Abstract
In abstinent alcoholics, stress induces negative affect—a response linked to craving and relapse. In rats, repeated stresses at weekly intervals before 5-day ethanol diet sensitize withdrawal-induced anxiety-like behavior (“anxiety”) that is blocked by a corticotrophin-releasing factor 1 (CRF-1)-receptor antagonist. Current experiments were performed to identify brain sites that support CRF involvement in stress sensitization of ethanol withdrawal-induced anxiety-like behavior. First, different doses of CRF microinjected weekly into the central amygdala (CeA) before ethanol exposure produced a dose-related sensitization of anxiety during ethanol withdrawal. Subsequently, CRF microinjection into the basolateral amygdala, dorsal raphe nucleus (DRN), or dorsal bed nucleus of the stria terminalis (d-BNST) also sensitized ethanol withdrawal-induced anxiety. In contrast, sensitization of ethanol withdrawal-induced anxiety was not observed after weekly CRF administration into the ventral-BNST, CA1-hippocampal region, or hypothalamic-paraventricular nucleus. Then, experiments documented the CRF receptor subtype responsible for CRF and stress sensitization of withdrawal-induced anxiety. Systemic administration of a CRF-1 receptor antagonist before CRF microinjection into the CeA, DRN, or d-BNST prevented CRF-induced sensitization of anxiety during ethanol withdrawal. Furthermore, repeated microinjections of urocortin-3, a CRF-2 receptor agonist, into the CRF-positive sites did not sensitize anxiety after withdrawal from ethanol. Finally, microinjection of a CRF-1 receptor antagonist into the CeA, DRN, or d-BNST before stress blocked sensitization of anxiety-like behavior induced by the repeated stress/ethanol withdrawal protocol. These results indicate that CRF released by stress acts on CRF-1 receptors within specific brain regions to produce a cumulative adaptation that sensitizes anxiety-like behavior during withdrawal from chronic ethanol exposure.
Considerable evidence supports involvement of corticotrophin-releasing factor (CRF), a 41-amino-acid peptide (Vale et al., 1981), in stress (Koob and Heinrichs, 1999; Bale and Vale, 2004), in production of anxiety (Spina et al., 2002), and in the expression of anxiety-like behavior (“anxiety”) during withdrawal from chronic ethanol (Baldwin et al., 1991; Overstreet et al., 2004). Previous work demonstrated that repeated stresses before the 5-day chronic ethanol diet (stress/withdrawal protocol) sensitized anxiety during withdrawal (Breese et al., 2004). Subsequently, a CRF-1 receptor antagonist prevented this sensitization (Breese et al., 2004), whereas repeated intracerebroventricular administrations of CRF before ethanol exposure substituted for stress to induce sensitization (Overstreet et al., 2004). Because it has been shown that corticosterone induced by CRF activation of the hypothalamic pituitary adrenal axis was not responsible for the stress/withdrawal-induced sensitization (Breese et al., 2004), these efforts provided critical support for an extrahypothalamic action of CRF being responsible for the sensitization.
An important aspect not previously explored is the neuroanatomical basis of CRF involvement in repeated stress sensitization. CRF (Cummings et al., 1983; Swanson et al., 1983) and CRF receptors (De Souza et al., 1985) are localized to regions of the extended amygdala (Alheid, 2003), sites that are reportedly related to anxiety-like behavior (Koob, 2008). Therefore, it was reasoned that sites that support CRF-induced sensitization could be identified by repeatedly microinjecting CRF into appropriate brain sites before chronic ethanol exposure. Furthermore, by administering a CRF-1 receptor antagonist into CRF-positive brain regions the role of CRF in repeated stress/withdrawal protocol sensitization of anxiety (Breese et al., 2004) could be confirmed.
Several brain regions known to be associated with anxiety-like behavior, including the central nucleus of the amygdala (CeA), the basolateral amygdala (BLA), the dorsal raphe nucleus (DRN), and the bed nucleus of the stria terminalis (BNST) contain CRF (Cummings et al., 1983; Swanson et al., 1983) and CRF receptors (De Souza et al., 1985; Van Pett et al., 2000). Because microinjection of a general CRF receptor antagonist into the CeA reversed the anxiogenic response to acute ethanol withdrawal (Baldwin et al., 1991; Rassnick et al., 1993), this site was the first to be chosen for investigation. The DRN was chosen because this site has been shown to have an association with sensitization of anxiety induced by repeated withdrawals (Overstreet et al., 2006). The BNST was chosen because it is linked to the amygdala (Dong et al., 2001) and has an association with fear- and anxiety-associated behaviors (Davis et al., 1997; Sahuque et al., 2006; Lee et al., 2008). In addition to these brain sites, the paraventricular nucleus (PVN) of the hypothalamus and the CA1 region of the hippocampus were tested with CRF. The PVN is a critical brain site for control of the hypothalamic pituitary adrenal axis (Rivier et al., 1983) and also contains CRF (Swanson et al., 1983) and CRF receptors (De Souza et al., 1985; Van Pett et al., 2000), but has not been associated with anxiety-like behavior. The CA1-hippocampal region was chosen because it has neural interactions with the amygdala (Akirav and Richter-Levin, 1999; Sheth et al., 2008) and there are CRF receptors present at this site (De Souza et al., 1985; Van Pett et al., 2000).
Even though CRF reportedly has approximately a 17-fold greater affinity for CRF-1 receptors than CRF-2 receptors (Vaughan et al., 1995; Hauger et al., 2003), we sought to confirm previous evidence for CRF-1 receptor involvement in the CRF/withdrawal (Overstreet et al., 2004) and stress/withdrawal (Breese et al., 2004) protocols. Testing for involvement of specific CRF-receptor subtypes in sensitization of withdrawal-induced anxiety was identified by administering a CRF-1 receptor antagonist systemically before repeated CRF microinjection or by substituting urocortin-3, a CRF-2 receptor agonist (Lewis et al., 2001), for CRF microinjection into selected brain sites before the 5 days of ethanol diet. Urocortin-3 has virtually no effect on CRF-1 receptors. In addition, to confirm the involvement of CRF-1 receptors in stress sensitization of withdrawal-induced anxiety (Breese et al., 2004), a CRF1-receptor antagonist was administered before each repeated stress application into sites where microinjected CRF was found to sensitize ethanol withdrawal-induced anxiety-like behavior.
Collectively, the present investigations sought to extend our understanding of the neuroanatomical basis of CRF involvement in the repeated stress sensitization of ethanol withdrawal-induced anxiety and document the CRF receptor subtype linked to this sensitization. In addition, these data were expected to confirm that stress and CRF induce a cumulative maladaptation that is made apparent only when followed by ethanol exposure and withdrawal.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, or Harlan, Indianapolis, IN) weighing 180 to 200 g were housed in groups of three or four for several days to acclimate to the local conditions (at 22°C and 40% humidity; dark/light cycle of 12:12 with lights on at 7:00 AM and off at 7:00 PM). After acclimation, the rats underwent surgery as described below. After surgery the animals were individually housed and, after several days of recovery, were placed on a nutritionally complete lactalbumin-dextrose diet and ultimately microinjected centrally as per the strategies outlined. All procedures for the animals were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Surgery for Cannula Implantation.
