Abstract
Many recombinant proteins have been successfully produced in silkworm larvae or pupae and used for academic and industrial purposes. Several recombinant proteins produced by silkworms have already been commercialized. However, construction of a recombinant baculovirus containing a gene of interest requires tedious and troublesome steps and takes a long time (3–6 months). The recent development of a bacmid, Escherichia coli and Bombyx mori shuttle vector, has eliminated the conventional tedious procedures required to identify and isolate recombinant viruses. Several technical improvements, including a cysteine protease or chitinase deletion bacmid and chaperone-assisted expression and coexpression, have led to significantly increased protein yields and reduced costs for large-scale production. Terminal N-acetyl glucosamine and galactose residues were found in the N-glycan structures produced by silkworms, which are different from those generated by insect cells. Genomic elucidation of silkworm has opened a new chapter in utilization of silkworm. Transgenic silkworm technology provides a stable production of recombinant protein. Baculovirus surface display expression is one of the low-cost approaches toward silkworm larvae-derived recombinant subunit vaccines. The expression of pharmaceutically relevant proteins, including cell/viral surface proteins, membrane proteins, and guanine nucleotide-binding protein (G protein) coupled receptors, using silkworm larvae or cocoons has become very attractive. Silkworm biotechnology is an innovative and easy approach to achieve high protein expression levels and is a very promising platform technology in the field of life science. Like the “Silkroad,” we expect that the “Bioroad” from Asia to Europe will be established by the silkworm expression system.
Keywords: Silkworm, Bombyx mori nucleopolyhedrovirus (BmNPV), Bacmid, Protein expression, Baculovirus display
Introduction
Various systems to produce recombinant proteins are available. Bacteria, especially Escherichia coli, are usually chosen first, due to the abundance of tools available for gene manipulations and the simple process for protein production. However, bacteria are severely limited because of their poor capacity to modify expressed proteins cotranslationally and posttranslationally, in spite of recent improvements of strains. Yeasts have some advantages over bacteria. Recombinant proteins expressed in yeast can be glycosylated, but certain modifications are not possible, especially in the case of complex form of N-glycosylation.
Insect cell systems are now popular and used widely to produce proteins from higher eukaryotes because insect cells have a similar pattern and capacity of cotranslational and posttranslational modifications as mammalian cells, including glycosylation, phosphorylation, and protein processing. The insect cell systems include the baculovirus expression system and the stably transformed cell system. In the baculovirus expression system, not only cultured cells but also insect larvae and pupae can be used for protein production.
The silkworm, Bombyx mori, has been used for silk production for centuries and recently also for protein production, as a bioreactor. Since Maeda et al. (1985) reported the production of human α-interferon in silkworm larvae, using recombinant B. mori nucleopolyhedrovirus (BmNPV), the production of many proteins has been achieved. TORAY Ind. Inc. (Tokyo, Japan) used silkworms to produce two recombinant proteins for veterinary use. Intercat, a drug principally composed of feline interferon, for feline calicivirus infections (commonly known as “cat flu”) was introduced in February 1994 as the world’s first antiviral drug for animal use. Interdog, a drug principally composed of canine interferon-γ for canine atopic dermatitis, was launched in December 2005. Nowadays, recombinant proteins produced from silkworms are being investigated for commercial purposes.
This review provides an overview of the production of recombinant proteins using silkworm larvae and pupae and the improvement of silkworm expression systems by the development of the BmNPV bacmid.
Silkworm genome sequence
Silkworms are the larvae of the silk moth, B. mori. B. mori was domesticated from its wild ancestor, Bombyx mandarina, and has been used for silk production for about 5,000 years. Silkworm silk in the natural form consists of silk fibroin, the core filaments, and silk sericin, a coating protein surrounding fibroin. Sericin is removed in commercial silk, and this silk has been used in textile production for centuries.
B. mori is an important model organism of Lepidoptera in the fields of genetics, physiology, and biochemistry. Two whole-genome shotgun (WGS) sequencing projects for B. mori were reported in 2004 (Mita et al. 2004; Xia et al. 2004), and its expressed sequence tag database was constructed (Mita et al. 2003). In 2008, two data sets from WGS projects were merged and assembled with new data (International Silkworm Genome Consortium 2008). The silkworm has 28 chromosomes and an estimated genome size of around 500 Mb. A large number of transposable elements (TEs) exist in the B. mori genome, accounting for 43.5% of the whole. TEs occupy from 2.7% to 25% in the whole genome in 12 Drosophila species and 47% in Aedes aegypti (Mita et al. 2004). The gene-finder algorithm BGF (BGI GeneFinder) predicted that approximately 18,510 genes are encoded in the silkworm genome. This gene number in the silkworm genome is higher than that in Drosophila melanogaster (13,379 genes), which belongs to Diptera (Drosophila 12 Genome Consortium 2007; Nene et al. 2007; International Silkworm Genome Consortium 2008). Moreover, orthology relationship of genes from B. mori, D. melanogaster, A. aegypti, and Caenorhabditis elegans by phylogenetic methods showed 13,450 gene families. Approximately 11.45% and 25.9% of the gene families are specific to insects and vertebrates, respectively, and 3,223 genes are silkworm specific (The International Silkworm Genome Consortium 2008). A genome-wide microarray analysis of fifth-instar larvae of the silkworm identified tissue- and developmental-stage-specific genes (Xia et al. 2007). Tissue-specific genes and genes differentially expressed in different tissues were identified. Moreover, to construct the secondary protein database of the silkworm, proteomic identification of protein-coding genes was performed by tandem mass spectrometry, using silkworm pupae as the sample (Zhang et al. 2009).
