Abstract
Glyceollins, a group of novel phytoalexins isolated from activated soy, have recently been demonstrated to be novel antiestrogens that bind to the estrogen receptor (ER) and inhibit estrogen-induced tumor progression. Our previous publications have focused specifically on inhibition of tumor formation and growth by the glyceollin mixture, which contains three glyceollin isomers (I, II, and III). Here, we show the glyceollin mixture is also effective as a potential antiestrogenic, therapeutic agent that prevents estrogen-stimulated tumorigenesis and displays a differential pattern of gene expression from tamoxifen. By isolating the individual glyceollin isomers (I, II, and III), we have identified the active antiestrogenic component by using competition binding assays with human ERα and in an estrogen-responsive element-based luciferase reporter assay. We identified glyceollin I as the active component of the combined glyceollin mixture. Ligand-receptor modeling (docking) of glyceollin I, II, and III within the ERα ligand binding cavity demonstrates a unique type II antiestrogenic confirmation adopted by glyceollin I but not isomers II and III. We further compared the effects of glyceollin I to the antiestrogens, 4-hydroxytamoxifen and ICI 182,780 (fulvestrant), in MCF-7 breast cancer cells and BG-1 ovarian cancer cells on 17β-estradiol-stimulated expression of progesterone receptor and stromal derived factor-1α. Our results establish a novel inhibition of ER-mediated gene expression and cell proliferation/survival. Glyceollin I may represent an important component of a phytoalexin-enriched food (activated) diet in terms of chemoprevention as well as a novel therapeutic agent for hormone-dependent tumors.
Breast cancer accounts for 25% of all female cancers, making it the most common cancer in women in the western world (Greenlee et al., 2000 Lester, 2007; Ali and Coombs, 2002). For patients with hormone receptor-positive breast cancer, several promising endocrine agents are currently available with promising results. Therapies have been developed to reduce estrogen levels or to block signaling through estrogen receptors (ER) (Pink and Jordan, 1996; Howell, 2006). These agents include tamoxifen, a selective estrogen receptor modulator; letrozole or anastrozole, aromatase inhibitors; and selective estrogen receptor down-regulators, such as fulvestrant (Ali and Coombs, 2002; Miller et al., 2007). However, because of the increased incidence of endometrial cancer associated with tamoxifen treatment, and other unwanted side effects, alternative therapies for postmenopausal breast cancer patients are required.
Interest in the physiological and pharmacological role of bioactive compounds present in plants has increased dramatically over the past decade (Koehn and Carter, 2005). Increasing epidemiological studies regarding consumption of dietary soy provide a rationale for various nutritional strategies designed to contribute to breast cancer prevention (Ganry, 2002; Limer and Speirs, 2004). One particular class of compounds, known as the phytoestrogens, embodies several groups of nonsteroidal estrogens that are widely distributed in nature, particularly soy-containing foods (Gikas and Mokbel, 2005; Usui, 2006). These compounds are rapidly gaining interest in both traditional and alternative medicine. Phytoestrogens can be divided into three main classes: isoflavones (genistein and daidzein), coumestans (coumestrol), and lignans (enterodiol and enterolactone). Because of their chemical structure, phytoestrogens compete with endogenous estrogens for binding to the ER, thereby reducing the hormonal effect of endogenous estrogens that are much more potent than phytoestrogens (Barnes, 1998; Magee and Rowland, 2004).
In response to pathogens and other stress stimuli (UV light, exposure to microorganisms, and low temperature), soybean tissues accumulate several phytoalexins, predominantly the glyceollins, which share structural similarities with the isoflavone daidzein (Ayers et al., 1976; Moesta et al., 1983; Hahn et al., 1985). In phytoalexin-enriched foods such as soy, we have identified three major forms of the glyceollins (I, II, and III). Our previous in vitro and in vivo breast cancer studies as well as our primate studies have collectively shown that glyceollins possess marked antiestrogenic effects (Burow et al., 2001; Salvo et al., 2006; Wood et al., 2006). The primate study also revealed mean serum glyceollin concentrations at 4 h after feeding of 134.2 ± 34.6 nM in the glyceollin group and negligible concentration in the soy protein isolate control group (p = 0.0007). Breast proliferation was significantly increased in the control group (+237%; p = 0.01) but not in the soy protein isolate group (+198%; p = 0.08) or glyceollin group (+36%; p = 0.18). Gene expression of trefoil factor 1 and progesterone receptor, two markers of estrogen receptor activity in breast epithelium, were also significantly higher in the control (p < 0.05 for both) but not in the glyceollin group. Taken together, these preliminary findings suggest that soybean glyceollins are natural compounds with potential estrogen-modulating properties in the breast. It is interesting to note that in our previous publication, we investigated a novel isoflavone, glycinol, a precursor to glyceollin that is produced in elicited soy, and we found it to be a potent phytoestrogen (Boue et al., 2009). In the current study, we demonstrate that glyceollin I is the most potent, antiestrogenic isomer of the glyceollin mixture and is distinctly different from its estrogenic precursor glycinol (Boué et al., 2009).
Glyceollins also have enhanced antagonism toward ERα relative to ERβ, and they lack any of the estrogen agonist activity of genistein and daidzein seen in low-estrogen conditions (Nikov et al., 2000; Burow et al., 2001). In the present study, we sought to define the antiestrogenic activity of the specific glyceollin isoforms. We used a combination of reporter gene assays, ER binding and molecular modeling to define the role for selective ER targeting by individual glyceollin isoforms. In addition, we investigated the effects of glyceollin I on proliferation and gene expression to explore potential mechanisms of action. Two estrogen-dependent cell lines (MCF-7 breast cancer cells and BG-1 ovarian cancer cells) were used to study the effects of the glyceollins and the regulation of ER in two different cellular contexts.
Materials and Methods
Chemicals.
4-Hydroxytamoxifen was purchased from Sigma-Aldrich (St. Louis, MO). Fulvestrant (ICI 182,780) was purchased from Tocris Bioscience (Ellisville, MO). Glyceollins I, II, and III were isolated using a procedure developed at the Southern Regional Research Center (U.S. Department of Agriculture–Agricultural Research Service, New Orleans, LA).
High-Performance Liquid Chromatography.