Surgery to implant stainless steel cannulae into brain sites was performed under 2.5% isoflurane anesthesia. While anesthetized, a rat was placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The dorsal surface of the skull was exposed, and holes were drilled over the appropriate region for placement of the cannulae. The cannulae (26-gauge stainless steel tubing) were directed dorsal to the brain site being microinjected with use of coordinates from a rat atlas (Paxinos and Watson, 2005). The rats received bilateral cannula implants over the CeA (AP = −2.3, ML = −4.5, DV = −5.5), BLA (AP = −2.3, ML = −5, DV = −6), the CA1 region of the hippocampus (AP = −4, ML = −3, DV −1.5), and either the dorsal (AP = −0.24, ML = −1.6, DV = −2) or the ventral (AP = −4, ML = −3, DV = −1.5) BNST. In addition, unilateral cannulae were implanted above the lateral ventricle for the intracerebroventricular injections (AP = −0.8, ML = −1.6, DV = −2). Because the DRN (AP = +1.56 from lambda, ML = −3.5, DV = −4.94) and the PVN of the hypothalamus (AP = −1.88, ML = 3, DV = −6.5) are midline structures, bilateral injections were not needed. All injections were angled to these midline locations from the drill holes on the right side of the skull. Insertion angles were 30° for the DRN and 20.7° for the PVN. The distances from the cannula tips to the injection sites were as follows: CeA, 2.5 mm; BLA, 2.5 mm; DRN, 2 mm; d-BNST, 4.5 mm; v-BNST, 4.5 mm; hippocampus, 2 mm; PVN, 2.05 mm; intracerebroventricular, 2.5 mm. Targeted brain sites and limits for acceptance of data are discussed below. Cannulae were secured to the skull with stainless steel screws and acrylic dental cement (Frye et al., 1983; Overstreet et al., 2006; Knapp et al., 2007). Once recovered from surgery, the rats were given acetaminophen (Children's Q-PAP, cherry flavored, 6 mg/ml) in the drinking water for 48 h. The rats were allowed to recover for at least 3 days before proceeding with further experimental procedures.
Procedures for Diet Administration.
After the recovery period, the rats were given a nutritionally complete control liquid diet (Frye et al., 1983; Overstreet et al., 2002). Rats received a calorically balanced and nutritionally complete control liquid diet for 12 days and were then placed on either a similar liquid diet containing 4.5% ethanol for 5 days or continued receiving the control diet (Overstreet et al., 2002). Rats were weighed at weekly intervals, and volumes of diet were adjusted to ensure that groups within a given investigation gained weight similarly. Previous reports (Overstreet et al., 2002; Breese et al., 2004) have demonstrated that this exposure to 4.5% ethanol diet produces blood ethanol levels of approximately 80 to 110 mg% before removal. The ethanol is reduced to near 0 by 5 to 6 h (Overstreet et al., 2002; Breese et al., 2004)—the time at which behaviors related to social interaction are monitored (see below).
Procedures for Microinjection of CRF and Urocortin-3 into Selected Brain Regions on Sensitization of Ethanol Withdrawal-Induced Anxiety.
Our laboratory prepares animals routinely for microinjection (Frye et al., 1983; Overstreet et al., 2006; Knapp et al., 2007). While on a liquid diet containing no ethanol, rats received microinjections of CRF (0.045–0.5 μg/0.5 μl per site) and urocortin-3 (0.5 μg/0.5 μl per site or 5 μg/5 μl i.c.v.) or vehicle (as appropriate) into the selected brain regions on days 6 and 12 in a temporal sequence comparable with that used with the repeated-stress exposure protocol (Breese et al., 2004). The general protocol for treatment is illustrated in Fig. 1. CRF was administered into the CeA, BLA, DRN, d-BNST, v-BNST, CA1 of the hippocampus, or the PVN of the hypothalamus. Urocortin-3 was microinjected intracerebroventricularly or into the CeA, DRN, and d-BNST. The doses of CRF and urocortin-3 were dissolved in an artificial cerebrospinal fluid (aCSF) and delivered into each brain site through 32-gauge injectors placed into the implanted cannula guides. During placement of the injector needle into a given brain site, the animals were gently restrained in a cotton towel. The maximal dose of CRF was administered weekly into the CeA before 5 days of control diet to demonstrate a lack of effect of this treatment on social interaction. A 1-μl syringe delivered the 0.5 μl of the drug solution into each of the brain sites (per side, if bilateral). In addition, a urocortin-3 dose (5 μg/5 μl i.c.v.) was administered into the ventricular cannula over a 1-min period by use of a 5-μl syringe. Urocortin-3 is active on CRF-2 receptors with virtually no affinity for CRF1 receptors (Lewis et al., 2001). The injector remained in place for 1 min after the end of the infusion. Five days of 4.5% ethanol diet was initiated 24 h after microinjection of CRF or urocortin-3 followed by determination of social interaction behavior during withdrawal from the ethanol as noted below.
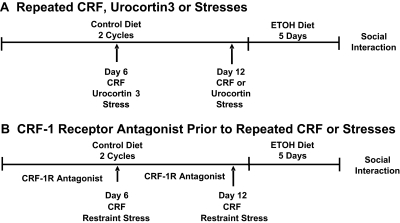
Fig. 1.
Protocol for repeated administration of CRF, urocortin 3, or repeated stresses before 5 days of chronic ED to assess for deficits in social interaction: drug treatments. A, at the arrows on days 6 and 12, CRF or urocortin 3 was microinjected weekly into selected brain regions or 60 min of restraint stress was applied followed by 5 days of 4.5% ethanol liquid diet. For these experiments, some groups received control diet for the entire study period and were given either CRF, urocortin 3, or vehicle. A group received vehicle (i.e., no other treatment) before the ethanol diet. Social interaction, a test of anxiety-like behavior, was measured 5 to 6 h after removal of the ethanol diet exposure. B, as a further approach to investigate the role of CRF receptor subtype involved in the repeated CRF or stress-induced sensitization of ethanol withdrawal anxiety-like behavior, a CRF-1 receptor (CRF-1R) antagonist was administered intraperitoneally 15 min before the microinjection of CRF or was microinjected into each of the CRF-positive sites 15 min before the application of restraint stress on days 6 and 12. As noted for A, some groups of rats received only control diet that had vehicle microinjected or vehicle applied before stress. Likewise, a group that received only 5 days of ethanol diet received vehicle. Social interaction test was conducted 5 to 6 h after removal of the ethanol diet exposures.
Testing of a CRF-1 Receptor Antagonist on CRF Sensitization of Ethanol Withdrawal-Induced Anxiety.
The CRF-1 receptor antagonist SSR125543 (10 mg/kg i.p.; sanofi-aventis, Bridgewater, NJ) (Griebel et al., 2002) or CP-154,526 (10 mg/kg i.p.; Tocris Bioscience, Ellisville, MO) (Schulz et al., 1996) was prepared as a suspension in 0.5% carboxymethylcellulose and 2.5% Tween 80. Both antagonists were used to confirm a blockade of CRF action. The CRF-1 receptor antagonist or the corresponding vehicle was given intraperitoneally 15 min before each of the repeated microinjections of CRF (0.5 μg in 0.5 μl prepared in aCSF) into designated brain sites. The 5 days of 4.5% ethanol diet started on day 13 as outlined in Fig. 1. Social interaction was measured between 5 and 6 h of withdrawal from the ethanol diet as noted below.
