in the binding of [11C]flumazenil to the benzodiazepine binding site in the baboon, which was essentially complete (i.e., 100% occupancy) at the highest TPA023 dose (0.32 mg/kg). In a physical dependence study, TPA023 (32 mg/kg/24 h) was delivered as a continuous intragastric drip. Neither flumazenil at 14 days nor stopping TPA023 after 30 to 31 days resulted in the marked withdrawal syndrome characteristic of benzodiazepines in baboons. In the context of other data, elimination of efficacy at the α1 subtype of the GABA/benzodiazepine receptor is not sufficient to eliminate abuse liability but may do so when coupled with reduced α2/3 subtype efficacy.
When they were introduced in the early 1960s, the benzodiazepines, typified by diazepam, represented a major advance in the treatment of generalized anxiety disorder relative to older treatments (Lader, 1993). They had a rapid onset of anxiolytic efficacy, were generally well tolerated, and relatively safe in overdose. Nevertheless, beginning in the late 1970s, concerns over the abuse potential and dependence liability of benzodiazepines resulted in more restrictive legislation (i.e., legal scheduling under the Controlled Substances Act in the United States in 1975 and by the World Health Organization in 1982) as well as a general reluctance of physicians to prescribe benzodiazepines (Williams and McBride, 1998). Benzodiazepines are abused by people who have a history of drug abuse, those on long-term benzodiazepines may independently escalate their dosage, patients can be overly reluctant to comply with physician recommendations to reduce benzodiazepine use, and there is considerable evidence of adverse effects on discontinuation of benzodiazepines (Higgitt and Fonagy, 1993; Lader, 1993; O'Brien, 2005).
In the search for new “anxioselective” drugs, which would retain the anxiolytic efficacy of the “classical” full-agonist benzodiazepines but without concomitant sedative effects, compounds with reduced intrinsic efficacy (e.g., such as bretazenil) were identified. For a variety of reasons, the distinct preclinical pharmacology of such compounds did not translate into clinical utility (Atack, 2005). A novel approach to the development of anxioselective drugs has been to identify compounds that bind with equal affinity to each of the four GABAA subtypes that contain a benzodiazepine binding site (i.e., α1, α2, α3, and α5 subunit-containing GABAA receptors) (Sieghart and Sperk, 2002) but that have different intrinsic efficacy at these subtypes (Atack, 2005), yielding compounds with novel pharmacological profiles (Dawson et al., 2005). Of great interest has been whether such selectively efficacious compounds would show reduced abuse liability and dependence potential in comparison with compounds that are full agonists at those subtypes, thereby providing a significant advance for the treatment of anxiety (Atack, 2005).
The prototypic “efficacy-selective” compound L-838,417, which is devoid of efficacy at the α1 subtype, showed characteristics of a nonsedating anxiolytic in rodent studies (McKernan et al., 2000), thus providing pharmacological confirmation of evidence from transgenic mice that the α1-GABAA subtype is responsible for the sedative effects of the classical nonselective benzodiazepine agonist diazepam (Rudolph et al., 1999; McKernan et al., 2000). L-838,417 did, however, retain the ability to maintain self-administration in nonhuman primates (Rowlett et al., 2005).
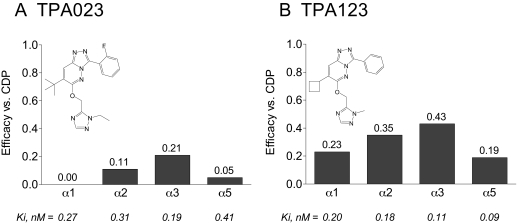
TPA123 and TPA023 are structurally related to L-838,417 and also bind with equivalently high affinity to the benzodiazepine binding site of the four GABAA receptor subtypes (Fig. 1), but they are subtly different from L-838,417 and from each other in their subtype-selective efficacy profiles. TPA123 has very low partial-agonist efficacy at the α1 subtype, with an efficacy relative to the nonselective full-agonist benzodiazepine chlordiazepoxide of 0.23, and low efficacy at the α2, α3, and α5 subtypes (relative efficacy values of 0.35, 0.43, and 0.19, respectively). TPA023, like L-838,417, is devoid of efficacy at the α1 subtype but has extremely low efficacy at the α2- and α3-containing subtypes (0.11 and 0.21, respectively) and virtually no efficacy (<5%) at the α5 subtype. TPA123 and TPA023 both were devoid of sedative effects and had effects in several rodent and nonhuman primate assays that have been predictive of anxiolytic efficacy in humans (Dawson et al., 2005; Atack et al., 2006; Atack, 2009). In clinical studies, TPA023 showed anxiolytic-like activity yet showed reduced sedative effects compared with lorazepam (de Haas et al., 2007; Atack, 2009).
Fig. 1.
Comparison of the structures, binding affinity (Ki), and efficacy profiles of TPA023 and TPA123. Efficacy values were measured against human recombinant GABAA receptors containing β3, β2, and either an α1, α2, α3, or α5 subunit using whole-cell patch-clamp electrophysiology and are expressed relative to the efficacy measured at each subtype using the nonselective full-agonist chlordiazepoxide (CDP). Data are modified from McCabe et al. (2004), Carling et al. (2005), and Atack et al. (2006).
Two primary pieces of data used to evaluate the abuse liability of psychoactive drugs are the ability of the compound to maintain self-administration and whether physical dependence develops after chronic administration (Ator and Griffiths, 2003). The purpose of the present study was to compare, in a nonhuman primate, the reinforcing and physical dependence-producing effects of TPA023 and TPA123 to determine whether the novel in vitro and in vivo partial-agonist profiles of these two compounds would translate into differences in those effects most relevant to predictions of abuse liability and dependence potential. The classic, nonselective benzodiazepine anxiolytic lorazepam served as a comparator in the self-administration study. The effects of substituting vehicle for self-administered doses of TPA123 permitted assessment of abrupt drug withdrawal. When TPA023 did not maintain self-injection, the ability of the compound to occupy the benzodiazepine site of brain GABAA receptors was measured using PET. Direct study of its physical dependence potential was initiated via methods used previously to characterize physical dependence on benzodiazepines and α1-selective compounds in baboons.
Materials and Methods
Behavioral Studies
Subjects.
Adult, male baboons (Papio hamadryas anubis, i.e., olive baboon) served as subjects. Water was available continuously; two pieces of fresh fruit and a children's chewable multivitamin were provided between 10:30 AM and 11:30 AM each day. Access to nutritionally balanced, banana-flavored 1-g food pellets (Bio-Serv, Frenchtown, NJ) is described below. The baboons had visual and auditory contact with other male baboons, multiple nonfood enrichment items, and frequent social interactions with two or more members of the laboratory 7 days/week. Each baboon was surgically implanted with either an intravenous (internal jugular or femoral vein) or intragastric chronically indwelling silastic catheter that exited in the midscapular region and was protected by a vest and tether system. If necessary to prevent the baboon's removing the vest, a custom-designed sleeveless mesh shirt was worn over it. Approximately every 2 to 3 weeks, except as noted below, a physical examination and care of the catheter exit site were carried out under intramuscular ketamine HCl anesthesia, preceded by atropine SO4 to control secretions.
Four baboons served in the study of intravenous drug self-administration. Baboon LC was pharmacologically naive. Baboon NG previously self-administered two opioids, which ended 6 months before the present study. Baboons GD and TZ previously self-administered two or three intravenous sedatives and had been physically dependent on methadone (Ator et al., 2005). Methadone dependence ended 3.5 months (GD) or 7 months (NG) before the present study, and self-administration of other drugs ended 3 weeks (GD) or 4 weeks (TZ) before the present study. Body weights (kilograms) fluctuated during the present study (30–33.9 for LC, 24.6–29.6 for NG, 29.1–34.5 for GD, and 24.4–28.6 for TZ).
Two baboons completed the study of physical dependence on TPA023; two other baboons began this study, but they had to have the intragastric catheter removed during the vehicle baseline condition. The two baboons (SHA and YO) that completed the dependence study had served in a similar study of intragastric delivery of the benzodiazepine ligand pagoclone, which ended 4.5 or 5 months before the start of the present study (N. A. Ator, unpublished data). Baboons SHA and YO weighed 15.8 and 26.4 kg, respectively, when the present study began; later weights are presented under Results.
Apparatus.
The surgery and apparatus for both intravenous and intragastric catheter systems are described in Lukas et al. (1982). In brief, the catheter was threaded through a fitting in the backplate of the vest, through a flexible, stainless steel cable, and onto a liquid swivel mounted at the top of the cage. For the intravenous catheters, tubing from the swivel was connected to a three-way valve. Tubing from one valve was connected to a source of heparinized (5 IU/ml) 0.9% saline, which was delivered continuously at a rate of approximately 150 ml/24 h via a peristaltic pump to maintain catheter patency. Tubing from the other two valves was connected to the source of drug and 0.9% saline flush, respectively, which also were delivered via peristaltic pumps under the drug reinforcement schedule described below. For the intragastric catheters, distilled water was delivered continuously from a calibrated glass aspirator bottle via peristaltic pump at a rate of approximately 500 ml/24 h to maintain catheter patency until the beginning of the vehicle baseline condition described below.
