Abstract
5-Aminolevulinic acid (ALA) is a prodrug used in photodynamic therapy, fluorescent diagnosis, and fluorescent-guided resection because it leads to accumulation of the photosensitizer protoporphyrin IX (PpIX) in tumor tissues. ALA has good oral bioavailability, but high oral doses are required to obtain selective PpIX accumulation in colonic tumors because accumulation is also observed in normal gut mucosa. Structural similarities between ALA and GABA led us to test the hypothesis that the H+-coupled amino acid transporter PAT1 (SLC36A1) will contribute to luminal ALA uptake. Radiolabel uptake and electrophysiological measurements identified PAT1-mediated H+-coupled ALA symport after heterologous expression in Xenopus oocytes. The selectivity of the nontransported inhibitors 5-hydroxytryptophan and 4-aminomethylbenzoic acid for, respectively, PAT1 and the H+-coupled di/tripeptide transporter PepT1 (SLC15A1) were examined. 5-Hydroxytryptophan selectively inhibited PAT1-mediated amino acid uptake across the brush-border membrane of the human intestinal (Caco-2) epithelium whereas 4-aminomethylbenzoic acid selectively inhibited PepT1-mediated dipeptide uptake. The inhibitory effects of 5-hydroxytryptophan and 4-aminomethylbenzoic acid were additive, demonstrating that both PAT1 and PepT1 contribute to intestinal transport of ALA. This is the first demonstration of overlap in substrate specificity between these distinct transporters for amino acids and dipeptides. PAT1 and PepT1 expression was monitored by reverse transcriptase-polymerase chain reaction using paired samples of normal and cancer tissue from human colon. mRNA for both transporters was detected. PepT1 mRNA was increased 2.3-fold in cancer tissues. Thus, increased PepT1 expression in colonic cancer could contribute to the increased PpIX accumulation observed. Selective inhibition of PAT1 could enhance PpIX loading in tumor tissue relative to that in normal tissue.
Photodynamic therapy of cancer involves the activation of a photosensitizer by light to induce localized cell death. The naturally occurring heme precursor 5-aminolevulinic acid (ALA) is used as a prodrug in photodynamic therapy of a wide variety of different solid tumors (Peng et al., 1997; Krammer and Plaetzer, 2008). ALA is converted by the heme biosynthesis pathway into the photosensitizer protoporphyrin IX (PpIX), which, when exposed to long-wavelength light, releases highly reactive singlet oxygen (Kennedy and Pottier, 1992). Topical or systemic application of ALA leads to selective accumulation of PpIX within tumor cells, which reduces nonspecific tissue damage during photodynamic therapy (Kennedy and Pottier, 1992). This selectivity has also been exploited in diagnosis and fluorescent-guided resection of tumors (e.g., malignant glioma), in which accumulated PpIX makes tumors fluoresce under illumination (Stummer et al., 2006). The molecular basis of the tumor-selective PpIX accumulation is not clear, although altered activity of enzymes in the heme biosynthesis pathway, which convert ALA to PpIX and PpIX to heme, has been suggested (Kennedy and Pottier, 1992; van Hillegersberg et al., 1992).
ALA is rapidly cleared from the body, which reduces potential side effects such as enduring cutaneous photosensitivity after treatment (Loh et al., 1993; van den Boogert et al., 1998). One of the key advantages of ALA-based therapy is its good oral bioavailability. In rats, oral ALA produces a plasma ALA level similar to that achieved by intravenous delivery of an equivalent dose within 1 h (van den Boogert et al., 1998). Oral dosage with ALA is also used in photodynamic therapy of gastrointestinal tumors. However, relatively high doses of ALA are required to produce adequate PpIX tumor/normal mucosa ratios, because of the high background accumulation in normal epithelial cells (Loh et al., 1993; Regula et al., 1995; Peng et al., 1997).
One of the unanswered questions of ALA-based therapy is how the hydrophilic ALA is absorbed from the lumen of the gastrointestinal tract. There is convincing evidence that the intestinal H+-coupled di/tripeptide transporter PepT1 (SLC15A1) can transport ALA (Döring et al., 1998) and so may be involved in ALA transport across the intestinal epithelial brush-border membrane. However, the structure of ALA is also analogous to that of the neuroactive amino acid GABA, suggesting that ALA may also interact with a GABA uptake system. To test this hypothesis, ALA transport by the H+-coupled amino acid transporter PAT1 (SLC36A1) (Boll et al., 2002; Chen et al., 2003) was measured. PAT1 mediates uptake of GABA, other small neutral amino acids (e.g., proline, glycine, and taurine), and a large number of related amino acid analogs across the apical membrane of intestinal epithelial cells (Thwaites et al., 1995, 2000; Anderson et al., 2004; Metzner et al., 2006; Thwaites and Anderson, 2007a). PAT1 can also transport therapeutic GABA analogs such as vigabatrin and gaboxadol (Abbot et al., 2006; Larsen et al., 2009), which may account for the excellent bioavailability of these drugs when given orally. PAT1 transport, like that of PepT1, is driven by the H+ electrochemical gradient that exists because of an area of low pH adjacent to the intestinal luminal surface, called the acid microclimate (McEwan et al., 1988; Thwaites and Anderson, 2007b). By using a combination of heterologous expression in Xenopus laevis oocytes and endogenous expression in Caco-2 cell monolayers, we show that PAT1 is a novel pH-dependent, rheogenic H+/ALA transporter and that both PAT1 and PepT1 contribute to ALA uptake across the brush-border membrane of intestinal epithelial cells. We also consider the possibility that ALA transport may contribute to selective PpIX accumulation within tumor cells after observing increased expression of PepT1, but not of PAT1, mRNA in colon cancer.
Materials and Methods
Materials.
[3H]GABA (90 Ci/mmol), [3H]glycine (16 Ci/mmol), and [3H]l-methionine (73 Ci/mmol) were from GE Healthcare (Little Chalfont, Buckinghamshire, UK). [3H]5-Aminolevulinic acid hydrochloride (30 Ci/mmol) and [3H]-β-alanine (50 Ci/mmol) were from American Radiolabeled Chemicals (St. Louis, MO). [3H]-l-Lysine (92–98.5 Ci/mmol) and [14C]-α-methylaminoisobutyric acid (MeAIB) (50.5 mCi/mmol) were from PerkinElmer Life and Analytical Sciences (Beaconsfield, UK). [3H]-l-Proline was from either PerkinElmer Life and Analytical Sciences (75 Ci/mmol) or GE Healthcare (43 Ci/mmol). [14C]Glycylsarcosine (Gly-Sar) (56.7 mCi/mmol) and [3H]-d-Phenylalanyl-l-glutamine (d-Phe-Gln) (17.4 Ci/mmol) were from Cambridge Research Biochemicals (Stockton-on-Tees, UK). All other chemicals were from Sigma-Aldrich (Poole, UK) or VWR (Lutterworth, UK).
