Abstract
Glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are gut-derived incretin hormones that regulate blood glucose levels. In addition to their widely accepted insulinotropic role, there is evidence that GLP-1 modulates feeding behavior and GIP regulates lipid metabolism, thereby promoting postprandial fat deposition. In this study, we investigated whether naturally occurring polymorphisms in the GLP-1 receptor (GLP-1R) and the GIP receptor (GIP-R) affect the pharmacological properties of these proteins. After transient expression of the receptors in human embryonic kidney 293 cells, basal and ligand-induced cAMP production were assessed by use of luciferase reporter gene assays. Our data reveal that the wild-type GIP-R displays a considerable degree of ligand-independent activity. In comparison, the GIP-R variants C46S, G198C, R316L, and E354Q show a marked decrease in basal signaling that may, at least in part, be explained by reduced cell surface expression. When stimulated with GIP, the C46S and R316L mutants display significantly reduced potency (>1000 and 25- fold, respectively) compared with wild type. Complementary competition binding assays further demonstrate that the C46S variant fails to bind radio-iodinated GIP, whereas all other GIP-R mutants maintain normal ligand affinity. In contrast to the GIP-R, the wild-type GLP-1R lacks constitutive activity. Furthermore, none of the 10 GLP-1R missense mutations showed an alteration in pharmacological properties versus wild type. The extent to which abnormalities in GIP-R function may lead to physiological changes or affect drug sensitivity in selected populations (e.g., obese, diabetic individuals) remains to be further investigated.
The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are homologous peptides released from intestinal enteroendocrine cells in response to food intake. Both hormones are important modulators of metabolic function. In the pancreas, GLP-1 and GIP potentiate nutrient-stimulated insulin secretion and promote the expansion of pancreatic islet mass via induction of β-cell proliferation and survival (Kim et al., 2005; Kim and Egan, 2008). In light of these insulinotropic actions, drugs that mimic or prolong the biological functions of GIP and GLP-1 have attracted considerable attention as treatment options for type 2 diabetes (T2D) (Lovshin and Drucker, 2009). Exendin-4 (Exenatide), a potent long-acting agonist of the GLP-1 receptor (GLP-1R), represents the first incretin-based pharmaceutical to reach the market for the treatment of T2D. Inhibitors of the enzyme dipeptidyl dipeptidase IV, which plays a major role in inactivating both incretin hormones, have also recently been approved as therapeutics for T2D.
Considerable efforts have focused on unraveling additional metabolic functions triggered by the incretins (Kim and Egan, 2008). Accumulating evidence supports that GIP modulates adipocyte metabolism, triggering fat deposition after feeding. Highlighting the physiological relevance of this function, previous studies have shown that targeted disruption of the GIP receptor (GIP-R) in mice results in protection from both diet-induced obesity and insulin resistance (Miyawaki et al., 2002). Consistent with these observations, inhibition of GIP-R signaling using a selective antagonist, or passive immunization against GIP, were both shown to decrease body weight and to protect against glucose intolerance in animals that were fed a high-fat diet (Gault et al., 2007; Fulurija et al., 2008).
GLP-1 also modulates metabolic function, in part, by acting on GLP-1Rs in extrapancreatic tissues (Kim and Egan, 2008). This peptide triggers delayed gastric emptying, which in turn slows the absorption of food, thus delaying the rise in blood glucose levels. In addition, GLP-1 has been shown to inhibit feeding behavior by stimulation of cognate receptors in the brain. Taken together, the incretin hormones and their receptors contribute at multiple levels to maintaining normal glucose homeostasis and regulating body weight.
Both the GIP-R and the GLP-1R belong to the glucagon subfamily of class B1 G protein-coupled receptors (GPCRs). These seven transmembrane domain proteins, when stimulated with ligand, undergo a conformational change from putative inactive to active conformations, thereby triggering a Gαs-mediated increase in cAMP production (Hoare, 2005). It has been observed with other wild-type and mutant GPCRs that partially active receptor conformations may occur even in the absence of agonist, leading to constitutive, ligand-independent signaling (Kenakin, 2004). Although engineered constitutively active incretin receptors have been generated (Tseng and Lin, 1997; M.B., unpublished data); the extent to which detectable basal signaling is influenced by naturally occurring polymorphic/mutant incretin receptors has not been investigated.
It is well established that missense mutations in GPCRs can result in a variety of pharmacological abnormalities (e.g., alterations in basal and ligand-dependent activity, receptor affinity, expression) which predispose to physiological changes or disease (Seifert and Wenzel-Seifert, 2002). In the current study, we examined the molecular pharmacological consequences of naturally occurring mutations/polymorphisms in the GIP-R and GLP-1R using a series of in vitro assays.
Materials and Methods
Generation of Incretin Receptor Variants.