Soybean seeds (1 kg) were sliced and inoculated with Aspergillus sojae. After 3 days, the glyceollins were extracted from the inoculated seeds with 1 l of methanol. A mixture of the glyceollins was isolated by a preparative-scale HPLC using two 25-mm 10-μm particle size μBondapak C18 radial compression column segments (Waters, Milford, MA) combined using an extension tube. HPLC was performed on a 600E System Controller combined with a UV-VIS 996 detector (both from Waters). Elution was carried out at a flow rate of 8.0 ml/min with the following solvent system (A, acetonitrile and B, water): 5% A for 10 min and then 5% A to 90% A in 60 min followed by holding at 90% A for 20 min. The injection volume was 20 ml. The fraction containing the glyceollins was concentrated under vacuum and freeze-dried. For the isolation of the individual glyceollins I, II, and III, semipreparative HPLC was used. We used an ODS-2 (10 × 500-mm) column (Whatman, Maidestone, UK) and a flow rate of 3.0 ml/min with the following solvent system (A, acetonitrile and B, water): 5% A for 15 min and then 5% A to 90% A in 40 min followed by holding at 90% A for 20 min. Glyceollins I, II, and III were confirmed by UV-visible spectrophotometry, mass spectrometry, and NMR analyses. The solvents acetonitrile (HPLC grade) and methanol were purchased from Aldrich Chemical Co. (Milwaukee, WI). Water was obtained using a Millipore system (Millipore, Billerica, MA) and was used during sample preparation procedures and HPLC analyses.
Cell Culture.
Human cancer cell lines derived from breast (MCF-7, ER-positive cells) and ovary (BG-1, ER-positive cells) were cultured in 75-cm2 culture flasks in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Invitrogen), basic minimal minimal essential medium essential (50×; Invitrogen) and essential medium nonessential (100×; Invitrogen) amino acids, sodium pyruvate (100×; Invitrogen), antimycotic-antibiotic [10,000 U/ml penicillin G sodium; 10,000 μg/ml streptomycin sulfate; and 25 μg/ml amphotericin B (Fungizone)], and human recombinant insulin (4 mg/ml; Invitrogen). The culture flasks were maintained in a tissue culture incubator in a humidified atmosphere of 5% CO2 and 95% air at 37°C. For estrogen studies, cells were washed with PBS three times and grown in phenol red-free DMEM supplemented with 5% dextran-coated charcoal-stripped FBS (5% CS-FBS) for 72 h before plating for each particular experiment.
Animals.
We obtained nu/nu immune-compromised female ovariectomized mice (29–32 days old) from Charles River Laboratories, Inc. (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment with phytoestrogen-free food and water ad libitum. Placebo or estradiol pellets (0.72 mg; 60-day release; Innovative Research of America, Inc, Sarasota, FL) were implanted subcutaneously in the lateral area of the neck in the middle point between the ear and shoulder by using a 10-gauge precision trochar, 10 mice each. MCF-7 cells in the exponential phase of growth were harvested using PBS/EDTA solution and washed. Viable cells (5 × 106) in a 50 μl of sterile PBS suspension were mixed with 100 μl of Matrigel Reduced Factors (BD Biosciences Discovery Labware, Bedford, MA). MCF-7 cells were injected in the mammary fat pad through a 5-mm incision at the hypogastric area, and the incision was closed using staples. All the procedures in animals were carried out under anesthesia using a mix of isoflurane and oxygen delivered by mask. Tumors were allowed to form over 10 days, and mice were randomized to four treatment groups with five mice per group: control (Con), estradiol (E2) + vehicle, glyceollin mixture only (Gly), and estradiol plus glyceollin mixture (E2 + Gly). The glyceollin mixture was suspended in a solution of DMSO (one-third volume) and propylene glycol (two-thirds volume) and was given intraperitoneally at 20 mg/kg/mouse/day for 15 days to Gly and E2 + Gly groups starting after tumors were measurable. Control and E2 groups were injected with vehicle daily for 15 days. Tumor size was measured every 2 days by using a digital caliper. The volume of the tumor was calculated using the following formula: 4/3LM2, where L is the larger radius and M is the smaller radius. At necropsy on day 15, animals were euthanized by decapitation after exposure to a CO2 chamber. Tumors, uteri, brain, livers, and lungs were removed and either frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Tulane University Animal Care and Use Committee. The facilities and laboratory animal program of Tulane University is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Real-Time RT-PCR.
Total cellular RNA was extracted using the RNeasy mini-column (QIAGEN, Valencia, CA), following the manufacturer's instructions. In brief, a maximum of 1 × 107 cells are disrupted in buffer containing guanidine isothiocyanate and homogenized. Ethanol is added to the lysate, creating conditions that promote selective binding of RNA to the RNeasy silica-gel membrane. The sample is then applied to the RNeasy mini-column. Total RNA binds to the membrane, and high-quality RNA is then eluted in RNase-free water. All binding, wash, and elution steps were performed by centrifugation in a microcentrifuge. The concentration of RNA was determined using an ultraviolet spectrophotometer. Reverse transcription (RT) was carried out using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). One microgram of total RNA was reverse-transcribed to cDNA following the manufacturer's instructions. For each RT, a blank was prepared using all the reagents except the RNA sample (for which an equivalent volume of diethyl pyrocarbonate-treated water was substituted). This blank was used as a nontemplate control in the real-time PCR experiments. The level of stromal-cell derived factor-1 (SDF-1) and progesterone receptor (PgR) transcripts was determined using a real-time quantitative PCR. Primers for PCR were designed to span intron/exon junctions to minimize amplification of residual genomic DNA. The primer sequences for PgR, SDF-1 and ERα are as follows (sense and antisense, respectively): PgR (5′-TACCCGCCCTATCTCAACTACC-3′ and 5′-TGCTTCATCCCCACAG-ATTAAACA-3′), SDF-1 (5′-AGTCAGGTGGTGGCTTAACAG-3′ and 5′-AGAGGAGGTGAAGGCAGTGG-3′), and ERα (5′-GCGATGGTGGAGATCTTCGA-3′ and 5′-CCTCTCCCTGCAGATTCATCA-3′). PCR mix contained optimal concentrations of primers, cDNA, and SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA). Quantification and relative gene expression were calculated with internal controls. The ratio between the values obtained provided the relative gene expression levels.
Gene Superarrays.
MCF-7 cells were seeded into 75-cm2 flasks in DMEM supplemented with 5% fetal bovine serum. On the following day, media were replaced with phenol red-free DMEM supplemented with 5% charcoal-stripped serum for 2 days. Cells were treated with DMSO (vehicle), 1 nM 17β-estradiol, 10 μM glyceollin mixture, and 100 nM tamoxifen. Total RNA was extracted. Each array profiles the expression of a panel of 96 genes. For each array, 4 μg of RNA was reverse-transcribed into cDNA in the presence of gene-specific oligonucleotide primers as described in the manufacturer's protocol. cDNA template was mixed with the appropriate ready-to-use PCR master mix. Equal volumes were measured (in aliquots) into each well of the same plate, and then the real-time PCR cycling program was run. Quantitative RT-PCR was performed using manufacturer's protocols for the RT2 Profiler PCR Array (Human Breast Cancer and Estrogen Receptor Signaling Superarray, Gaithersburg, MD). Relative gene expressions were calculated by using the 2−ΔΔCt method, in which Ct indicates the fractional cycle number where the fluorescent signal reaches detection threshold. The “delta-delta” method (described by Pfaffl, 2001) uses the normalized ΔCt value of each sample, calculated using a total of five endogenous control genes (18S rRNA, HPRT1, RPL13A, GAPDH, and ACTB). -Fold change values are then presented as average -fold change = 2−(average ΔΔCt) for genes in treated relative to control samples. Clinical variables were characterized using descriptive statistics, and the statistical significance of differences in gene expression between groups was calculated using Student's t test.