Expression in X. laevis Oocytes.
Oocytes were isolated as described previously (Anderson et al., 2004). cRNA was synthesized by in vitro transcription either with the mMessage mMachine T7 kit (Ambion, Warrington, UK) (PAT1) or as described previously (Kennedy et al., 2002) (PepT1). Oocytes were injected with 50 nl of cRNA (1 mg/ml) or water (as a control) and maintained for at least 2 days before use, as described previously (Anderson et al., 2004).
Transport Measurements in Oocytes.
Uptake of [3H]ALA, [3H]-β-alanine, or [14C]Gly-Sar (all 5 μCi/ml, 100 μM) was measured at 22°C for 30 to 40 min, essentially as described previously (Anderson et al., 2004). In brief, oocytes were washed in Na+-free uptake solution (100 mM choline chloride, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.4 with Tris base), and then uptake was measured at pH 5.5, 6.5, or 7.4. For pH 5.5, HEPES was replaced by MES. After uptake, oocytes were washed three times in ice-cold uptake buffer (without radiolabel) and lysed in 10% SDS, and radioactivity was measured by scintillation counting.
Trans-stimulation of [3H]-β-alanine efflux from oocytes was determined after injection of oocytes with 50 nl of [3H]-β-alanine (50 mM, 0.1 μCi/μl). If we assume an effective oocyte volume of 250 nl (You et al., 1993), this would equate to an intracellular β-alanine concentration of approximately 8 mM. After injection, oocytes were allowed to recover for 15 min before washing and measurement of efflux in uptake solution (as above, pH 5.5) for 10 min at 22°C. Efflux was measured in the presence and absence of potential trans-stimulating compounds (20 mM). The amount of radioactivity effluxed into the uptake solution was then measured by scintillation counting.
Two-Electrode Voltage Clamp.
Oocytes were clamped at −60 mV while being superfused with Na+-free (pH 5.5) buffer in the presence or absence of various compounds (see figure legends for details). Current changes associated with H+-coupled transport were measured using a Geneclamp 500 amplifier, Digidata 1200 (Axon Instruments, Molecular Devices, Sunnyvale, CA) and pClamp software. To determine the current evoked by a substrate, the current measured over the last 15 s of the exposure time was averaged. The baseline current (taken as the average current over the 15 s before exposure to the substrate) was then subtracted. Current-voltage (I-V) relationships were determined by clamping the membrane potential at −60 mV and then stepping sequentially between −100 and +80 mV in 20-mV steps (each for 200 ms). I-V measurements made in the absence of a substrate were subtracted from those made when substrate-induced current flow reached steady state.
Uptake Measurements in Caco-2 Cell Monolayers.
Caco-2 cells were grown as confluent monolayers of polarized cells on Transwell polycarbonate filters (Corning, Schiphol-Rijk, The Netherlands), as described previously (Thwaites et al., 1995). Before uptake, Caco-2 cell monolayers were washed (four 500-ml washes) and bathed in Na+-free Krebs' solution (137 mM choline chloride, 5.4 mM KCl, 1 mM MgSO4, 0.34 mM KH2PO4, 2.8 mM CaCl2, 10 mM glucose, and 10 mM HEPES, adjusted to pH 7.4 with Tris base). Apical uptake of various radiolabeled amino acids (0.8 μCi/ml, all 10 μM concentrations except [14C]MeAIB, which was 26 μM), [3H]ALA, [3H]-β-alanine, or [3H]-d-Phe-Gln (all 0.5 μCi/ml, 100 μM) was measured over 5 to 15 min at 37°C, apical pH 7.4 or pH 5.5 (HEPES substituted with MES) and basolateral pH 7.4. Monolayers were washed immediately in ice-cold solution (three 500-ml washes) and removed from the plastic inserts to allow radioactivity to be measured by scintillation counting.
Tissue Collection.
Eighteen adult patients with colorectal cancer were included after obtaining their informed consent and approval from the institutional review board of the Medical College of Georgia (Gupta et al., 2006). Samples 1 to 6 were from patients GM1 to GM6, sample 7 was from patient GM18, samples 8 to 16 were from patients GM24 to GM32, sample 17 was from patient GM34, and sample 18 was from patient GM36 (see Gupta et al., 2006, for patient details). Total RNA from mucosal samples (paired normal and cancer samples) was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions.
Reverse Transcriptase-PCR.
RNA was reverse-transcribed using the GeneAmp PCR system (Applied Biosystems, Branchburg, NJ). PCR was performed using the following primers: PepT1, forward GGCTGGACTGGGCTAAAGAGAAATACGATG and reverse GTTGGCCCTGCTTGAAGTCGTCAGTTAC; and PAT1, forward CCACCAATAACTGCCACAACAATGAGACGG and reverse TGCGCACAAACAGGTCCACCACTAACTCAC. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a universal control. The levels of PAT1 and PepT1 were quantified by densitometry scanning and normalized to the intensities of the corresponding GAPDH bands.
Data Analyses.
Data are expressed as mean ± S.E.M. For all radiolabel transport measurements, experiments were repeated two to three times using at least 9 to 10 oocytes per condition within each replicate or 5 to 6 Caco-2 cell monolayers per condition within each replicate. For two-electrode voltage-clamp experiments, measurements were made on 4 to 5 individual oocytes from at least two frogs. Statistical comparisons of mean values were made using an unpaired, two-tailed Student's t test or one-way analysis of variance as appropriate. Significance was assumed if p < 0.05. Kinetic analyses to determine Km and Vmax values were carried out by fitting Michaelis-Menten kinetics (describing a single saturable transport system) and confirmed by linear regression analysis using the Eadie-Hofstee equation. Km is the Michaelis constant, the concentration of substrate necessary for half-maximal transport. IC50 values (concentration of inhibitor necessary to produce 50% inhibition) were estimated from semilogarithmic plots of the inhibitor concentrations versus substrate uptake relative to the uninhibited control. Nonlinear and linear regressions were fitted using Prism 4 (GraphPad Software Inc., San Diego, CA).