Baculovirus expression system in silkworm larvae or pupae
Silkworm larvae have been used as a bioreactor for recombinant protein production for decades. Maeda et al. (1985) first reported the production of human interferon alpha (IFN-α) in the hemolymph of silkworm larvae using BmNPV, containing the gene encoding human α-interferon driven by the polyhedrin promoter. Recombinant proteins expressed in silkworm larvae are shown in Table 1. Many proteins from eukaryotes were expressed in silkworm larvae and purified. In general, the expression level of recombinant proteins in silkworm larvae is higher than that in cultures cells from insect and animals. The mouse interleukin-3 activity in the hemolymph of silkworm larvae was about 20-fold and 10,000-fold higher than those in the culture supernatants of BmN cells and COS7 cells, respectively (Miyajima et al. 1987). The hemolymph of infected silkworms showed human butyrylcholinesterase activities 23- and 280-fold higher than those in BmN cells and Chinese hamster ovary (CHO) cells, respectively (Wei et al. 2000). One milligram of purified human macrophage colony-stimulating factor (CSF) was obtained from the hemolymph of ten silkworm larvae, and 160 μg of human growth factor was purified from 1 ml of hemolymph (Kadono-Okuda et al. 1995; Qiu et al. 1994).
Table 1.
Expression of recombinant proteins in silkworm larvae and pupae
| Proteins | Used viruses or bacmids | Expression level | References |
|---|---|---|---|
| Intracellular protein | |||
| Firefly luciferase | BmNPV | 13 mg per larva | Palhan et al. (1995) |
| Secretory proteins | |||
| Human interferon-α | BmNPV | 50 mg in hemolymph | Maeda et al. (1985) |
| Human macrophage colony-stimulating factor | BmNPV | 1 mg/10 larvae (after purification) | Qiu et al. (1994) |
| Human growth factor | BmNPV | 160 μg/ml hemolymph (after purification) | Kadono-Okuda et al. (1995) |
| Rat interleukin-5 | Cysteine protease depleted BmNPV | 1 mg/ml hemolymph 51 mg/4 larvae (after purification) | Ishihara et al. (1999) |
| Human butyrylcholinesterase | BmNPV | 35 μg/ml hemolymph | Wei et al. (2000) |
| Bovine interleukin-21 | HyNPV | 50 μg/ml hemolymph | Muneta et al. (2004) |
| Bovine interferon-t | Cysteine protease depleted BmNPV | 4.6 mg/100 larvae (after purification) | Nagaya et al. (2004) |
| Porcine lactoferrin | HyNPV | 20.5 mg/100 pupae (after purification) | Wang et al. (2005) |
| Human granulocyte macrophage colony-stimulating factor | BmNPV | 100 μg per pupa | Chen et al. (2006) |
| GFPuv-β3GnT2 fusion protein | BmNPV bacmid | 91 μg/ml hemolymph | Park et al. (2007) |
| EGFP-spider dragline silk fusion protein | BmNPV bacmid | 6 mg/a larva | Zhang et al. (2008) |
| Cholera toxin B | BmNPV | 54.4 μg/ml hemolymph | Gong et al. (2005) |
| Human stem cell factor | BmNPV | 3 μg/ml hemolymph | Han et al. (2004) |
| anti-BSA scFV | Cysteine protease and chitinase depleted BmNPV | 188 μg/ml hemolymph | Ishikiriyama et al. (2009) |
| Human anti-BSA IgG1 | Cysteine protease and chitinase depleted BmNPV | 36 μg per larva | Park et al. (2009) |
| Human α2,6-sialyltransferase | Cysteine protease and chitinase depleted BmNPV | 2.2 mg/11 larvae (after purification) | Ogata et al. (2009b) |
| Transmembrane proteins | |||
| Human (pro)renin receptor | Cysteine protease depleted BmNPV | 31 μg per larva (after purification) | Du et al. (2008) |
| Human prorenin-(pro)renin receptor complex | Cysteine protease depleted BmNPV | 70 μg/15 larvae (after purification) | Du et al. (2009b) |
The degradation of the expressed proteins is often observed in silkworm larvae. This is probably caused by a baculovirus cysteine protease, v-cath, in the baculovirus-insect cell expression system (Kadono-Okuda et al. 1995). To overcome this problem, BmNPV lacking the cysteine protease gene (BmNPV-CP −) was constructed by cotransfection with BmNPV genome DNA and pBmFCPdLZ containing the cysteine protease gene partially substituted with the β-galactosidase gene fused with the hsp70 promoter. Efficient production of undegraded firefly luciferase was achieved (Suzuki et al. 1997). Using this BmNPV-CP − strain, 4.6 mg of bovine interferon-τ was purified from the hemolymph of 100 silkworm larvae (Nagaya et al. 2004). This expression level is at least tenfold higher than those of other expression systems. Moreover, insect-derived cellulose was expressed using BmNPV in which the cysteine protease and chitinase genes (BmNPV-CP −-Chi −) were deleted, to suppress larvae liquefaction caused by baculovirus infection (Lee et al. 2006).