The complementary DNA encoding the GIP-R was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org) and subcloned into pcDNA1.1. The human GLP-1R cDNA was reported previously (Tibaduiza et al., 2001). Single-amino-acid substitutions and a hemagglutinin (HA) tag were introduced into the receptor sequence by use of oligonucleotide-directed site-specific mutagenesis as described previously (Fortin et al., 2009). The nucleotide sequences of all receptor coding regions were confirmed by automated DNA sequencing.
Cell Culture and Transfection.
Human embryonic kidney (HEK) 293 cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin. The cells were maintained at 37°C in a humidified environment containing 5% CO2.
Luciferase Reporter Gene Assay.
Receptor-mediated signaling was assessed by use of a previously described luciferase assay (Fortin et al., 2009). In brief, HEK293 cells were plated at a density of 2000 to 3000 cells/well onto clear-bottom, white 96-well plates and grown for 2 days to ∼80% confluence. Cells were then transiently transfected by use of Lipofectamine reagent (Invitrogen) with cDNAs encoding 1) a GPCR (or empty expression vector), 2) a cAMP responsive element-luciferase reporter gene (CRE6X-luc), and 3) β-galactosidase to enable correction for interwell variability in transfection efficiency and cell survival. Forty-eight hours after transfection, cells were incubated for 6 h with or without the appropriate peptide ligand (American Peptide Company Inc., Sunnyvale, CA) in serum-free medium. After agonist treatment, the medium was gently aspirated, the cells were lysed, and luciferase activity was measured by use of Steadylite reagent (PerkinElmer Life and Analytical Sciences, Waltham, MA). A β-galactosidase assay was then performed after adding the enzyme substrate, 2-nitrophenyl β-d-galactopyranoside. After incubation at 37°C for 30 to 60 min, substrate cleavage was quantified by measurement of optical density at 420 nm using a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA). Corresponding values were used to normalize the luciferase data.
Assessment of Receptor Expression with Use of ELISA.
The surface expression levels of the HA-tagged GIP-Rs were assessed by use of a previously described approach (Shinyama et al., 2003). In agreement with earlier reports (Lee et al., 1994; Qi et al., 1997), two independent predictor tools (http://bmbpcu36.leeds.ac.uk/prot_analysis/Signal.html and http://www.cbs.dtu.dk/services/) supported the presence of a signal sequence in the GIP-R extracellular domain that is cleaved during receptor maturation. An HA tag was thus inserted immediately downstream of the putative 24-amino acid GIP-R signal peptide (shown in Fig. 1B). HEK293 cells grown in 96-well clear Primaria plates (BD Biosciences Discovery Labware, Bedford, MA) were transiently transfected with increasing amounts of either pcDNA1.1 or a cDNA encoding the HA-tagged GIP-R. Forty-eight hours after transfection, the cells were washed once with phosphate-buffered saline (PBS), pH 7.4, and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After washing with 100 mM glycine in PBS, the cells were incubated for 30 min in blocking solution (PBS containing 20% bovine serum). A horseradish peroxidase-conjugated antibody directed against the HA epitope tag (Roche; clone 3F10, monoclonal, 1:500 in blocking buffer) was then added to the cells. After 1 h, the cells were washed five times with PBS. Fifty microliters per well of a solution containing the peroxidase substrate BM-blue (3.3′-5,5′-tetramethylbenzidine; Roche Applied Science, Indianapolis, IN) was then added. After incubation for 30 min at room temperature, conversion of this substrate by antibody-linked horseradish peroxidase was terminated by adding 2.0 M sulfuric acid (50 μl/well). Results were quantified by measuring light absorbance at 450 nm.
Fig. 1.
Localization of the GLP-1R and GIP-R missense mutations within the receptor protein. Residues flanking the GIPR epitope tag are also shown. Schematic illustration of the location of amino acid substitutions within the 7-transmembrane domain structure of the human GLP-1R (A) and GIP-R (B). Respective residues in the wild-type proteins are indicated by the single letter code.
Radioligand Binding Studies.
HEK293 cells were plated at a density of 30,000 cells/well onto 24-well plates coated with poly-l-lysine, and grown for 18 to 24 h to ∼80% confluence. Cells were then transiently transfected by use of lipofectamine with receptor cDNA (100 ng/well) and grown for an additional 18 to 24 h. Whole cell binding studies were initiated by washing cells twice with cold (4°C) assay buffer (Dulbecco's modified Eagle's medium with 0.1% bovine serum albumin and 15 mM HEPES), followed by addition of the same media with 20,000 cpm of 125I-GIP (PerkinElmer Life and Analytical Sciences) and varying concentrations of unlabeled GIP. After an 8-h incubation period at 4°C, the cells were washed twice with cold assay buffer and solubilized in 0.1 N NaOH. The lysates were then counted by use of a Packard Cobra Quantum γ-counter to determine cell-associated radioactivity.
Data and Statistical Analysis.
GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA) was used for sigmoidal curve fitting. Half-maximal effective concentrations (EC50 values) were calculated as an index of ligand potency, and half-maximal inhibitory concentrations (IC50 values) were calculated as an index of receptor binding affinity. pEC50, basal activity and surface expression values for each of the mutants were compared with the corresponding control value at the wild-type receptor by use of one-way analysis of variance followed by Dunnett's post test (GraphPad INSTAT software).
Results
Human Incretin Receptor Variants.
Receptor constructs containing naturally occurring missense mutations of the human GLP-1R (P7L, R20K, R44H, R131Q, G168S, F260L, A316G, A316T, S333C, and R421C) and human GIP-R (C46S, R136W, G198C, A207V, L262V, R316L, E354Q, and E463Q) were generated for investigation. The position of each amino acid substitution is illustrated in cartoons of the GLP-1R and GIP-R (Fig. 1, A and B, respectively). Each incretin receptor variant appeared in the NaVa (Natural Variants) database that catalogs known human GPCR polymorphisms (frequency >1%), as well as rarer mutations (Kazius et al., 2008). As outlined in the discussion, three GIP-R variants have been described previously in the literature (Kubota et al., 1996; Almind et al., 1998). Site-directed mutagenesis was used to introduce amino acid substitutions corresponding to the receptor variants. Each of the mutant receptor constructs or corresponding wild-type proteins were expressed in HEK293 cells and pharmacologically characterized.
Missense Variants of the GLP-1R Exhibit Normal Basal and Agonist-Induced Signaling.
Basal signaling in cells expressing the wild-type GLP-1R (assessed by use of a cAMP-responsive luciferase construct) was indistinguishable from that observed in cells transfected with the empty expression vector, pcDNA1.1 (data not shown). This observation confirms that the GLP-1R lacks constitutive activity. In addition, none of the 10 GLP-1R variants showed a significant level of basal signaling (Table 1).
Table 1.
Agonist potency and basal activity at wild-type vs. mutant GLP-1Rs
All values represent the mean ± S.E.M. from four independent experiments.
| Receptor | GLP-1 |
Exendin-4 |
Basal Activity a , b | ||
|---|---|---|---|---|---|
| EC50 | pEC50 a | EC50 | pEC50 a | ||
| pM | pM | ||||
| hGLP-1R | 1.8 | 11.75 ± 0.04 | 1.3 | 11.90 ± 0.04 | 0.9 ± 0.2 |
| P7L | 2.0 | 11.71 ± 0.06 | 1.4 | 11.87 ± 0.06 | 1.4 ± 0.5 |
| R20K | 1.7 | 11.73 ± 0.07 | 1.1 | 11.95 ± 0.08 | 1.4 ± 0.5 |
| R44H | 2.0 | 11.70 ± 0.07 | 1.6 | 11.79 ± 0.08 | 1.0 ± 0.3 |
| R131Q | 1.8 | 11.74 ± 0.09 | 1.1 | 11.96 ± 0.08 | 1.4 ± 0.4 |
| G168S | 1.6 | 11.79 ± 0.09 | 1.3 | 11.89 ± 0.09 | 1.1 ± 0.4 |
| F260L | 2.2 | 11.65 ± 0.11 | 1.4 | 11.85 ± 0.12 | 1.3 ± 0.5 |
| A316G | 2.0 | 11.58 ± 0.07 | 2.4 | 11.63 ± 0.63 | 0.9 ± 0.3 |
| A316T | 2.6 | 11.70 ± 0.11 | 1.6 | 11.79 ± 0.10 | 1.4 ± 0.5 |
| S333C | 2.2 | 11.66 ± 0.10 | 1.7 | 11.76 ± 0.10 | 1.2 ± 0.5 |
| R421C | 2.6 | 11.58 ± 0.11 | 1.7 | 11.78 ± 0.09 | 1.1 ± 0.4 |
No significant difference vs. wild type.
Percentage of the corresponding GLP-1-induced maximum.
Agonist-induced GLP-1R function was assessed by use of two structurally related agonists, GLP-1 and exendin-4 (illustrated for the wild-type receptor and representative variants in Fig. 2). At each mutant receptor, both peptides demonstrate potency and efficacy values that are comparable with wild type (Table 1).
Fig. 2.
All GLP-1R variants show a pharmacological response to GLP-1 and exendin-4 that is similar to wild type. HEK293 cells were transiently transfected with a receptor-encoding cDNA and a CRE-Luc reporter gene construct. Forty-eight hours after transfection, cells were stimulated for 4 h with media containing either no peptide (basal) or increasing concentrations of GLP-1 (A) or exendin-4 (B). After stimulation, luciferase activity was quantified as described under Materials and Methods. All activity values were normalized relative to the GLP-1- or exendin-4-induced maximal stimulation (A or B) at the wild-type GLP-1R. Average values for basal and GLP-1/exendin-4-induced maximum luciferase activity were 3.51 ± 0.62 × 104 and 2.38 ± 0.34 × 106/2.34 ± 0.21 × 106 cps, respectively. Data represent the mean ± S.E.M. from at least three independent experiments, each performed in triplicate. Representative variants are shown.