ERE-Luciferase Assay.
As described previously (Klotz et al., 1996; Burow et al., 1999), the cells were plated in 24-well plates at a density of 5 × 105 cells/well in the same medium and allowed to attach overnight. After 18 h, cells were transfected for 5 h in serum-free DMEM with 300 μg of pGL2-ERE2X-TK-luciferase plasmid, by using 6 μl of Effectene transfection reagent (QIAGEN) per microgram of DNA. After 5 h, the transfection medium was removed and replaced with phenol red-free DMEM supplemented with 5% CS-FBS containing vehicle, E2, Gly I, or E2 + Gly I, and incubated at 37°C. After 18 h, the medium was removed, and 100 μl of lysis buffer was added per well and then incubated for 15 min at room temperature. Cell debris was pelleted by centrifugation at 15,000g for 5 min. Cell extracts were normalized for protein concentration by using reagent according to the protocol supplied by the manufacturer (Bio-Rad Laboratories). Luciferase activity for the cell extracts was determined using luciferase substrate (Promega, Madison, WI) in an Auto Lumat Plus luminometer (Berthold, Oak Ridge, TN).
ERα Binding Assays.
Receptor binding determinations of glyceollin were achieved using the method of Bolger et al. (1998). In this method, recombinant ER is in equilibrium with a fluorescent ligand (ES2) and a concentration of the competitor (glyceollin). The relative displacement of the ES2 is measured as a change in polarization anisotropy. Serial dilutions of competitors (glyceollin and estradiol) were prepared from DMSO stock solutions in screening buffer at the desired concentrations. The ER and ES2 were combined with each competitor aliquot to a final concentration of 2 nM ER and 3 nM ES2, respectively. In addition, both a no binding control (ER + ES2 only) equivalent to 0% competitor inhibition) and a 100% binding control (only free ES2, no ER, equivalent to 100% competitor inhibition) were prepared. All competitor and controls were prepared in duplicate within a binding experiment. After a 2-h incubation at room temperature, the anisotropy value for each sample and control was measured using the Beacon 2000 Fluorescence Polarization System (Invitrogen, Carlsbad, CA). Anisotropy values were converted to percent inhibition using the following formula: I% = (A0 − A)/(A0 − A100) × 100, where I% is the percentage of inhibition, A0 is 0% inhibition, A100 is 100% inhibition, and A represents the observed value. This conversion to percentage of inhibition makes the data more intuitive and normalizes the day-to-day differences of multiple experiments. The percentage of inhibition versus competitor concentration curves was analyzed by nonlinear least-squares curve fitting (Prism 5.0a; GraphPad Software Inc., San Diego, CA; www.graphpad.com) to yield IC50 values (the concentration of competitor needed to displace half of the bound ligand). To compare binding affinities of the test compounds to those reported in the literature, IC50 values were converted to relative binding affinities (RBA) using E2 as a standard. The E2 RBA was set equal to 100 RBA = (IC50/IC50 of E2) × 100.
Molecular Modeling.
The three glyceollin isomers (I, II, and III) used in this study maintain two chiral centers within their structure resulting in four possible enantiomers of the compound. Of these configurations, only the SS configuration is found in nature, and this correct structure was thus used in ligand-receptor modeling (docking) studies. The SS configuration of each glyceollin was converted to a unique SMILE string with ChemDraw (CambridgeSoft Corporation, Cambridge, MA; www.cambridgesoft.com) and then converted to a three-dimensional structure by using CONCORD (R. S. Pearlman, distributed by Tripos, St. Louis, MO). The initial three-dimensional model was then optimized in SYBYL 8.0 (Tripos) using the MMFF94 force field and the conjugated gradient method with a termination of 0.005 kcal/mol. After optimization, the glyceollin structures were assigned AM1 charges by using MOPAC 6.0 distributed with SYBYL.
Docking and scoring of the three glyceollins was performed using Surflex-Dock in SYBYL 8.0. The crystal structure of the human estrogen receptor-a ligand-binding domain in complex with 4-OH-tamoxifen (Protein Data Bank: 3ert), which is in the antagonist configuration, was used for docking studies. Only the A Chain of the crystal structure was retained for docking and preparation of the receptor included extraction of 4-OH-tamoxifen, addition of hydrogens to the protein, and the generation of a protomol surface of the binding cavity. The models of the three glyceollins were then docked into the crystal structure protein model with Surflex-Dock (default settings with reign flexibility sampled), and the resulting 10 best poses were sorted by the Surflex-Dock scoring function in −log10(Kd) units to simulate binging affinities. The “best” overall scoring pose of each of the three glyceollins docked with the tamoxifen-induced, antagonist form of the estrogen receptor α is reported in this study.
Cell Viability and Proliferation Assay.
MCF-7 and BG-1 cells were placed in phenol red-free DMEM supplemented with 5% dextran-CS-FBS for 72 h before plating. Cells (1 × 103 MCF-7 or BG-1 cells/well) were plated in six-well plates containing phenol red-free DMEM 10% charcoal-stripped FBS. Forty-eight hours later, cells were treated with glyceollin I from 10−8 to 10−5 M, with and without estrogen (10−10 M) or vehicle (DMSO) stimulation. Cells were incubated at 37°C, 5% CO2 in a humidified incubator for 10 to 14 days. Ten to 14 days latter, cells were fixed with glutaraldehyde for 30 min and then stained with 0.1% crystal violet in methanol 20% for 30 min. The number of colonies was counted by an automatic colony counter.
Statistical Analysis.
Data were summarized as the mean ± S.E.M. by using the Prism version 4 software program (GraphPad Software Inc.). Analysis of variance models were used to compare relative cell proliferation or gene expression between control versus different doses of glyceollin I with and without E2 stimulation. A Tukey post-test analysis was performed to compare differences between groups, where a p value <0.05 was considered significant. All treatment groups compared with control are designated as “a,” whereas all treatment groups compared with estrogen are designated as “b.” Results are expressed as the mean unit ± S.E.M. (***, p < 0.001; **, p < 0.01; and *, p < 0.05).
Results
Tumor Growth in MCF-7 Xenograft Mice.