Several rounds of virus amplification steps are needed for BmNPV because the virus titer from B. mori cells is lower than that of Sf-9 cells, and it is more difficult to obtain a high titer of BmNPV than Autographa californica multiple nucleopolyhedrovirus (AcMNPV), which is generally used in the baculovirus expression system. To amplify BmNPV rapidly, a hybrid virus of AcMNPV and BmNPV was isolated and applied to the expression of recombinant proteins in silkworm larvae (Mori et al. 1992). A BmScH DNA fragment, the 572-bp SacI–HindIII fragment of the DNA helicase gene from BmNPV, determines the host specificity of BmNPV, and AcMNPV with the recombinant BmScH DNA fragment from BmNPV can infect BmN cells (Maeda et al. 1993). Two adjacent nucleotides (A and T) are the minimal essential sequence necessary to expand the host range of AcMNPV (Kamita and Maeda 1997). Muneta et al. (2004) reported that 500 μg of bovine interleukin-21 (IL-21) was purified from 30 ml of hemolymph using a hybrid baculovirus. Silkworm pupae have also been used as a bioreactor for recombinant protein production. Pupae can survive at 4°C for a long time and do not require mulberry leaves or an artificial diet during their growth. Human granulocyte macrophage colony-stimulating factor (hGM-CSF) was produced in pupae and purified from a homogenate (Chen et al. 2006). The hGM-CSF expressed in pupae had a molecular mass of 29 kDa, which is greater than that produced in the hemolymph of silkworm larvae. Moreover, the hGM-CSF expressed in pupae was glycosylated without its signal peptide. These results suggest that some comodification and postmodifications are different between silkworm larvae and pupae.
BmNPV bacmid development and its applications
To construct a recombinant baculovirus containing a gene of interest, cultured cells must be transfected with the baculovirus and the transfer vector. Moreover, this system requires the isolation and amplification of the recombinant baculovirus. It takes a long time (3–6 months) to prepare the high-titer recombinant baculovirus solution, and these steps are very tedious and troublesome. A novel system for the efficient production of recombinant AcMNPV was reported, which is based on site-specific transposition in Escherichia coli (Luckow et al. 1993). Luckow and colleagues constructed a recombinant baculovirus vector (bacmid) that can replicate in E. coli as a large plasmid. This system is known as the Bac-to-Bac baculovirus expression system, and various kits based on this Bac-to-Bac system have been distributed from Invitrogen Corp. This system reduces the time required to isolate and purify a recombinant baculovirus and allows the simultaneous construction of multiple recombinant baculoviruses.
Motohashi et al. (2005) constructed a BmNPV bacmid and established the Bac-to-Bac system using BmNPV. GFPuv was expressed with only the injection of BmNPV bacmid DNA into silkworm larvae and pupae. This bacmid system provided rapid expression of recombinant proteins, since it did not require the preparation of a baculovirus solution by transfection, as compared to the baculovirus expression system using cultured cells (Fig. 1). Furthermore, this BmNPV bacmid system dramatically reduced the time needed for recombinant protein production by silkworm expression.
Fig. 1.
Baculovirus gene expression system in insect cells and silkworm larvae. a AcMNPV expression system using the AcMNPV bacmid. The target gene was incorporated into the AcMNPV bacmid. This extracted recombinant AcMNPV bacmid from E. coli was transfected into insect cells. The resulting recombinant AcMNPV was purified, amplified, and increased in titer and then was used for insect cell infection. b Conventional BmNPV expression system. The target gene and the wild-type BmNPV gene were cotransfected into B. mori cells, and the recombinant BmNPV was obtained. This recombinant BmNPV bacmid from E. coli was injected directly into silkworm larvae. After 4–6 days postinjection, the recombinant protein was harvested
Improved protein expression by a modified BmNPV bacmid
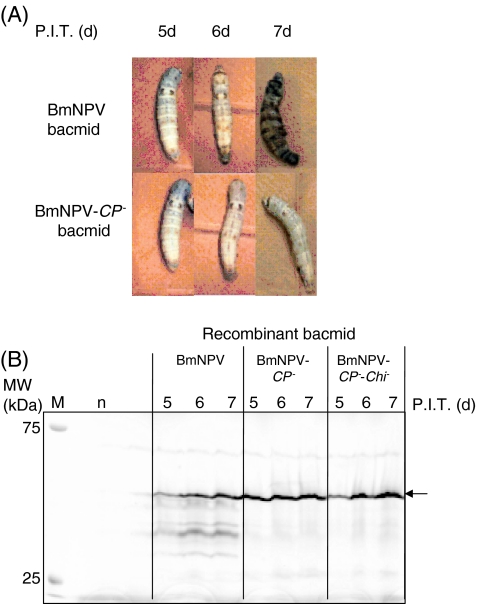
Several proteins were produced using this BmNPV bacmid in silkworm larvae (Yue et al. 2006; Zhang et al. 2008). However, the degradation of recombinant proteins by baculoviral cysteine protease also occurred (Park et al. 2007). Therefore, the BmNPV-CP − bacmid was constructed, and the GFPuv-β1,3-N-acetylglucosaminyltransferase2 (β3GnT2) fusion protein (GGT2) was expressed successfully using this bacmid (Hiyoshi et al. 2007). The protease activity in the hemolymph of silkworm larvae injected with the BmNPV-CP − bacmid was reduced by 85%, as compared to that in the hemolymph of BmNPV-bacmid-injected silkworm larvae. GGT2 degradation was also suppressed by using the BmNPV-CP − bacmid, and the β3GnT activity was improved by 30%. Moreover, the β3GnT activity of the hemolymph was improved 2.8-fold by using the BmNPV-CP −-Chi − bacmid over that obtained with the unmodified BmNPV bacmid (Park et al. 2008). Liquefaction of larvae was delayed by using the BmNPV-CP − and BmNPV-CP −-Chi − bacmids (Fig. 2). The expression level of human anti-bovine-serum-albumin (BSA) single-chain Fv (scFv) fragment in hemolymph was 3.8–4.3-fold higher with the BmNPV-CP − or BmNPV-CP −-Chi − bacmid than that with the unmodified BmNPV because of the suppression of degradation by the cysteine protease from BmNPV (Fig. 2). However, no difference in the scFv expression levels between the modified and unmodified bacmids was observed in pupae (Ishikiriyama et al. 2009). This result suggests that the baculoviral cysteine protease, v-cath, may not be activated or only a small portion of the cysteine protease may be expressed in pupae. Pupae may be useful for the production of cysteine protease-sensitive proteins.