Results
PAT1 Is a Novel H+-Coupled ALA Transporter.
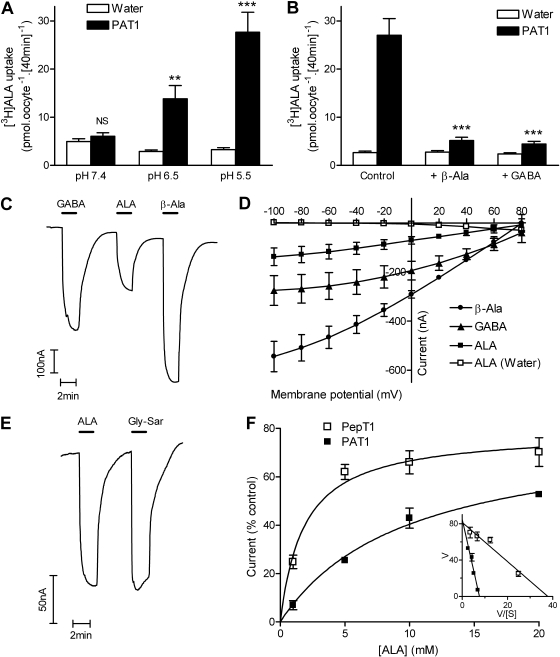
pH-dependent, H+-coupled ALA uptake was measured in PAT1-expressing oocytes (Fig. 1). At pH 5.5 and 6.5, but not at pH 7.4, there was significantly greater [3H]ALA uptake in PAT1-expressing oocytes than in control (water-injected) oocytes (Fig. 1A). ALA uptake at pH 5.5 was inhibited by the PAT1 substrates β-alanine and GABA (both 20 mM) (Fig. 1B). To characterize PAT1-mediated ALA uptake, rheogenic transport (consistent with H+/zwitterion symport) was measured as inward positive charge movement by the two-electrode voltage-clamp technique (Fig. 1, C and D). ALA caused inward current in oocytes heterologously expressing PAT1 but not in control oocytes (Fig. 1D). The current induced by ALA was consistently smaller than that for GABA and β-alanine (Fig. 1, C and D). The affinity of PAT1 for ALA (Km = 10.4 ± 5.6 mM) (shown in Fig. 1F) was also lower than that for GABA (Km = 1.1 ± 0.5 mM; data not shown).
Fig. 1.
PAT1 and PepT1 are both H+-coupled ALA transporters. A, [3H]ALA uptake (100 μM) into PAT1-expressing oocytes was pH-dependent. ***, p < 0.001; **, p < 0.01; NS, p > 0.05, all versus water-injected (control) oocytes. B, [3H]ALA uptake at pH 5.5 (control) was inhibited by the PAT1 substrates β-alanine and GABA (both 20 mM). ***, p < 0.001 versus control. C, Two-electrode voltage-clamp was used to measure PAT1-mediated H+-coupled transport. Superfusion of GABA, β-alanine (β-Ala), or ALA (all 20 mM) in pH 5.5 buffer for 2 min caused inward current in PAT1-expressing oocytes. D, current-voltage (I-V) relationship for GABA, β-alanine, and ALA (all 10 mM) in PAT1-expressing oocytes. ALA caused no current in water-injected control oocytes. E, ALA and Gly-Sar (20 mM) both induced an inward current in PepT1-expressing oocytes. F, concentration-dependent current induced by ALA in PAT1- or PepT1-expressing oocytes relative to the appropriate control (20 mM GABA for PAT1 and 20 mM Gly-Sar for PepT1). Data are fitted with Michaelis-Menten kinetics. Inset, Eadie-Hofstee plot [current (V) versus current/ALA concentration (V/[S])].
Transport of ALA via PepT1.
A previous study by Daniel and colleagues (Döring et al., 1998) showed that the intestinal di/tripeptide transporter PepT1 could transport ALA. To compare ALA transport via PepT1 and PAT1, PepT1 was expressed in oocytes. At pH 5.5, significantly more [3H]ALA uptake was measured in oocytes injected with PepT1 cRNA than in water-injected controls [(uptake being 43.5 ± 3.8 and 4.6 ± 0.4 pmol · oocyte−1 · [40 min]−1 (p < 0.001), in PepT1-expressing and control oocytes, respectively]. Like PAT1, PepT1 is a H+-coupled transporter, and rheogenic transport can be measured by the two-electrode voltage-clamp technique. ALA induced similar levels of current in PepT1-expressing oocytes as the prototypical substrate Gly-Sar (Fig. 1E) and the affinity of PepT1 for ALA (Km = 1.6 ± 0.9 mM) (Fig. 1F) was comparable with that reported previously for Gly-Sar (Km = 1.9 mM) (Fei et al., 1994).
Characterization of Nontransported Inhibitors of PAT1 and PepT1.
Figure 1 shows clearly that, despite their distinct substrate specificities, both the amino acid transporter PAT1 and the di/tripeptide PepT1 can transport ALA in a pH-dependent, H+-coupled manner. Previous studies have identified both PAT1 and PepT1 at the apical membrane of the small intestinal epithelium (and the model cell line Caco-2) (Walker et al., 1998; Chen et al., 2003) where they may both play important roles in nutrient and drug absorption (Rubio-Aliaga and Daniel, 2002; Thwaites and Anderson, 2007a). To identify whether both PAT1 and PepT1 play roles in ALA uptake across the brush-border membrane of the small intestinal epithelium it was necessary to use nontransported inhibitors of each transporter. Such inhibitors will mitigate any potential interaction caused by changes in pHi associated with the saturating concentrations of transported substrates normally used in competition experiments.
