Abstract
The discriminative stimulus effects of dopamine (DA) D3/D2 receptor agonists are thought to be mediated by D2 receptors. To maintain responding, access to food is often restricted, which can alter neurochemical and behavioral effects of drugs acting on DA systems. This study established stimulus control with quinpirole in free-feeding rats and tested the ability of agonists to mimic and antagonists to attenuate the effects of quinpirole. The same antagonists were studied for their ability to attenuate quinpirole-induced yawning and hypothermia. DA receptor agonists apomorphine and lisuride, but not amphetamine and morphine, occasioned responding on the quinpirole lever. The discriminative stimulus effects of quinpirole were attenuated by the D3 receptor-selective antagonist N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide HCl (PG01037) and the nonselective D3/D2 receptor antagonist raclopride, but not by the D2 receptor-selective antagonist 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]methyl-1H-indole (L-741,626); the potencies of PG01037 and raclopride to antagonize this effect of quinpirole paralleled their potencies to antagonize the ascending limb of the quinpirole yawning dose-response curve (thought to be mediated by D3 receptors). L-741,626 selectively antagonized the descending limb of the quinpirole yawning dose-response curve, and both L-741,626 and raclopride, but not PG01037, antagonized the hypothermic effects of quinpirole (thought to be mediated by D2 receptors). Food restriction (10 g/day/7 days) significantly decreased quinpirole-induced yawning without affecting the quinpirole discrimination. Many discrimination studies on DA receptor agonists use food-restricted rats; together with those studies, the current experiment using free-feeding rats suggests that feeding conditions affecting the behavioral effects of direct-acting DA receptor agonists might also have an impact on the effects of indirect-acting agonists such as cocaine and amphetamine.
Many drugs of abuse as well as many drugs used in the clinic have actions on dopamine (DA) systems; however, there are a number of different DA receptors [i.e., D1-like (D1 and D5) and D2-like (D2, D3, and D4)], and the relative contribution of each receptor subtype to the abuse or therapeutic effects of drugs is not known. For example, DA D3 and D2 receptors are thought to mediate many of the behavioral effects of cocaine because some D3/D2 receptor agonists share behavioral (e.g., discriminative stimulus) effects with cocaine (Caine and Koob, 1993; Acri et al., 1995; Spealman, 1996; Sinnott et al., 1999). Drug discrimination procedures have been used extensively to investigate the role of different DA receptors, particularly D3 and D2, in mediating the behavioral effects of drugs such as cocaine that act indirectly through DA receptors. Collectively, studies conducted with rats suggest that the discriminative stimulus effects of a number of DA receptor agonists that bind to both D3 and D2 receptors are mediated through D2 receptors (Appel et al., 1988; Kleven and Koek, 1997; Bristow et al., 1998; Baker et al., 1999; Katz and Alling, 2000; Millan et al., 2000, 2007; Christian et al., 2001; Koffarnus et al., 2009). For example, the discriminative stimulus effects of the D3/D2 receptor agonist S32504 are antagonized by the D2 receptor-selective antagonist L-741,626 but not by the D3 receptor-selective antagonist S33084 (Millan et al., 2007), suggesting that the selective binding of S32504 to D3 receptors does not correlate with receptors mediating its discriminative stimulus effects. Moreover, the discriminative stimulus effects of the D3/D2 receptor agonist (+)-PD 128,907 are antagonized by the D2 receptor-selective antagonist L-741,626 and not by the D3 receptor-selective antagonist GR 103,691 (Bristow et al., 1998).
Most drug discrimination studies with DA receptor agonists in rats used food to maintain responding, thereby necessitating food restriction throughout the experiment. However, it is becoming clear that restricting access to food can change the behavioral and neurochemical effects of many drugs, including drugs acting on DA systems. For example, food restriction decreases extracellular DA (Pothos et al., 1995), increases D2 receptor binding (Thanos et al., 2008), and increases coupling between DA receptors and G proteins (Carr et al., 2003). Food restriction also decreases the sensitivity of rats to yawning induced by DA receptor agonists including quinpirole and pramipexole (Collins et al., 2008; Sevak et al., 2008; Baladi and France, 2009). DA receptor agonist-induced yawning generates an inverted U-shaped dose-response curve, with the ascending limb thought to be mediated by D3 receptors and the descending limb by D2 receptors, and the decreased sensitivity to agonist-induced yawning in food-restricted rats is thought to be due to increased sensitivity of D2 receptors (Collins et al., 2008). Although the discriminative stimulus effects of pramipexole are thought to be mediated by D2, but not D3, receptors (Koffarnus et al., 2009), it is unclear whether food restriction that modifies some effects of DA receptor agonists (e.g., yawning) also modifies the discriminative stimulus effects of DA receptor agonists.
In this study, stimulus control was established with the DA receptor agonist quinpirole in free-feeding rats responding under a schedule of shock avoidance. DA receptor agonists were studied for their ability to mimic the quinpirole discriminative stimulus, and DA receptor antagonists were studied for their ability to attenuate the quinpirole discriminative stimulus. The antagonists studied vary in selectivity for different DA receptor subtypes as follows: the D2 receptor-selective antagonist L-741,626 [15-fold selective for D2 over D3 receptors in vitro (Grundt et al., 2007a)], the D3 receptor-selective antagonist PG01037 [133-fold selective for D3 over D2 receptors in vitro (Grundt et al., 2005, 2007b)], and the nonselective D3/D2 receptor antagonist raclopride. The same antagonists were also studied for their ability to attenuate quinpirole-induced yawning (presumably the ascending limb reflecting activity at D3 receptors and descending limb reflecting activity at D2 receptors) and quinpirole-induced hypothermia (thought to be mediated by D2 receptors). Finally, feeding conditions that decrease DA agonist-induced yawning were examined to see whether the discriminative stimulus effects of quinpirole were similarly decreased by food restriction. It was expected that the discriminative stimulus effects of quinpirole in free-feeding rats, presumably lacking increased D2 receptor sensitivity that occurs during ongoing food restriction, would be mediated by D3 receptors or by a combination of D3 and D2 receptors, and, to the extent that food restriction selectively increases sensitivity of D2 receptors, it was expected that the D3 receptor-mediated discriminative stimulus effects of quinpirole would not be affected by food restriction.