Fig. 2.
a Comparison of larval liquefaction. Silkworm larvae were injected with BmNPV (WT) and BmNPV-CP − bacmids. b GFPuv fluorescence of the GFPuv-13CG2 (scFv) fusion protein expressed in the BmNPV/bx-GFPuv-13CG2-, BmNPV-CP -/bx-GFPuv-13CG2-, and BmNPV-CP −-Chi −/bx-GFPuv-13CG2 bacmid-injected silkworm larvae. Loaded protein concentrations were 65–95 μg. The arrow indicates the molecular mass of the GFPuv-13CG2 fusion protein
A hybrid nucleopolyhedrovirus (HyNPV) bacmid was also constructed, by cotransfection with the AcMNPV bacmid and pUC18/BmScH containing the BmScH fragment of the DNA helicase gene from BmNPV (Deo et al. 2006). This bacmid allows baculoviral amplification in AcMNPV and protein expression in BmNPV. To obtain a high virus titer, this HyNPV bacmid is very helpful.
Molecular chaperones play an important role in protein folding at the endoplasmic reticulum. Bacmids allow easy coexpression of a chaperone with a target protein. The expression level of a recombinant GFPuv-human α1,4-N-acetylglucosaminyltransferase (α4GnT) fusion protein was enhanced in silkworm larvae, by coexpression of a human molecular chaperone, human calnexin (CNX), or immunoglobulin heavy-chain binding protein (BiP; Nakajima et al. 2009). The expression of CNX and BiP under the control of the polyhedrin promoter enhanced the α4GnT activity by 1.4- and 2.0-fold, respectively. When CNX or BiP under the control of the ie-2 promoter was expressed during the early stage of baculovirus infection, the α4GnT activity in the hemolymph was also enhanced significantly. In silkworm larvae, the coexpression of a chaperone with the target protein effectively increases the quality of the recombinant protein.
Protein production using transgenic silkworms
Transgenic silkworms have also been used for recombinant protein production. To establish transgenic silkworms, two different systems, involving the use of an attenuated recombinant baculovirus or a piggyBac transposon-derived vector, were adopted (Tamura et al. 2000; Yamao et al. 1999). A method combining the two systems was also established (Yamamoto et al. 2004). Human type III procollagen and feline interferon were produced in cocoons using transgenic silkworms (Kurihara et al. 2007; Tomita et al. 2003). Human μ-opioid receptor was expressed in the silk glands and fat bodies of transgenic silkworms, which were screened by the GAL4/UAS system (Tateno et al. 2009). Its expression level was comparable to that obtained in the baculovirus expression system using Sf-9 cells.
N-glycan structure produced by silkworm larvae
Insect cells can assemble N-glycan precursors to produce high mannose or paucimannosidic end products. However, the cells failed to elongate the trimmed N-glycan to produce complex products containing terminal galactose and sialic acid (SA) residues (Kost et al. 2005). Enzyme assays revealed that the insect cell lines had little or none of the galactosyltransferase and sialyltransferase activities involved in N-glycan elongation.
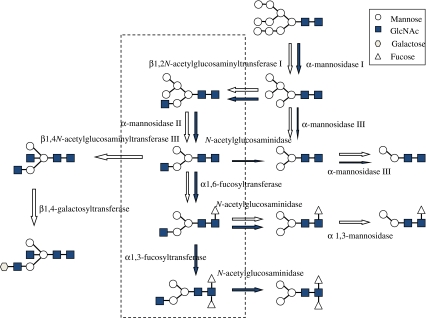
When human anti-BSA IgG1 was expressed in hemolymph, using the BmNPV-CP −-Chi − bacmid, its main N-glycan structure was paucimannosidic (Park et al. 2009). In accordance with this observation, human killer cell Ig-like receptor 2DL1 was expressed using the BmNPV bacmid (see details below), and the protein thus obtained harbored the paucimannose-type oligosaccharides, Manalpha1-6Manbeta1-4GlcNAcbeta1-4GlcNAc and Manalpha1-6Manbeta1-4GlcNAcbeta1-4(Fucalpha1-6)GlcNAc (Sasaki et al. 2009). However, the N-glycan of β3GnT2 purified from the hemolymph of larvae contained terminal N-acetylglucosamine (GlcNAcβ1,2Manα1,3(GlcNAcβ1,4)(Manα1,6)Manβ1,4GlcNAcβ1,4GlcNA) and galactose residues (Galβ1,4GlcNAcβ1,2Manα1,3(GlcNAcβ1,4)(Manα1,6)Manβ1,4GlcNAcβ1,4GlcNAc), which were not observed in samples from cultured cells (Dojima et al. 2009). These results indicate that β1,2N-acetylglucosaminyltransferase I (GlcNAcT I), β1,4N-galactosyltransferase (GalT), and β1,4N-acetylglucosaminyltransferase III (GlcNAcT III) might exist in silkworm larvae, but not in insect cells (Fig. 3). Therefore, silkworm larvae would be useful for the production of human glycoproteins, if the glycosylation pathway was reinforced with GlcNAcT II and sialyltransferase.
Fig. 3.