The individual home cages had the experimental panel incorporated into the rear wall. The panel contained operanda, stimulus lights, and a food hopper. Other equipment (e.g., water bottle, pellet feeder, and peristaltic pump) was on a grating that ran from wall to wall above the cages (Ator, 2000; Ator et al., 2000). The experimental panel was connected via an interface to a computer, which was custom-programmed to control experimental conditions and collect data (equipment and software from MED Associates, St. Albans, VT). For the self-administration experiment, cumulative recorders (Gerbrands, Arlington, MA) also collected responses and reinforcer deliveries under the schedule of drug reinforcement.
Self-Administration Procedures.
A single-subject design was used, in which each baboon served as his own control; and each additional baboon served as a replication. A self-administration procedure was used that involves establishing self-administration with a standard dose of cocaine and then substituting each test dose of the drug of interest for cocaine (Ator and Griffiths, 2003). This procedure was chosen for the present study because it has been used to assess and compare self-administration of a wide range of psychoactive drugs in the baboon, including those with which it is most relevant to compare TPA023 and TPA123, such as benzodiazepines and novel ligands for the benzodiazepine site (e.g., Griffiths et al., 1991, 1992; Sannerud et al., 1992; Ator, 2000). TPA123 was studied first and then lorazepam and TPA023. Dose conditions, including vehicle, were studied in a mixed order within and across baboons, except that vehicle was the first condition for all baboons in study of TPA123. Given the low partial agonist efficacy profile of TPA123, most doses were studied twice in each baboon to assess reliability of the reinforcing effects under the cocaine baseline condition. To assess drug reinforcement compared with vehicle more directly, and to assess the effects of abrupt withdrawal of TPA123, vehicle was substituted for a TPA123 dose that was reinforcing under the cocaine baseline procedure. Once injections/day was stable (no increasing or decreasing trends over at least 4 days), the same or another reinforcing TPA123 dose was substituted for vehicle to determine sensitivity to TPA123 reinforcement when baseline rate of self-injection was low. During the lorazepam condition, one reinforcing dose was studied a second time in baboons NG and LC but not in the other baboons due to time constraints for completing the entire study.
Schedules of drug and food reinforcement were in effect 24 h/day. The experimental panel contained two operanda, each with a cue light mounted above it. An amber cue light over a custom-made lever was continuously illuminated, correlated with an FR-10 schedule of food reinforcement being in effect. That is, 10 lever presses produced a 1-g food pellet, accompanied by illumination of the food hopper for 1 s. When the green cue light over the other device, which was a Lindsley operandum, was illuminated, a 5-s tone sounded, a low level of white noise was continuously emitted through a speaker, and an FR-160 schedule of drug reinforcement was in effect. That is, each pull and release of the operandum produced a 100-ms feedback tone; completion of the 160th response turned off the cue light and the white noise, a square Plexiglas panel was transilluminated with green light, and a peristaltic pump delivered drug in a volume of 5 ml over 85 to 90 s (depending on the baboon). The drug delivery was followed by 5 ml of 0.9% saline from another peristaltic pump over 85 to 90 s. Due to solubility limitations, however, 0.1 mg/kg TPA123 was delivered in 10 ml over approximately 170 to 175 s. There was a delay of approximately 20 s between completion of the response requirement and delivery of drug to the vein, which was bridged by the immediate illumination of the green panel and the sound of the pump motor. A 3-h time-out, during which the green panel remained illuminated for the 1st h, also began with the completion of the response requirement; during time-out, responses on the Lindsley operandum were counted but had no programmed consequences. Under this arrangement of contingencies, the maximal number of self-injections that could be produced per 24 h was eight.
Each test dose was substituted for a dose of cocaine (0.32 mg/kg) that reliably maintained a criterion level of self-injection (6–8/24 h). The criterion performance was required for three consecutive days before test dose substitution. This ensured a high probability that the baboon would self-inject the test dose soon after substitution and thus come into contact with its effects early in the period of availability. Each test dose (or vehicle) remained available for 15 days to permit the transition from cocaine and relative stabilization of the rate of self-injection of the test dose itself. Solutions were changed, if required, and data for the preceding 24 h were collected by 8:30 AM. The volume of water consumed in the preceding 24 h and whether any food pellets earned remained uneaten also were recorded. The physical examination described above occurred either between days 6 and 10 or after the 15-day period ended. In the latter case, the drug reinforcement schedule was suspended at the end of the 15 days, but the food reinforcement schedule remained in effect. The interval between the end of a 15-day assessment period and the beginning of the next cocaine baseline period usually was 0 to 5 days.
Blood level determinations.
During study of TPA023, two doses that had been available for self-injection were delivered to the baboon under the same parameters as for self-injection, and then the baboon was anesthetized with intravenous ketamine to be able to obtain blood between 15 and 30 min after completion of the injection sequence. No other drug injections had occurred in the preceding 4 days. Blood was collected from a saphenous vein, injected into a heparinized tube, and centrifuged at approximately 3200 rpm for 12 min. The plasma was drawn off into a polypropylene tube with a 23-gauge needle, and it was frozen at −20°C until shipment for analysis within a month.
Data analysis.
Consistent with a single-subject design, demonstration of drug effects was assessed for each baboon. Group data are presented for the purpose of summarizing drug reinforcement and effects of drug taking on food pellets per day. The mean self-injections in the last 5 days of each 15-day period of test dose availability were assessed in relation to self-injection of the drug vehicle. That a drug dose was reinforcing was concluded if the mean self-injections exceeded by at least one injection 2 S.D.s for an individual baboon and two S.E.M.s for the group of the vehicle mean (i.e., analogous to a one-tailed statistical test since prediction of drug reinforcement requires an increase in injections/day compared with vehicle). Number of food pellets/day was similarly plotted, and a significant change compared with the vehicle condition was concluded if mean pellets per day during self-injection exceeded, by at least one pellet, ±1 S.D. for an individual baboon and 1 S.E.M. for the group (i.e., analogous to a two-tailed test since it was possible this measure could either decrease or increase as a function of self-injected drug).
Chronic Delivery and Withdrawal of Intragastric TPA023 Procedures.
As in self-administration, a single-subject design was used. The three successive experimental conditions differed in terms of the fluid delivered 24 h/day via the intragastric catheter: 1) TPA023 vehicle baseline for at least 18 days; 2) TPA023 (32 mg/kg/24 h) delivery for 30 days; and 3) TPA023 vehicle for 15 days (followed by distilled water thereafter), with assessment of the effects of TPA withdrawal for 30 days. During the TPA023 vehicle baseline and during the TPA023 (32 mg/kg/24 h) conditions, the effects of flumazenil vehicle and of flumazenil itself were assessed, respectively, on days 10 and 14.
TPA023 vehicle or TPA023 (32 mg/kg) suspension was delivered continuously across 24 h each day from a glass aspirator bottle via a peristaltic pump. Due to the viscosity of the suspension, and in an effort to avoid the catheter's becoming blocked, the volume was adjusted upward during the vehicle baseline (i.e., by increasing the pump speed) to achieve a volume of 600 ml/24 h. Fresh suspension was put in place each day at approximately 8:30 AM, which defined the beginning of that 24-h period of data collection (e.g., day 1 of drug delivery began at 8:30 AM and ended at 8:29 AM the following calendar day). Volume of water consumed in the preceding 24-h period and whether any food pellets that had been delivered remained uneaten also were recorded by 8:30 AM. If catheter blockage was discovered, an amount equal to what should have been received by that time of day was given as a bolus after the catheter was cleared.
A Lindsley operandum was mounted on the experimental panel and an FR-10 schedule of reinforcement with the same 1-g food pellets used in the self-administration study was in effect 20 h/day beginning, with few exceptions, at 9:00 AM (±15 min). A cue light above the operandum was continuously illuminated during that time; and a 1-s illumination of the food hopper accompanied delivery of each pellet. Between 8:30 AM and 9:30 AM, a fine motor coordination task was presented to each baboon by a veterinary technician familiar with the baboon. For this task, a custom-made Plexiglas board, onto which a line of six equally spaced shallow Plexiglas cups had been glued (Weerts et al., 1998), was positioned against the bars of the baboon's cage. A single raisin was placed in each cup, and the technician timed how long it took for the baboon to retrieve all six raisins; the time limit was 120 s. Afterward, the technician recorded whether any raisins were dropped and whether there was tremor or lack of coordination in performing the task.