Materials and Methods
Subjects.
Fourteen male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 250 to 300 g upon arrival, were housed individually in an environmentally controlled room (24 ± 1°C, 50 ± 10% relative humidity) under a 12-h light/dark cycle. Six rats were used in a discrimination study, and eight rats were used in a study on yawning and body temperature. All rats had unlimited access to standard laboratory chow and water, except during experimental sessions (for all rats) and on two occasions when rats in the drug discrimination study were restricted to 10 g/day of food for 7 days. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, the University of Texas Health Science Center at San Antonio, and with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Apparatus.
Experimental sessions were conducted in sound-attenuating, ventilated enclosures (models ENV-018ME and ENV-008CT; Med Associates Inc., St. Albans, VT), which contained an operant chamber. One side of the chamber was a stainless steel response panel equipped with two metal levers and stimulus lights 11.5 cm apart and separated by a clear Plexiglas partition (1.4 × 5.2 × 20.4 cm high) that extended from the floor to the ceiling of the chamber. The grid floor of the chamber was 19 stainless steel rods, 4.8 mm in diameter, spaced 1.6 cm apart, and oriented parallel to the response panel. A constant current generator (Med Associates, Inc.) delivered a scrambled electric current to the grid floor of the chamber. Data were collected using MED-PC IV software (Med Associates, Inc.) and a PC interface.
Drug Discrimination.
Six rats were trained to discriminate 0.032 mg/kg quinpirole (intraperitoneal) from vehicle (i.e., saline) under a schedule of stimulus shock termination. Discriminative control was first established with an acute-dosing, single-cycle procedure that consisted of 21 trials and began with a 10-min timeout period, during which stimulus lights were not illuminated and responding had no programmed consequence. The timeout period was followed by illumination of a house light that signaled the delivery of a brief (250 ms) shock stimulus (1.5 mA) every 10 s; a response on the injection-appropriate (correct) lever or the passage of 50 s turned off the house light, ended the trial, and initiated a 50-s timeout. Vehicle or 0.032 mg/kg quinpirole was administered immediately before the session. Stimulus control was considered adequate for testing when the following criteria were satisfied for four consecutive or five of six sessions: 1) the first response of the cycle was made on the correct lever and 2) at least 80% of the trials were completed by a response on the correct lever. Test sessions were identical to training sessions except that a response on either lever postponed shock and different doses of quinpirole were administered before the session. After a quinpirole dose-response curve was determined under the single-cycle procedure, the experimental conditions were changed to a cumulative-dosing, multiple-cycle procedure consisting of one to four 20-min cycles. Each cycle consisted of 10 trials and began with a 10-min timeout period, during which stimulus lights were not illuminated, and responding had no programmed consequence. The timeout period was followed by illumination of the house light signaling scheduled delivery of a brief electric stimulus every 10 s; a response on the injection-appropriate (correct) lever or the passage of 30 s turned off the house light, ended the trial, and initiated a 30-s timeout. If fewer than five trials were completed by a response on the correct lever in any cycle, the session ended. For vehicle training sessions, animals received an intraperitoneal injection of vehicle before one cycle followed by between one and three sham (no injection) cycles. For drug training sessions, animals received an intraperitoneal injection of 0.032 mg/kg quinpirole before one cycle followed by a single sham injection. The cycle during which quinpirole was administered was preceded by zero to two cycles during which vehicle or sham injections were administered. Testing resumed after animals satisfied the following criteria for four consecutive or five of six sessions under the multiple-cycle procedure: 1) the first response of all cycles was on the correct lever and 2) at least 80% of the trials were completed by a response on the correct lever. Thereafter, tests were conducted whenever animals satisfied these same criteria for two consecutive sessions. Multiple-cycle test sessions were identical to training sessions except that a response on either lever postponed shock and either vehicle or increasing doses of drug were administered across cycles. For substitution studies, vehicle was administered before the first cycle, followed by increasing doses of drug before subsequent cycles, with the cumulative dose increasing by 0.5 log unit per cycle. Drugs were studied up to doses that occasioned greater than 80% responding on the quinpirole lever. For drug combination studies, a single dose of antagonist was administered (subcutaneously) 10 min before the first (saline) test cycle (i.e., 30 min before the first dose of quinpirole).
To test whether food restriction alters the discriminative stimulus effects of quinpirole, dose-response curves were determined for quinpirole the day before and the day after a 7-day period when training was suspended and rats continued to have unlimited access to food in the home cage. The similarity in ED50 values of these two dose-response curves [mean (95% CL) = 0.016 (0.013–0.018) mg/kg before and 0.018 (0.015–0.021) mg/kg after] demonstrated that suspension of training did not adversely affect stimulus control with quinpirole. Next quinpirole discrimination dose-response curves were determined the day before and the day after a 7-day period when training was suspended and rats received only 10 g/day of food in the home cage. On different occasions, dose-response curves for quinpirole-induced yawning were determined (see below) in the rats used in the discrimination study: once when they had unlimited access to food and once when they received 10 g/day of food for 7 days (no discrimination studies were conducted during these yawning studies).
Yawning.
Yawning was defined as an opening of the mouth such that the lower incisors were completely visible (Kurashima et al., 1995; Sevak et al., 2008; Baladi and France, 2009). On the day of testing, rats were transferred from their home cage to a test cage (same dimensions as the home cage but with no food, water, or bedding) and allowed to habituate for 15 min. Cumulative dose-response curves were generated for quinpirole (0.0032–1.0 mg/kg i.p.) with increasing doses administered every 30 min. Beginning 20 min after each injection, the total number of yawns was recorded for 10 min.