Proposed N-glycan processing pathway in the T. ni cell line and silkworm larvae. Open and closed arrows indicate silkworm larvae and the T. ni cell line, respectively. N-glycans enclosed by dotted lines were not detected by the HPLC mapping
Expression of glycosyltransferase in silkworm larvae and its application to the synthesis of glycomimetics to block infections by avian and human influenza viruses
Recently, the glycosyltransferase produced by silkworm larvae was applied to the synthesis of sialoglycopolypeptides for blocking influenza virus infection. Influenza viruses infect host cells through the binding of viral hemagglutinins (HAs) to sialoglycoproteins or sialoglycopeptides of the receptors on the host cell surfaces (Suzuki et al. 1986). Since the molecular recognition process leads to the adhesion of the host cells and virus, molecules with a high affinity for the viral HA act as potent inhibitors of infection by influenza viruses (Ogata et al. 2007; Schengrund 2003). The host cell specificity of the virus is dependent not only on the linkage of SAs to the penultimate galactose but also on the number of SA residues and the precise nature of the core structure (Gambaryan et al. 2004; Yamada et al. 2006). In order to synthesize an artificial sialoglycoprotein, rat α2,6-sialyltransferase (ST6Gal I) was expressed in fifth-instar silkworm larval hemolymph, using a recombinant bacmid. Approximately 2.2 mg of ST6GalI was purified from the hemolymph of 11 larvae. The substrate specificity of the ST6GalI purified from hemolymph was the same as that of the native rat ST6GalI. By using this ST6GalI, the synthesis of a α2,6-sialoglycopolypeptide as a glycoprotein mimetic was successfully achieved. The synthesized α2,6-sialoglycopolypeptide selectively inhibited hemagglutination induced by Sambucus nigra lectin, which specifically binds to the Siaα2-6Gal(GalNAc) structure, and showed approximately 780-fold higher affinity than fetuin as a control (Ogata et al. 2009b). Moreover, Ogata et al. (2009a) designed a series of γ-polyglutamic acid (γ-PGA)-based glycopolypeptides carrying long/short R2,3/6 sialylated glycans to act as inhibitors of the influenza virus. As an alternative design, sialoglycopolypeptides carrying long-spacer-linked glycans were engineered, by the replacement of the N-acetyllactosamine (LN) unit by an alkyl chain. The avian viruses specifically bound to glycopolypeptides carrying a short sialoglycan with higher affinity than to a long glycan. In contrast, human viruses preferentially bound not only to a long R2,3/6 sialylated glycan with LN repeats in the receptors but also to more spacer-linked glycans, in which the inner sugar was replaced by a nonsugar structural unit, such as a pentylamido group (Hidari et al. 2007). A spaced tandem/triplet pentylamido repeat is a good mimetic of a tandem/triplet LN repeat and thus provides a facile way to design strong polymeric inhibitors of infection by avian and human influenza viruses (Fig. 4).
Fig. 4.
Blocking influenza virus infection using an α2,6-sialoglycopolypeptide. The α2,6-sialoglycopolypeptide was enzymatically synthesized from an (γ-PGA)-based glycopolypeptides carrying α2,6-sialylated glycans acted as inhibitors of influenza virus because human viral HAs bind to α2,6-linked sialosides in the core glycan structures
Surface display system and subunit vaccine production in silkworm
Baculovirus particles have become valuable tools in biotechnology and medicine. Even a small virus provides a huge surface, which can be engineered in many ways. Heterologous peptides or proteins can be displayed on the surface of baculovirus particles by fusing the peptide or protein to the baculovirus surface glycoprotein, gp64 (Boublik et al. 1995). Baculoviruses displaying gp64 fusion proteins have proven to be very effective immunogens (Kost et al. 2005). Since this approach was first used to raise monoclonal antibodies against the nuclear receptors LXRβ and FXR (Lindley et al. 2000), it has been used for the development of low-cost insect larvae-derived recombinant subunit vaccines. These include H5N1 influenza vaccine (Jin et al. 2008), as well as subunit vaccines against rabbit hemorrhagic disease virus (Pérez-Filgueira et al. 2007) and pancreatic necrosis virus (Shivappa et al. 2005).
Recently, Li et al. (2008) reported that the capsid proteins of foot-and-mouth disease virus (FMDV) were produced in the hemolymph of silkworm larvae, and cattle vaccinated with hemolymph, diluted 30-fold, were protected from challenge with FMDV.
The ability of BmNPV to display gp64 fusion proteins also offers the possibility of using this silkworm as a host for the production and display of a wide range of antigenically important proteins on the cell or virus surface. Using silkworm larvae, rather than insect cell lines, as a host for recombinant virus amplification and recombinant protein production has advantages, in terms of their size and easiness to rear. The purified virus particles displaying the cloned foreign protein on the viral envelope could be used directly for functional analysis, without isolating the proteins. Since the presence of the gp64 fusion protein did not alter the growth and yield of the virus, the availability of the gp64-fusion-protein-displayed virus will also provide insight into the virus–host cell interaction, and this system may provide a fundamental platform for large-scale vaccine production. The BmNPV-based system is an economic process for the large-scale production of surface-displayed baculoviruses and safe subunit vaccines for animals, since the silkworm larvae, which are easy to rear on a synthetic or natural diet, can be used instead of the cultured cell lines.
Expression of pharmaceutically relevant proteins, including cell/viral surface proteins
The infection of silkworms with recombinant BmNPV virus is a very attractive strategy for protein production, especially for cell surface receptors and secreted proteins, which often require posttranslational modifications, including sugar modifications. Silkworm expression is used for the expression of pharmaceutically relevant proteins. Immune cell and viral surface molecules and guanine nucleotide-binding protein (G protein) coupled receptors (GPCR) are the main drug targets because they regulate a broad range of physiological functions (Kristiansen 2004). The production of cell surface proteins using the silkworm-BmNPV expression system is summarized in Table 2. The expression levels ranged from several micrograms to 0.5 mg per larva. The proteins normally had signal sequences and thus were secreted in the hemolymph when properly processed. The obtained proteins were basically functional, as confirmed by either ligand binding assays or ELISA. Human killer cell immunoglobulin-like receptor, KIR2DL1, which regulates natural killer cell function by recognizing human leukocyte antigen C molecules, was expressed successfully (Sasaki et al. 2009). The expression level was high, 0.2 mg/larva, either by direct injection of bacmid DNA or by injection of collected body fluid containing the recombinant virus. Furthermore, the protein was easily purified using Ni-NTA affinity chromatography. The sugar modifications of the obtained KIR2DL1 protein revealed that the protein harbored the relatively small paucimannose-type oligosaccharides and thus seemed to be appropriate for structural and binding studies of cell surface receptors.