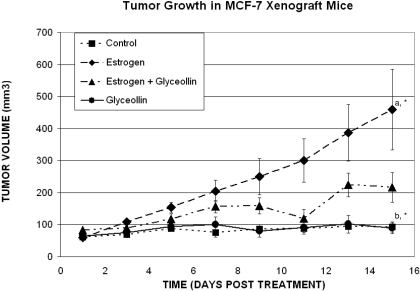
Previously, we identified the phytoalexin compound glyceollin that exhibited marked antiestrogenic effects on ER function and estrogen-dependent tumor growth in vivo. These findings identified glyceollins as antiestrogenic agents that may be useful in the prevention of breast and ovarian carcinoma. Here, we sought to further understand the antitumorigenic properties of glyceollin beyond its ability to prevent tumor growth and investigate whether glyceollin would be a useful agent for breast cancer treatment in vivo. Therefore, in vivo studies were performed on MCF-7 cells inoculated into female nude mice treated with vehicle, E2, glyceollin, or E2 + glyceollin once tumors were measurable and continued for 15 days. As shown in Fig. 1, the results demonstrate there are significant differences in the growth rates fitted for the two E2 groups, with the rate for E2 + glyceollin group approximately 3.5 times that of the control group and the rate for E2 group approximately 6 times that of the control group. The growth rate for the E2 group is approximately 1.7 times higher than the E2 + glyceollin group. No significant difference was observed between the glyceollin group and the control. However, we can conclude that both the E2 and E2 + glyceollin groups have significantly higher growth rates than both the control and the glyceollin groups. It is most important to note that glyceollin inhibited estrogen-stimulated tumor growth, suggesting that it may be useful in the treatment of breast cancer.
Fig. 1.
Effects of the glyceollin mixture on breast tumor growth in vivo. MCF-7 cells were injected in the mammary fat pad. Tumors were allowed to form over 10 days, and mice were randomized to four treatment groups with five mice per group: Con, E2 + vehicle, Gly, and E2 + Gly. The glyceollin mixture was suspended in a solution of DMSO (one-third volume) and propylene glycol (two-thirds volume) and was given intraperitoneally at 20 mg/kg/mouse/day for 15 days to Gly and E2 + Gly groups starting after tumors were measurable. Control and E2 groups were injected with vehicle daily for 15 days. Tumor size was measured every 2 days. Treatment, trial day, and treatment by trial day interactions are statistically significant for tumors (p < 0.001), and the tumors of all groups are significantly smaller than those of the E2 only group on every treatment day measured.
Effects of Glyceollin on ERα, PgR, and SDF-1 Gene Expression.
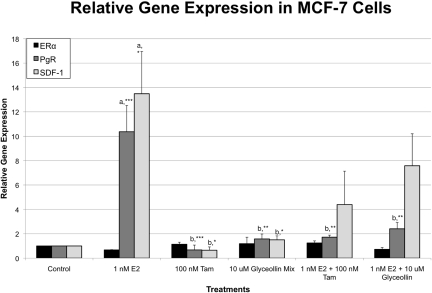
Three differentially expressed genes were selected for analysis by quantitative real-time RT-PCR: ERα, SDF-1, and PgR. These genes have been shown to have functions that are associated with cell proliferation and/or tumorigenesis. SDF-1 has been identified as a novel ER-regulated gene and mediator of the mitogenic effects of estrogen in ovarian and breast cancer cells (Hall and Korach, 2003). In the present study, we examined the expression of these three genes in the presence of vehicle (DMSO), 1 nM E2, 100 nM tamoxifen, 10 μM glyceollin, 1 nM E2 + 100 nM tamoxifen, and 1 nM E2 + 10 μM glyceollin treatment for 4 h in MCF-7 cells (Fig. 2). Our results demonstrate that estrogen treatment caused an increase in PgR and SDF-1 expression, whereas treatment with tamoxifen alone or in combination with estrogen caused a decrease in PgR and SDF-1 expression. Treatment with estradiol and glyceollin reduced the estrogen response, whereas glyceollin alone caused very little changes in PgR and SDF-1 gene expression.
Fig. 2.
Effects of glyceollin mixture on ERα, SDF-1, and PgR mRNA gene expression in MCF-7 cells. E2 treatment (1 nM) increased PgR and SDF-1 expression, whereas 10 μM glyceollin and tamoxifen treatment decreased PgR and SDF-1 gene expression ± E2 treatment.
Glyceollin-Induces Differential Gene Expression in MCF-7 Cells Compared with Tamoxifen.
Upon examination of the differential effects of glyceollin and tamoxifen treatment on both SDF-1 and PgR gene expression, we sought to further investigate the differences between the two compounds by using a more extensive panel of genes that are commonly altered in breast cancer and estrogen signaling by performing a superarray analyses (Table 1). Based on the above-mentioned real-time RT-PCR data, we chose to treat the MCF-7 cells for 4 h with DMSO (vehicle), 1 nM E2, 100 nM tamoxifen, or 10 μM glyceollin. Total RNA was extracted, quantitated, and a real-time PCR array was performed. As expected E2 treatment caused a 30.76-fold increase in PgR gene expression, whereas glyceollin treatment caused a 2.28-fold induction in PgR gene expression, consistent with real-time RT-PCR results. We identified several genes regulated by E2 alone: PgR, BAG1, C3, pS2, bcl-2, CCND1, IL6ST, STC2, RAC2, and SCGB1D2. We also identified one gene uniquely regulated by glyceollin, nerve growth factor receptor (NGFR), a member of the tumor necrosis factor receptor superfamily. It is interesting to note that NGFR exhibited a differential pattern of expression compared with tamoxifen and estrogen. Glyceollin caused a 3.29-fold increase in NGFR, whereas tamoxifen and estrogen treatments did not cause a significant change in expression.
Table 1.
SuperArray analysis of genes altered by estradiol, glyceollin mixture, and tamoxifen treatment
Gene expression of select genes from SuperArray analysis that exhibited significant changes in expression with E2 treatment, glyceollin mixture treatment, and tamoxifen treatment is shown. Numbers in bold indicate -fold changes in gene expression greater than 2.
| Gene Name | Gene Symbol | E2 Treatment | Glyceollin Mixture Treatment | Tamoxifen Treatment |
|---|---|---|---|---|
| Progesterone receptor | PGR | 30.76 | 2.28 | −1.68 |
| BCL2-associated athanogene | BAG1 | 2.24 | −1.24 | −1.40 |
| Complement component 3 | C3 | 3.84 | 1.20 | 1.33 |
| Trefoil factor 1 | TFF1 or pS2 | 2.94 | −1.03 | −1.27 |
| B-cell CLL/lymphoma 2 | BCL2 | 2.58 | −1.03 | 1.02 |
| Cyclin D1 | CCND1 | 2.32 | −1.32 | −1.74 |
| Interleukin-6 signal transducer (gp130, oncostatin M receptor) | IL6ST | 3.06 | 1.23 | 1.31 |
| Stanniocalcin 2 | STC2 | 4.50 | 1.05 | −1.57 |
| Ras-related C3 botulinum toxin substrate 2 (rho family, small GTP-binding protein Rac2) | RAC2 | 3.11 | −1.17 | −1.09 |
| Secretoglobin, family 2A, member 1 | SCGB1D2 | 2.35 | 1.07 | −2.23 |
| Nerve growth factor receptor (tumor necrosis factor receptor superfamily, member 16) | NGFR | 1.50 | 3.29 | 1.75 |
| Fibronectin leucine rich transmembrane protein 1 | FLRT1 | −1.53 | −3.46 | −4.21 |
| Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | PTGS2 | −1.70 | −4.79 | −4.47 |
| Cytochrome P450, family 19, subfamily A, polypeptide 1 | CYP19A1 | −1.28 | −2.22 | −3.06 |
Glyceollin Structure and Biosynthetic Pathway.