The six rats in the discrimination study were studied twice, once when they had unlimited access to food and once when they received 10 g/day of food for 7 days. A separate group of eight free-feeding rats were studied with cumulative doses of quinpirole alone and in combination with several different DA receptor antagonists: the D3 receptor-selective antagonist PG01037 (32.0 and 56.0 mg/kg s.c.), the D2 receptor-selective antagonist L-741,626 (1.0 and 3.2 mg/kg s.c.), and the nonselective D3/D2 receptor antagonist raclopride (0.032 and 0.056 mg/kg s.c.). The doses of L-741,626, PG01037, and raclopride studied were shown by others to have antagonist actions at dopamine receptors (Collins et al., 2005; Sevak et al., 2007). Antagonists were administered 30 min before administration of the first dose of quinpirole. Experimental sessions were separated by at least 48 h.
Body Temperature.
In the same eight free-feeding rats that were studied for quinpirole-induced yawning, body temperature was measured in a temperature-controlled room (24 ± 1°C and 50 ± 10% relative humidity) by inserting a lubricated thermal probe (attached to a thermometer) 3 cm into the rectum. Animals were adapted to the experimental procedure by measuring body temperature on multiple occasions before studies with drug commenced. Body temperature was measured during yawning experiments, after completion of each 10-min observation period and before the next injection.
Data Analyses.
Drug discrimination data are expressed as a percentage of the total responses made on the quinpirole-associated lever averaged among six rats (±S.E.M.) and are plotted as a function of dose. Also plotted as a function of dose is the percentage of trials completed (±S.E.M.). When a rat completed fewer than five trials, discrimination data from that test were not included in the average, although data for the percentage of trials completed were included in the group average. Yawning data are expressed as the average (±S.E.M.) number of yawns during the 10-min observation period and plotted as a function of dose. Body temperature data are expressed as a change in degrees Centigrade from baseline (i.e., body temperature determined after vehicle administration) averaged among eight rats (±S.E.M.) and plotted as a function of dose.
A two-way repeated-measures analysis of variance with quinpirole dose and feeding condition as factors was used to determine whether yawning was different in the same group of rats before and during food restriction; post hoc multiple comparisons were made with the Bonferroni test. For each group, differences between quinpirole dose-response curves in the presence and absence of antagonist were analyzed by simultaneously fitting straight lines to the linear portion of the dose-response curves by means of GraphPad Prism (GraphPad Software Inc., San Diego, CA). The linear portion included doses that spanned the 50% level of effect and included not more than one dose with greater than 75% effect and not more than one dose with less than 25% effect. Differences between slopes and intercepts of the curves were analyzed with the F ratio test (GraphPad Prism), as detailed elsewhere (Koek et al., 2006). ED50 values were calculated for individual rats using linear regression when at least three appropriate data points were available and otherwise by interpolation. To calculate ED50 values for quinpirole-induced yawning and hypothermia in the absence and presence of antagonists, a common maximum effect was selected for individual rats. The 95% CLs were calculated from ED50 values averaged among rats. To evaluate changes in potency as a result of antagonist treatment, a dose ratio was calculated for each rat by dividing the ED50 obtained in the presence of antagonist by the ED50 value obtained in the absence of antagonist. When the 95% CLs of the dose ratio did not include 1, antagonists were considered to significantly change the potency of the drug relative to its potency in the absence of antagonist.
Drugs.
The following compounds were purchased from Sigma-Aldrich (St. Louis, MO): (−)-quinpirole [trans-(−)-(4aR)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline HCl]; S-(−)-raclopride (+)-tartrate salt [3,5-dichloro-N-(1-ethylpyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxybenzamide]; and S-(+)-apomorphine hydrochloride. Lisuride hydrogen maleate was purchased from Alfarma Research and Development (Prague, Czech Republic), and L-741,626 was purchased from Tocris Bioscience (Ellisville, MO). Morphine sulfate and d-amphetamine sulfate were provided by the Research Technology Branch, National Institute of Drug Abuse (Rockville, MD). PG01037 (N-“4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl”-4-pyridine-2-yl-benzamide HCl) was synthesized by Jianjing Cao (Medicinal Chemistry Section, National Institute on Drug Abuse, Baltimore, MD) using methods reported previously (Grundt et al., 2005). The vehicle for all drugs was sterile 0.9% saline with the exceptions of L-741,626 (dissolved in 5% ethanol with 1 M HCl) and PG01037 (dissolved in 10% β-cyclodextrin). L-741,626, PG01037, and raclopride were administered subcutaneously, typically in a volume of 1 ml/kg; other drugs were administered intraperitoneally in a volume of 1 ml/kg.
Results
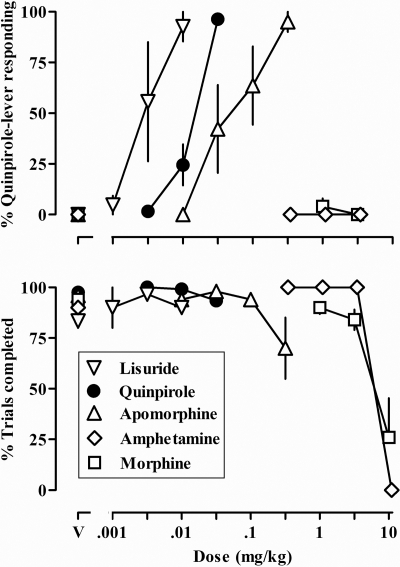
Rats satisfied the testing criteria under the single-cycle procedure after an average of 32 ± 5 (mean ± S.E.M.) training sessions (range = 19 to 51). Conditions were changed to a multiple-cycle procedure, and rats satisfied the testing criteria again after an average of 15 ± 6 (range = 6 to 42) training sessions. Vehicle (Fig. 1, top panel, data above V) and small doses of quinpirole occasioned responding predominantly on the vehicle-associated lever, whereas larger doses of quinpirole increased responding on the quinpirole-associated lever [mean ED50 = 0.015 (95% CL 0.012–0.018)] (Fig. 1, top panel). Lisuride and apomorphine also increased responding on the quinpirole-associated lever in a dose-related manner (Fig. 1, top panel) with the largest dose of each occasioning more than 80% drug-lever responding [lisuride ED50 = 0.004 mg/kg (0.001–0.012) and apomorphine ED50 = 0.090 mg/kg (0.017–0.164)]. Quinpirole, lisuride, and apomorphine did not markedly alter the percentage of trials completed (Fig. 1, bottom panel). Amphetamine and morphine did not occasion responding on the quinpirole-associated lever up to doses of each that markedly decreased the percentage of trials completed (Fig. 1).