Testing of a CRF-1 Receptor Antagonist into Selected Brain Sites on Stress Sensitization of Ethanol Withdrawal-Induced Anxiety.
To test CRF involvement in the CeA, DRN, or d-BNST in the sensitization of ethanol withdrawal-induced anxiety elicited by the stress/withdrawal protocol (Breese et al., 2004), rats were treated with either the CRF1-receptor antagonist SSR125543 (10 μg/0.5 μl) or vehicle in each brain site 15 min before each stress session during exposure to the control liquid diet. The SSR125543 was dissolved in the aCSF containing 2.5% Tween 80. The stress sessions consisted of restraining rats in plastic conical decapicones for 60 min on days 6 and 12. Either a single 5-day cycle of 4.5% ethanol diet was initiated 24 h after the final stress or the control diet was continued (Breese et al., 2004). Social interaction changes were measured during withdrawal from ethanol diet as noted below.
Social Interaction Test.
The social interaction test was given 5 h after the ethanol diet was withdrawn—a time at which the ethanol levels reach zero (Overstreet et al., 2002; Breese et al., 2004). In contrast to the original testing method of File (1980) that emphasized the score of the rat pair (see review by File and Seth, 2003), the social interaction measure for this investigation used the behavior of each rat in the pair, as established previously (Overstreet et al., 2002, 2003). For testing, each rat was placed in a test box (65 × 65 cm with 47-cm walls) with an unfamiliar test partner for 5 min to evaluate social interaction (Overstreet et al., 2002). An observer blind to treatments scored the time rats spent in active social contact. Behaviors monitored included sniffing, nipping, grooming, mounting, kicking, wrestling, jumping on, and crawling under or over the partner (Overstreet et al., 2002; File and Seth, 2003). A locomotor activity score was also obtained based on crossings over a grid (Overstreet et al., 2002).
Histological Confirmation of Injection Sites.
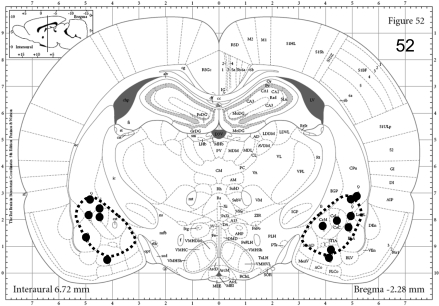
To confirm appropriate placement, the cannula sites were defined histologically at the end of each experimental series. In this case, 0.5 μl of methyl green dye was microinjected into the brain site as described by Knapp et al. (2007) with use of the same injectors used to microinject CRF, urocortin-3, and the CRF-1 receptor antagonist. After euthanizing the rat, the brain was removed, frozen on dry ice, and stored at −80°C until sections were cut on a microtome to confirm the site of injection by an individual blind to the treatments. To provide a representation of placement of microinjections, Supplemental Material is provided noting the limits for acceptance. Figure 3 demonstrates specific injection sites for CRF identified in the CeA. Only animals with the correct cannula locations were used in the statistical analysis. In general, proper cannula placement was between 80% and 90%.
Fig. 3.
Representative dye spots derived from post-mortem histological assessments of 0.5-μl injections for the 0.5-μg CRF injections into the CeA. The image is adapted from Paxinos and Watson (2005). The hatched lines represent approximate limits of injection variability deemed acceptable. The dots represent the estimated centers of dye spots found upon histological examination. See Materials and Methods for the targeted coordinates for this region. The other brain sites of focus are included in the Supplemental Material.
Statistics.
The measures obtained from the testing of anxiety (seconds of social interaction), locomotor test (crossings), ethanol intake, and body weight were analyzed with analysis of variance followed by Fisher's protected least significant difference tests for comparisons of individual groups within an investigation. When data for differing brain sites for either the CD-vehicle or the ED-vehicle groups were combined (see Figs. 4, 5, and 8), no significant difference was observed in social interaction across the different brain regions for CD vehicle [F(5,61) = 0.748; P > 0.1] and for ED vehicle [F(5,66) = 0.696; P > 0.1]. Therefore, vehicle data for either the CD-vehicle group or the ED-vehicle group from data sets that contained results from different brain sites were combined for the individual experimental illustrations (figures).
Fig. 4.
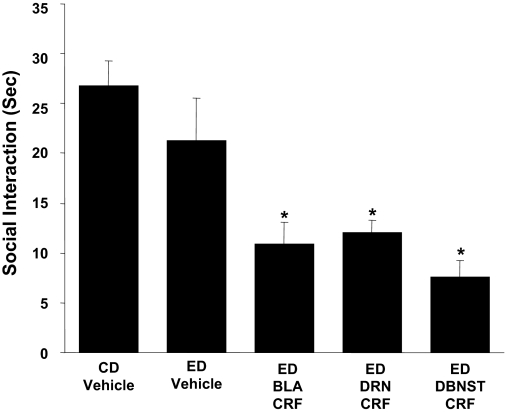
Repeated CRF into the BLA, DRN, and d-BNST before ethanol diet reduces social interaction behaviors during withdrawal from chronic ethanol. The CRF dose (0.5 μg/0.5 μl) was microinjected twice at weekly intervals into the BLA, DRN, or d-BNST before exposure to 5 days of 4.5% ED (see Fig. 1 for protocol). A representation of the site and limits at which CRF was administered into each of these brain sites is presented in supplementary material. In the CD-vehicle and ED-vehicle groups, vehicle was administered into each of the brain sites (n = 3–4 for each site) and data for these vehicle injections were combined because a significant change across sites was not observed for these groups. When social interaction for the CD-vehicle group was compared with the ED-vehicle group, no significant effect was observed (P > 0.05). A group that received CRF and was on control diet only was not included for each of the present sites because previous data demonstrated that intracerebroventricular administration of CRF to rats that received control diet does not induce sensitization (Overstreet et al., 2004) and the repeated CRF in the CeA of control diet-treated animals likewise did not sensitize withdrawal-induced anxiety (Fig. 2). Social interaction was measured 5 to 6 h after the ethanol diet removal. The number of rats for each group is listed in Table 1, part 2. Representative sites where CRF was microinjected are presented in Supplemental Material. *, Significantly different from CD-vehicle and ED-vehicle [F(4,42) = 9.227, P < 0.001].
Fig. 5.
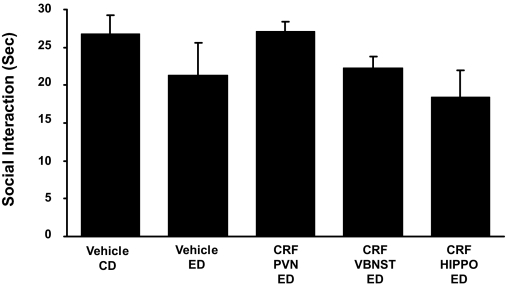
Absence of effect of repeated CRF microinjections into the PVN, v-BNST, or the CA1 region of the hippocampus (HIPPO) on social interaction behaviors during withdrawal from chronic ethanol. The CRF dose (0.5 μg), which sensitized ethanol withdrawal-induced anxiety after injection into CeA (Fig. 2) and other sites (Fig. 4), was microinjected twice at weekly intervals into the PVN, v-BNST, or the CA1 of the HIPPO before exposure to 5 days of 4.5% ED (see Fig. 1 for protocol). In the CD-vehicle and the ED-vehicle groups, vehicle was administered into each of the brain sites (n = 3 or 4/site). Because a significant change was not observed across sites, the data for the sites were combined. No significant effect on social interaction (P > 0.05) was observed during ethanol withdrawal when the CD-vehicle group was compared with the ED-vehicle group. A representation of brain region site at which CRF injections were aimed for each of these brain sites is presented in Supplemental Material. Social interaction was measured 5 to 6 h after the ethanol diet removal. The number of rats for each group is listed in Table 1, part 3. None of the CRF treatments caused a significant change in social interaction [F(4,40) = 1.487, P > 0.05].