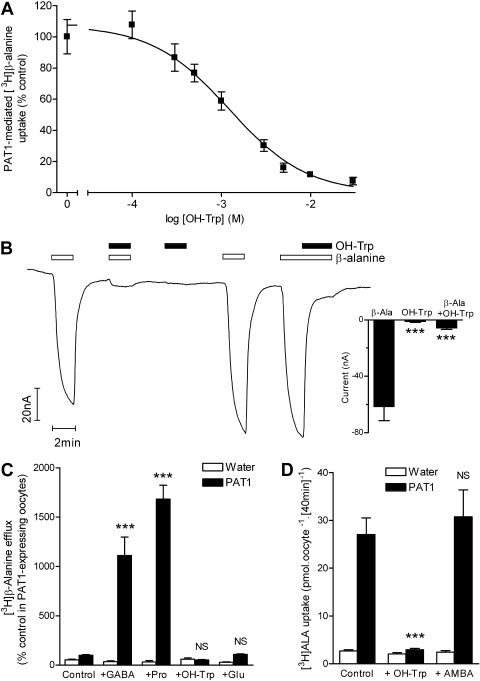
5-Hydroxy-l-tryptophan (OH-Trp) has been proposed as a nontransported inhibitor of PAT1 (Metzner et al., 2005). Uptake of [3H]-β-alanine by PAT1 expressed in oocytes was inhibited in a concentration-dependent manner by OH-Trp (IC50 = 1.2 ± 0.3 mM) (Fig. 2A). OH-Trp (20 mM) caused no current change in PAT1 oocytes but could inhibit current induced by β-alanine (2 mM) by 89% (p < 0.001) (Fig. 2B). This observation suggests that OH-Trp is either a nontransported inhibitor or possibly a substrate transported in a non-rheogenic manner. Consistent with being a nontransported inhibitor, extracellular OH-Trp (20 mM) was unable to trans-stimulate PAT1-specific efflux of β-alanine from oocytes (p > 0.05), whereas the PAT1 substrates GABA and proline (20 mM) caused significant trans-stimulation (p < 0.001) (Fig. 2C). As a control, the amino acid glutamate (20 mM), which is not a substrate for PAT1 (Thwaites et al., 1995), was included and did not cause trans-stimulation. PAT1-mediated [3H]ALA uptake was reduced by 96% by OH-Trp (20 mM) (Fig. 2D). Likewise, current induced by ALA (10 mM) was reduced to 11.4 ± 1.8% (p < 0.001) by OH-Trp (20 mM).
Fig. 2.
OH-Trp is a nontransported inhibitor of PAT1. A, concentration-dependent inhibition of PAT1-mediated [3H]-β-alanine (100 μM) uptake (40 min, pH 5.5) by OH-Trp (0.1–30 mM). Data represent uptake after subtraction of uptake into water-injected control oocytes measured under identical conditions. B, OH-Trp (20 mM) induced no current in PAT1-expressing oocytes but significantly reduced the current associated with β-alanine (β-Ala) (2 mM). ***, p < 0.001 versus β-alanine. C, extracellular OH-Trp (20 mM) did not trans-stimulate PAT1-specific [3H]-β-alanine efflux, whereas the PAT1 substrates GABA and proline (Pro) (both 20 mM) caused significant trans-stimulation. Glutamate (20 mM), which is not a PAT1 substrate, did not trans-stimulate PAT1-specific [3H]-β-alanine efflux. ***, p < 0.001; NS, p > 0.05, versus control. D, inhibition of PAT1-mediated [3H]ALA (100 μM) uptake (pH 5.5) by OH-Trp (20 mM) but not by the PepT1 inhibitor AMBA (30 mM). ***, p < 0.001; NS, p > 0.05, versus control.
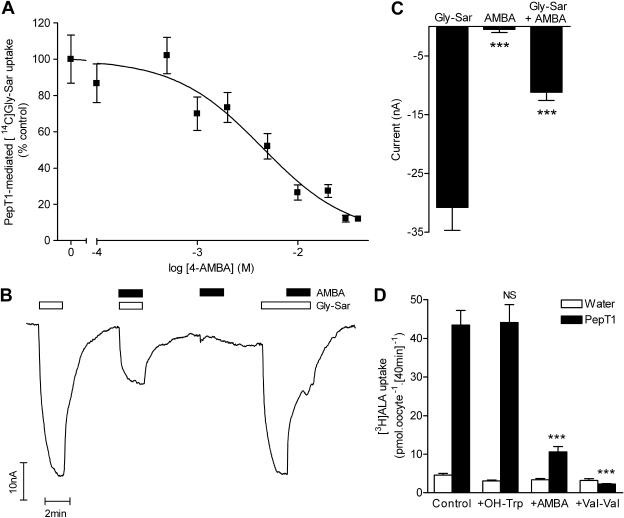
A nontransported inhibitor of PepT1 was discovered because of its ability to inhibit dipeptide uptake but inability to cause trans-stimulation (Meredith et al., 1998). 4-Aminomethylbenzoic acid (AMBA) inhibited [14C]Gly-Sar uptake into PepT1-expressing oocytes (IC50 = 4.4 ± 1.5 mM) (Fig. 3A). AMBA (30 mM) induced no current in PepT1-expressing oocytes suggesting that PepT1 does not transport AMBA (Fig. 3B). At 30 mM, AMBA significantly inhibited (p < 0.001) current caused by 1 mM Gly-Sar (Fig. 3, B and C). Likewise, 30 mM AMBA inhibited PepT1-mediated [3H]ALA uptake by 82% (Fig. 3D). The dipeptide Val-Val (20 mM) completely inhibited PepT1-mediated [3H]ALA uptake (Fig. 3D). It is noteworthy that the PAT1 inhibitor OH-Trp had no effect (p > 0.05) on PepT1-mediated [3H]ALA uptake (Fig. 3D), and the PepT1 inhibitor AMBA had no effect (p > 0.05) on PAT1-mediated [3H]ALA uptake (Fig. 2D), making these tools suitable for investigation of the contribution of PAT1 and PepT1 to ALA uptake across the luminal membrane of the intestinal epithelium.
Fig. 3.
AMBA is a nontransported inhibitor of PepT1. A, concentration-dependent inhibition of [14C]Gly-Sar (100 μM) uptake via PepT1 by the inhibitor AMBA (0.1–40 mM). Data represent uptake after subtraction of uptake into water-injected control oocytes measured under identical conditions. B and C, AMBA (30 mM) inhibited current induced by Gly-Sar (1 mM) in PepT1-expressing oocytes but did not induce any current when superfused alone. ***, p < 0.001 versus Gly-Sar. D, AMBA (30 mM) and the dipeptide Val-Val (20 mM) both inhibited PepT1-mediated [3H]ALA (100 μM) uptake (pH 5.5), whereas the PAT1 inhibitor OH-Trp (20 mM) had no effect. ***, p < 0.001; NS, p > 0.05, versus control.