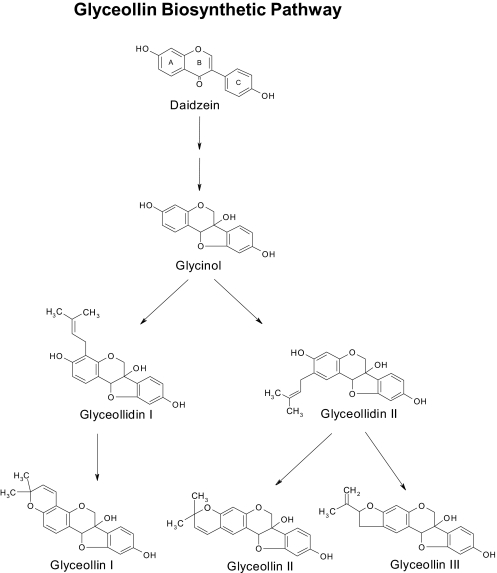
Our previous publications (Burow et al., 2001; Salvo et al., 2006) have examined the antiestrogenic effects of glyceollin in breast and ovarian cancer; however, the active component of the glyceollin mixture has not been identified. In this study, we investigated the ability of the phytoalexin glyceollin I to suppress breast cancer growth and gene expression. The structures and biosynthetic pathway of glyceollins I, II, and III are shown in Fig. 3. Glyceollins are derived from the precursor molecule diadzein that is then converted into glycinol followed by conversion to glyceollidins I and II, ultimately forming glyceollins I, II, and III.
Fig. 3.
Flavonoid pathway showing the biosynthetic route from daidzein to glyceollins I to III.
Effects of Glyceollins on Luciferase Activity in MCF-7 Cells.
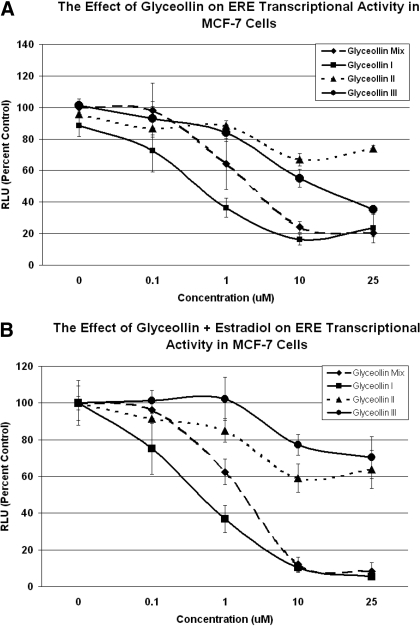
Previous studies in our laboratory have shown antagonistic activity of the glyceollin mixture on ERα and ERβ (Burow et al., 2001). However, until recently it remained unclear as to the specific component of the glyceollin mixture responsible for its antiestrogenic properties. In this study, we examined the ER transcriptional activity induced by the individual glyceollin isomers (I, II, and III) in comparison with the glyceollin mixture by using an estrogen-responsive element (ERE)-based luciferase reporter gene assay. MCF-7 cells were treated with glyceollin I, II, III, and the mixture and were examined for ERE transcriptional activity. Results demonstrated a dose-dependent decrease in estrogen activity associated with glyceollin I and glyceollin mixture treatment (Fig. 4). ERE activity was inhibited below control levels with increasing concentrations of glyceollin I, similar to the inhibition obtained with the glyceollin mixture, reaching a maximal inhibition at 25 μM. In contrast, glyceollin isomers II and III showed no significant inhibition of ERE potentiation compared with glyceollin I or the glyceollin mixture (Fig. 4), suggesting that glyceollin I is the active component of the three glyceollin isomers in the mixture.
Fig. 4.
Glyceollin I Inhibits the ER transcriptional activity in MCF-7 cells. Cells were transfected with pGL2-ERE2x-TK-luciferase plasmid. After 5 h, the transfection medium was removed and replaced with phenol red-free DMEM supplemented with 5% CS-FBS containing vehicle, glyceollin I, glyceollin II, glyceollin III, and glyceollin mixture (A) and vehicle, E2 + glyceollin I, E2 + glyceollin II, E2 + glyceollin III, and E2 + glyceollin mixture (B). After 18 h, the medium was removed and 100 μl of lysis buffer was added per well and incubated for 15 min at room temperature.
Relative Affinity of Glyceollins I, II, and III for ERα.
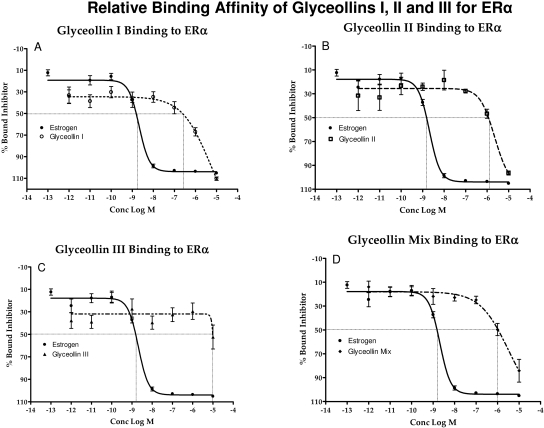
In breast cancer cells, ERα is thought to mediate the mitogenic actions of estrogen by inducing the expression of genes involved in cell proliferation. ERα is also expressed in 70% of ovarian tumors. In previous studies, the glyceollin mixture was found to possess a 3-fold higher affinity for ERα than ERβ (Nikov et al., 2000; Burow et al., 2001). In the present study, we evaluated the differences in binding affinity between the three glyceollin isomers. The ability of glyceollin I, II, III, and the mixture to bind to ERα was examined by competitive binding assays with fluorescent detection (Fig. 5). Analysis of the competition binding curve yielded IC50 values (concentration of unlabeled ligand required to displace 50% of the tracer from the ERα). A displacement of 50% E2 bound to ERα occurred at a concentration of 2.05 nM. Among the three individual isomers tested, the glyceollin I isomer displayed the highest affinity for ERα, with an IC50 value of 1.68 μM. The IC50 values of glyceollins II and III were 6.57 and greater than 10 μM, respectively, and the glyceollin mixture value was 1.46 μM. This indicated that the ability of glyceollin I to act as an ER antagonist occurred through ER binding and supported our hypothesis that the glyceollin I isomer is the active component of the mixture.