Fig. 8.
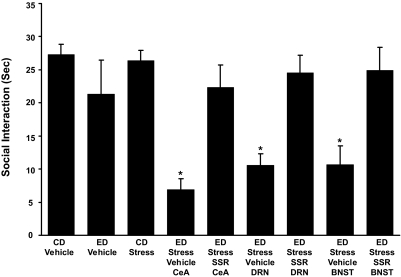
Microinjection of CRF-1 receptor antagonist (SSR) into the CeA, DRN, or d-BNST before restraint stress prevents sensitization of ethanol withdrawal-induced anxiety-like behavior. The CRF-1 receptor antagonist SSR125543 (SSR; 10 μg/0.5 μl) was microinjected into the CeA, DRN, or the d-BNST 15 min before the two weekly 60-min restraint stresses before exposure to 5 days of 4.5% ED (see Fig. 1 for protocol). Social interaction was measured 5 to 6 h after the ethanol diet removal. The anxiogenic effect of stress on ethanol withdrawal-induced anxiety was blocked by the SSR125543 microinjected in the selected brain sites. The number of rats for each group is listed in Table 1, part 6. For the CD-vehicle and ED-vehicle groups, vehicle was administered into each of the brain sites (n = 3–6 for each site). The vehicle data for each of these controls were combined because a significant change across sites was not observed. No significant effect on social interaction (P > 0.05) was observed during ethanol withdrawal when the CD-vehicle group was compared with the ED-vehicle group. A representation of brain site at which CRF injections were aimed for each site is presented in Supplemental Material. *, P < 0.001 compared with the CD-vehicle, ED-vehicle, and CD stress groups as well as the groups that received the CRF-1 receptor antagonist before the repeated stresses. [F(8, 75) = 7.385, *, P < 0.001].
Results
CRF Dose Effect in the CeA on Sensitization of Ethanol Withdrawal-Induced Anxiety.
Repeated microinjections of CRF into the CeA before 5 days of ethanol diet were used to assess the potential action of this peptide within this site to sensitize ethanol withdrawal-induced anxiety. As shown in Fig. 2, the repeated CRF microinjections into the CeA reduced social interaction in a dose-related fashion indicating that the CeA can support the action of CRF to induce sensitization of withdrawal-induced anxiety. The location of the injection sites for the 0.5-μg CRF dose administered in the CeA is illustrated (Fig. 3).
Fig. 2.
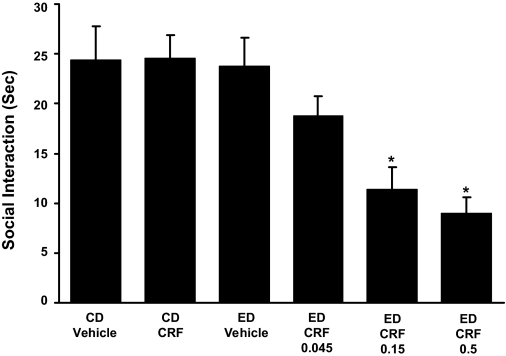
Repeated administration of CRF into the CeA before ethanol diet reduces social interaction behaviors during withdrawal from chronic ethanol: dose-effect relationship. CRF (0.045 μg, 0.15 μg, or 0.5 μg) was given twice at weekly intervals into the CeA before exposure to 5 days of 4.5% ED (see Fig. 1 for illustration of the protocol). A representation of the sites at which CRF (0.5 μg) was administered into the CeA is presented in Fig. 3. Social interaction, as a measure of anxiety-like behavior, was measured 5 to 6 h after ethanol diet removal. No effect on social interaction was observed during ethanol withdrawal in rats that received only 5 days of 4.5% ethanol diet with vehicle injections (ED vehicle) or that received the two injections of CRF (0.5 μg) into the CeA while animals were consuming CD. The number of rats in each group is provided in Table 1, part 1. *, Significantly different from CD-vehicle, CD-CRF, and ED-vehicle groups [F(5,41) = 7.278, P < 0.001].
Consistent with early studies of intracerebroventricular injections of CRF (Overstreet et al., 2004), two 0.5-μg doses of CRF, which produced a maximal effect on social interaction when administered before ethanol (Fig. 1), did not alter social interaction when microinjected into the CeA before 5 days of control diet (CD-CRF). Because the lowest dose of CRF was without effect in alcohol-exposed rats, it would unlikely that lower doses would have an action in controls. Likewise, repeated vehicle microinjections before the 5 days of ethanol diet were without effect (ED vehicle). These findings collectively demonstrate the need for the weekly doses of CRF to be given in combination with ethanol diet to produce the reflection of adaptive change (i.e., a reduction in social interaction). In addition, consistent with previous data which showed that social interaction scores for vehicle are equivalent to values in rats that do not receive central microinjections (Overstreet et al., 2002; Breese et al., 2004), unpublished work showed that the social interaction score for CD vehicle was not different from that of rats that receive no surgery or vehicle. However, when results for the ED-CRF groups are compared with the social interaction score obtained with the repeated CRF dosing alone before control diet (CD-CRF) or the ethanol diet alone (ED vehicle), it is apparent that the combination of CRF and ethanol is required for induction of sensitization of withdrawal-induced anxiety. The first part of Table 1 indicates the number of rats in each group for Fig. 2 and demonstrates that measures for locomotor activity, body weight, and alcohol intake for groups did not differ.
Table 1.
Ethanol intakes, body weights, and locomotor activity for groups in Figs. 2 and 4 through 8
In the Treatment Group column, the number in parentheses is the n per group.