Selected GIP-R Variants Show Altered Basal and/or GIP-Mediated Activity.
Basal and GIP-induced signaling was examined at each GIP-R isoform (Fig. 3). In contrast to the GLP-1R, the wild-type GIP-R showed constitutive activity (∼25.0 ± 4.8% of the GIP-induced maximum) that markedly exceeded control values (determined by use of vector-transfected cells). Four GIP-R variants, C46S, G198C, R316L, and E354Q, showed a significant reduction in basal activity (Table 2). Of these functionally abnormal receptors, two also showed a marked decrease in GIP potency. The C46S variant showed a greater than 1000-fold reduction, whereas R316L had a ∼25-fold decrease in GIP potency compared with that at the wild-type GIP-R. In contrast, the EC50 values for GIP at the R136W, G198C, A207V, L262V, E354Q, and E463Q mutants were comparable with the reference value at the wild-type receptor (Table 2).
Fig. 3.
Selected GIP-R mutations alter GIP-induced signaling. HEK293 cells were transiently transfected with the empty vector pcDNA1.1 or a receptor-encoding cDNA, together with a CRE-Luc reporter gene construct. Forty-eight hours after transfection, cells were stimulated for 6 h with media containing either no peptide (basal) or increasing concentrations of GIP. After stimulation, luciferase activity was quantified as described in Materials and Methods. All activity values were normalized relative to the GIP-stimulated maximum at the wild-type GIP-R. Average values for basal and GIP-induced maximum luciferase activity were 1.50 ± 0.22 × 106 and 5.62 ± 0.70 × 106 cps, respectively. Data represent the mean ± S.E.M. from at least three independent experiments, each performed in quadruplicate.
Table 2.
Pharmacological properties of wild-type vs. mutant GIP-Rs
All values represent the mean ± S.E.M. from at least three independent experiments. Functionally abnormal variants are highlighted.
| Receptor | GIP |
GIP binding Kd | Basal Activity b | Surface Expression c | Slope d | |
|---|---|---|---|---|---|---|
| EC50 | pEC50 | |||||
| pM | nM | |||||
| hGIP-R | 0.9 | 12.06 ± 0.11 | 3.1 ± 0.6 | 100 | 100 | 1.0 |
| C46S | >1000 | <7 a | >1000 a | 34 ± 4 a | 31 ± 6 a | 1.1 |
| R136W | 1.8 | 11.70 ± 0.19 | 3.9 ± 0.6 | 102 ± 1 | 102 ± 8 | 0.9 |
| G198C | 2.0 | 11.75 ± 0.12 | 1.7 ± 0.8 | 22 ± 4 a | 56 ± 9 a | 0.3 |
| A207V | 1.0 | 11.97 ± 0.05 | 2.6 ± 0.9 | 104 ± 10 | 99 ± 5 | 1.1 |
| L262V | 1.0 | 12.06 ± 0.12 | 2.9 ± 0.4 | 99 ± 11 | 97 ± 13 | 1.0 |
| R316L | 24.3 | 10.75 ± 0.20 a | 2.7 ± 0.4 | 3 ± 1 a | 37 ± 7 a | 0.01 |
| E354Q | 0.7 | 12.22 ± 0.11 | 2.2 ± 0.5 | 15 ± 6 a | 60 ± 10 a | 0.2 |
| E463Q | 1.3 | 11.96 ± 0.13 | 2.7 ± 0.9 | 100 ± 11 | 99 ± 5 | 1.0 |
Value significantly different (P < 0.01) vs. wild-type GIP-R value.
Percentage of basal signaling activity of the wild-type GIP-R.
Percentage of wild type GIP-R surface expression.
Slope of regression line in Fig. 5C.
Impaired Binding Affinity of GIP at the C46S GIP-R Variant.
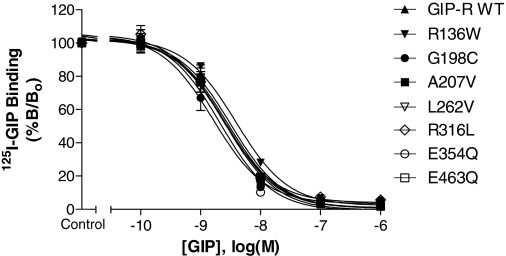
To complement the functional studies of GIP-R mutants, we evaluated the affinity of GIP at each receptor variant by radioligand competition binding assays (Fig. 4, Table 2). In agreement with previous work using other cell lines (Manhart et al., 2003), equilibrium binding of the radioligand to HEK293 cells expressing the recombinant GIP-R was reached within a 7-h incubation period at 4°C (data not shown). Consistent with the marked reduction in agonist potency at the C46S isoform (Fig. 3), no specific binding of 125I-GIP was detectable for this variant (not shown). In contrast, competition assays revealed that each of the other variants, R136W, G198C, A207V, L262V, R316L, E354Q, and E463Q, had an affinity for GIP comparable with that observed at the wild-type receptor.