Fig. 5.
Competition binding curves of glyceollin I, II, III, and glyceollin mixture to ERα. Increasing concentrations of glyceollin I, II, III, and the glyceollin mixture were added to the ERα complex and compared with E2. Data points and error bars represent the mean ± S.E.M. of at least three experiments (n = 3) for each concentration tested. (p < 0.05).
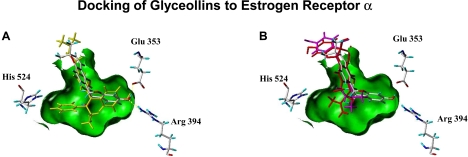
Docking and Scoring of Glyceollins to Estrogen Receptor α.
Evaluation of the ligand-receptor docking of the glyceollins to the tamoxifen-induced antagonist form of ER presents a binding mode model where glyceollin I interacts more like tamoxifen with ER, whereas the glyceollins II and III can only bind in a completely different way. Figure 6 depicts the docking pose of glyceollin I to ERα in relation to tamoxifen, which suggests how glyceollin I exerts antagonist properties. Also shown is the completely different docking poses for the glyceollin isomers II and III. In this figure, a Connolly channel, colored green, represents the surface of the binding cavity of the ER is depicted with the three essential amino acids within the binding cavity: Arginine 394 and glutamate 353 on the right and histidine 524 on the left. The experimentally defined binding pose of tamoxifen is shown in yellow, whereas the binding pose of glyceollin I suggested by docking is shown in atom colors. The docked binding pose of glyceollin I uses the same phenolic hydrogen bond interactions to arginine 394 and glutamate 353 that are found in the actual 4-hydroxytamoxifen binding within the ER. At the same time, the glyceollin I pose has no interactions with histidine 524 as is observed in the binding of most estrogen agonists (17β-estradiol, diethylstilbestrol). In Figure 6B, the “best” fit poses for glyceollins II and III are shown in magenta and red, respectively, to contrast with the atom colored docking pose of glyceollin I. The best docking poses of glyceollin II and III can not interact with arginine 394 and glutamate 353 or histidine 524 and thus take on only a weak and superficial stearic interaction within the binding channel induced by the tamoxifen side chain. Glyceollin II and III docking poses are also in the opposite orientation compared with glyceollin I and the phenolic group of these glyceollins can not fit inside the ER ligand binding cavity to stabilize a binding interaction. The more linear overall ring linkage in the glyceollin I structure (Fig. 3) is the primary contributing feature of its unique ability to fit well into the tamoxifen induced antagonist form of the ER.
Fig. 6.
A and B, 4-hydroxytamoxifen and glyceollin I in the binding cavity of the ligand binding domain of ERα interacting with histidine 524, arginine 394, and glutamate 353; glyceollin I (atom is colored yellow) (A) and glyceollin II (magenta) and glyceollin III (red) in the binding cavity of the ligand binding domain of the ERα interacting with histidine 524, arginine 394, and glutamate 353 (B).
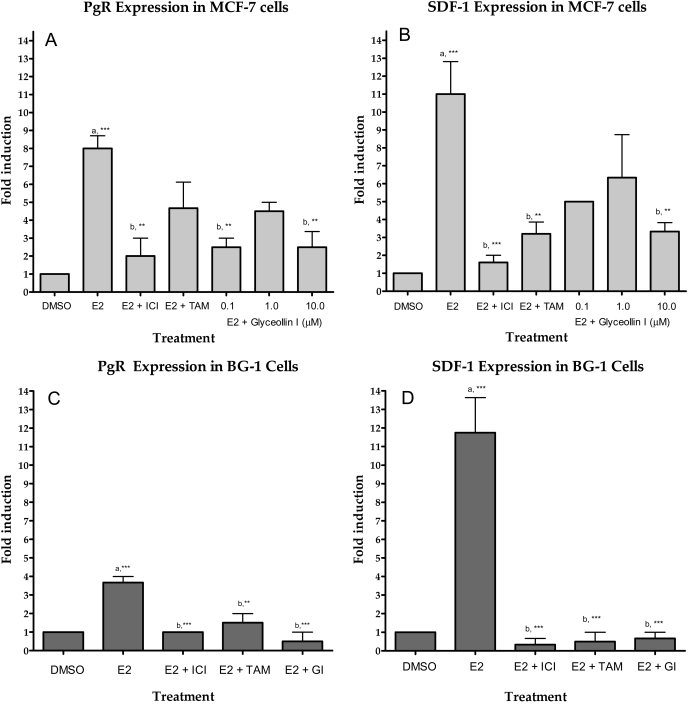
Glyceollin I Inhibits Estrogen-Stimulated SDF-1 and PgR Expression in MCF-7 and BG-1 Cells.
In the present study, we examined the correlation between SDF-1 and PgR expression in the presence of glyceollin I with and without estrogen stimulation on two estrogen-responsive cell lines: MCF-7 breast cancer cells and BG-1 ovarian cancer cells. To determine whether glyceollin I inhibited estrogen-induced expression of both genes, MCF-7 and BG-1 cells were treated with various concentrations (0.1–10 μM) of glyceollin I. Both cell lines showed a significant dose-dependent decrease in gene expression compared with the E2 alone-treated cells. In MCF-7 cells, glyceollin I at 0.1, 1, and 10 μM inhibited PgR expression induced with 100 nM estrogen by 68.75 (p < 0.01), 43.75, and 68.75% (p < 0.01), respectively, compared with the estrogen (Fig. 7A). Similar to PgR expression, glyceollin I at 0.1, 1, and 10 μM inhibited SDF-1 expression induced by 1 nM estrogen by 56.52 (p < 0.01), 65.22 (p < 0.01), and 73.9% (p < 0.001), respectively, compared with the control (Fig. 7B). In addition BG-1 cells treated with glyceollin I at 10 μM inhibited PgR expression in 86.38% (p < 0.001) and SDF-1 in 94.4% (p < 0.001) in the presence of estrogen stimulation (Fig. 7, C and D, respectively). This further suggests that glyceollin I is the isomer most responsible for the antiestrogenicity of the glyceollin mixture.
Fig. 7.
PgR and SDF-1 expression. Total RNA was isolated from MCF-7 and BG-1 cells, reverse-transcribed into cDNA, and subjected to real-time RT-PCR analysis for quantitation. Treatment on MCF-7 cells was as follows: DMSO (vehicle), E2 alone, E2 + ICI, E2 + 4-OH-tamoxifen (TAM) and glyceollin I (0.1, 1, and 10 μM with stimulation (A and B). Treatment on BG-1 cells was as follows: DMSO (vehicle), E2 alone, E2 + ICI 182,780 (ICI), E2 + 4-OH TAM, and glyceollin I (10 μM + E2) (C and D). Results are expressed as the mean unit ± S.E.M. (***, p < 0.001; **, p < 0.01; and *, p < 0.05), where “a” represents treatments compared with control and “b” represents treatments compared with estrogen.