Fig. 1.
Discriminative stimulus effects and the percentage of trials completed for lisuride, quinpirole, apomorphine, amphetamine, and morphine in six rats with unlimited access to food and discriminating 0.032 mg/kg quinpirole. Abscissa, dose in milligrams per kilogram of body weight; V, vehicle. Ordinates, mean (±S.E.M.) percentage of responses on the quinpirole lever (top) and mean (±S.E.M.) percentage of trials completed (bottom).
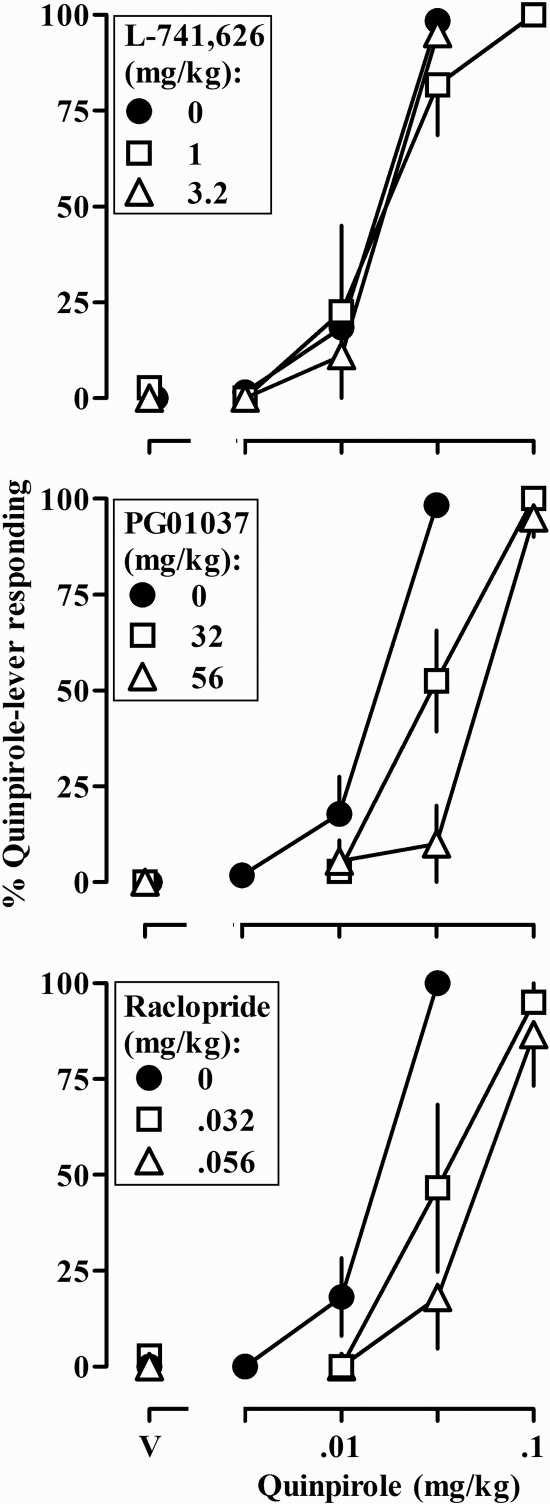
At doses of 1.0 and 3.2 mg/kg, L-741,626 did not clearly alter the discriminative stimulus effects of quinpirole (Fig. 2, top panel). In contrast, PG01037 (32 and 56 mg/kg) and raclopride (0.032 and 0.056 mg/kg) antagonized the discriminative stimulus effects of quinpirole, in each case shifting the dose-response curve to the right in a dose-related manner (Fig. 2, middle and bottom panels, respectively). Under control conditions, a dose of 0.032 mg/kg quinpirole occasioned greater than 80% drug-lever responding; in the presence of PG01037 or raclopride, a 3-fold larger dose of quinpirole (0.1 mg/kg) was required to obtain greater than 80% drug-lever responding. When administered alone, none of the antagonists produced responding on the quinpirole lever (Fig. 2, data above V) or markedly altered the percentage of trials completed (data not shown).
Fig. 2.
Discriminative stimulus effects of quinpirole administered alone (●) and in combination with different doses of L-741,626 (top), PG01037 (middle), and raclopride (bottom). See legend to Fig. 1 for other details.