Both PAT1 and PepT1 Mediate ALA Uptake at the Intestinal Brush-Border Membrane.
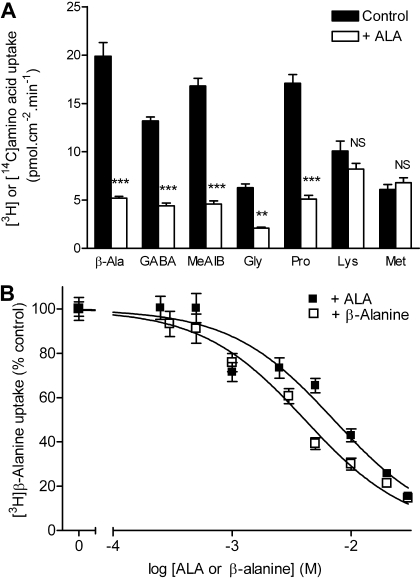
Caco-2 cell monolayers grown on permeable supports are frequently used as a model of the small intestinal epithelium. The interaction of ALA with amino acid transport at the intestinal brush-border membrane was assessed (Fig. 4A). Uptakes of the PAT1 substrates β-alanine, GABA, glycine, l-proline, and MeAIB were all significantly reduced by ALA (10 mM; p < 0.01) (Fig. 4A). In contrast, the uptakes of amino acids not transported by PAT1, such as lysine and methionine (Thwaites et al., 1995), were unchanged in the presence of ALA (p > 0.05). The inhibition of [3H]-β-alanine uptake by ALA was concentration-dependent (Fig. 4B). The IC50 for ALA inhibition of apical amino acid uptake in Caco-2 cells (7.1 ± 0.9 mM) was close to the affinity constant estimated by electrophysiology in PAT1-expressing oocytes (Fig. 1).
Fig. 4.
ALA inhibition of PAT1-mediated amino acid uptake at the apical membrane of the human intestinal cell line Caco-2. The cells were grown as confluent monolayers of polarized cells on Transwell polycarbonate filters. A, apical uptake of the PAT1 substrates [3H]-β-alanine (10 μM), [3H]GABA (10 μM), [14C]MeAIB (26 μM), [3H]glycine (10 μM), and [3H]proline (10 μM) and the nonsubstrates [3H]lysine (10 μM) and [3H]methionine (10 μM) measured in the presence and absence of apical ALA (10 mM). Uptake was measured at apical pH 5.5 and basolateral pH 7.4. ***, p < 0.001; **, p < 0.01; NS, p > 0.05, all versus control. B, concentration-dependent inhibition of [3H]-β-alanine (100 μM) uptake measured at apical pH 5.5 and basolateral pH 7.4 by unlabeled ALA or β-alanine.
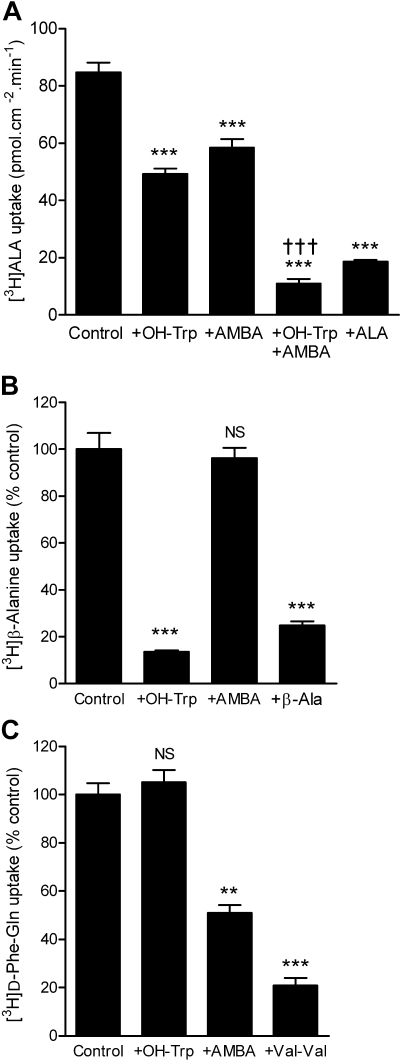
[3H]ALA uptake was measured across the apical membrane of Caco-2 cell monolayers. [3H]ALA uptake was significantly greater in the presence of a pH gradient consistent with both PAT1- and PepT1-mediated transport (uptake being 9 ± 1 and 47 ± 2 pmol · cm−2 · min−1 at apical pH 7.4 and 5.5, respectively; p < 0.001). [3H]ALA uptake was inhibited by either AMBA (30 mM) or OH-Trp (20 mM) when tested individually (Fig. 5A). Inclusion of both OH-Trp and AMBA lead to significantly greater (additive) inhibition of apical [3H]ALA uptake than either compound caused individually (p < 0.001) (Fig. 5A). The nontransported PAT1 inhibitor, OH-Trp (20 mM) reduced uptake of the PAT1 substrate [3H]-β-alanine to a degree similar to that observed with unlabeled β-alanine (30 mM) (Fig. 5B) but had no effect on uptake of the PepT1 substrate [3H]-d-Phe-Gln (Fig. 5C). The nontransported PepT1 inhibitor AMBA (30 mM) inhibited uptake of [3H]-d-Phe-Gln but to a lesser extent than the dipeptide Val-Val (30 mM) (Fig. 5C) and had no effect on uptake of [3H]-β-alanine (Fig. 5B). These data confirm that the two inhibitors, OH-Trp and AMBA, are selective for PAT1 and PepT1, respectively, in a cell system in which both transporters are coexpressed in the same cell membrane. Therefore, these data demonstrate that both PAT1 and PepT1 contribute to ALA transport across the brush-border membrane of intestinal epithelial cells (Fig. 5A). It should be noted that AMBA (30 mM) does not completely inhibit dipeptide uptake (Fig. 5C). Therefore, the degree of inhibition by AMBA observed in Fig. 5A will underestimate the PepT1 contribution to [3H]ALA uptake. If AMBA were to inhibit PepT1-mediated [3H]ALA uptake with an efficiency similar to that observed with Val-Val on [3H]-d-Phe-Gln, then we estimate that the [3H]ALA uptake after inhibition of PepT1 (in the studies reported in Fig. 5A) would be reduced to approximately 42 pmol · cm−2 · min−1. This level of [3H]ALA uptake is similar to that measured after inhibition of PAT1 (as observed in the presence of OH-Trp), which was 49 pmol · cm−2 · min−1 (Fig. 5A). Competition experiments using substrates rather than nontransported inhibitors are complicated by the effects of substrate-induced pHi changes. Therefore, although AMBA is only a low-affinity inhibitor, it has been a useful experimental tool in this investigation, because it negates potential nonspecific effects on driving force.