Between 9:30 AM and 10:30 AM on days 2 to 6 of TPA023 vehicle baseline and TPA023 (32 mg/kg/24 h) delivery, one of three trained observers sat in front of the baboon's cage with a laptop computer on a small typing table and recorded frequencies of behaviors in 1-min intervals for 15 min by use of a customized observation program (for information on the program and the definitions of the behaviors, see Weerts et al., 1998). The observers had practiced conducting these structured observational sessions in front of each baboon before the TPA023 vehicle baseline began to habituate the baboons to the individuals and the procedures and to conduct interobserver reliability assessments. On day 10 and day 14 of TPA023 vehicle baseline and TPA023 (32 mg/kg/24 h) delivery, flumazenil vehicle and 5 mg/kg flumazenil, respectively, were injected intramuscularly at approximately 9:30 AM, and the observation lasted 60 rather than 15 min. On day 15 of TPA023 vehicle baseline and day 16 of the TPA023 (32 mg/kg/24 h) condition, each baboon was anesthetized for the physical examination. Blood samples also were taken, and blood was handled as described above. A series of five more daily 15-min observations occurred at the end of the period of TPA023 delivery.
After 30 (baboon YO) or 31 (baboon SHA) days of drug delivery, TPA023 vehicle was substituted for the drug and was delivered for approximately 2 weeks before the fluid was changed to distilled water. Observations (15 min each) began the morning after drug delivery ended (at the end of the first day of drug withdrawal) and continued daily for 15 days. A physical examination occurred and blood was drawn on day 20 of this condition. Evaluation of raisin task performance and daily pellet delivery continued for 30 days after drug delivery ended.
Data analysis.
A withdrawal score (Weerts et al., 1998; Ator et al., 2000) to summarize the appearance of signs characteristic of withdrawal from barbiturates and benzodiazepines was derived to characterize flumazenil-precipitated and spontaneous withdrawal. The components of the withdrawal score were as follows: 1) “pellets” (decrease in number of pellets delivered); 2) “raisin” (increase in time to complete the raisin retrieval task); 3) “postures” (increase in postures other than the normal posture and/or increase in periods in which the eyes were closed); 4) “locomotion” (increase or decrease in locomotion); 5) “self-directed” (increase in self-directed behavior, i.e., scratching, nose-wiping, nose-rubbing, masturbation, or “wet-dog shakes”); 6) “aggression” (aggressive movement toward the observer or another baboon, bruxism, yawn); 7) “tremor/jerk” (increase in tremor and/or spontaneous jerking movements of the limbs); 8) “vomit/retch” (increase in vomiting/retching); and 9) “seizure” (occurrence of a convulsion). To arrive at the score for spontaneous withdrawal, a mean and S.D. were calculated for each of the dependent measures taken during the TPA023 vehicle baseline. These values were used to solve for x in the formula t = (x − mean)/S.D., where t is the tabled t value needed for significance of a one- or two-tailed probability (p) level at the appropriate df (i.e., number of scores used in the calculation minus 1). For all measures except locomotion, a one-tailed p level was selected since the prediction was that the behavior would change in a particular direction for a drug withdrawal syndrome. The value of x was rounded to the nearest whole number since 1) it was impossible to observe a fraction of the behavioral signs or delivery of less than a single pellet and 2) a difference of less than a second in the raisin retrieval task was not considered meaningful. The value of x provided the t score that needed to be exceeded for the particular measure to be significant on any given day of withdrawal, provided one additional constraint was met. That is, the value of an observation in the drug withdrawal period was not considered indicative of a drug abstinence syndrome if the same value had occurred during the last five observations before TPA023 delivery ended. For flumazenil-precipitated withdrawal, the effects of 5 mg/kg flumazenil on day 14 of TPA023 delivery were considered significant for a measure if its value exceeded that after the flumazenil injection on day 14 of the TPA023 vehicle baseline and also after flumazenil vehicle injections on both day 10 of TPA023 vehicle baseline and day 10 of TPA023 delivery.
PET Scanning
Subjects.
Three adult male baboons (olive baboons), weighing 16, 22, and 24 kg, were anesthetized with ketamine HCl (10 mg/kg i.m.) followed by propofol (2 mg/kg/i.v. bolus plus a constant intravenous infusion of 0.4 mg/kg/min), intubated, and then ventilated using medical grade compressed air at approximately 100 ml/breath at a rate of 25 respirations/min. Body temperature was maintained with circulating water heating pads; and temperature, O2, and end tidal CO2 were monitored for the duration of the study.
[11C]Flumazenil Synthesis.
Desmethyl flumazenil (0.5 mg) was dissolved in 250 μl of anhydrous N,N-dimethylformamide in an autosampler vial, and cooled to 0°C in an ice bath. Fifteen microliters of 0.1 M NaOH was added 5 min before distillation of [11C]methyl iodide (produced by a TRACERlab FXc synthesizer; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) into the solution. Further manipulations were performed by an SK233 automated sample injector (Gilson, Middleton, WI). When the level of [11C]methyl iodide in the solution plateaued (as measured by a photodiode detector), the solution was transferred to an empty vial preheated to 80°C. After 5 min, the reaction was quenched by the addition of 750 μl of water, loaded into the high-performance liquid chromatographic injection loop of the autosampler, and injected onto an Xterra high-performance liquid chromatographic column (7.8 × 150 mm) equilibrated at 20% MeCN, 80% (0.1% trifluoroacetic acid). A gradient was run to 90% MeCN, 10% (0.1% trifluoroacetic acid) over a period of 15 min at 3 ml/min. The desired product peak (retention time, 5–6 min) was diverted directly into a flask connected to a modified rotary evaporator. The organic solvent was evaporated at reduced pressure with the aid of a warm water bath for <1 min, and the [11C]flumazenil was transferred into a sterile vial via 1/16-inch Teflon tubing.
Procedures.
PET acquisitions were performed in three-dimensional mode using the ECAT EXACT HR+ (CTi/Siemens, Waltham, MA). Emission data were acquired in three-dimensional (retracted septa) mode; transmission data (for subsequent attenuation correction) were acquired in two-dimensional mode before injection of the radiopharmaceutical. Emission scans were corrected for attenuation, scatter, and dead-time, and then reconstructed with a ramp filter, resulting in transverse and axial spatial resolutions of approximately 5 mm at full-width at half-maximum.
After preparation, the animals were positioned in the PET camera gantry in supine position, with the head in the center of the scanner field of view. A bolus injection of approximately 5 mCi of [11C]flumazenil was injected intravenously over 15 s with emission imaging initiating at the time of injection. TPA023 (0.0032, 0.032, or 0.32 mg/kg) was administered 40 min later over 90 s, followed by 5 ml of saline. The vehicle, injection duration, and saline flush mimicked conditions of intravenous injection in the self-administration study. Additional scans were performed to estimate [11C]flumazenil-specific binding under baseline conditions and test-retest variability. Two baseline scans were acquired on the same day for each animal, separated by at least 2 h. In two baboons, two baseline scans were acquired on each of 2 days. The total time for each scan was 90 min and consisted of 22 frames (4 × 15 s, 4 × 60 s, 5 × 180 s, 4 × 300 s, and 5 × 600 s).
Data Analysis and Estimation of Receptor Availability Change.
For each baboon, a static PET image was obtained by averaging the dynamic frames in the baseline studies. Regions of interest were drawn on the static image in frontal, parietal, temporal, and occipital cortices; cerebellum; and pons. All PET studies were aligned to the static PET image, and regions of interest were projected into the dynamic scans to obtain the corresponding time-activity curves (TACs). Static PET images and TACs were expressed in standard uptake value (SUV) units using the baboon's body weight and the injected tracer dose as follows: TAC (SUV) = 1000 × TAC (Bq) × weight (kg)/injected tracer dose (Bq).
The regional [11C]flumazenil specific binding (SB) was calculated using the area under the TACs from 60 to 90 min after tracer injection (i.e., 20–50 min after TPA023 administration). The nonsaturable binding was estimated from the pons region after administration of 0.32 mg/kg TPA023 in the same time interval: SB = TAC(60–90 min)/pons(60–90 min); 0.32 mg/kg TPA023)−1.
The SB variability between test and retest was calculated as the absolute value of the difference between test and retest, expressed in percentage of the mean value of both measurements. For each region, the percentage occupancy by TPA023 was calculated as follows: 100 × (1 − SBBL/SBTPA023), where SBBL corresponds to the average SB calculated under baseline conditions and SBTPA023 corresponds to the SP calculated after different doses of TPA023.