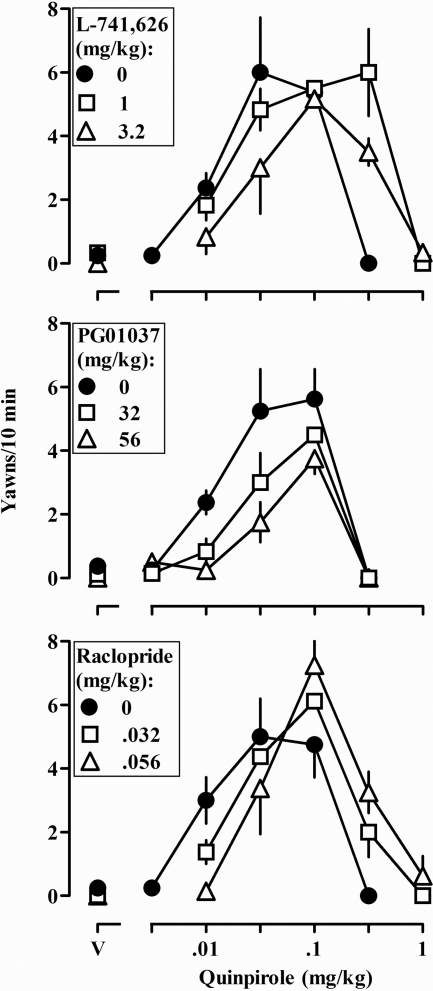
Increasing doses of quinpirole first increased then decreased yawning, resulting in an inverted U-shaped dose-response curve (Fig. 3, all panels, ●). A dose of 1.0 mg/kg L-741,626 shifted the descending, but not the ascending, limb of the yawning dose-response curve to the right (Fig. 3, top panel, compare ● with □). A larger dose of L-741,626 (3.2 mg/kg) shifted both limbs of the yawning dose-response curve to the right (Fig. 3, top panel). A dose of 32.0 or 56.0 mg/kg PG01037 shifted the ascending, but not the descending, limb of the yawning dose-response curve to the right (Fig. 3, middle panel, compare ● with □ and △). Finally, doses of 0.032 or 0.056 mg/kg raclopride shifted both the ascending and descending limbs of the quinpirole yawning dose-response curve to the right (Fig. 3, bottom panel). When administered alone, none of the antagonists produced yawning (Fig. 3, data above V).
Fig. 3.
Quinpirole-induced yawning when quinpirole was administered alone (●) and in combination with different doses of L-741,626 (top), PG01037 (middle), and raclopride (bottom) in eight rats with unlimited access to food. Ordinate, mean (±S.E.M.) number of yawns in the 10-min observation period. See legend to Fig. 1 for other details.
The same data shown in Figs. 2 and 3 are presented in Table 1 as dose ratios expressing the magnitude of shift to the right in the quinpirole discrimination and yawning dose-response curves. A dose of L-741,626 (1.0 mg/kg) that antagonized the descending limb of the quinpirole yawning dose-response curve did not significantly affect the discriminative stimulus effects of quinpirole or the ascending limb of the yawning dose-response curve. In contrast, doses of PG01037 that antagonized the discriminative stimulus effects of quinpirole and the ascending limb of the yawning dose-response curve did not significantly affect the descending limb of the yawning dose-response curve (Table 1). The same doses of raclopride antagonized the discriminative stimulus effects and both limbs of the yawning dose-response curve.
Table 1.
Antagonism of the discriminative stimulus effects and of yawning by quinpirole: dose ratios
| Antagonist Dose | Dose Ratio |
||
|---|---|---|---|
| Discrimination | Yawning (Ascending) | Yawning (Descending) | |
| L-741,626 | |||
| 1 mg/kg | 1.55a (0.82–2.29) | 1.71b (0.20–3.22) | 3.64c (1.70–5.57)d |
| 3.2 mg/kg | 1.25 (0.38–2.13) | 3.12 (1.14–5.11)d | 2.96 (1.09–4.83)d |
| PG01037 | |||
| 32 mg/kg | 2.60 (1.58–3.62)d | 2.48 (1.28–3.68)d | 1.01 (0.67–1.35) |
| 56 mg/kg | 3.57 (2.85–4.28)d | 3.82 (2.23–5.41)d | 1.13 (0.96–1.27) |
| Raclopride | |||
| 0.032 mg/kg | 2.06 (1.15–2.96)d | 1.81 (1.10–2.52)d | 1.87 (1.33–2.77)d |
| 0.056 mg/kg | 3.20 (1.80–4.60)d | 2.78 (1.30–3.81)d | 2.60 (1.23–3.93)d |
Dose ratio (95% CL) for antagonizing the discriminative stimulus effects of quinpirole in six rats.
Dose ratio (95% CL) for antagonizing the ascending limb of the quinpirole dose-response curve for yawning in eight rats.
Dose ratio (95% CL) for antagonizing the descending limb of the quinpirole dose-response curve in eight rats.
95% CL does not include 1.
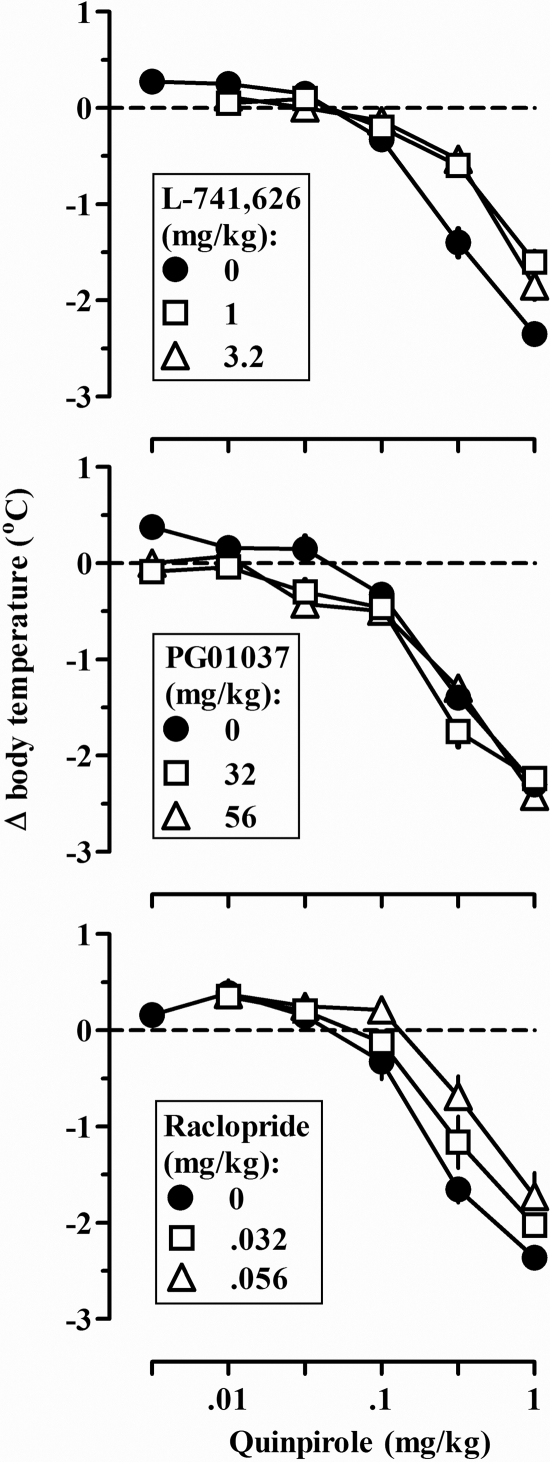
Quinpirole dose dependently decreased body temperature (Fig. 4, all panels, ●). L-741,626 and raclopride, but not PG01037, antagonized the hypothermic effects of quinpirole (Fig. 4). For example, 1.0 and 3.2 mg/kg L-741,626 shifted the quinpirole dose-response curve for hypothermia 2.26- and 2.81-fold to the right, respectively, and 0.032 and 0.056 mg/kg raclopride shifted the quinpirole dose-response curve 2.50- and 3.60-fold to the right, respectively. Administration of the antagonists alone did not significantly change body temperature (data not shown).