Fig. 5.
Both PAT1 and PepT1 mediate ALA uptake across the intestinal brush-border membrane. A, apical uptake of [3H]ALA (100 μM) was measured at apical pH 5.5 and basolateral pH 7.4 in the presence and absence of excess ALA (20 mM), the PAT1 inhibitor OH-Trp (20 mM), and/or the PepT1 inhibitor AMBA (30 mM). ***, p < 0.001 versus control; †††, p < 0.001 versus either +AMBA or +OH-Trp. B, apical uptake of the PAT1 substrate [3H]-β-alanine (β-Ala) (100 μM) in the presence of the PAT1 inhibitor OH-Trp (20 mM), the PepT1 inhibitor AMBA (30 mM), or unlabeled β-alanine (30 mM). ***, p < 0.001; NS, p > 0.05, versus control. C, apical uptake of the PepT1 substrate [3H]-d-Phe-Gln (100 μM) in the presence of the PAT1 inhibitor OH-Trp (20 mM), the PepT1 inhibitor AMBA (30 mM), or the dipeptide Val-Val (30 mM). ***, p < 0.001; **, p < 0.01; NS, p > 0.05, versus control.
Expression of PAT1 and PepT1 in Colon Cancer.
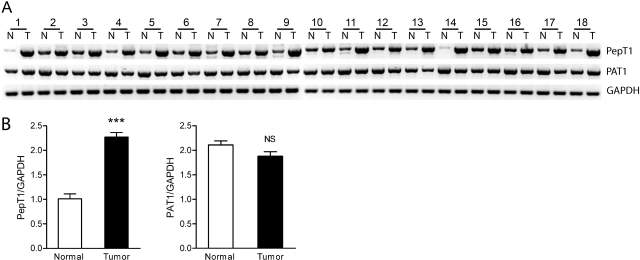
For increased conversion of oral ALA to PpIX to occur in tumors, accumulation of ALA within tumor cells is first required. Therefore, expression of PAT1 and PepT1 in colon cancer and normal tissue was investigated (Fig. 6). Eighteen paired biopsy samples from patients with colorectal cancer were screened for PAT1 and PepT1 expression at the mRNA level (Fig. 6A). The relative expression of PAT1 was not significantly different between normal and tumor tissue (Fig. 6B), which may indicate that PAT1 contributes to ALA accumulation in both normal and tumor intestinal mucosa. However, PepT1 expression was increased in all 18 tumor samples, with an average increase of 2.3 ± 0.1-fold (Fig. 6B). Therefore, it is conceivable that increased PepT1 expression could contribute to increased ALA and subsequent PpIX accumulation in colon cancer and other tumors.
Fig. 6.
PepT1 expression is increased in colon cancer. A, expression of PepT1 and PAT1 in normal colon (N) and paired colon tumor (T) tissues from 18 patients (see Materials and Methods) assessed by reverse transcriptase-PCR and compared with GAPDH as a control. B, semiquantitative analysis of expression of PepT1 and PAT1 in normal and tumor tissues shown in A as a ratio to GAPDH expression. ***, p < 0.001; NS, p > 0.05.
Discussion
The H+-coupled amino acid transporter PAT1 acts as a high-capacity, absorptive route for small neutral amino acids, such as GABA, and many orally active analogs across the brush-border membrane of the small intestine (Thwaites et al., 2000; Chen et al., 2003; Anderson et al., 2004; Abbot et al., 2006; Metzner et al., 2006; Thwaites and Anderson, 2007a; Larsen et al., 2009). PAT1 can also be considered a novel intestinal transporter of the heme precursor ALA, which is used extensively in photodynamic therapy. PAT1 has a relatively low affinity for ALA (Km 10.4 mM) (Fig. 1A) compared with GABA (Km 1.1 mM), but the affinity is within the range of that for other naturally occurring PAT1 substrates such as glycine, l-proline, l-alanine, and taurine (Km ∼2–10 mM) (Thwaites et al., 1995; Boll et al., 2002; Metzner et al., 2006; Anderson et al., 2009).
During photodynamic therapy, ALA is typically given orally in the range of 10 to 60 mg/kg b.wt., in small amounts of fluid, either as a bolus or in fractionated doses. The low molecular weight of ALA (mol. wt. 167) makes it conceivable that concentrations around the Km of PAT1 are achieved in the intestinal lumen.
The data presented here show that ALA transport across the brush-border membrane of the human small intestinal epithelium is via both PAT1 and the H+-coupled di/tripeptide transporter PepT1 (Fig. 5). Understanding the mechanisms by which ALA is transported across the brush-border membrane is essential when one is considering the potential for patient variability either through polymorphisms, drug-drug interactions, or pathophysiological regulation of intestinal function. Both PAT1 and PepT1 are regulated by a range of dietary, (patho)physiological, and neurohormonal pathways (Anderson et al., 2003; Daniel, 2004; Thwaites and Anderson, 2007a), which in turn may alter the efficacy of photodynamic therapy.