Fig. 4.
Effect of GIP-R mutations on GIP binding affinity. 125I-GIP radioligand binding with increasing concentrations of unlabeled GIP was evaluated in HEK293 cells transiently expressing either the wild-type or a mutant GIP-R. The cells were incubated in the presence of radioligand with indicated concentrations of unlabeled GIP for 8 h at 4°C. Data represent the mean ± S.E.M. from at least three independent experiments, each performed in quadruplicate.
GIP-R Variants with Decreased Basal Activity Also Show Reduced Cell Surface Expression.
Reduced basal activity of several GIP-R variants (C46S, G198C, R316L, and E354Q; Fig. 3) was confirmed in experiments where ligand-independent signaling was measured after transfecting cells with increasing concentrations of respective receptor cDNAs (Fig. 5A). In a parallel experiment using the same transfection protocol, receptor expression levels at the cell surface were determined by ELISA (Fig. 5B). These studies revealed that, at each cDNA level, receptors with reduced basal signaling were also expressed at a significantly decreased density relative to the wild-type protein (100%). In contrast, the GIP-R variants where basal activity was not altered (R136W, A207V, L262V, and E463Q) showed normal expression levels (Fig. 5, Table 2). The reduced cell expression of the C46S, G198C, R316L, and E354Q variants was also confirmed by confocal microscopy (Supplemental Fig. 1).
Fig. 5.
Selected GIP-R missense mutations alter basal signaling and cell surface expression. A, basal activity of multiple GIP-Rs increases as a function of cDNA concentration. HEK 293 cells were transfected with increasing amounts of plasmid encoding either the wild-type or a mutant GIPR, together with a CRE-Luc reporter gene construct. After 48 h, ligand-independent luciferase activity was measured as described under Materials and Methods. B, cell surface expression of HA-tagged GIP-Rs increases as a function of cDNA concentration. HEK 293 cells were transfected with increasing amounts of plasmid encoding either the wild type or a mutant HA-tagged GIP-R. After 48 h, surface expression was measured by ELISA as described in Materials and Methods. C, surface expression (corresponding to data in B) and basal activity (corresponding to data in A) of wild-type and mutant GIPRs show a linear correlation. The slope of the correlation lines for most mutants approximates the wild-type value, with the exception of the G198C, R316L, and E354Q variants (see Table 2). Basal signaling and expression data are shown as a percentage of the maximal value observed at the wild-type GIP-R (transfection of 2 ng of cDNA/well). Each data point represents the mean ± S.E.M. from at least three independent experiments, each performed in triplicate.
Figure 5C illustrates the linear relationship that exists between expression level and basal activity of corresponding GIP-R isoforms. The slope of the regresssion line of the C46S mutant is similar to wild type, suggesting that the decreased basal activity observed with this receptor variant is largely attributable to its diminished expression. In contrast, the lower slope value of the G198C, R316L, and E354Q variants suggests that basal activity in these cases is disproportionately reduced relative to corresponding expression level (Table 2). It therefore seems that, with the latter three receptors, additional mutation-induced changes (e.g., diminished G protein affinity) contribute to the loss of ligand-independent signaling.
Discussion
The related peptides, GIP and GLP-1, play important physiological roles in maintaining blood glucose homeostasis, most notably by potentiating glucose-stimulated insulin secretion by pancreatic β-cells (Kim and Egan, 2008; Lovshin and Drucker, 2009). These peptides have additional peripheral and central functions, including the regulation of fat metabolism in adipocytes (GIP) and the induction of satiety (GLP-1). To investigate the effect of naturally occurring polymorphisms on the function of cognate incretin receptors, we compared the pharmacological properties of known human GIP-R and GLP-1R missense variants with those of corresponding wild-type GPCRs.
Introduction of 10 naturally occurring mutations in the human GLP-1R sequence did not interfere with the ability of GLP-1 or exendin-4 to trigger receptor-mediated activity. This is the case despite the occurrence of variants in domains which are susceptible to mutation-induced pharmacological alteration. These include the N terminus, a well established site of GLP-1 binding (Runge et al., 2008), and multiple intracellular domains (loops 1, 2, and 3 and the C terminus), implicated in G protein coupling (Mathi et al., 1997). These new findings complement an earlier report by our laboratory describing a GLP-1R variant, T149M, which decreases endogenous agonist (GLP-1) as well as exendin-4 affinity and potency (Beinborn et al., 2005).
Studies of the GIP-R revealed that two variants (C46S and R316L), although largely preserving GIP efficacy, result in >1000-fold and 25-fold reduced agonist potency compared with wild-type values. Mutation-induced abnormalities in agonist potency may be triggered by two distinct mechanisms: 1) alteration of the hormone binding site and/or 2) defective GPCR transitioning from the inactive to the active receptor state (the conformation triggering G protein activation) (Beinborn et al., 2004).