Fig. 4.
Quinpirole-induced hypothermia when quinpirole was administered alone (●) and with different doses of L-741,626 (top), PG01037 (middle), and raclopride (bottom). Ordinate, mean (±S.E.M.) change in body temperature (degrees Centigrade). See legend to Fig. 1 for other details.
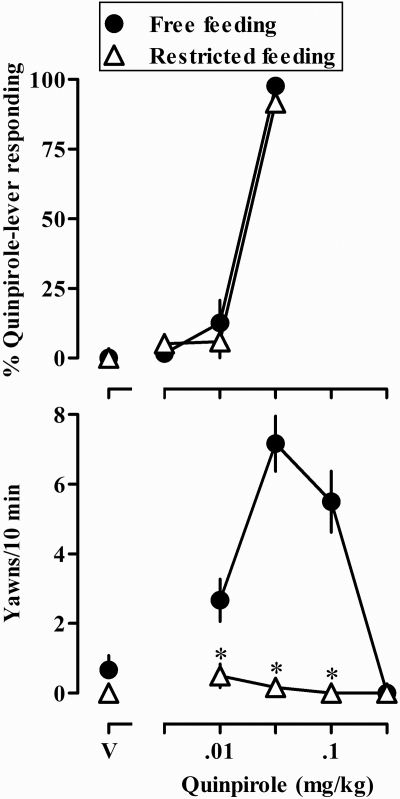
Restricting rats to 10 g/day of food for 7 days significantly decreased (P < 0.05) sensitivity to quinpirole-induced yawning (Fig. 5, bottom panel) without affecting sensitivity to the discriminative stimulus effects of quinpirole (Fig. 5, top panel). When rats had unlimited access to food, the training dose of quinpirole (0.032 mg/kg) occasioned responding on the drug lever and maximally increased yawning (Fig. 5, ●). When rats were restricted to 10 g/day of food, the same dose of quinpirole continued to occasion responding on the drug lever but failed to significantly increase yawning (Fig. 5, ▵).
Fig. 5.
Dose-response curves for the discriminative stimulus effects of quinpirole (top) and quinpirole-induced yawning (bottom) when rats had unlimited access to food (free feeding, ●) and when they were restricted to 10 g/day of food for 7 days (restricted feeding, ▵). Ordinates, mean (±S.E.M.) percentage of responses on the quinpirole lever (top) and mean (±S.E.M.) number of yawns occurring in the 10-min observation period (bottom panel). *, P < 0.05 compared with the same rats when consuming a free-feeding diet at the corresponding dose of quinpirole. See legends to Figs. 1 and 3 for other details.
Discussion
The current study established stimulus control between 0.032 mg/kg quinpirole and vehicle in free-feeding rats responding under a two-choice, multiple-cycle, cumulative-dosing procedure. Others have established stimulus control with similar doses of quinpirole [0.03 mg/kg (Katz and Alling, 2000) and 0.025 mg/kg (Weathersby and Appel, 1986; Appel et al., 1988; Huffman et al., 1995)], although in those studies rats had restricted access to either food or water. Nevertheless, the DA receptor agonists apomorphine and lisuride produced responding on the quinpirole lever in the current study with free-feeding rats (and free access to water) as well as in prior studies with food- or water-restricted rats (Weathersby and Appel, 1986; Appel et al., 1988; Widzowski and Cory-Slechta, 1993). Substitution for quinpirole (D3/D2 receptor agonist) by apomorphine and lisuride confirms a role for DA receptors in this effect but fails to address which receptor(s) mediates the quinpirole discriminative stimulus in free-feeding rats because apomorphine and lisuride are reported to be D3/D2 receptor agonists in other discrimination studies (Woolverton et al., 1985; Cunningham et al., 1987; Kamien et al., 1987).
The discriminative stimulus effects of a variety of DA receptor agonists that bind to both D3 and D2 receptors are thought to be mediated by D2 receptors (Kleven and Koek, 1997; Bristow et al., 1998; Baker et al., 1999; Katz and Alling, 2000; Millan et al., 2000, 2007; Christian et al., 2001; Koffarnus et al., 2009) based on results of studies that used DA receptor antagonists to attenuate the effects of DA receptor agonists; however, most studies used food-restricted rats. In the current study, quinpirole dose-response curves were determined in the presence of different doses of antagonists that vary in their selectivity for D3 and D2 receptors. The D3 receptor-selective antagonist PG01037 and the nonselective D3/D2 receptor antagonist raclopride, but not the D2 receptor-selective antagonist L-741,626, antagonized the discriminative stimulus effects of quinpirole in free-feeding rats, shifting the dose-response curve to the right. These data suggest that the discriminative stimulus effects of quinpirole in free-feeding rats are mediated predominantly, if not exclusively, by D3 receptors.