Because ALA readily appears in the plasma after oral dosage (van den Boogert et al., 1998), it must also be transported, intact, across the basolateral membrane of the small intestinal epithelium. There is some evidence that this may be via the as yet unidentified basolateral di/tripeptide transporter described in cell cultures (Irie et al., 2001). In other cells, ALA transport may be via either amino acid or peptide transporters. A common transport system for ALA and the structurally analogous GABA was first shown in Saccharomyces cerevisiae (Bermúdez Moretti et al., 1995). At around the same time, studies in bacteria identified ALA transport via a dipeptide permease (Elliott, 1993). Daniel and colleagues (Döring et al., 1998) were the first to show that ALA is an excellent substrate not only for PepT1 but also for nonintestinal PepT2 (SLC15A2). Subsequently, PepT1 was shown to mediate ALA transport in cholangiocytes, in which its affinity for the transporter (Km 2.1 mM) is similar to that for PepT1 in this study (Fig. 2) (Neumann and Brandsch, 2003). Studies using knockout mice demonstrate that PepT2 plays a key role not only in ALA reabsorption in the kidney but also in limiting ALA neurotoxicity by removal of ALA from the cerebrospinal fluid (Hu et al., 2007). In certain cell lines, ALA transport has been attributed to members of the SLC6 family of Na+- and Cl−-coupled amino acid transporters, particularly the high-affinity GAT transporters (Rud et al., 2000; Bermúdez Moretti et al., 2002). Therefore, ALA transport will vary depending on the complement of amino acid and dipeptide transporters expressed in each cell type. The work presented here is the first study to demonstrate overlapping substrate specificity of distinct transporters for dipeptides and amino acids coexpressed in the same functional compartment of a cell.
Substantial transport of ALA into intestinal epithelial cells after oral ALA is evident by the accumulation of PpIX within normal intestinal mucosal tissue measured by fluorescent imaging (Loh et al., 1993; Regula et al., 1995). However, the high background accumulation of PpIX in gastrointestinal epithelia is also a limiting factor in achieving sufficient PpIX tumor/normal mucosa ratios necessary for effective photodynamic therapy of gastrointestinal tumors. Thus, much higher doses of ALA are required (40–60 mg/kg b.wt.) to achieve reasonable tumor selectivity in colon cancer compared with the oral doses used successfully in the detection of other tumors such as malignant glioma (10–20 mg/kg b.wt.) (Loh et al., 1993; Regula et al., 1995, Peng et al., 1997; Stummer et al., 2006). The variation in accumulation of PpIX after ALA dosage, both between normal and tumor tissue and between different types of tumors, is still a matter of debate and is likely to be multifactorial. It is clear that the first step required for in situ PpIX synthesis is the effective transport of ALA into the tumor cell. Therefore, increased ALA transport capacity may facilitate PpIX accumulation in combination with downstream effects such as the reduced ferrochelatase activity (which converts PpIX to heme) reported in some tumors (van Hillegersberg et al., 1992). Figure 6 shows that both PepT1 and PAT1 are expressed in human colon cancer. The consistent increase in PepT1 expression in all tumor samples investigated is the first evidence that an ALA uptake mechanism may be altered in colon cancer. Up-regulation of PepT1 in cancer may not be restricted to colonic tumors. [11C]Gly-Sar has been found to accumulate in mouse tumor xenografts originating from human pancreatic, prostate, and gastric cancer cells (Mitsuoka et al., 2008). In addition to influencing photodynamic therapy, increased PepT1 expression in cancer would be of potential therapeutic advantage in the delivery of the anticancer PepT1 substrate bestatin (Rubio-Aliaga and Daniel, 2002). It is tempting to speculate that the aberrant expression of H+-coupled transporters could provide a selective advantage for cancer cells by providing nutrient uptake pathways that can exploit the acid extracellular milieu associated with many solid tumors (Gerweck and Seetharaman, 1996). Thus, selective inhibitors for such transporters could be a means to limit tumor nutritional supply. Further investigation is required to determine whether PepT1 functional capacity is increased in colon cancer.
It is interesting to note that previous work has demonstrated that expression of the PpIX efflux pump BCRP (breast cancer resistance protein, ABCG2) is down-regulated in colon cancer (Gupta et al., 2006). Thus, a combination of increased PepT1 expression and decreased BCRP expression could produce a selective increase in PpIX accumulation in cancer tissues. In addition, we demonstrate that coadministration of nontransported inhibitors of either PAT1 or PepT1 results in selective reduction of ALA uptake in small intestinal epithelial cells (without any inhibition of the second transporter) (Figs. 2, 3, and 5). These observations reveal a potential method for improvement in selective ALA loading in colon cancer cells compared with normal mucosa. Coadministration of a selective nontransported PAT1 inhibitor with ALA, as demonstrated in vitro here using OH-Trp, could reduce background PpIX accumulation in normal mucosa. The relatively high expression of PepT1 in cancerous tissue would then favor ALA uptake into cancer cells. Further experimentation is required to test these possibilities.
In conclusion, this study shows that PAT1 is a novel transporter for ALA and that both PAT1 and PepT1 will contribute to the absorption of oral ALA across the brush-border membrane of the human small intestinal epithelium. The role of H+-coupled nutrient transporters such as PAT1 and PepT1 in cancer biology and anticancer therapeutic regimens merits further investigation.
Acknowledgments
We thank Lisa Burdis for excellent technical assistance.
This work was supported in part by the Wellcome Trust [Grant 078640/Z/05/Z]; the Biotechnology and Biological Sciences Research Council [Grants BB/B512008/1 and BB/D5526596/1] (Ph.D. studentships to M.J. and N.E.); and the Medical Research Council [Grant G0800128] (Ph.D. studentship to N.J.C.).
Parts of this work were previously presented as follows: Anderson CMH, Jevons M, and Thwaites DT (2009) Luminal uptake of the photodynamic therapy agent 5-aminolevulinic acid via the proton-coupled amino acid transporter PAT1 (SLC36A1) in human intestinal epithelial cells. Digestive Disease Week; 2009 May 31–Jun 3; Chicago, IL. Gastroenterology 136 (Suppl 1):A-570.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.159822
- ALA
- 5-aminolevulinic acid
- PpIX
- protoporphyrin IX
- PepT1
- H+-coupled di/tripeptide transporter 1
- PAT1
- H+-coupled amino acid transporter 1
- Gly-Sar
- glycylsarcosine
- MeAIB
- α-methylaminoisobutyric acid
- d-Phe-Gln
- d-phenylalanyl-l-glutamine
- MES
- 2-(N-morpholino) ethanesulfonic acid
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- OH-Trp
- 5-hydroxy-l-tryptophan
- AMBA
- 4-aminomethylbenzoic acid
- PepT2
- H+-coupled di/tripeptide transporter 2.