| Treatment Group | Alcohol Intake | Body Weight | Locomotor Activity |
|---|---|---|---|
| g/kg/day | g | crosses/5 min | |
| 1. CRF Dose Response on Social Interaction (Fig. 2) | |||
| CD Veh (7) | – | 336 ± 5 | 112 ± 8 |
| CD CRF-0.5 (9) | – | 324 ± 6 | 131 ± 7 |
| ED Veh (7) | 7.42 ± 0.24 | 328 ± 5 | 99 ± 7 |
| ED CRF-0.045 (8) | 7.87 ± 0.14 | 328 ± 6 | 100 ± 10 |
| ED CRF-0.15 (8) | 7.70 ± 0.19 | 331 ± 6 | 113 ± 13 |
| ED CRF-0.5 (8) | 7.76 ± 0.18 | 330 ± 7 | 96 ± 10 |
| NS | NS | NS | |
| 2. CRF Microinjections into the BLA, DRN, and d-BNST on Social Interaction (Fig. 4) | |||
| CD Veh (12) | – | 320 ± 9 | 88 ± 15 |
| ED Veh (10) | 7.08 ± 0.20 | 341 ± 5 | 83 ± 18 |
| ED BLA CRF (7) | 7.53 ± 0.06 | 330 ± 4 | 97 ± 7 |
| ED DRN CRF (8) | 7.36 ± 0.36 | 325 ± 8 | 69 ± 8 |
| ED d-BNST CRF (10) | 7.23 ± 0.27 | 302 ± 6 | 63 ± 7 |
| NS | NS | NS | |
| 3. CRF Microinjections into the v-BNST, PVN, and CA1-Hippocampus on Social Interaction (Fig. 5) | |||
| CD Veh (9) | – | 312 ± 11 | 95 ± 20 |
| ED Veh (10) | 7.56 ± 0.49 | 325 ± 6 | 92 ± 15 |
| ED V BNST CRF (8) | 8.56 ± 0.18 | 304 ± 12 | 72 ± 10 |
| ED PVN CRF (9) | 8.45 ± 0.21 | 292 ± 10 | 90 ± 12 |
| ED Hippo CRF (9) | 8.17 ± 0.18 | 340 ± 7 | 72 ± 10 |
| NS | NS | NS | |
| 4. CRF-Induced Sensitization of Anxiety Blockade by CRF-1 Receptor Antagonists (Fig. 6) | |||
| CD Veh (13) | – | 315 ± 9 | 91 ± 14 |
| ED Veh (22) | 7.47 ± 0.17 | 333 ± 7 | 94 ± 10 |
| ED CeA CRF (9) | 6.91 ± 0.24 | 321 ± 10 | 89 ± 12 |
| ED SSR i.p. CeA CRF (8) | 8.35 ± 0.22 | 317 ± 7 | 119 ± 11 |
| ED DRN CRF (8) | 8.52 ± 0.20 | 306 ± 8 | 94 ± 10 |
| ED CP i.p. DRN CRF (8) | 7.75 ± 0.19 | 315 ± 9 | 81 ± 13 |
| ED d-BNST CRF (8) | 7.32 ± 0.14 | 312 ± 11 | 77 ± 16 |
| ED SSR i.p. d-BNST CRF (8) | 7.24 ± 0.26 | 326 ± 5 | 80 ± 12 |
| NS | NS | NS | |
| 5. Urocortin-3 into CeA, DRN, and d-BNST on Social Interaction (Fig. 7) | |||
| CD Veh (8) | – | 306 ± 4 | 119 ± 10 |
| CD UCN-3 (8) | – | 319 ± 5 | 121 ± 10 |
| ED Veh (14) | 7.37 ± 0.18 | 323 ± 5 | 111 ± 8 |
| ED CeA UCN-3 (8) | 7.97 ± 0.28 | 327 ± 5 | 109 ± 4 |
| ED DRN UCN-3 (11) | 7.60 ± 0.26 | 328 ± 8 | 102 ± 8 |
| ED d-BNST UCN-3 (8) | 7.60 ± 0.20 | 306 ± 11 | 122 ± 7 |
| ED i.c.v. UCN-3 (9) | 7.90 ± 0.18 | 304 ± 9 | 116 ± 7 |
| NS | NS | NS | |
| 6. Blockade of Stress Sensitization by CRF-1 Antagonist into the CeA, DRN, or d-BNST (Fig. 8) | |||
| CD Veh (18) | – | 310 ± 7 | 127 ± 10 |
| CD Stress (10) | – | 298 ± 6 | 132 ± 10 |
| ED Veh (9) | 7.57 ± 0.32 | 314 ± 9 | 112 ± 17 |
| ED Stress Veh CeA (7) | 8.24 ± 0.19 | 295 ± 8 | 111 ± 16 |
| ED Stress SSR CeA (8) | 8.26 ± 0.21 | 303 ± 6 | 135 ± 16 |
| ED Stress Veh DRN (8) | 7.70 ± 0.30 | 312 ± 8 | 132 ± 11 |
| ED Stress SSR DRN (11) | 8.20 ± 0.25 | 309 ± 11 | 129 ± 13 |
| ED Stress Veh d-BNST (6) | 8.01 ± 0.24 | 300 ± 12 | 130 ± 12 |
| ED Stress SSR d-BNST (7) | 8.33 ± 0.24 | 285 ± 7 | 129 ± 6 |
| NS | NS | NS | |
N.S., no significant difference noted among the groups for any of the measures for each of the six experiments (1–6); Hippo, hippocampus; Veh, vehicle; SSR, SSR125543.
Repeated CRF Microinjections into the BLA, DRN, or d-BNST Sensitize Ethanol Withdrawal-Induced Anxiety.
To evaluate whether CRF would induce sensitization when microinjected into other brain sites that contained CRF receptors (De Souza et al., 1985; Van Pett et al., 2000) and that have been associated with anxiety-like behaviors (Davis et al., 1997; Overstreet et al., 2006; Lee et al., 2008), CRF was microinjected into the BLA, DRN, and d-BNST. As shown in Fig. 4, a 0.5-μg CRF dose repeatedly microinjected into either the BLA, the DRN, or the d-BNST at a weekly interval before 5 days of ethanol diet decreased social interaction during ethanol withdrawal—a response indicative of enhanced anxiety-like behavior. Thus, just as seen when CRF was microinjected into the CeA (Fig. 2), CRF injected into each of these additional brain sites also decreased social interaction during ethanol withdrawal. See Supplemental Data for additional information on these sites.
Figure 4 also demonstrates that microinjection of vehicle into the selected brain sites before ethanol diet (ED vehicle) or in the control diet controls (CD vehicle) was without effect on social interaction (see Materials and Methods for details). Because repeated intracerebroventricular administration of CRF (Overstreet et al., 2004) and repeated administration of CRF into the CeA (Fig. 2) before control diet did not sensitize ethanol withdrawal-induced anxiety, the repeated CRF administration into all of the brain sites before control diet was not deemed necessary. See Table 1, part 2, for the numbers of rats in each group and data illustrating that treatments had no significant effect on locomotion during testing and showing the equal weight gain for these groups.
Repeated CRF Microinjections into the PVN of the Hypothalamus, v-BNST, or the CA1 Region of the Hippocampus Do Not Sensitize Ethanol Withdrawal-Induced Anxiety.
In addition to the brain regions described above, CRF (0.5 μg) action on sensitization of ethanol withdrawal-induced anxiety was investigated in other brain areas. Because the BNST has two distinct regions (Egli and Winder, 2003), CRF was repeatedly microinjected into the v-BNST before administration of 5 days of ethanol diet. The other brain regions included the PVN of the hypothalamus and the CA1 region of the hippocampus. Apparent from Fig. 5 and in contrast to the brain regions described previously (Figs. 2 and 4), the repeated CRF microinjections into these brain sites (i.e., PVN, v-BNST, and CA1) before the 5 days of 4.5% ethanol diet did not reduce social interaction during withdrawal from the 5-day single cycle of ethanol diet compared with the groups that received vehicle into sites—a reflection of a lack of sensitization of ethanol withdrawal-induced anxiety-like behavior by CRF in these brain regions. See Supplementary Data for further information on these sites. As before, alcohol intake, body weight, and locomotor activity for groups did not differ (Table 1, part 3).
Systemic Administration of CRF-1 Receptor Antagonists Prevents Microinjected CRF-Induced Sensitization of Withdrawal-Induced Anxiety from the CeA, DRN, and d-BNST.