Yawning induced by DA receptor agonists yields an inverted U-shaped dose-response curve, and it is thought that the ascending limb of this curve (induction of yawning) is mediated by actions at D3 receptors and the descending limb (inhibition of yawning) is mediated by actions at D2 receptors (Collins et al., 2005). The same antagonists that were compared for their ability to antagonize the discriminative stimulus effects of quinpirole also were compared for their ability to antagonize quinpirole-induced yawning. The D3 receptor-selective antagonist PG01037 attenuated the ascending limb of the quinpirole dose-response curve in a dose-related manner without affecting the descending limb. In contrast, a dose of the D2 receptor-selective antagonist L-741,626 (1.0 mg/kg) that attenuated the descending limb of the quinpirole dose-response curve had no effect on the ascending limb. The nonselective D3/D2 receptor antagonist raclopride dose dependently attenuated both limbs of the dose-response curve for quinpirole-induced yawning. The ability of these antagonists to attenuate the discriminate stimulus effects of quinpirole in free-feeding rats parallels their ability to attenuate the ascending and, presumably, D3 receptor-mediated limb of the dose-response curve for quinpirole-induced yawning. In particular, the potencies of PG01037 and raclopride, but not of L-741,626, to antagonize the discriminative stimulus effects of quinpirole parallel their relative potencies in attenuating the ascending limb of the yawning dose-response curve (Table 1), further supporting the view that the discriminative stimulus effects of quinpirole in free-feeding rats are mediated predominantly, if not exclusively, by D3 receptors.
Based on the effects of various DA receptor agonists administered alone and in combination with different DA receptor antagonists, it is thought that the hypothermic effects of D3/D2 receptor agonists (e.g., including quinpirole) are mediated by D2 receptors (Nunes et al., 1991; Chaperon et al., 2003; Collins et al., 2007). Consistent with that view, quinpirole-induced hypothermia was attenuated by the D2 receptor-selective antagonist L-741,626 and the nonselective D3/D2 receptor antagonist raclopride but not by the D3 receptor-selective antagonist PG01037.
Food restriction markedly decreases sensitivity of rats to quinpirole-induced yawning (Sevak et al., 2008; Baladi and France, 2009); interpretations of that observation might include decreased sensitivity of D3 receptors to agonists, increased sensitivity of D2 receptors to agonists, or both decreased sensitivity of D3 and increased sensitivity of D2 receptors. However, results of behavioral as well as molecular studies indicate that the effect of food restriction is to increase sensitivity of D2 receptors (Carr et al., 2003; Collins et al., 2008; Thanos et al., 2008). If the discriminative stimulus effects of DA receptor agonists in free-feeding rats are mediated by D2 receptors, then it might be expected that food restriction would increase sensitivity to those effects, as reflected by a leftward shift in the dose-response curve. However, food restriction (10 g/day for 7 days) that markedly decreased sensitivity to quinpirole-induced yawning had no effect on the discriminative stimulus effects of quinpirole in the same group of rats, supporting the hypothesis that D2 receptors are not involved in the discriminative stimulus effects of quinpirole in free-feeding rats. Food restriction in the current study reduced body weight to approximately 90% of free-feeding weight, somewhat less than body weight loss reported in other studies using food restriction and studying dopamine drugs (e.g., 80–85%; Weathersby and Appel, 1986; Carr et al., 2003; Collins et al., 2008; Koffarnus et al., 2009). Together with the current antagonism studies, these data support the view that D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats and that sensitivity of D3 receptors to agonists is not markedly affected by food restriction. Future studies might include establishing a discrimination with a highly selective D2 receptor agonist in free-feeding rats (i.e., acute food restriction might shift the dose-response curve leftward) or with a highly selective D3 receptor agonist in food-restricted rats (i.e., D3 and not D2 receptor antagonists should block the training stimulus). Moreover, it is not clear whether the reinforcer used to maintain responding (i.e., shock) influences the contribution of different DA receptors in the discriminative stimulus effects of agonists.
In summary, although D2 receptors are thought to mediate the discriminative stimulus effects of quinpirole in food-restricted rats, D3 receptors seem to mediate the discriminative stimulus effects of quinpirole in free-feeding rats. Thus, feeding conditions can affect the contribution of D3 and D2 receptors to the discriminative stimulus effects of quinpirole. Several mechanisms are thought to underlie the effects of food restriction on DA receptors, including increased DA receptor number and signaling and reduced plasma levels of hormones such as insulin and leptin that can directly affect DA systems. Understanding the link between feeding condition and DA neurotransmission might be critical for understanding the comorbidity of eating disorders and drug abuse and also has implications for understanding how feeding condition might affect the behavioral effects of other drugs acting on DA systems, including drugs of abuse such as cocaine and amphetamine.
Acknowledgments
We acknowledge Jianjing Cao in the Medicinal Chemistry Section, National Institute on Drug Abuse-Intramural Research Program, for synthesizing the PG01037 used in this study.
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse (to A.H.N.); and the National Institutes of Health National Institute on Drug Abuse [Grant DA17918].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158394
- DA
- dopamine
- S32504
- (+)-trans-3,4,4a,5,6,10b-hexahydro-9-carbamoyl-4-propyl-2H-naphth[1,2-b]-1,4-oxazine
- L-741,626
- 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]methyl-1H-indole
- S33084
- (3aR,9bS)-N-[4-(8-cyano-1,3a,4,9b-tetrahydro-3H-benzopyrano[3,4-c]pyrrole-2-yl)-butyl]-(4-phenyl)benzamide
- PD 128907
- S(+)-(4aR,10bR)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-(1)benzopyrano(4,3-b)-1,4-oxazin-9-ol
- GR 103,691
- “4′-acetyl-N-“4-[(2-methoxy-phenyl)-piperazin-1-yl]-butyl”-biphenyl-4-carboxamide”
- PG01037
- N-“4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl”-4-pyridine-2-yl-benzamide HCl
- CL
- confidence limit.