References
- Abbot EL, Grenade DS, Kennedy DJ, Gatfield KM, Thwaites DT. (2006) Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+-coupled amino-acid transporter hPAT1. Br J Pharmacol 147:298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, et al. (2004) H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology 127:1410–1422 [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Howard A, Walters JRF, Ganapathy V, Thwaites DT. (2009) Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl−-dependent TauT (SLC6A6). J Physiol 587:731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Mendoza ME, Kennedy DJ, Raldua D, Thwaites DT. (2003) Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2). Br J Pharmacol 138:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez Moretti M, Correa García SR, Chianelli MS, Ramos EH, Mattoon JR, Batlle A. (1995) Evidence that 4-aminobutyric acid and 5-aminolevulinic acid share a common transport system into Saccharomyces cerevisiae. Int J Biochem Cell Biol 27:169–173 [DOI] [PubMed] [Google Scholar]
- Bermúdez Moretti M, Correa García S, Perotti C, Batlle A, Casas A. (2002) δ-Aminolevulinic acid transport in murine mammary adenocarcinoma cells is mediated by β transporters. Br J Cancer 87:471–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. (2002) Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem 277:22966–22973 [DOI] [PubMed] [Google Scholar]
- Chen Z, Fei YJ, Anderson CMH, Wake KA, Miyauchi S, Huang W, Thwaites DT, Ganapathy V. (2003) Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol 546:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H. (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361–384 [DOI] [PubMed] [Google Scholar]
- Döring F, Walter J, Will J, Föcking M, Boll M, Amasheh S, Clauss W, Daniel H. (1998) δ-Aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest 101: 2761–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. (1993) Transport of 5-aminolevulinic acid by the dipeptide permease in Salmonella typhimurium. J Bacteriol 175:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. (1994) Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 368:563–566 [DOI] [PubMed] [Google Scholar]
- Gerweck LE, Seetharaman K. (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56:1194–1198 [PubMed] [Google Scholar]
- Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, Podolsky R, Ganapathy V. (2006) Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem Biophys Res Commun 343:571–577 [DOI] [PubMed] [Google Scholar]
- Hu Y, Shen H, Keep RF, Smith DE. (2007) Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J Neurochem 103:2058–2065 [DOI] [PubMed] [Google Scholar]
- Irie M, Terada T, Sawada K, Saito H, Inui K. (2001) Recognition and transport characteristics of nonpeptidic compounds by basolateral peptide transporter in Caco-2 cells. J Pharmacol Exp Ther 298:711–717 [PubMed] [Google Scholar]
- Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. (2002) Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflügers Archiv 445:139–146 [DOI] [PubMed] [Google Scholar]
- Kennedy JC, Pottier RH. (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14:275–292 [DOI] [PubMed] [Google Scholar]
- Krammer B, Plaetzer K. (2008) ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 7:283–289 [DOI] [PubMed] [Google Scholar]
- Larsen M, Holm R, Jensen KG, Brodin B, Nielsen CU. (2009) Intestinal gaboxadol absorption via PAT1 (SLC36A1): modified absorption in vivo following co-administration of L-tryptophan. Br J Pharmacol 157:1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh CS, MacRobert AJ, Bedwell J, Regula J, Krasner N, Bown SG. (1993) Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer 68:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan GTA, Daniel H, Fett C, Burgess MN, Lucas ML. (1988) The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B 234:219–237 [DOI] [PubMed] [Google Scholar]
- Meredith D, Boyd CAR, Bronk JR, Bailey PD, Morgan KM, Collier ID, Temple CS. (1998) 4-Aminomethylbenzoic acid is a non-translocated competitive inhibitor of the epithelial peptide transporter PepT1. J Physiol 512:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner L, Kottra G, Neubert K, Daniel H, Brandsch M. (2005) Serotonin, l-tryptophan, and tryptamine are effective inhibitors of the amino acid transport system PAT1. FASEB J 19:1468–1473 [DOI] [PubMed] [Google Scholar]
- Metzner L, Neubert K, Brandsch M. (2006) Substrate specificity of the amino acid transporter PAT1. Amino Acids 31:111–117 [DOI] [PubMed] [Google Scholar]
- Mitsuoka K, Miyoshi S, Kato Y, Murakami Y, Utsumi R, Kubo Y, Noda A, Nakamura Y, Nishimura S, Tsuji A. (2008) Cancer detection using a PET tracer, 11C-glycylsarcosine, targeted to H+/peptide transporter. J Nucl Med 49:615–622 [DOI] [PubMed] [Google Scholar]
- Neumann J, Brandsch M. (2003) δ-Aminolevulinic acid transport in cancer cells of the human extrahepatic biliary duct. J Pharmacol Exp Ther 305:219–224 [DOI] [PubMed] [Google Scholar]
- Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, Nesland JM. (1997) 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 79:2282–2308 [DOI] [PubMed] [Google Scholar]
- Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, Spencer GM, Hatfield ARW, Bown SG. (1995) Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX—a pilot study. Gut 36:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. (2002) Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci 23:434–440 [DOI] [PubMed] [Google Scholar]
- Rud E, Gederaas O, Høgset A, Berg K. (2000) 5-Aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem Photobiol 71:640–647 [DOI] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJALA-Glioma Study Group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401 [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. (2007a) Deciphering the mechanisms of intestinal imino (and amino) acid transport: the redemption of SLC36A1. Biochim Biophys Acta 1768:179–197 [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. (2007b) H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92:603–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Basterfield L, McCleave PMJ, Carter SM, Simmons NL. (2000) Gamma-Aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol 129:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Simmons NL. (1995) The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol 145:245–256 [DOI] [PubMed] [Google Scholar]
- van den Boogert J, van Hillegersberg R, de Rooij FWM, de Bruin RWF, Edixhoven-Bosdijk A, Houtsmuller AB, Siersema PD, Wilson JHP, Tilanus HW. (1998) 5-Aminolaevulinic acid-induced protoporphyrin IX accumulation in tissues: pharmacokinetics after oral or intravenous administration. J Photochem Photobiol B 44:29–38 [DOI] [PubMed] [Google Scholar]
- Van Hillegersberg R, Van den Berg JWO, Kort WJ, Terpstra OT, Wilson JHP. (1992) Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 103:647–651 [DOI] [PubMed] [Google Scholar]
- Walker D, Thwaites DT, Simmons NL, Gilbert HJ, Hirst BH. (1998) Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J Physiol 507:697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. (1993) Cloning and characterization of the vasopressin-regulated urea transporter. Nature 365:844–847 [DOI] [PubMed] [Google Scholar]