Drugs
TPA023 (Atack et al., 2006) and TPA123 (TP13, McCabe et al., 2004; MRK-067, Atack, 2009) were synthesized as described by Carling et al. (2005), where they were identified as compounds 17 and 15, respectively). For intravenous administration, they first were dissolved in polyethylene glycol 400, which then was diluted 50% with 0.9% sterile saline. Lorazepam (Wyeth-Ayerst, now Wyeth, Princeton, NJ) first was dissolved in 20:80 polyethylene glycol 400/propylene glycol, and then diluted 50% with 0.9% sterile saline. Cocaine HCl (Research Triangle Institute, Research Triangle Park, NC) for intravenous self-administration was dissolved in 0.9% saline. The drugs prepared for intravenous delivery were filter-sterilized (22-μm filter; Millipore, Bedford, MA). TPA023 for intragastric administration was suspended, by use of an electric blender, in a matrix prepared by blending 2 g of suspending agent K (Bio-Serve, Frenchtown, NJ) in distilled water. The matrix was prepared no more than 48 h before use and refrigerated; when drug powder was added, it was blended for 5 min before use. Flumazenil (F. Hoffman-La Roche, Basel, Switzerland) for intramuscular injection was prepared immediately before use by dissolving powder in 2.5 ml of 20:80 95% (v/v) ethanol and propylene glycol; this was diluted with 2.5 ml of sterile water for a total injection volume of 5 ml delivered in two sites on the thigh. The same vehicle and volumes of injection preceded control observations.
Results
Lorazepam
Self-Administration.
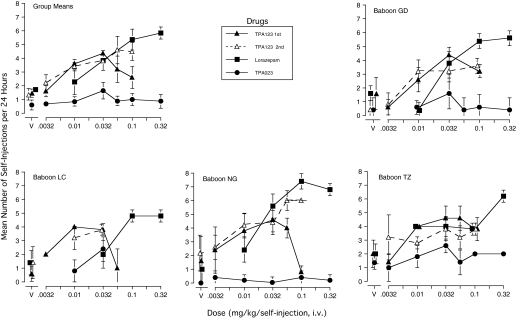
Lorazepam self-injection was an ascending function of dose across the range tested (Fig. 2). The 0.032 mg/kg dose maintained self-injection at rates significantly greater than vehicle in three baboons, and the two higher doses did so in all four baboons. Notes in the records indicated slight sedative effects on the first day of self-injection of 0.032 mg/kg and higher doses for baboons LC and TZ (e.g., “very slight swaying while sitting on the bench”). The highest mean self-injection rates ranged from approximately five to seven per day, out of the possible maximum of eight. Reinforcement by 0.032 mg/kg was replicated for baboon NG (4.4 injections) and by 0.1 mg/kg was replicated for baboon LC (4.0 injections).
Fig. 2.
Mean daily number of self-injections delivered on the last 5 days of the 15-day period of availability of TPA123, lorazepam, TPA023, or their vehicles (V) in each of four baboons and for the group. Each self-injection was available under an FR-160 schedule of reinforcement; a time-out of 180 min followed completion of the response requirement, which limited maximal injections/day to eight. Each test dose or V was substituted for 0.32 mg/kg cocaine, which had maintained six to eight self-injections/day for the three preceding days (data not shown). Vertical bars indicate 1 S.D. for the individual baboons and 1 S.E.M. for the group means. Food pellets were concurrently available under a separate schedule of reinforcement (Fig. 3). TPA123 V for the second determinations for baboons GD and TZ are for the last 5 days in which vehicle was substituted directly for a dose of TPA123 (Fig. 4). TPA023 V for LC was not included due to his removal from the study for treatment of infection during that condition.
Food-Maintained Responding.
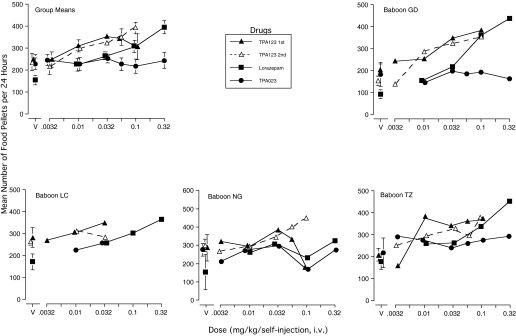
When lorazepam was being self-administered, food-maintained responding increased dose-dependently in three baboons; the function was relatively flat for the fourth (Fig. 3). Notes indicated that operating the food lever and drinking water were observed soon after self-injections occurred.
Fig. 3.
Mean number of 1-g food pellets per day produced by four baboons during the evaluation of self-administration of TPA123, TPA023, and lorazepam. Food pellets were available 24 h/day under an FR-20 schedule of reinforcement. Data are for the same periods of time as the self-injection data in Fig. 2. For the individual baboons, vertical bars around the vehicle (V) means indicate 1 S.D., and those around group means represent 1 S.E.M. Bars were omitted around the drug dose means for individual baboons for clarity. Pellet data for 0.056 mg/kg TPA123 were omitted for LC because pellet intake dropped and he received supplemental food.
As described under Materials and Methods, a physical examination (under ketamine anesthesia) occurred after each test drug condition ended and before return to the cocaine baseline. When one or more days elapsed between the end of drug access and the physical examination, we were able to examine food-maintained responding after drug withdrawal. When lorazepam access periods ended, pellets/day generally decreased below not only what they had been during the just-ended self-administration period but also below what they had been in the vehicle condition, and they did so in a dose-dependent manner. For example, for baboon GD the day after access to 0.032 mg/kg lorazepam ended, the number of pellets was only 32 compared with 112 the day after the 0.01 mg/kg condition ended. Likewise, for the other baboons, after the 0.1 mg/kg condition ended, pellets earned dropped to 37 (compared with 66 after 0.01 and 48 after 0.032 mg/kg) for TZ, 19 (compared with 198 after vehicle) for LC, and four (compared with 75 after 0.032 mg/kg) for NG.
TPA123
Self-Administration.
TPA123 self-administration generally was an inverted U-shaped function of dose during the first dose-effect determination, but it became an ascending function of dose during the second determination (Fig. 2). Self-injection rates of one to four TPA123 doses met the criterion for reinforcement for three baboons (not NG) in the initial determinations and for all four baboons in the redeterminations. Peak self-injection rate was approximately four to five per day in all four baboons in both the first and second determinations, except that baboon NG peaked at six injections per day in the second determination. The notes made on baboon appearance and behavior after the first self-injections of TPA123 (0.032 mg/kg) and/or higher doses indicated slight sedative effects (e.g., in the first 10 min after the first self-injections of 0.032 and 0.056 mg/kg, baboon LC's eyes were half-closed, and he was sitting “swaying” on the bench or had difficulty maintaining balance when he stood up). These effects disappeared with subsequent self-injections.
To further determine the intrasubject reliability of TPA123 reinforcement, given its novel partial agonist profile, vehicle was substituted directly for a reinforcing dose of TPA123 during the second set of dose-effect determinations. Then, the same or another dose that had been reinforcing was substituted for vehicle. During the 3-day period of cocaine availability, responding met the criterion of six to eight self-injections/day (data not shown). On the first day of TPA123 availability, self-injection decreased to three or less; it then increased across days to predominantly four/day or, for NG, six/day (Fig. 4). When vehicle was substituted for TPA123 (i.e., an extinction condition began), self-injection remained high the first day but decreased across the next 3 days to be clearly less than had been maintained by TPA123 for three of the baboons (i.e., number of injections ranged from zero to approximately two or three in the last 5 days of vehicle). The fourth baboon (TZ) showed greater resistance to extinction (i.e., injections per day did not show the same decreasing trend as for the other baboons), but the probability/frequency of taking more than two injections per day clearly decreased in the vehicle condition. When the same or another previously reinforcing dose replaced vehicle, response rates increased higher than maintained by vehicle. Another replication of TPA123 reinforcement was obtained for baboon LC when vehicle again was substituted for 0.032 mg/kg, and responding again decreased to a rate lower than when TPA123 was available (Fig. 4). The effects of these manipulations were less definitive for baboon TZ; it is likely that the period of vehicle substitution was terminated prematurely. Note that recovery of self-injection of 0.032 mg/kg for baboon LC did not occur until the period of availability was extended. Overall, mean injections/day in the last 5 days when TPA123 was substituted for vehicle (Fig. 4) was the same as or very similar to the mean when the same TPA123 dose had been substituted off the cocaine baseline (Fig. 2).
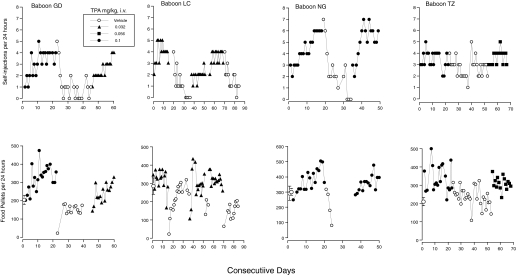
Fig. 4.
Number of self-injections (top) and pellets (bottom) per day for each baboon when a reinforcing dose of TPA123 was substituted for cocaine for 15 days (for the second determination of the effects of those doses), followed by substitution of vehicle (V) until extinction of self-injection was shown, followed by substitution of the same or another reinforcing dose of TPA123 for at least 15 days (for baboon LC, vehicle substitution and return to TPA123 was replicated; note that 3 days in which only pellets were available occurred after the first 15 days of return to 0.032 mg/kg). Data for days on which the baboon was anesthetized for the physical exam or on which equipment malfunctioned are omitted from both panels. Data for days on which supplementation with monkey chow occurred are omitted from the lower panels.