The C46 substitution is found in the N terminus of the GIP-R, a domain that plays an important role in ligand binding (Parthier et al., 2007). It is thus likely that the observed decrease in potency and the absence of radioligand binding to the C46S variant (Table 2) is due to a mutation-induced alteration of the hormone binding domain. This conclusion is supported by analysis of the recently obtained crystal structure of the GIP-R extracellular domain bound to GIP. Experimental evidence from this study suggests that three conserved disulfide bridges, including a link between C46 and C70, stabilize the secondary structure of the extracellular domain (Parthier et al., 2007). Furthermore, mutation of homologous cysteine residues in other class B1 GPCRs have been shown to disrupt ligand affinity (Lee et al., 1994; Gaudin et al., 1995; Qi et al., 1997; Lisenbee et al., 2005).
In contrast to C46S, the other GIP-R polymorphism that decreases agonist potency (R316L) is found in the third intracellular loop. Because this receptor region is far removed from the ligand binding domains, it is unlikely that the reduced GIP potency observed at the R316L variant results from a direct change in the hormone docking site. Consistent with this conclusion, the R316L mutant maintains normal affinity for radioiodinated GIP (which reflects the initial step of ligand-receptor interaction) despite reduced agonist potency (a measure of subsequent ligand-induced receptor activation). It is noteworthy that previous structure-function studies on the related GLP-1R and parathyroid hormone receptor revealed that important G protein coupling determinants localize in the N-terminal section of the third intracellular loop of these receptors (i.e., the region where R316L is found in the GIP-R) (Huang et al., 1996; Mathi et al., 1997). It is thus probable that the reduction in GIP potency at the R316L isoform reflects an altered ability of this variant to couple and/or activate stimulatory G proteins.
Our studies revealed normal GIP potency and affinity for the G198C mutant (Table 2). It is noteworthy that this result contrasts with an earlier study that reported lower potency for this GIP-R variant relative to wild type (Kubota et al., 1996). The basis for this discrepancy is not clear, but it is possible that the divergent findings are at least in part explained by differences in methodologies used for receptor characterization (including the choice of cells for cDNA expression and the type of signaling assay). Whereas in vitro findings provide valuable insight into the potential of mutations to affect receptor function (Seifert and Wenzel-Seifert, 2002), they do not necessarily cover the full range of possible mutation-induced changes. Some alterations in receptor-mediated function may only be detectable when using a particular experimental setup and/or with specific functional readouts.
Illustrating this limitation, the current study is the first to clearly demonstrate that the human wild-type GIP-R is constitutively active (Figs. 3 and 5). There is only one prior report in the literature suggesting that the GIP-R has a low degree of constitutive activity (Almind et al., 1998). Our ability in the current study to readily detect a pronounced elevation in GIP-R basal activity is likely explained by the sensitivity of the luciferase-based system that was used to assess receptor-mediated signaling. For the broader group of class B1 GPCRs (i.e., the secretin-glucagon family), there are few reports of significant ligand-independent signaling of unmodified wild-type receptors (Seifert and Wenzel-Seifert, 2002; Hoare et al., 2008). Our demonstration of GIP-R constitutive activity provided the basis on which to define the effects of specific missense mutations on this receptor property.
Four GIP-R variants (C46S, G198C, R316L, and E354Q) are characterized by a significant reduction in ligand-independent signaling relative to wild type (Fig. 5, Table 2). For one of these mutants (C46S), this functional change appears to be largely accounted for by reduced cell surface expression, whereas additional factors may underlie the decreased basal activity of the G198C, R316L, and E354Q variants. As a contributing mechanism, mutation-induced structural changes may shift the putative equilibrium between active and inactive receptor conformations (Lefkowitz et al., 1993) and/or may alter G protein-receptor interaction (as discussed above for the R316L mutant). Given that the G198C and E354Q substitutions are localized outside the intracellular receptor portion (in EC loop I and transmembrane domain VI), it is possible that these mutations induce structural changes that primarily shift the receptor equilibrium and thereby indirectly compromise G protein interaction.
Loss of function in the GIP-R could provide a potential mechanism for altered glucose homeostasis or fat deposition (Miyawaki et al., 2002; Gault et al., 2007; Fulurija et al., 2008; Kim and Egan, 2008). It is noteworthy that a previous study reported that glucose-tolerant subjects homozygous for the E354Q polymorphism (a variant which in our hands showed reduced basal activity) had a decreased serum C-peptide concentration (an index of insulin secretion) (Almind et al., 1998). This abnormality was observed under fasting conditions and after an oral glucose load, relative to subjects with the wild-type GIP-R. Future efforts will explore whether functional abnormalities of GIP-R variants, including the E354Q polymorphism, contribute to metabolic phenotypes.