Table 2.
Expression of cell/viral surface proteins and GPCRs in silkworm
| Receptor | Gene transfer | Yield | Expression level | Application | Functional assay | Function | Reference |
|---|---|---|---|---|---|---|---|
| KIR2DL1 | BmNPV Bacmid | ~0.2 mg/larva | SPR, sugar characterization | Confirmed | Sasaki et al. 2009 | ||
| IL4-Rα | BmNPV | 0.11 mg/ml hemolymph | SPR, sugar characterization | Confirmed | Honjo et al. 2008 | ||
| IL4-Rα-Fca | 0.053 mg/ml hemolymph | Gel filtration | |||||
| IL13-Rα1 | 0.55 mg/ml hemolymph | ||||||
| IL13-Rα1-Fca | 0.33 mg/ml hemolymph | ||||||
| Influenza hemagglutinin | BmNPV | 0.4–4 μg/larva | ELISA | Not confirmed | Sugiura et al. 2001 | ||
| Nociceptin receptor (Giα1)b | BmNPV bacmid | Not determined | [35S]GTPγS binding assay (EC50=9.3 ± 3.4 nM) | Kajikawa et al. 2009 | |||
| µ-Opioid receptors | Transgenic | 150–250 ng/larva | [3H]diprenorphine saturation analysis (K d=1.4~2.1 nM) | Tateno et al. 2009 |
Expression of immune cell and viral surface receptors using silkworm
SPR surface plasmon resonance analysis
aFc fusion proteins
bGα-fusion proteins
GPCRs are seven-transmembrane proteins and thus are quite difficult to express due to their low solubility and instability. The expression of a GPCR (human μ-opioid receptor) in silkworms was first reported using a transgenic technique, but the expression level was rather low, and many technical steps were required (Tateno et al. 2009). The expression of other GPCRs using other expression systems including Sf9 and CHO cells was reviewed by Massotte (2003) and Sarramegna et al. (2003). Human GPCR (human nociceptin receptor) expression was reported recently by the simple injection of BmNPV bacmid DNA into the silkworm (Kajikawa et al. 2009). Human nociceptin receptor, which is an inhibitory GPCR for an exogenous opioid peptide, nociceptin, involved in pain control ( Meunier 1997), was actually produced as a membrane protein in microsomal fractions of the fat bodies of silkworms. The microsomal fractions including functional GPCRs could be very easily prepared by a simple operation and centrifugation. Interestingly, the protein was also expressed in the BmNPV viral fraction, which was consistent with that previously reported for the GPCR expression in Sf9 cells (Masuda et al. 2003). GPCRs physiologically bind at the cytoplasmic region to a trimeric G protein, composed of alpha (Gα), beta (Gβ), and gamma (Gγ) subunits (Wess 1997). The ligand binding to GPCRs enhances the guanosine triphosphatase (GTPase) activity of the trimeric G protein, resulting in its dissociation from GPCRs by exchanging Gα-bound GTP to GDP, which triggers the G-protein-mediated signal transduction (Liebmann and Bohmer 2000). The nociceptin receptor fused with the G protein alpha subunit (Giα), whose ligand recognition can be detected by measuring the [35S]GTPγS binding, was also successfully expressed in the same system as the nonfused protein as described above. This expression system exhibited sufficient production for 500 binding assays using the radiolabeled compound, [35 S]GTPγS. The expression level of the nonfusion type of nociceptin receptor was much higher than that of the fusion type.
Silkworms also have the capacity to produce functional membrane proteins in the same way as secretory proteins. Many membrane proteins have been produced in the hemolymph and pupae of silkworms as secretory proteins. Recently, human (pro)renin receptor (hPRR) and its complex with human prorenin were expressed in silkworm larvae, with the same expression level as some secretory proteins, and were purified from the fat bodies of larvae (Du et al. 2008, 2009a). This hPRR may be helpful for the development of an hPRR blocker.
Conclusions and future developments
The BmNPV bacmid system is innovative and easy to use for producing large amount of recombinant protein, without any laborious techniques. Many BmNPV DNAs for recombinant protein expression can be constructed rapidly by the BmNPV bacmid system, based on the Bac-to-Bac system. Moreover, expression of multi-subunit complexes can be achieved simply by injection of a mixture of BmNPV bacmids into silkworms, without prior virus amplification using cultured cells. The protein expression levels in bacmid-injected silkworm larvae are very high, which reduces the cost of large-scale production. This BmNPV bacmid-silkworm expression strategy is very successful for expression of membrane proteins including GPCRs which are major drug targets. Therefore, this system is very promising and will be useful for high-throughput drug screening of many transmembrane proteins and orphan GPCRs for which physiological ligands have not yet been identified (Takeda et al. 2002; Wise et al. 2004).