Food-Maintained Responding.
Compared with the vehicle condition, responding maintained by food pellets increased in all four baboons during the conditions in which peak self-injection of TPA123 occurred (Fig. 3). Comparison of Figs. 3 and 4 shows, for example, that self-injection of 0.032 mg/kg three to four times/day was associated with an approximately 33% increase in pellets for the baboons (GD and TZ) whose pellet intake in the vehicle condition was approximately 200/day. Pellets/day for baboons LC and NG approached 300/day, and the increase in this value during TPA123 self-injection was smaller. Pellets/day generally was higher than 1 S.D. of the appropriate vehicle condition if some self-injection was occurring, even if the rate of self-injection was not greater than vehicle. Written notes indicated that the baboons often began operating the food-paired lever soon after a TPA123 injection was received. That the pellets earned were consumed was supported by the fact that no more than one or two were found in the pan under the cage each morning.
The close relationship between self-injection rate and increase in food-maintained responding is shown across days in Fig. 4. When cocaine was available before the first manipulation shown in Fig. 4, food-maintained responding often was lower than when vehicle was available (data not shown). Pellets/day thus tended to increase the first day the TPA123 dose was available and then increased higher than in the TPA123 vehicle condition (cf., Fig. 3) and remained the same or continued to increase across the entire 15 days the dose was self-injected. When vehicle was substituted directly for the TPA123 dose, food-maintained responding decreased, precipitously for three baboons (Fig. 4). As the vehicle condition continued, pellets/day increased for two of the three. Given the decreasing trend for NG, daily supplementation with standard monkey chow occurred for the remainder of the vehicle condition, which precluded evaluation of the food-maintained responding (data omitted in Fig. 4). When a TPA123 dose was made available again, pellets/day again increased concomitantly with self-injections in all four baboons. For LC, pellets/day also decreased dramatically when vehicle was resubstituted for 0.032 mg/kg.
As with lorazepam, pellet data were examined for the days after a TPA023 self-administration condition ended but the physical examination had not yet occurred. There were one or more such occasions for each baboon. Pellets/day always decreased by 95 to 278 pellets (median decrease, 199) the first day after termination of access to doses of TPA123 0.01 mg/kg and higher (13 observations), which put the number of pellets obtained below the range of pellets/day across the last 5 days of the just-ended TPA123 dose condition. After the 0.0032 mg/kg condition ended, in contrast, pellets per day were not decreased (three observations) except for baboon TZ's second exposure to that dose condition, in which he self-administered two to six injections per day (Fig. 2); and pellets dropped to 19 when access to the dose ended. Other evidence of a TPA123 withdrawal effect was seen when baboon GD's catheter failed during the second determination of 0.032 mg/kg. Pellets/day dropped from 215 to 1, and notes indicated that the baboon was “not acting normal; lying on bench or at bottom of cage”; “failed to eat apple”; no infection or other illness was diagnosed; and the symptoms dissipated across a few days. Finally, when baboon GD was changed to the 0.0032 mg/kg condition after running the 0.032 and then the 0.01 conditions, both of which maintained approximately three or more injections/day, pellets/day plummeted, and the baboon failed to eat one of the two pieces of fruit given on the second day; evidence of vomiting also was observed. Feeding recovered over the next few days. Thus, abrupt termination of the opportunity to self-inject most doses at a rate of three or more injections/day generally resulted in a decrease in pellets/day compared with feeding during vehicle or drug conditions studied off the cocaine baseline.
TPA023
Self-Administration.
The TPA023 self-injection dose-response curve was essentially flat (Fig. 2). The individual functions for TPA023 were lower than for lorazepam and TPA123 in all four baboons. The peak rate of TPA023 self-injection was approximately two to three injections/day for three baboons, and less than one injection for the fourth baboon (NG). Notes indicated that only baboon TZ showed any sign of sedation, which was “slight” after the first self-injection of 0.32 mg/kg. Number of self-injections generally dropped to three or less on the first day of TPA023 dose availability and did not rise above that rate during the 15 days of the condition (data not shown).
Food-Maintained Responding.
Responding maintained by food pellets remained fairly stable and comparable with that during vehicle condition(s) for all periods of TPA023 availability (Fig. 3). No pattern of increasing or decreasing food-maintained responding occurred.
Blood Levels of TPA023.
Plasma samples of TPA023 in three baboons were taken after intravenous injection under the same parameters as during self-injection, and analysis revealed that concentration was dose-dependent. Plasma concentrations were as follows: baboon GD, 15.51 ng/ml 30 min after 0.01 mg/kg and 57.59 ng/ml 24 min after 0.032 mg/kg; baboon NG, 5.87 ng/ml 21 min after 0.01 mg/kg and 27.73 ng/ml 22 min after 0.032 mg/kg; and baboon TZ, 22.95 ng/ml 23 min after 0.01 mg/kg and 37.76 ng/ml 24 min after 0.032 mg/kg.
PET Scanning after Intravenous TPA023.
The intravenous administration of 0.032 and 0.32 mg/kg TPA023 resulted in a rapid washout of the specifically bound [11C]flumazenil in cortical regions and cerebellum. On average, [11C]flumazenil binding was reduced by 61 ± 9% after administration of 0.032 mg/kg and essentially by 100% at 0.32 mg/kg. The kinetics of [11C]flumazenil were not clearly affected after administration of 0.0032 mg/kg TPA023. The estimated occupancy, 10 ± 4%, was very close to the level of reliable detection.
Despite the low levels of GABAA receptor in the pons, a clear [11C]flumazenil washout was observed after administration of both 0.032 and 0.32 mg/kg TPA023. Given the high occupancy observed with the latter dose in the three baboons (all curves practically collapse together by 20 min after TPA023 administration, which was 60 min after [11C]flumazenil injection), the area under the pons curve from 60 to 90 min after tracer injection provided a good estimate of nonspecific uptake (Fig. 5).
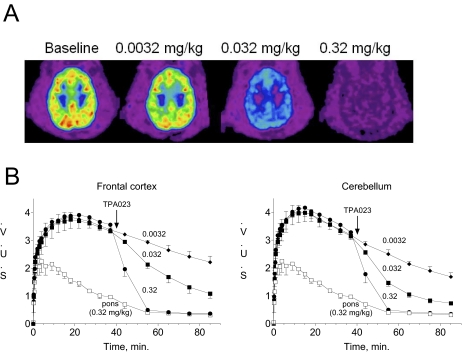
Fig. 5.
A, pseudocolor images of [11C]flumazenil binding to the benzodiazepine binding sites of GABAA receptors in the baboon brain. Images were collected and averaged 30 to 45 min after intravenous administration of either 0.0032, 0.032, or 0.32 mg/kg TPA023 (i.e., 70–85 min after commencement of scanning). Baseline scans were collected and averaged over a 10-min period (scan times, 20–30 min) before drug administration (which occurred at a scan time of 40 min). Red and orange colors represent areas of highest [11C]flumazenil binding, whereas blue and purple areas have lowest binding. B, time-activity curves for [11C]flumazenil in baboon frontal cortex, cerebellum, and pons. Each baboon received three separate scans during which they received either 0.0032, 0.032, or 0.32 mg/kg i.v. TPA023, 40 min after scanning commenced. For each dose group, the SUVs at each time point were averaged across the three baboons. The data for the pons (acquired during the 0.32 mg/kg TPA023 scan) are the same in each panel and represent the level of nonspecific uptake of [11C]flumazenil. Horizontal axis corresponds to mean frame time. Values shown are mean ± S.E.M.
The summed PET images in the interval 60 to 90 min after [11C]flumazenil injection after administration of 0.0032, 0.032, and 0.32 mg/kg i.v. TPA023 clearly show the dose-dependent displacement of [11C]flumazenil from the benzodiazepine binding site throughout the cortex and cerebellum (Fig. 5). At a dose of 0.32 mg/kg, TPA023 had reduced essentially all the specific uptake of [11C]flumazenil. The extent of the displacement of [11C]flumazenil seemed comparable in the examined brain regions (Table 1).
Table 1.