The relatively high rate of mutation-induced functional changes in the GIP-R (four of the eight known variants were pharmacologically distinct from wild type) contrasts with our parallel analysis of the GLP-1R where none of the 10 variants that were investigated showed detectable abnormalities. On this background, it is noteworthy that the GIP-R is constitutively active, whereas the GLP-1R is not, raising the possibility that constitutively active receptors are more sensitive to polymorphism-induced alterations in pharmacology. In fact, such a parallel is also suggested by our recent study of the dopamine D1R and D2R (Al-Fulaij et al., 2008). Reminiscent of our current findings, this prior study revealed that several variants of the constitutively active D1R were associated with decreased basal activity and/or expression, whereas all missense mutants of the D2R, which displayed no ligand-independent signaling, appeared pharmacologically normal. We also recently reported that a majority of missense variants found in the constitutively active ghrelin receptor lead to alterations in ligand-independent signaling, potency, and/or expression (Liu et al., 2007). The human melanocortin-4 receptor provides an additional well known example of a constitutively active GPCR for which a high number of naturally occurring missense mutants with altered function have been identified (Vaisse et al., 2000). As a group, loss-of-function melanocortin-4 receptor missense mutations constitute the most frequent monogenic cause of obesity.
It is noteworthy that the low expression level of selected wild-type and mutant GPCRs displaying high level of basal signaling has been explained by structural instability of corresponding proteins (Gether et al., 1997; Samama et al., 1997; Alewijnse et al., 1998). It is possible, therefore, that the sensitivity of constitutively active GPCRs, like the GIP-R, to mutation-induced functional alterations in part reflects higher structural fragility compared with receptors that lack agonist-independent signaling.
Taken together, our current findings and earlier observations suggest an emerging trend that constitutive receptor activation may increase the likelihood of mutation-induced functional abnormalities. This apparent link could have important implications for predicting a subset of receptors that are likely to show missense variant-induced changes in signaling with consequent alterations in physiological response.
Supplementary Material
This work was supported by the Fonds de la Recherche en Santé du Québec and the Canadian Institutes of Health Research (Fellowship Awards to J.-P.F.); the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK072497]; and the American Diabetes Association [Grant 7-05-RA-08].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160531
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- GIP
- glucose-dependent insulinotropic peptide
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- glucagon-like peptide 1 receptor
- GIP-R
- glucose-dependent insulinotropic peptide receptor
- GPCR
- G protein-coupled receptor
- HA
- hemagglutinin
- CRE
- cAMP-responsive element
- T2D
- type 2 diabetes
- ELISA
- enzyme-linked immunosorbent assay
- HEK
- human embryonic kidney
- PBS
- phosphate-buffered saline.
References
- Alewijnse AE, Smit MJ, Hoffmann M, Verzijl D, Timmerman H, Leurs R. (1998) Constitutive activity and structural instability of the wild-type human H2 receptor. J Neurochem 71:799–807 [DOI] [PubMed] [Google Scholar]
- Al-Fulaij MA, Ren Y, Beinborn M, Kopin AS. (2008) Pharmacological analysis of human D1 AND D2 dopamine receptor missense variants. J Mol Neurosci 34:211–223 [DOI] [PubMed] [Google Scholar]
- Almind K, Ambye L, Urhammer SA, Hansen T, Echwald SM, Holst JJ, Gromada J, Thorens B, Pedersen O. (1998) Discovery of amino acid variants in the human glucose-dependent insulinotropic polypeptide (GIP) receptor: the impact on the pancreatic beta cell responses and functional expression studies in Chinese hamster fibroblast cells. Diabetologia 41:1194–1198 [DOI] [PubMed] [Google Scholar]
- Beinborn M, Ren Y, Bläker M, Chen C, Kopin AS. (2004) Ligand function at constitutively active receptor mutants is affected by two distinct yet interacting mechanisms. Mol Pharmacol 65:753–760 [DOI] [PubMed] [Google Scholar]
- Beinborn M, Worrall CI, McBride EW, Kopin AS. (2005) A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept 130:1–6 [DOI] [PubMed] [Google Scholar]
- Fortin JP, Zhu Y, Choi C, Beinborn M, Nitabach MN, Kopin AS. (2009) Membrane-tethered ligands are effective probes for exploring class B1 G protein-coupled receptor function. Proc Natl Acad Sci U S A 106:8049–8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulurija A, Lutz TA, Sladko K, Osto M, Wielinga PY, Bachmann MF, Saudan P. (2008) Vaccination against GIP for the treatment of obesity. PLoS One 3:e3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin P, Couvineau A, Maoret JJ, Rouyer-Fessard C, Laburthe M. (1995) Mutational analysis of cysteine residues within the extracellular domains of the human vasoactive intestinal peptide (VIP) 1 receptor identifies seven mutants that are defective in VIP binding. Biochem Biophys Res Commun 211:901–908 [DOI] [PubMed] [Google Scholar]