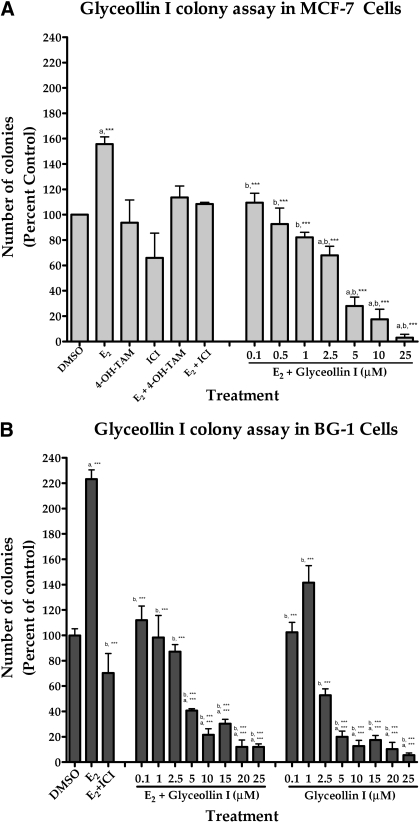
Glyceollin I Inhibits Viability and Proliferation.
Glyceollins have been shown to possess marked antiestrogenic activity in vitro (Burow et al., 2001) and in vivo (Salvo et al., 2006; Wood et al., 2006). To test our hypothesis that the glyceollin I isomer is the active component of the glyceollin mixture and is also an antiestrogen, two ERα-positive cell lines, MCF-7 and BG-1, were treated with glyceollin I at concentrations ranging from 0.1 to 10 μM, either in the absence or in the presence of E2. Cells were also treated with 100 pM E2, 100 nM ICI 182,780, and 100 nM 4-OH-tamoxifen as controls to validate their responses to known antiestrogenic compounds and compare it with glyceollin I. Proliferation and viability were measured using a colony assay (Fig. 8). The addition of 100 pM E2 to the culture media caused a 2-fold increase in the growth of both the MCF-7 and BG-1 cell lines compared with DMSO-treated controls. The addition of 100 nM ICI 182,780 and 100 nM 4-OH-tamoxifen inhibited E2-stimulated proliferation of both MCF-7 and BG-1 cells. Glyceollin I inhibited the viability and proliferation of MCF-7 and BG-1 cells in the presence and absence of E2 in a dose-dependent manner. Reductions in cell viability compared with E2-treated cells ranged from 50% (p < 0.01) at 0.1 μM glyceollin I to 86% (p < 0.001) at 10 μM glyceollin I on MCF-7 cells and from 49.82% (p < 0.01) at 0.1 μM glyceollin I to 90.32% (p < 0.001) at 10 μM glyceollin I on BG-1 cells.
Fig. 8.
Effects of glyceollin I on colony formation on MCF-7 and BG-1 cells. MCF-7 (A) and BG-1 (B) cells were placed in phenol red-free DMEM supplemented with 5% dextran-coated charcoal-treated FBS for 48 h before plating. MCF-7 or BG-1 cells (1000) were plated in six-well plates. Forty-eight hours later, cells were treated with DMSO (vehicle) or E2 + glyceollin I (0.1–10 μM). Colonies of ≥50 cells were counted as positive. Results are normalized to percentage of clonogenic survival from DMSO control cells. Results are expressed as the mean unit ± S.E.M. (***, p < 0.001; **, p < 0.01; and *, p < 0.05), where “a” represents treatments compared with control and “b” represents treatments compared with estrogen.
Discussion
The glyceollins constitute a unique class of flavonoid phytochemicals produced in certain legumes in response to stress (Burow et al., 2001). Specifically, the activation of soy yields an increase in the production of glyceollins I to III. The biosynthesis of all three glyceollin isomers occurs within soybean tissues starting with daidzein through several intermediate structures, including glycinol shown in Fig. 3 (Banks and Dewick, 1983). Glycinol, which we have shown previously to be estrogenic (Boué et al., 2009), is derived from daidzein via a pterocarpan by cyclization and 6α-hydroxylation with retention of configuration (Banks and Dewick, 1983; Hagmann et al., 1984). For the synthesis of the glyceollins, glycinol is prenylated to produce glyceollidin I and II, followed by cyclization into the corresponding glyceollins (Zähringer et al., 1981). Although these function normally as a plant defense response, only recently have their effects on mammalian hormonal signaling been characterized (Burow et al., 2001; Salvo et al., 2006).
In this study, we initially examined the effects of the glyceollin mixture on MCF-7 tumor formation in nude mice. Our data demonstrate that the glyceollin mixture inhibited the estrogen-stimulated tumor growth.
In this study, we investigated for the first time the effects of the individual glyceollin isomers (I, II, and III) on ERE transcriptional activity, ER binding, ER docking, gene expression analysis, and cell colony formation. Our results demonstrate that of the three glyceollin isomers studied, glyceollin I decreases the proliferation of ER-positive breast and ovarian cancer cells (MCF-7 and BG-1, respectively) at physiologically relevant concentrations (0.1–10 μM). Glyceollin I seems to be more efficient in this regard due to its higher binding affinity for the receptor. The three glyceollin isomers have similarities in their core ring structure (rings A, B, and C), but they differ in the size and position of the last ring formed attached at ring A during the last step of biosynthesis (Fig. 3). These minor structural differences lead to differential binding affinities within the ER cavity. The differences in IC50 values between the three glyceollins obtained with ERα binding confirm the finding that glyceollin I has the highest affinity for ERα. The modeling of glyceollin I is predicted to be in an appropriate orientation to interact with histidine524, arginine 394, and glutamate 353 of the ER ligand binding domain. However, this interaction is less evident with glyceollin II and III. The importance of this interaction for the onset of an estrogenic response explains the stronger antiestrogenic activity of glyceollin I compared with glyceollin II and III isomers.