GABAA/benzodiazepine receptor occupancy after intravenous administration of TPA023 in baboons
Specific binding was estimated 20 min after TPA023 administration and 60 min after [11C]flumazenil injection.
| Baboon | TPA023 Dose | Frontal Cortex | Parietal Cortex | Temporal Cortex | Occipital Cortex | Cerebellum | Avg. ± S.D. |
|---|---|---|---|---|---|---|---|
| mg/kg | % | ||||||
| 1 | 0.0032 | 11 | 10 | 16 | 13 | 19 | 14 ± 4 |
| 0.032 | 63 | 68 | 70 | 69 | 76 | 69 ± 5 | |
| 0.32 | 99 | 98 | 98 | 97 | 99 | 98 ± 1 | |
| 2 | 0.0032 | 5 | 5 | 4 | 6 | 12 | 6 ± 3 |
| 0.032 | 58 | 64 | 65 | 62 | 70 | 64 ± 5 | |
| 0.32 | 98 | 99 | 99 | 97 | 99 | 98 ± 1 | |
| 3 | 0.0032 | 8 | 8 | 5 | 10 | 16 | 9 ± 4 |
| 0.032 | 44 | 46 | 50 | 51 | 64 | 51 ± 8 | |
| 0.32 | 95 | 95 | 95 | 94 | 96 | 95 ± 1 | |
Test-retest reproducibility of SB estimates under baseline conditions was mostly below 10% for all regions examined in the three animals (mean, 10 ± 2%). Table 1 shows the benzodiazepine-binding-site occupancy by TPA023 in the regions examined in the three baboons. Figure 5 shows the average time activity curves for [11C]flumazenil in the frontal cortex, cerebellum, and pons of three animals.
Assessment of Chronic Delivery and Withdrawal of Intragastric TPA023
Food-Maintained Responding.
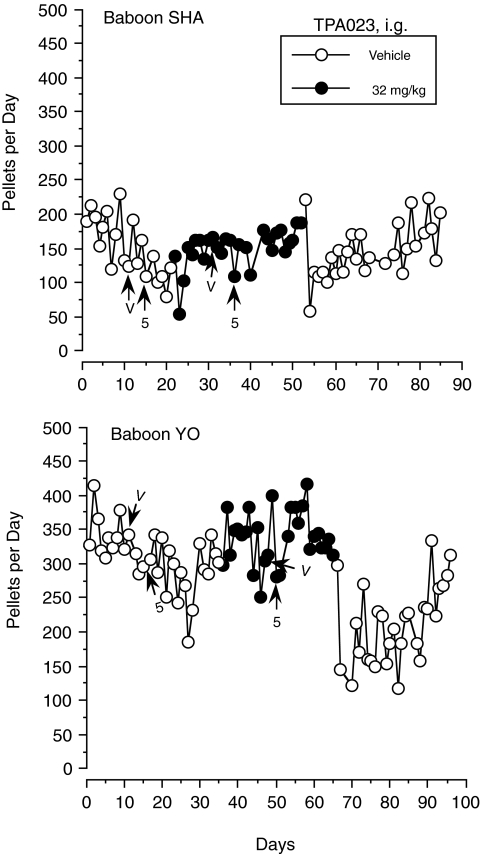
In the vehicle baseline condition in Fig. 6, pellets/day ranged from 79 to 230 for baboon SHA (mean, 157; S.D., 43; for 21 days) and 186 to 415 for YO (mean, 309; S.D., 45; for 35 days). Both baboons showed a decreasing trend across baseline, but the baseline period for YO was longer (for logistical reasons) and the trend reversed. When TPA023 (32 mg/kg/24 h) was delivered by slow chronic intragastric drip, pellets/day remained within the range of the vehicle baseline throughout the 31 days of drug delivery for baboon SHA (mean, 157; S.D., 19) and the 30 days for baboon YO (mean, 341; S.D., 39) (Fig. 6).
Fig. 6.
Numbers of 1-g food pellets earned per day by each baboon during (left to right) the TPA023 vehicle baseline, the period of continuous delivery of TPA023 32 mg/kg/24 h, and after TPA023 vehicle was substituted to assess the effects of drug withdrawal. Pellets were available for 20 consecutive hours each day under an FR-10 schedule of reinforcement beginning at approximately 9:00 AM. Shaded symbols indicate days on which either 5 mg/kg flumazenil (5) or its vehicle (V) was injected intramuscularly approximately 30 to 60 min after the period of pellet availability began. Data were omitted for days on which interpretation was confounded by ketamine administration for physical examination or an equipment problem that interrupted the period of pellet availability (i.e., vehicle baseline: day 16 for SHA; days 12, 16, and 29 for YO; TPA023 delivery: day 17 for both; and days 20 and 21 for SHA; vehicle substitution: day 21 for both; days 17–19 and 28 for SHA and days 3 and 4 for YO).
Baboons SHA and YO weighed 15.8 and 24.3 kg, respectively, when the vehicle baseline began, and they weighed 16.3 and 26.5 kg 6 or 7 days before beginning chronic drug delivery. After 17 days on TPA023 (32 mg/kg/24 h), baboon SHA's weight was the same (16.3 kg) and YO had gained slightly (28.1 kg). Blood samples were taken on day 17 as well. Unfortunately, the analysis for baboon YO was lost, but the value of TPA023 for SHA was 151.92 ng/ml. Given baboon YO's much higher weight, it is likely his value was as high or higher than SHA's value.
When chronic drug delivery ended (at 8:30 AM on withdrawal day 1), pellets/day for baboon SHA increased to 221 on that day, decreased to 58 for day 2, was at the lower end of the vehicle baseline range on days 3 to 6, but began an increasing trend on day 7, stabilizing at the higher end of the baseline range during the 33 days in which withdrawal was assessed (Fig. 6). For baboon YO, pellets/day dropped to 146 on day 2 of drug withdrawal and was generally low across the next 18 days (ranging from 121 to 270) before beginning an increasing trend and regaining vehicle baseline range in the last six of the 30 days in which withdrawal was assessed (Fig. 6). On day 20 of withdrawal, baboon SHA weighed 16 kg and YO weighed 27.6 kg, which were slightly lower than weights during the drug condition but higher than during vehicle baseline. Blood samples taken that day indicated that levels of TPA023 were below the limit of quantitation (5 ng/ml) for both baboons.
Withdrawal Scores.
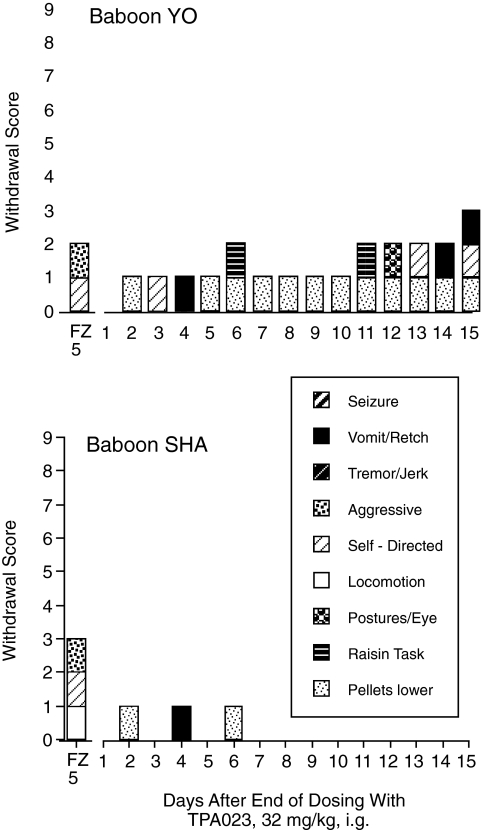
When flumazenil was administered after 14 days of TPA023 (32 mg/kg/24 h), withdrawal scores were 2 or 3 out of a possible 9 for the two baboons (Fig. 7). Baboon SHA showed a slight increase in locomotion; both baboons showed increases in self-directed behaviors (scratching and nose-wiping) and in aggression (lunging, and also the “yawn” threat for SHA). When the TPA023 vehicle was substituted for 32 mg/kg/24 h after 30 (YO) or 31 (SHA) days of continuous drug delivery, withdrawal score for the first 24 h after drug delivery ended was 0 for both baboons (Fig. 7). Withdrawal scores then were 0 to 1 for SHA and 1 to 3 for YO on days 2 through 15 of withdrawal. These scores were largely a function of the lowered number of food pellets described above, but each baboon also had one (SHA) or three (YO) observations during which vomit/retch occurred. Baboon YO also was slower than normal in completing the test of fine motor control on days 6 and 11 and spent time on day 12 in a head-lower-than-torso posture that has been correlated with nausea (Fig. 2 in Lukas and Griffiths, 1982).
Fig. 7.
Withdrawal scores for assessment of withdrawal from continuous intragastric administration of TPA023 32 mg/kg/24 h. Precipitated withdrawal was assessed in a 60-min observation session that followed injection of 5 mg/kg i.m. flumazenil (FZ) (bar over FZ 5). Spontaneous withdrawal was assessed in daily 15-min observation sessions beginning the day after substitution of TPA023 vehicle for TPA023 (32 mg/kg/24 h i.g.) (bars over days 1–15). The maximal possible score was 9; 1 point was assigned for each of the nine behaviors (listed in the key) only if its frequency during the observation session was significantly different from control (see text).