- Gault VA, McClean PL, Cassidy RS, Irwin N, Flatt PR. (2007) Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 50:1752–1762 [DOI] [PubMed] [Google Scholar]
- Gether U, Ballesteros JA, Seifert R, Sanders-Bush E, Weinstein H, Kobilka BK. (1997) Structural instability of a constitutively active G protein-coupled receptor. Agonist-independent activation due to conformational flexibility. J Biol Chem 272:2587–2590 [DOI] [PubMed] [Google Scholar]
- Hoare SR. (2005) Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today 10:417–427 [DOI] [PubMed] [Google Scholar]
- Hoare SR, Fleck BA, Gross RS, Crowe PD, Williams JP, Grigoriadis DE. (2008) Allosteric ligands for the corticotropin releasing factor type 1 receptor modulate conformational states involved in receptor activation. Mol Pharmacol 73:1371–1380 [DOI] [PubMed] [Google Scholar]
- Huang Z, Chen Y, Pratt S, Chen TH, Bambino T, Nissenson RA, Shoback DM. (1996) The N-terminal region of the third intracellular loop of the parathyroid hormone (PTH)/PTH-related peptide receptor is critical for coupling to cAMP and inositol phosphate/Ca2+ signal transduction pathways. J Biol Chem 271:33382–33389 [DOI] [PubMed] [Google Scholar]
- Kazius J, Wurdinger K, van Iterson M, Kok J, Bäck T, Ijzerman AP. (2008) GPCR NaVa database: natural variants in human G protein-coupled receptors. Hum Mutat 29:39–44 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. (2005) Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 280:22297–22307 [DOI] [PubMed] [Google Scholar]
- Kim W, Egan JM. (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60:470–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A, Yamada Y, Hayami T, Yasuda K, Someya Y, Ihara Y, Kagimoto S, Watanabe R, Taminato T, Tsuda K, et al. (1996) Identification of two missense mutations in the GIP receptor gene: a functional study and association analysis with NIDDM: no evidence of association with Japanese NIDDM subjects. Diabetes 45:1701–1705 [DOI] [PubMed] [Google Scholar]
- Lee C, Gardella TJ, Abou-Samra AB, Nussbaum SR, Segre GV, Potts JT, Jr, Kronenberg HM, Jüppner H. (1994) Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology 135:1488–1495 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Samama P, Costa T. (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14:303–307 [DOI] [PubMed] [Google Scholar]
- Lisenbee CS, Dong M, Miller LJ. (2005) Paired cysteine mutagenesis to establish the pattern of disulfide bonds in the functional intact secretin receptor. J Biol Chem 280:12330–12338 [DOI] [PubMed] [Google Scholar]
- Liu G, Fortin JP, Beinborn M, Kopin AS. (2007) Four missense mutations in the ghrelin receptor result in distinct pharmacological abnormalities. J Pharmacol Exp Ther 322:1036–1043 [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 [DOI] [PubMed] [Google Scholar]
- Manhart S, Hinke SA, McIntosh CH, Pederson RA, Demuth HU. (2003) Structure-function analysis of a series of novel GIP analogues containing different helical length linkers. Biochemistry 42:3081–3088 [DOI] [PubMed] [Google Scholar]
- Mathi SK, Chan Y, Li X, Wheeler MB. (1997) Scanning of the glucagon-like peptide-1 receptor localizes G protein-activating determinants primarily to the N terminus of the third intracellular loop. Mol Endocrinol 11:424–432 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al. (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8:738–742 [DOI] [PubMed] [Google Scholar]
- Parthier C, Kleinschmidt M, Neumann P, Rudolph R, Manhart S, Schlenzig D, Fanghänel J, Rahfeld JU, Demuth HU, Stubbs MT. (2007) Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci U S A 104:13942–13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LJ, Leung AT, Xiong Y, Marx KA, Abou-Samra AB. (1997) Extracellular cysteines of the corticotropin-releasing factor receptor are critical for ligand interaction. Biochemistry 36:12442–12448 [DOI] [PubMed] [Google Scholar]
- Runge S, Thøgersen H, Madsen K, Lau J, Rudolph R. (2008) Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem 283:11340–11347 [DOI] [PubMed] [Google Scholar]
- Samama P, Bond RA, Rockman HA, Milano CA, Lefkowitz RJ. (1997) Ligand-induced overexpression of a constitutively active beta2-adrenergic receptor: pharmacological creation of a phenotype in transgenic mice. Proc Natl Acad Sci U S A 94:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416 [DOI] [PubMed] [Google Scholar]
- Shinyama H, Masuzaki H, Fang H, Flier JS. (2003) Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology 144:1301–1314 [DOI] [PubMed] [Google Scholar]
- Tibaduiza EC, Chen C, Beinborn M. (2001) A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. J Biol Chem 276:37787–37793 [DOI] [PubMed] [Google Scholar]
- Tseng CC, Lin L. (1997) A point mutation in the glucose-dependent insulinotropic peptide receptor confers constitutive activity. Biochem Biophys Res Commun 232:96–100 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. (2000) Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.