In Asian countries, silkworms are abundantly available, and many laboratories have experience in rearing and maintaining larvae. Thus, the opportunity to utilize the long-forgotten resourceful silkworm for producing therapeutically important proteins, vaccines, and biomaterials is here. By the spurt in influenza viruses, vaccines for influenza viruses are being actively produced in insect cell system. More and more patents are being procured for customized insect cell lines and their products are making inroads into the market which until now had been dominated by other production systems. With the genomic elucidation of silkworm, even more laboratories around the world will be interested in its utilization. We expect that the glory of silkworm is returning back, and it will be once more associated with wealth and prosperity.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Boublik Y, Bonito PD, Jones IM. Eukaryotic virus display: engineering the major surface glycoprotein of the Autographa californica nuclear polyhedrosis virus (AcNPV) for the presentation of foreign proteins on the virus surface. Bio/Technol. 1995;13:1079–1084. doi: 10.1038/nbt1095-1079. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu XF, Zhang YZ. Expression, purification and characterization of human GM-CSF using silkworm pupae (Bombyx mori) as a bioreactor. J Biotechnol. 2006;123:236–247. doi: 10.1016/j.jbiotec.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Deo VK, Hiyoshi M, Park EY. Construction of hybrid Autographa californica nuclear polyhedrovirus bacmid by modification of p143 helicase. J Virol Methods. 2006;134:212–216. doi: 10.1016/j.jviromet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Dojima T, Nishina T, Kato T, Uno T, Yagi H, Kato K, Park EY. Comparison of the N-linked glycosylation of human β1,3-N-acetylglucosaminyltransferase 2 expressed in insect cells and silkworm larvae. J Biotechnol. 2009;143:27–33. doi: 10.1016/j.jbiotec.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Drosophila 12 Genome Consortium Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Du D, Kato T, Nurun Nabi AHM, Suzuki F, Park EY. Expression of functional human (pro)renin receptor in silkworm (Bombyx mori) larvae using BmMNPV bacmid. Biotechnol Appl Biochem. 2008;49:195–202. doi: 10.1042/BA20070136. [DOI] [PubMed] [Google Scholar]
- Du D, Kato T, Suzuki F, Park EY. Binding affinity of full length and extracellular domains of recombinant human (Pro)renin receptor to human renin when expressed in the fat body and hemolymph of silkworm larvae. J Biosci Bioeng. 2009;108:304–309. doi: 10.1016/j.jbiosc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Du D, Kato T, Suzuki F, Park EY. Expression of protein complex comprising the human prorenin and (pro)renin receptor in silkworm larvae using Bombyx mori nucleopolyhedrovirus (BmNPV) bacmids for improving biological function. Mol Biotechnol. 2009;43:154–161. doi: 10.1007/s12033-009-9183-7. [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Tuzikov AB, Pazynina GV, Webster RG, Matrosovich MN, Bovin NV. H5N1 chicken influenza viruses display a high binding affinity for Neu5AcR2-3Gal_1-4(6-HSO3)GlcNAc-containing receptors. Virol. 2004;326:310–316. doi: 10.1016/j.virol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gong ZH, Jin HQ, Jin YF, Zhang YZ. Expression of cholera toxin B subunit and assembly as functional oligomers in silkworm. J Biochem Mol Biol. 2005;38:717–724. doi: 10.5483/bmbrep.2005.38.6.717. [DOI] [PubMed] [Google Scholar]
- Han J, Zang Y, Lu H, Zhu J, Qin J. A novel recombinant dual human SCF expressed in and purified from silkworm, Bombyx mori, possesses higher bioactivity than recombinant monomeric human SCF. Eur J Haematol. 2004;72:273–279. doi: 10.1111/j.1600-0609.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Hidari KI-PJ, Shimada S, Suzuki Y, Suzuki T. Binding kinetics of influenza viruses to sialic acid-containing carbohydrates. Glycoconj J. 2007;24:583–590. doi: 10.1007/s10719-007-9055-y. [DOI] [PubMed] [Google Scholar]
- Hiyoshi M, Kageshima A, Kato T, Park EY. Construction of a cysteine protease deficient Bombyx mori multiple nucleopolyhedrovirus bacmid and its application to improve expression of a fusion protein. J Virol Methods. 2007;144:91–97. doi: 10.1016/j.jviromet.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Honjo E, Shoyama Y, Tamada T, Shigematsu H, Hatanaka T, Kanaji S, Arima K, Ito Y, Izuhara K, Kuroki R. Expression of the extracellular region of the human interleukin-4 receptor alpha chain and interleukin-13 receptor alpha1 chain by a silkworm-baculovirus system. Protein Expr Purif. 2008;60:25–30. doi: 10.1016/j.pep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- International Silkworm Genome Consortium The genome of lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol. 2008;38:1036–1045. doi: 10.1016/j.ibmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Satoh I, Nittoh T, Kanaya T, Okazaki H, Suzuki T, Koyama T, Sakamoto T, Ide T, Ohuchi K. Preparation of recombinant rat interleukin-5 by baculovirus expression system and analysis of its biological activities. Biochim Biophys Acta. 1999;1451:48–58. doi: 10.1016/S0167-4889(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Ishikiriyama M, Nishina T, Kato T, Ueda H, Park EY. Human single-chain antibody expression in the hemolymph and fat body of silkworm larvae and pupae using BmNPV bacmids. J Biosci Bioeng. 2009;107:67–72. doi: 10.1016/j.jbiosc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Jin R, Lv Z, Chen Q, Quan Y, Zhang H, Li S, Chen G, Zheng Q, Jin L, Wu X, Chen J, Zhang Y. Safety and immunogenicity of H5N1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS ONE. 2008;3:e3933. doi: 10.1371/journal.pone.0003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono-Okuda K, Yamamoto M, Higashino Y, Taniai K, Kato Y, Chowdhury S, Xu J, Choi SK, Sugiyama M, Nakashima K, Maeda S, Yamakawa M. Baculovirus-mediated production of the human growth hormone in larvae of the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1995;213:389–396. doi: 10.1006/bbrc.1995.2144. [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Sasaki K, Wakimoto Y, Toyooka M, Motohashi T, Shimojima T, Takeda S, Park EY, Maenaka K. Efficient silkworm expression of human (nociceptin receptor) by a Bombyx mori bacmid DNA system. Biochem Biophys Res Commun. 2009;385:375–379. doi: 10.1016/j.bbrc.2009.05.063. [DOI] [PubMed] [Google Scholar]