References
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, Katz JL, Witkin JM. (1995) Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol 281:R7–R9 [DOI] [PubMed] [Google Scholar]
- Appel JB, Weathersby RT, Cunningham KA, Callahan PM, Barrett RL. (1988) Stimulus properties of dopaminergic drugs: comparisons involving selective agonists and antagonists. Psychopharmacol Ser 4:44–56 [DOI] [PubMed] [Google Scholar]
- Baker LL, Hood CA, Heidema AM. (1990) Assessment of D3 versus D2 receptor modulation of the discriminative stimulus effects of (+)-7-OH-DPAT in rats. Behav Pharmacol 10:717–722 [DOI] [PubMed] [Google Scholar]
- Baladi MG, France CP. (2009) High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol 610:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LJ, Cook GP, Patel S, Curtis N, Mawer I, Kulagowski JJ. (1998) Discriminative stimulus properties of the putative dopamine D3 receptor agonist, (+)-PD 128907: role of presynaptic dopamine D2 autoreceptors. Neuropharmacology 37:793–802 [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816 [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. (2003) Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 119:1157–1167 [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. (2003) Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 44:1047–1053 [DOI] [PubMed] [Google Scholar]
- Christian AJ, Goodwin AK, Baker LE. (2001) Antagonism of the discriminative stimulus effects of (+)-7-OH-DPAT by remoxipride but not PNU-99194A. Pharmacol Biochem Behav 68:371–377 [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. (2008) Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther 325:691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 193:159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. (2005) Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM, Appel JB. (1987) Discriminative stimulus properties of lisuride revisited: involvement of dopamine D2 receptors. J Pharmacol Exp Ther 241:147–151 [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. (2005) Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem 48:839–848 [DOI] [PubMed] [Google Scholar]
- Grundt P, Husband SL, Luedtke RR, Taylor M, Newman AH. (2007a) Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR. Bioorg Med Chem Lett 17:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. (2007b) Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem 50:4135–4146 [DOI] [PubMed] [Google Scholar]
- Huffman EM, Caul WF, Strand EJ, Jones JR, Barrett RJ. (1995) D2-specific discriminative stimuli: parameters, blocking, and rebound. Pharmacol Biochem Behav 51:77–82 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kamien JB, Goldberg LI, Woolverton WL. (1987) Discriminative stimulus properties of D1 and D2 dopamine agonists in rats. J Pharmacol Exp Ther 242:804–811 [PubMed] [Google Scholar]
- Katz JL, Alling KL. (2000) Discriminative stimulus effects of putative D3 dopamine receptor agonists in rats. Behav Pharmacol 11:483–493 [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1997) Dopamine D2 receptors play a role in the (−)-apomorphine-like discriminative stimulus effects of (+)-PD 128907. Eur J Pharmacol 321:1–4 [DOI] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. (2006) Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with γ-hydroxybutyrate and its precursors 1,4-butanediol and γ-butyrolactone. Behav Pharmacol 17:239–247 [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Greedy B, Husbands SM, Grundt P, Newman AH, Woods JH. (2009) The discriminative stimulus effects of dopamine D2- and D3-preferring agonists in rats. Psychopharmacology (Berl) 203:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima M, Yamada K, Nagashima M, Shirakawa K, Furukawa T. (1995) Effects of putative dopamine D3 receptor agonists, 7-OH-DPAT, and quinpirole, on yawning, stereotypy, and body temperature in rats. Pharmacol Biochem Behav 52:503–508 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Girardon S, Monneyron S, Dekeyne A. (2000) Discriminative stimulus properties of the dopamine D3 receptor agonists, PD128,907 and 7-OH-DPAT: a comparative characterization with novel ligands at D3 versus D2 receptors. Neuropharmacology 39:586–598 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Iob L, Péglion JL, Dekeyne A. (2007) Discriminative stimulus properties of S32504, a novel D3/D2 receptor agonist and antiparkinson agent, in rats: attenuation by the antipsychotics, aripiprazole, bifeprunox, N-desmethylclozapine, and by selective antagonists at dopamine D2 but not D3 receptors. Psychopharmacology (Berl) 191:767–782 [DOI] [PubMed] [Google Scholar]
- Nunes JL, Sharif NA, Michel AD, Whiting RL. (1991) Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem Res 16:1167–1174 [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. (1995) Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15:6640–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Galli A, France CP. (2007) Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J Pharmacol Exp Ther 320:1216–1223 [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. (2008) Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol 592:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. (1999) Dopamine D2/D3 receptors modulate cocaine's reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend 54:97–110 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1996) Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 278:1128–1137 [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. (2008) Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 62:50–61 [DOI] [PubMed] [Google Scholar]
- Weathersby RT, Appel JB. (1986) Dopamine D2 receptor mediation of the discriminative stimulus properties of LY 171555 (quinpirole). Eur J Pharmacol 132:87–91 [DOI] [PubMed] [Google Scholar]
- Widzowski DV, Cory-Slechta DA. (1993) Apparent mediation of the stimulus properties of a low dose of quinpirole by dopaminergic autoreceptors. J Pharmacol Exp Ther 266:526–534 [PubMed] [Google Scholar]
- Woolverton WL, Kamien JB, Goldberg LI. (1985) Effects of selective dopamine receptor agonists in rats trained to discriminate apomorphine from saline. Pharmacol Biochem Behav 22:577–581 [DOI] [PubMed] [Google Scholar]