Discussion
The purpose of the present study was to establish whether the self-administration and dependence potential previously reported for a variety of nonselective full-agonist benzodiazepines and for compounds with selective affinity for the α1-GABAA/benzodiazepine receptor subtype (such as zolpidem) also would occur with the GABAA/benzodiazepine receptor subtype-selective compounds TPA123 and TPA023. TPA123, as well as the nonselective full-agonist comparator lorazepam, maintained dose-dependent self-administration; and increased food-reinforced behavior, which is consistent with the effects of benzodiazepine agonists. Evidence for development of physical dependence during TPA123 self-administration also was found. In marked contrast, TPA023 failed to maintain self-administration even under conditions of probable full receptor occupancy; and assessment of month-long, high-dose TPA023 administration revealed neither adverse effects nor more than a mild withdrawal syndrome. The data suggest that TPA023's unique GABAergic efficacy profile is sufficient to eliminate the reinforcing efficacy of a benzodiazepine agonist and to diminish dependence potential.
Self-Administration.
The TPA123 data are consistent with those from self-administration study of the nonselective benzodiazepine partial agonist bretazenil, but they show more reliable self-injection than those with the partial agonist imidazenil studied under comparable conditions (Ator, 2002) (N. A. Ator, unpublished data). In comparison, the nonselective full benzodiazepine agonist lorazepam not only served as a reinforcer, thus replicating a previous finding with lorazepam in baboons (Griffiths et al., 1991), but also maintained a higher rate of self-injections/day than TPA123. The total lack of self-administration of TPA023 in the present study is relatively unique among benzodiazepine ligands in our experience. Only the β-carboline partial agonist abecarnil has failed to maintain self-administration (Sannerud et al., 1992).
PET Findings.
To rule out the possibility that lack of self-administration of TPA023 might be due to failure to penetrate brain and occupy benzodiazepine binding sites, PET experiments examined its ability to displace in vivo binding of [11C]flumazenil. These studies clearly showed a dose-dependent displacement of [11C]flumazenil by TPA023 such that at the highest dose tested there was essentially complete inhibition in the specific binding of the radiotracer, which indicates that TPA023 was occupying all the GABAA/benzodiazepine receptor binding sites. Moreover, this occupancy is probably due to TPA023 rather than any metabolite(s) because the major metabolites (the isobutanol and NH-triazole) have very poor brain penetration (Atack, 2009).
The extent of occupancy of benzodiazepine binding sites seen at either 0.032 (60–70%) or 0.32 mg/kg (∼100%) TPA023 was much higher than that achieved in humans by using clinically relevant doses of lorazepam (6%) (Lingford-Hughes et al., 2005) or hypnotic doses of midazolam (≤35%) (Malizia et al., 1996), diazepam (24%) (Pauli et al., 1991), clonazepam (15–24%) (Shinotoh et al., 1989), or zolpidem (21%) (Abadie et al., 1996). These data are consistent with the facts that full-efficacy agonists require much lower levels of occupancy than partial agonists to produce their pharmacological effects (Brouillet et al., 1991) and that TPA023 behaves like a weak partial agonist in vivo (Atack et al., 2006).
Food-Maintained Responding.
Benzodiazepine agonists characteristically increase food-maintained responding in laboratory studies (Cooper and Estall, 1985), and this has been reported in the context of self-administration of zaleplon, zolpidem, and triazolam in baboons as well as during chronic intragastric administration of those compounds (Griffiths et al., 1992; Weerts et al., 1998; Ator, 2000; Ator et al., 2000). In the present study, self-administered lorazepam dose-dependently increased food-maintained responding compared with vehicle. TPA123 also increased food-maintained responding during self-injection conditions, even when the dose was not self-administered at a rate significantly higher than vehicle. Thus, selective partial agonism per se did not eliminate this benzodiazepine effect, which is consistent with feeding literature on nonselective benzodiazepine partial agonists (Yerbury and Cooper, 1987). It is interesting, however, that chronic intragastric TPA023 administration did not increase (or decrease) food-maintained responding, although day-to-day variability was reduced compared with baseline.
Physiological Dependence.
Previous studies of benzodiazepine ligands in baboons have found that chronic administration of nonselective and αl-selective full agonists yielded evidence for a dependence process after intragastric diazepam, lorazepam, triazolam, zolpidem, and zaleplon (Lukas and Griffiths, 1982; Lamb and Griffiths, 1984; Weerts et al., 1998; Ator et al., 2000) and intravenous zolpidem (Griffiths et al., 1992). In the present study, feeding decreased in a dose-dependent manner when vehicle was substituted for self-administered lorazepam and TPA123; other occasions in which intravenous TPA123 access ended abruptly also were accompanied by signs consistent with a withdrawal syndrome. Thus, it seems that the α2/α3-selective partial agonist efficacy profile of TPA123 is sufficient to support development of physiological dependence in the context of chronic administration. Whether the spontaneous withdrawal profile after chronic intragastric delivery would be milder than occurs in withdrawal from full benzodiazepine agonists remains to be determined.
Because TPA023 was not self-administered, we could not evaluate effects of drug withdrawal as with TPA123. Rather, a study of chronic intragastric delivery of a high TPA023 dose was carried out with methods comparable with those in studies cited above. Flumazenil, given after 2 weeks of round-the-clock intragastric TPA123, produced behaviors characterized as “mild” withdrawal signs among those that characterize benzodiazepine or barbiturate withdrawal in monkeys (Ator et al., 2000). When TPA023 delivery ended after 30 or 31 days, generally mild withdrawal signs also occurred in the first 2 weeks. The only “moderate” withdrawal sign observed was retching/vomiting, which occurred for each baboon on at least one occasion. Also unique is the fact that the moderate withdrawal sign of tremors/jerks never was observed in either precipitated or spontaneous TPA023 withdrawal. In contrast, assessments of flumazenil-precipitated and spontaneous withdrawal with full benzodiazepine agonists have found vomit/retch and tremor/jerk to be prominent and repeated signs in each animal. Although feeding behavior in the present study decreased in spontaneous withdrawal, the extent and duration of the decrease was markedly less than with the benzodiazepine full agonists mentioned above.
Mechanisms of Benzodiazepine Reinforcing and Dependence-Producing Efficacy.
The present data with TPA123 suggest that selectively reducing GABAergic efficacy at α2 and α3 subtypes below 50% and reducing α1 and α5 efficacy even more is not sufficient to eliminate reinforcing efficacy of a benzodiazepine agonist, nor its ability to produce physiological dependence.
Compared with the nonselective full agonists and to TPA123, TPA023 differs in two important respects. First, it only potentiates the actions of GABA at GABAA receptors containing an α2 or α3 subunit. Second, its efficacy at the α2 and α3 subtypes is much lower than a full agonist, such as lorazepam, and is lower than that of TPA123. It is not possible to discriminate which of these factors is primarily responsible for the reinforcing efficacy of benzodiazepines per se and for the negligible self-administration of TPA023.
The fact that zolpidem lacks affinity for the α5 subtype yet was robustly self-administered in nonhuman primates (Griffiths et al., 1992; Ator, 2002; Rowlett et al., 2005) strongly suggests that α5-containing GABAA receptors are not related to benzodiazepine abuse potential. It initially seemed to us (Ator, 2005) that the α1 subtype must play a prominent role in mediating reinforcing effects of benzodiazepine agonists given the high rates of self-administration of the α1-preferring compounds zolpidem and zaleplon in contrast to the lack of TPA023 self-administration. L-838,417 has no α1 efficacy, however, and has only partial agonism at the α2, α3, and α5 subtypes (McKernan et al., 2000), but it did maintain self-administration (Rowlett et al., 2005). Thus, although elimination of α1 efficacy may play a role in reinforcing efficacy of benzodiazepine agonists and elimination of α5 efficacy seems not to be influential, reduction of efficacy at α2/α3 subtypes below 35% may be the critical element of TPA023's profile of GABA modulation that was responsible for lack of self-administration.
It is interesting that chronic high-dose administration of TPA023 for 30 or 31 days failed to produce more than a mild withdrawal syndrome, and a syndrome that was absent the usual tremor/jerks component of benzodiazepine withdrawal in nonhuman primates. Thus, it seems that the selective efficacy profile for TPA023 not only does not produce drug reinforcement but also results in diminished propensity for physiological dependence.
Implications.
Whether the lack of abuse-liability and reduced dependence potential of TPA023 in baboons is a result of no α1 efficacy and/or reduced α2/α3 efficacy, the key issue is whether this profile translates into humans. In this regard, baboon self-administration studies agree well with measures of drug-liking in subjects with a history of drug abuse. For example, the classical full-agonist benzodiazepines diazepam, lorazepam, flunitrazepam, and triazolam as well as the α1-selective drugs zaleplon and zolpidem, all self-administered in baboons, were “liked” by drug abusers. Conversely, the β-carboline benzodiazepine agonist abecarnil was not self-administered in baboons and was not liked in the same-day assessment in human drug abusers (for review, see Ator and Griffiths, 2003). By analogy, the present data suggest that TPA023 may also have a much reduced abuse liability relative to nonselective benzodiazepines when tested in human studies. Coupled with its nonsedating anxiolytic properties (Atack et al., 2006; Atack, 2009) and evidence that TPA023 has reduced potential for producing physiological dependence, TPA023, or drugs of a similar selective efficacy profile (Mirza et al., 2008), may be the route to a significant advance in the treatment of generalized anxiety disorder.