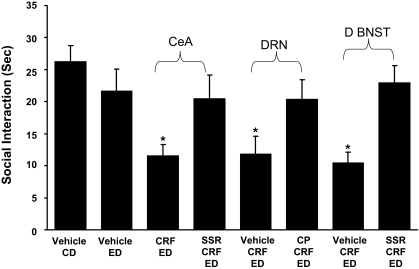
Previous work has demonstrated that CRF has greater affinity for and more functional activity from CRF-1 receptors than from CRF-2 receptors (Vaughan et al., 1995; Hauger et al., 2003). To address whether CRF-1 receptors, but not CRF-2 receptors, were involved in the CRF-induced sensitization of ethanol withdrawal-induced anxiety-like behavior from the CeA, DRN, and d-BNST, CRF-1 receptor antagonists were given 15 min before each of the CRF microinjections. The CRF-1 receptor antagonist SSR125543 (10 mg/kg i.p.; Griebel et al., 2002) was given before each of the CRF microinjections into the CeA and d-BNST. In addition, CP154526 (10 mg/kg i.p.; Schulz et al., 1996) was administered before each of the CRF microinjections into the DRN to confirm that a different CRF-1 receptor antagonist would have an effect similar to the SSR125543 in another CRF-sensitive brain site. Results confirm that CRF into each of these brain sites sensitizes ethanol withdrawal-induced anxiety as shown in Figs. 2 and 4. In each of the cases, this action of CRF to induce sensitization of ethanol withdrawal-induced anxiety was prevented by the CRF-1 receptor antagonists (Fig. 6). These findings are consistent with CRF action on CRF-1 receptors being critical to the sensitization induced from these brain sites. As before, locomotor activity, body weight, and alcohol intake were not significantly affected by any of these treatments (Table 1, part 4).
Fig. 6.
CRF-1 receptor antagonist blockade of CRF-induced sensitization of ethanol withdrawal-induced anxiety-like behavior from the central amygdala, dorsal raphe, and d-BNST. A 10 mg/kg dose of the CRF-1 receptor antagonist, SSR125543 (SSR), was administered intraperitoneally 15 min before the microinjection of CRF (0.5 μg) into either the central amygdala or the d-BNST followed by the 5 days of ethanol diet. Meanwhile, another CRF-1 receptor antagonist CP154526 (CP; 10 mg/kg i.p.) was administered 15 min before each of the CRF (0.5 μg) microinjections into the dorsal raphe. See Fig. 1 for protocol. The action of CRF in these brain sites to induce sensitization of withdrawal-induced anxiety-like behavior was prevented by the CRF-1 receptor antagonist. Social interaction was measured 5 to 6 h after the ethanol diet removal. The number of rats for each group is listed in Table 1, part 4. In the CD-vehicle group and the ED-vehicle group, vehicle was administered into each of the brain sites (n = 4–6 for each site), and data were combined because a significant change across sites was not observed. No significant difference in social interaction during ethanol withdrawal (P > 0.05) was observed when the CD-vehicle group was compared with the ED-vehicle group. A representation of brain region at which CRF injections were aimed for each brain site is presented in Supplemental Material. *, P < 0.01 compared with vehicle CD- and vehicle ED-treated groups and the groups that received the SSR125543 systemically [F(7,76) = 3.005, P < 0.01].
Lack of Effect of Urocortin-3 on Sensitization of Ethanol Withdrawal-Induced Anxiety.
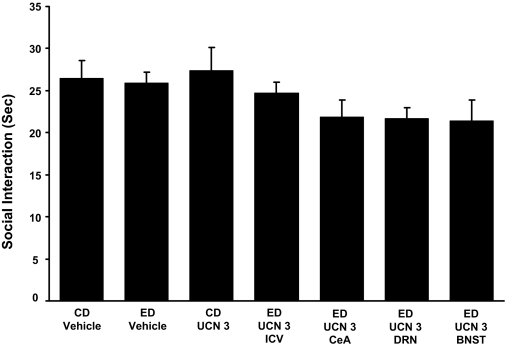
To confirm the conclusion that CRF-1, but not CRF-2 receptors, were critical to the action of repeated CRF administration before ethanol diet, we tested whether repeated microinjections of a dose of the CRF-2 receptor agonist, urocortin-3 (UCN-3) (Lewis et al., 2001) equivalent to that of the highest dose of CRF administered into the CeA (Fig. 2), the DRN, or the d-BNST would, like CRF, sensitize ethanol withdrawal-induced anxiety-like behavior. Urocortin-3 has virtually no measurable effect on the CRF-1 receptor (Lewis et al., 2001). As shown in Fig. 7, this dose of UCN-3 was without a significant effect when microinjected into these brain sites that supported the action of CRF. Finally, to rule out the possibility that UCN-3 might have an effect in other brain sites than those that support the CRF sensitization, UCN-3 was repeatedly injected intracerebroventricularly before the 5-day ethanol exposure. Again, UCN-3 showed no effect on sensitization of ethanol withdrawal-induced anxiety. These findings provided further evidence for CRF-1 receptors being critical to the action of CRF to sensitize ethanol withdrawal-induced anxiety from the CeA, DRN, and the d-BNST. Locomotor activity, body weight, and alcohol intake were not significantly different for any of the treatments (Table 1, part 5).
Fig. 7.
Repeated UCN-3 microinjected intracerebroventricularly, or into the central amygdala or the dorsal raphe, before ethanol diet was without effect on social interaction during withdrawal from chronic ethanol. The UCN-3 dose (0.5 μg/0.5 μl) was microinjected twice at weekly intervals into the CeA, DRN, or the d-BNST before exposure to 5 days of 4.5% ED (see Fig. 1). Meanwhile, UCN-3 (5 μg/5 μl) was microinjected intracerebroventricularly in another group. Social interaction was measured 5 to 6 h after the ethanol diet removal. In ED-vehicle group, vehicle was administered into each of the brain sites (n = 4–6 for each site), and data were combined because a significant change across sites was not observed. No significant difference in social interaction during ethanol withdrawal (P > 0.05) was observed when the CD-vehicle group was compared with the ED-vehicle group. No significant difference on social interaction was observed during ethanol withdrawal between rats that received UCN-3 or the ones that received vehicle injections (ED vehicle and CD vehicle). UCN-3 by itself did not induce anxiety either (CD UCN 3). [F(6,59) = 1.838, P > 0.05]. The n for each group is listed in Table 1, part 5.
CRF-1 Receptor Antagonist Blocks Anxiogenic Effect of Stress after Microinjection into the CeA, DRN, or the d-BNST.
Breese et al. (2004) showed that two applications of 60-min restraint stresses at weekly intervals preceding 5 days of 4.5% ethanol diet significantly reduced social interaction upon ethanol withdrawal. To confirm that this anxiogenic-like effect of stress before ethanol was mediated by CRF at CRF-positive brain sites, the CRF-1 receptor antagonist SSR125543 (10 μg/μl) was microinjected into the CeA, DRN, or the d-BNST 15 min before the initiation of each of the 60-min restraint stresses. As shown in Fig. 8, the anxiogenic effect of ethanol withdrawal from the stress/withdrawal protocol was completely blocked by the CRF-1 receptor antagonist at each of the brain sites. Previous work has demonstrated that a CRF-1 receptor antagonist given before ethanol has no effect on social interaction during withdrawal from 5 days of ethanol diet (Overstreet et al., 2004). In agreement with results from other treatments, the locomotion, body weight, and alcohol intake for the groups were not significantly affected by treatments (Table 1, part 6).