The ability of glyceollin I to inhibit the expression of an ERE-luciferase reporter gene in MCF-7 cells suggests that they may exert their antiestrogenic activity, at least in part, via an ER-dependent mechanism. In this context, we confirm here that glyceollin I down-regulates endogenous E2-responsive gene transcription, i.e., SDF-1 and PgR in a dose-dependent manner. In addition, glyceollins exhibit antagonism of ERα without agonist activity, a mechanism similar to that of the pure antiestrogen fulvestrant (ICI 182,780). Based upon the activity and structural characteristics, the potential exists that glyceollins may represent lead compounds for the development of novel antiestrogens that may prove useful in the prevention and/or treatment of breast carcinoma. We also demonstrate that glyceollin I regulates genes differentially compared with tamoxifen as demonstrated by the gene superarray analysis (Table 1), suggesting an alternate mechanism of action for glyceollin I. This finding in addition to the ability of glyceollin I to inhibit proliferation, E2-responsive gene expression, and bind ERα is a significant finding in that glyceollin I may offer an alternate treatment strategy for postmenopausal women with breast cancer who eventually become resistant to tamoxifen therapy. In addition, glyceollins, particularly glyceollin I, may represent an important component of a soy-based diet in terms of chemoprevention and treatment of estrogen-related cancer.
Acknowledgments
We thank Dr. Paul Erhardt for critical reading of the manuscript.
This work was supported by a cooperative agreement with the U.S. Department of Agriculture [Grants 58-6435-7-019, 58-6435-7-019]; the United States Department of Defense Breast Cancer Research Program [Grants DAMD17-01-1-0655, DAMD17-01-1-0655 ]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK059389]; The Tulane/Xavier Center for Bioenvironmental Research; and the National Institutes of Health National Heart, Lung, and Blood Institute [Grant T32-HL00797305] (Training Grant in Lung Molecular and Cell Pathobiology).
M.C.Z. and S.L.T. share co-first authorship.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160382
- ER
- estrogen receptor(s)
- ICI 182,780
- fulvestrant
- HPLC
- high-performance liquid chromatography
- DMEM
- Dulbecco's modified Eagle's medium
- 4-OH-tamoxifen
- 4-hydroxytamoxifen
- FBS
- fetal bovine serum
- PBS
- phosphate-buffered saline
- CS
- charcoal-stripped
- Con
- control
- E2
- 17β-estradiol
- Gly
- glyceollin mixture
- DMSO
- dimethyl sulfoxide
- RT
- reverse transcription
- PRC
- polymerase chain reaction
- SDF-1
- stromal cell-derived factor-1
- PgR
- progesterone receptor
- Ct
- fractional cycle number
- ES2
- fluorescent ligand
- RBA
- relative binding affinity(ies)
- NGFR
- nerve growth factor receptor
- ERE
- estrogen-responsive element.
References
- Ali S, Coombes RC. (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112 [DOI] [PubMed] [Google Scholar]
- Ayers AR, Ebel J, Finelli F, Berger N, Albersheim P. (1976) Host-pathogen interactions. IX. Quantitative assays of elicitor activity and characterization of the elicitor present in the extracellular medium of cultures of Phytophthora megasperma var. sojae. Plant Physiol 57:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SW, Dewick PM. (1983) Biosynthesis of glyceollins I, II and III in soybean. Phytochemistry 22:2729–2733 [Google Scholar]
- Barnes S. (1998) Phytoestrogens and breast cancer. Baillieres Clin Endocrinol Metab 12:559–579 [DOI] [PubMed] [Google Scholar]
- Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W. (1998) Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect 106:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boué SM, Tilghman SL, Elliott S, Zimmerman MC, Williams KY, Payton-Stewart F, Miraflor AP, Howell MH, Shih BY, Carter-Wientjes CH, et al. (2009) Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max). Endocrinology 150:2446–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow ME, Boue SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA. (2001) Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab 86:1750–1758 [DOI] [PubMed] [Google Scholar]
- Burow ME, Tang Y, Collins-Burow BM, Krajewski S, Reed JC, McLachlan JA, Beckman BS. (1999) Effects of environmental estrogens on tumor necrosis factor alpha-mediated apoptosis in MCF-7 cells. Carcinogenesis 20:2057–2061 [DOI] [PubMed] [Google Scholar]
- Ganry O. (2002) Phytoestrogen and breast cancer prevention. Eur J Cancer Prev 11:519–522 [DOI] [PubMed] [Google Scholar]
- Gikas PD, Mokbel K. (2005) Phytoestrogens and the risk of breast cancer: a review of the literature. Int J Fertil Womens Med 50:250–258 [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA. (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33 [DOI] [PubMed] [Google Scholar]
- Hagmann ML, Heller W, Grisebach H. (1984) Induction of phytoalexin synthesis in soybean. Stereospecific 3,9-dihydropterocarpan 6a-hydroxylase from elicitor-induced soybean cell cultures. Eur J Biochem 142:127–131 [DOI] [PubMed] [Google Scholar]
- Hahn MG, Bonhoff A, Grisebach H. (1985) Quantitative localization of the phytoalexin glyceollin I in relation to fungal hyphae in soybean roots infected with Phytosphthora megasperma f. sp. glycinea. Plant Physiol 77:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Korach KS. (2003) Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol 17:792–803 [DOI] [PubMed] [Google Scholar]
- Howell A. (2006) Pure oestrogen antagonists for the treatment of advanced breast cancer. Endocr Relat Cancer 13:689–706 [DOI] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220 [DOI] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. (1996) Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect 104:1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester J. (2007) Breast cancer in 2007: incidence, risk assessment, and risk reduction strategies. Clin J Oncol Nurs 11:619–622 [DOI] [PubMed] [Google Scholar]
- Limer JL, Speirs V. (2004) Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res 6:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee PJ, Rowland IR. (2004) Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr 91:513–531 [DOI] [PubMed] [Google Scholar]
- Miller WR, Bartlett JM, Canney P, Verrill M. (2007) Hormonal therapy for postmenopausal breast cancer: the science of sequencing. Breast Cancer Res Treat 103:149–160 [DOI] [PubMed] [Google Scholar]
- Moesta P, Hahn MG, Grisebach H. (1983) Development of a radioimmunoassay for the soybean phytoalexin glyceollin I. Plant Physiol 73:233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikov GN, Hopkins NE, Boue S, Alworth WL. (2000) Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environ Health Perspect 108:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink JJ, Jordan VC. (1996) Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res 56:2321–2330 [PubMed] [Google Scholar]
- Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, et al. (2006) Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res 12:7159–7164 [DOI] [PubMed] [Google Scholar]
- Usui T. (2006) Pharmaceutical prospects of phytoestrogens. Endocr J 53:7–20 [DOI] [PubMed] [Google Scholar]
- Wood CE, Clarkson TB, Appt SE, Franke AA, Boue SM, Burow ME, McCoy T, Cline JM. (2006) Effects of soybean glyceollins and estradiol in postmenopausal female monkeys. Nutr Cancer 56:74–81 [DOI] [PubMed] [Google Scholar]
- Zähringer U, Schaller E, Grisebach H. (1981) Induction of phytoalexin synthesis in soybean. Structure and reactions of naturally occurring and enzymatically prepared prenylated pterocarpans from elicitor-treated cotyledons and cell cultures of soybean. Z Naturforsch C 36:234–241 [Google Scholar]