Acknowledgments
We thank Christine Ryan, Sandra Sanabria-Bohórquez, and Eric Hostetler at Merck Research Laboratories (West Point, PA) for expert assistance with the PET studies. At Johns Hopkins, we thank Christine Ebaugh and Samuel Womack for technical assistance with the self-administration studies; Kelly Lane for technical assistance with the TPA023 dependence study; Michael McDermott, April Jones, and Dr. Amy Goodwin for the observations for the dependence study; and Susan James for preparation of graphics for behavioral study data. Special acknowledgment goes to the members of the GABA drug development research team at Merck Sharp & Dohme Research Laboratories (Neuroscience Research Centre, Terlings Park, Harlow, Essex, UK) without whom these studies would not have been possible.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA04133] (for the study of lorazepam and manuscript preparation by N.A.A.); Merck Research Laboratories (West Point, PA) for PET studies and blood plasma analyses; and Merck, Sharp & Dohme for behavioral studies with TPA123 and TPA023.
Portions of these data were presented: Ator NA (2002) Differential abuse liability profiles of GABA-A subtype selective compounds. Annual Meeting of the American College of Neuropsychopharmacology; 2002 Dec 7–11; San Juan, Puerto Rico. American College of Neuropsychopharmacology, Nashville, TN; and conclusions on self-administration were reviewed in Ator (2005).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158303
- L-838417
- 7-(1,1-dimethylethyl)-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2,5-difluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine
- TPA123
- 7-Cyclobutyl-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-3-phenyl-1,2,4-triazolo[4,3-b]pyridazine
- TPA023
- 7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine
- PET
- positron emission tomography
- FR
- fixed ratio
- TAC
- time-activity curve
- SUV
- standard uptake value
- SB
- specific binding.
References
- Abadie P, Rioux P, Scatton B, Zarifian E, Barré L, Patat A, Baron JC. (1996) Central benzodiazepine receptor occupancy by zolpidem in the human brain as assessed by positron emission tomography. Eur J Pharmacol 295:35–44 [DOI] [PubMed] [Google Scholar]
- Atack JR. (2005) The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs 14:601–618 [DOI] [PubMed] [Google Scholar]
- Atack JR. (2009) Subtype-selective GABAA receptor modulation yields a novel pharmacological profile: the design and development of TPA023. Adv Pharmacol, in press [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, et al. (2006) TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a non-sedating anxiolytic in rodents and primates. J Pharmacol Exp Ther 316:410–422 [DOI] [PubMed] [Google Scholar]
- Ator NA. (2000) Zaleplon and triazolam: drug discrimination, plasma levels, and self-administration in baboons. Drug Alcohol Depend 61:55–68 [DOI] [PubMed] [Google Scholar]
- Ator NA. (2002) Relation between discriminative and reinforcing effects of midazolam, pentobarbital, chlordiazepoxide, zolpidem, and imidazenil in baboons. Psychopharmacology 163:477–487 [DOI] [PubMed] [Google Scholar]
- Ator NA. (2005) Contributions of GABAA receptor subtype selectivity to abuse liability and dependence potential of pharmacological treatments for anxiety and sleep disorders. CNS Spectr 10:31–39 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70 ( Suppl 3): S55–S72 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, Weerts EM. (2005) Self-injection of flunitrazepam alone and in the context of methadone maintenance in baboons. Drug Alcohol Depend 78:113–123 [DOI] [PubMed] [Google Scholar]
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. (2000) Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend 61:69–84 [DOI] [PubMed] [Google Scholar]
- Brouillet E, Chavoix C, Bottlaender M, Khalili-Varasteh M, Hantraye P, Fournier D, Dodd RH, Mazière M. (1991) In vivo bidirectional modulatory effect of benzodiazepine receptor ligands on GABAergic transmission evaluated by positron emission tomography in non-human primates. Brain Res 557:167–176 [DOI] [PubMed] [Google Scholar]
- Carling RW, Madin A, Guiblin A, Russell MGN, Moore KW, Mitchinson A, Sohal B, Pike A, Cook SM, Ragan IC, et al. (2005) 7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine: a functionally selective γ-aminobutyric acidA (GABA(A)) α2/α3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models. J Med Chem 48:7089–7092 [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Estall LB. (1985) Behavioural pharmacology of food, water and salt intake in relation to drug actions at benzodiazepine receptors. Neurosci Biobehav Rev 9:5–19 [DOI] [PubMed] [Google Scholar]
- Dawson GR, Collinson N, Atack JR. (2005) Development of subtype selective GABAA modulators. CNS Spectr 10:21–27 [DOI] [PubMed] [Google Scholar]
- de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, Cohen AF, Vega JM, Agrawal NG, Goel TV, et al. (2007) Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol 21:374–383 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. (1991) Self-injection of barbiturates, benzodiazepines and other sedative-anxiolytics in baboons. Psychopharmacology 103:154–161 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. (1992) Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther 260:1199–1208 [PubMed] [Google Scholar]
- Higgitt A, Fonagy P. (1993) Benzodiazepine dependence syndromes and syndromes of withdrawal, in Benzodiazepine Dependence (Hallström C. ed) pp 58–70, Oxford Medical Publications, Oxford, UK [Google Scholar]
- Lader M. (1993) Historical development of the concept of tranquilizer dependence, in Benzodiazepine Dependence (Hallström C. ed) pp 46–57, Oxford Medical Publications, Oxford, UK [Google Scholar]
- Lamb RJ, Griffiths RR. (1984) Precipitated and spontaneous withdrawal in baboons after chronic dosing with lorazepam and CGS 9896. Drug Alcohol Depend 14:11–17 [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Wilson SJ, Feeney A, Grasby PG, Nutt DJ. (2005) A proof-of-concept study using [11C]flumazenil PET to demonstrate that pagoclone is a partial agonist. Psychopharmacology 180:789–791 [DOI] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR. (1982) Precipitated withdrawal by a benzodiazepine receptor antagonist (Ro 15-1788) after 7 days of diazepam. Science 217:1161–1163 [DOI] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR, Bradford LD, Brady JV, Daley L. (1982) A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol Biochem Behav 17:823–829 [DOI] [PubMed] [Google Scholar]
- Malizia AL, Gunn RN, Wilson SJ, Waters SH, Bloomfield PM, Cunningham VJ, Nutt DJ. (1996) Benzodiazepine site pharmacokinetic/pharmacodynamic quantification in man: direct measurement of drug occupancy and effects on the human brain in vivo. Neuropharmacology 35:1483–1491 [DOI] [PubMed] [Google Scholar]
- McCabe C, Shaw D, Atack JR, Street LJ, Wafford KA, Dawson GR, Reynolds DS, Leslie JC. (2004) Subtype-selective GABAergic drugs facilitate extinction of mouse operant behaviour. Neuropharmacology 46:171–178 [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor α1 subtype. Nat Neurosci 3:587–592 [DOI] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, Nielsen AN, Troelsen KB, Nielsen EØ, Ahring PK. (2008) NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther 327:954–968 [DOI] [PubMed] [Google Scholar]
- O'Brien CP. (2005) Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 66( Suppl 2): 28–33 [PubMed] [Google Scholar]
- Pauli S, Farde L, Halldin L, Sedvall G. (1991) Occupancy of the central benzodiazepine receptors during benzodiazepine treatment determined by PET. Eur J Neuropsychopharmacol 1:229 [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. (2005) Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A 102:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H. (1999) Benzodiazepine actions mediated by specific γ-aminobutyric acid(A) receptor subtypes. Nature 401:796–800 [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Ator NA, Griffiths RR. (1992) Behavioral pharmacology of abecarnil in baboons: self-injection, drug discrimination and physical dependence. Behav Pharmacol 3:507–516 [PubMed] [Google Scholar]
- Shinotoh H, Iyo M, Yamada T, Inoue O, Suzuki K, Itoh T, Fukuda H, Yamasaki T, Tateno Y, Hirayama K. (1989) Detection of benzodiazepine receptor occupancy in the human brain by positron emission tomography. Psychopharmacology 99:202–207 [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795–816 [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. (1998) Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther 285:41–53 [PubMed] [Google Scholar]
- Williams DD, McBride A. (1998) Benzodiazepines: time for reassessment. Br J Psychiatry 173:361–362 [DOI] [PubMed] [Google Scholar]
- Yerbury RE, Cooper SJ. (1987) The benzodiazepine partial agonists, Ro 16-6028 and Ro 17-1812, increase palatable food consumption in nondeprived rats. Pharmacol Biochem Behav 28:427–431 [DOI] [PubMed] [Google Scholar]