Discussion
Ballenger and Post (1978) hypothesized that multiple detoxifications worsen symptoms of withdrawal. In support of this hypothesis, animal studies demonstrated that repeated withdrawals from chronic ethanol increase seizure susceptibility (McCown and Breese, 1990) and sensitize withdrawal-induced anxiety-like behavior (Overstreet et al., 2002). Subsequently, Overstreet et al. (2007) reported that CRF-1 receptor antagonist administration before the first and second withdrawals of the multiple withdrawal protocol prevented sensitization of anxiety-like behavior by this protocol, an observation implicating CRF in the worsening symptoms of ethanol withdrawal from chronic exposure. Based on a relationship of CRF to stress, it was reported that repeat stresses given before a single 5-day exposure to ethanol diet (stress/withdrawal protocol) substituted for repeated withdrawals to sensitize anxiety-like behavior (“anxiety”) during withdrawal from a single 5-day exposure to chronic ethanol exposure that alone was without effect (Breese et al., 2004). To assess whether the stress-induced sensitization of withdrawal-induced anxiety was related to a central action of CRF, CRF was repeatedly administered intracerebroventricularly to substitute for the stress exposure before the 5 days of ethanol diet (Overstreet et al., 2004). This repeated intracerebroventricular CRF administration sensitized withdrawal-induced anxiety—a finding suggesting that stress involves a central, not a peripheral mechanism, to induce this emotional change during ethanol withdrawal (Overstreet et al., 2004). This conclusion is consistent with the finding that repeated peripheral administration of a glucocorticoid before chronic ethanol exposure does not sensitize withdrawal-induced anxiety (Breese et al., 2004).
The concept that a cumulative adaptation induced by the repeated chronic ethanol exposures was responsible for the negative consequences during withdrawal was initially proposed by Koob (2003) to be a change in allostasis—a concept consistent with that proposed by Ballenger and Post (1978). The observation that CRF-1 receptor antagonist administration before the first and second withdrawals of the multiple withdrawal protocols prevented sensitization of withdrawal-induced anxiety was consistent with this concept (Overstreet et al., 2004). In the present investigation a CRF-1 receptor antagonist administered before each of the stress and CRF applications also prevented sensitization of withdrawal-induced anxiety-like behavior. These findings are consistent with the idea that both CRF and stress promote a cumulative adaptive mechanism that affects the subsequent magnitude of ethanol withdrawal—an adaptation that can also enhance the negative affect induced by a future stress during abstinence from ethanol (Valdez et al., 2003; Breese et al., 2005a,b,c).
Because others demonstrated that CRF administered into the CeA was related to the anxiety-like behavior seen during ethanol withdrawal (Baldwin et al., 1991; Rassnick et al., 1993), it was next considered whether this or other regions of brain were involved in the cumulative adaptation by stress and CRF that supported sensitization of ethanol withdrawal-induced anxiety-like behavior. When microinjected into the CeA, CRF, as expected, induced a dose-related sensitization of withdrawal-induced anxiety. Furthermore, repeated CRF administrations into the BLA had a sensitizing effect similar to that for CRF microinjection into the CeA (Figs. 2 and 4). Collectively, the positive action of repeated CRF administrations into these differing components of the amygdala to induce sensitization of anxiety during ethanol withdrawal clearly demonstrates the probable importance of CRF receptors in the amygdala in the cumulative adaptation associated with the repeated stress/withdrawal protocol sensitization of symptoms during ethanol withdrawal. Based on these findings in the amygdala, additional brain sites were investigated for CRF-induced sensitization of ethanol withdrawal-induced anxiety. Repeated CRF exposure of the DRN likewise sensitized withdrawal-induced anxiety-like behavior. Because the BNST has been overlooked in evaluations of involvement in sensitization of withdrawal-induced anxiety, both the dorsal and ventral components of the BNST (Egli and Winder, 2003) were individually microinjected with CRF. Consistent with previous work showing that CRF induces anxiety-like behavior when microinjected into this site (Sahuque et al., 2006), repeated CRF microinjection into the d-BNST before ethanol diet sensitized withdrawal-induced anxiety (Fig. 4), although CRF administered into the v-BNST did not (Fig. 5). Collectively, these data indicate that CRF is capable of acting within several regions of the brain to sensitize withdrawal-induced anxiety-like behavior. Consequently, in contrast to other investigations, the focus of the present study provided an unexpected outcome in that several brain sites injected with CRF supported equally the degree to which an adaptive action resulted in sensitization of withdrawal-induced anxiety-like behavior. The relevance and neural basis of several sites being equally effective for CRF to induce sensitization of ethanol withdrawal-induced anxiety will need clarification in future experiments.
Despite CRF presence in the hippocampus and the PVN, microinjection of CRF into these sites did not support the CRF-induced sensitization of withdrawal-induced anxiety (Fig. 5). A sensitization by CRF into the PVN might not have been expected, because repeated administration of corticosterone does not substitute for repeated stresses to sensitize withdrawal-induced anxiety (Breese et al., 2004). However, this finding also suggests that any action of CRF in the PVN by stress does not activate other brain regions that support the stress-induced anxiety-like behavior that follows ethanol withdrawal. Despite evidence that CA1 hippocampal pyramidal cells have been investigated extensively with respect to plasticity changes by stress (McEwen, 2001) and chronic ethanol (Liang et al., 2006), repeated CRF into this brain site did not support sensitization of withdrawal-induced anxiety. Therefore, the plasticity of this brain site associated with chronic ethanol and stress probably supports other functions not associated with the adaptation that results in sensitization of anxiety during ethanol withdrawal (e.g., depressive-like behavior).
To confirm that the sensitization of ethanol withdrawal-induced anxiety by the repeated CRF exposures was related to activation of a specific CRF-receptor subtype, two approaches were undertaken (Figs. 6 and 7). First, systemic administration of a CRF-1 receptor antagonist before repeated CRF microinjection into the CeA, the DRN, or the d-BNST was capable of preventing the CRF-induced sensitization of anxiety. In further support of the repeated CRF acting on CRF-1 and not CRF-2 receptors to induce sensitization, UCN-3, an endogenous agonist specific for CRF-2 receptors (Lewis et al., 2001), did not sensitize withdrawal-induced anxiety when microinjected into the CeA, DRN, and d-BNST, the three brain sites associated with CRF-induced sensitization of anxiety during ethanol withdrawal. In addition, UCN-3, when injected intracerebroventricularly, did not elicit ethanol withdrawal-induced anxiety either. Collectively these results indicate that CRF-2 receptor activation in the CeA, DRN, or d-BNST is not responsible for the CRF-induced sensitization of anxiety during ethanol withdrawal. Likewise, because intracerebroventricular administration of UCN-3 was without effect, CRF-2 receptors from other brain regions do not seem to be involved in the stress sensitization either.
To define whether CRF release at the CeA, DRN, or d-BNST sites was activated by the repeated stress/withdrawal protocol to induce sensitization of withdrawal-induced anxiety, the CRF-1 receptor antagonist SSR 125543 was microinjected into each of these sites before each stress (Fig. 8). The anxiogenic response during ethanol withdrawal from repeated stresses was completely blocked by the CRF-1 receptor antagonist microinjected into each of these brain sites, a finding that strongly supports the stress/withdrawal protocol inducing anxiety through activation of CRF-1 receptors in the CeA, DRN, or d-BNST. Thus, these results are consistent with CRF-1 receptors being the primary target for sensitization of anxiety during withdrawal from ethanol induced by the CRF/withdrawal and stress/withdrawal protocols (Breese et al., 2004; Overstreet et al., 2004).
The tension-reduction hypothesis of alcoholism was published several decades ago to suggest that the consequence of stress per se increased alcohol drinking (Conger, 1956); however, more recent studies (Breese et al., 2004; Overstreet et al., 2007) raised the possibility that adaptation induced by stresses before a bout of alcohol abuse can also contribute to adaptive change much as repeated exposures to ethanol (Ballenger and Post, 1978; Overstreet et al., 2002). Bale and Vale (2004) have reviewed the evidence for CRF involvement in stress. The present effort provides evidence that stress exposure before ethanol depends on release of CRF onto CRF-1 receptors in specific brain sites that are capable of facilitating adaptive change induced by a lesser amount of ethanol—a change that results in a negative outcome during withdrawal from chronic ethanol (Breese et al., 2004, 2005a,b,c; Overstreet et al., 2004). In this respect, previous work has provided support for the conclusion that CRF contributes to adaptive change related to persistent alcohol exposure (Overstreet et al., 2004; Heilig and Koob, 2007). Furthermore, basic data (Breese et al., 2005a,b) indicate that stress after chronic ethanol can precipitate negative affect, a response which one could speculate is comparable with the negative affect observed in the abstinent alcoholic that results in craving (Sinha, 2001; 2008; Sinha et al., 2009). Both basic (Breese et al., 2005a,b) and clinical findings (Heilig et al., 2007) indicate that a CRF-1 receptor antagonist will prevent the negative consequences of stress after chronic ethanol exposure. Future investigations will be required to address whether chronic treatment of alcoholics administered a CRF-1 receptor antagonist could be a successful approach to minimize the increased susceptibility alcoholics display for increased craving to stress (Sinha, 2001; Yoon et al., 2006; Sinha et al., 2009).
Supplementary Material
Acknowledgments
We thank Kui-Ling Huang for assistance with the preparation and administration of liquid diets.
This work was supported by the National Institutes of Health [Grants AA11605, AA12655, AA14949, AA014284, AA007573, AA167704, and DK26741]; and by the University of North Carolina Bowles Center for Alcohol Studies.
Data presented in part as an abstract in Huang et al. (2008).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.159186
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- CRF
- corticotropin-releasing factor
- aCSF
- artificial cerebrospinal fluid
- BLA
- basolateral amygdala
- BNST
- dorsal bed nucleus of the stria terminalis
- d-BNST
- dorsal BNST
- v-BNST
- ventral BNST
- CeA
- central amygdala
- CD
- control diet
- DRN
- dorsal raphe nucleus
- ED
- ethanol diet
- i.c.v.
- intracerebroventricular
- PVN
- paraventricular nucleus
- AP
- anterior-posterior
- ML
- medial-lateral
- DV
- dorsal-ventral
- UCN-3
- urocortin-3
- SSR125543
- 4-(chloro-4-methoxy-5-methylphenyl)-N-((1S)-2-cyclopropylfluoro-4-methylphenyl)ethyl)5-methyl-N-(2-propynyl)-1,3-thiazamine
- CP154526
- butyl-(2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo(2,3-d)pyrimidin-4-yl)ethylamine.
References
- Akirav I, Richter-Levin G. (1999) Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci 19:10530–10535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. (2003) Extended amygdala and basal forebrain. Ann N Y Acad Sci 985:185–205 [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. (1991) CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 103:227–232 [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. (2004) CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557 [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. (1978) Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry 133:1–14 [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Lê DA, O'Dell LE, Overstreet DH, Roberts AJ, et al. (2005a) Stress enhancement of craving during sobriety: Risk of relapse. Alcohol Clin Exp Res 29:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. (2004) Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology 29:470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. (2005b) Conceptual framework for the etiology of alcoholism—a “kindling”/stress hypothesis. Psychopharmacology (Berl) 178:367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. (2005c) Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 30:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ. (1956) Alcoholism: theory, problem and challenge. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol 17:296–305 [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. (1983) Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci 3:1355–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. (1997) Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety-like behavior measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci 352:1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. (1985) CRF receptors are widely distributed with the rat central nervous system: an autoradiographic study. J Neurosci 5:3189–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38:192–246 [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. (2003) Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol 90:405–414 [DOI] [PubMed] [Google Scholar]
- File S. (1980) The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth 2:219–238 [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53 [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. (1983) Characterization of susceptibility of audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra, or medial septum. J Pharmacol Exp Ther 227:663–670 [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrié P. (2002) 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther 301:333–345 [DOI] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. (2003) International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55:21–26 [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurol Sci 30:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Cippitelli A, Gehlert D, Ciccocioppo R. (2007) Negative affect and excessive alcohol drinking driven by central CRF systems: Reversal by the novel CRF1 antagonist MTIP. Alcoholism Clin Exp Res 31:283A [Google Scholar]
- Huang M, Navarro M, Wills T, Knapp DJ, Overstreet DH, Angel R, Breese GR. (2008) Corticotropin releasing factor (CRF) has brain region specific effects on ethanol withdrawal-induced anxiety: relation to stress-induced drinking. Alcohol Clin Exp Res 32:79A [Google Scholar]
- Knapp DJ, Overstreet DH, Angel RA, Navarro M, Breese GR. (2007) The amygdala regulates the antianxiety sensitization effect of flumazenil during repeated chronic ethanol or repeated stress. Alcohol Clin Exp Res 31:1872–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2008) A role for brain stress systems in addiction. Neuron 59:11–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. (1999) A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848:141–152 [DOI] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. (2008) Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology 33:2586–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, et al. (2001) Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A 98:7570–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. (2006) Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci 26:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. (1990) Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res 14:394–399 [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2001) Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–277 [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. (2006) Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 187:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res 26:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. (2004) Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav 77:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. (2007) Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res 31:1473–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. (2003) A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology 167:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates, 5th ed., Elsevier Academic Press, Oxford, United Kingdom [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. (1993) Microinjection of CRF antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res 605:25–32 [DOI] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Vale W. (1983) Characterization of the rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci U S A 80:4851–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. (2006) Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 186:122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, et al. (1996) CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A 93:10477–10482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth A, Berretta S, Lange N, Eichenbaum H. (2008) The amygdala modulates neuronal activation in the hippocampus in response to spatial novelty. Hippocampus 18:169–181 [DOI] [PubMed] [Google Scholar]
- Sinha R. (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359 [DOI] [PubMed] [Google Scholar]
- Sinha R. (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. (2009) Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34:1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, Britton KT, Rivier J, Vale WW, Koob GF. (2002) Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl) 160:113–121 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. (2003) Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol 29:55–60 [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotrophin and beta-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212 [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. (1995) Urocortin, a mammalian neuropeptide related to fish urotensin I and the corticotopin-releasing factor. Nature 378:287–292 [DOI] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Grant JE, Westermeyer J. (2006) Alcohol craving in outpatients with alcohol dependence: rate and clinical correlates. J Stud Alcohol 67:770–777 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.