- Kamita SG, Maeda S. Sequencing of the putative DNA helicase-encoding gene of the Bombyx mori nuclear polyhedrosis virus and fine-mapping a region involved in host range expansion. Gene. 1997;190:173–179. doi: 10.1016/S0378-1119(96)00671-3. [DOI] [PubMed] [Google Scholar]
- Kost TA, Contreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Sezutsu TT, Yamada K. Production of an active feline interferon in the cocoon of transgenic silkworms using the fibroin H-chain expression system. Biochem Biophys Res Commun. 2007;355:976–980. doi: 10.1016/j.bbrc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Lee KS, Je YH, Woo SD, Sohn HD, Jin BR. Production of a cellulose in silkworm larvae using a recombinant Bombyx mori nucleopolyhedrovirus lacking the virus-encoded chitinase and cathepsin genes. Biotechnol Lett. 2006;28:645–650. doi: 10.1007/s10529-006-0030-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Yi Y, Yin X, Zhang Z, Liu J. Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS ONE. 2008;3:e2273. doi: 10.1371/journal.pone.0002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann C, Bohmer FD. Signal transduction pathways of G protein-coupled receptors and their cross-talk with receptor tyrosine kinases: lessons from bradykinin signaling. Curr Med Chem. 2000;7:911–943. doi: 10.2174/0929867003374589. [DOI] [PubMed] [Google Scholar]
- Lindley KM, Su JL, Hodges PK, Wisely GB, Bledsoe RK, Condreay JP, Winegar DA, Hutchins JT, Kost TA. Production of monoclonal antibodies using recombinant baculovirus displaying gp64-fusion proteins. J Immunol Methods. 2000;234:123–135. doi: 10.1016/S0022-1759(99)00133-7. [DOI] [PubMed] [Google Scholar]
- Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, Saeki Y, Sato Y, Furusawa M. Production of human α-interferon in silkworm using a baculovirus vector. Nature. 1985;315:592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamita SG, Kondo A. Host range expansion of Autographa californica nuclear polyhedrosis virus (NPV) following recombination of a 0.6-kilobase-pair DNA fragment originating from Bombyx mori NPV. J Virol. 1993;67:6234–6238. doi: 10.1128/jvi.67.10.6234-6238.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massotte D. G protein-coupled receptor overexpression with the baculovirus-insect cell system: a tool for structural and functional studies. Biochim Biophys Acta. 2003;1610:77–89. doi: 10.1016/S0005-2736(02)00720-4. [DOI] [PubMed] [Google Scholar]
- Masuda K, Itoh H, Sakihama T, Akiyama C, Takahashi K, Fukuda R, Yokomizo T, Shimizu T, Kodama T, Hamakubo T. A combinatorial G protein-coupled receptor reconstitution system on budded baculovirus. Evidence for Galpha and Galphao coupling to a human leukotriene B4 receptor. J Biol Chem. 2003;278:24552–24562. doi: 10.1074/jbc.M302801200. [DOI] [PubMed] [Google Scholar]
- Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/S0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- Mita K, Morimyo M, Okano K, Koike Y, Nohata J, Kawasaki H, Kadono-Okuda K, Yamamoto K, Suzuki MG, Shimada T, Goldsmith MR, Maeda S. The construction of an EST database for Bombyx mori and its application. Proc Natl Acad Sci U S A. 2003;100:14121–14126. doi: 10.1073/pnas.2234984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita K, Kasahara M, Sasaki S, Nagayasu Y, Yamada T, Kanamori H, Namiki N, Kitagawa M, Yamashita H, Yasukochi Y, Kadono-Okuda K, Yamamoto K, Ajimura M, Ravikumar G, Shimomura M, Nagamura Y, Shin-i T, Abe H, Shimada T, Morishita S, Sasaki T. The genome sequence of silkworm, Bombyx mori. DNA Res. 2004;11:27–35. doi: 10.1093/dnares/11.1.27. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Schreurs J, Otsu K, Kondo A, Arai K, Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level of expression and secretion of biologically active mouse inteleukin-3. Gene. 1987;58:273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Mori H, Nakazawa H, Shirai N, Shibata N, Sumida M, Matsubara F. Foreign gene expression by a baculovirus vector with an expanded host range. J Gen Virol. 1992;73:1877–1880. doi: 10.1099/0022-1317-73-7-1877. [DOI] [PubMed] [Google Scholar]
- Motohashi T, Shimojima T, Fukagawa T, Maenaka K, Park EY. Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nuclear polyhedrosis virus bacmid system. Biochem Biophys Res Commun. 2005;326:564–569. doi: 10.1016/j.bbrc.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Muneta Y, Nagaya H, Minagawa Yu, Enomoto C, Matsumoto S, Mori Y. Expression and one-step purification of bovine interleukin-21 (IL-21) in silkworm using a hybrid baculovirus expression system. Biotechnol Lett. 2004;26:1453–1458. doi: 10.1023/B:BILE.0000045643.66758.9d. [DOI] [PubMed] [Google Scholar]
- Nagaya H, Kanaya T, Kaki H, Tobita Y, Takahashi M, Takahashi H, Yokomizo Y, Inumaru S. Establishment of a large-scale purification procedure for purified recombinant bovine interferon-τ produced by a silkworm-baculovirus gene expression system. J Vet Med Sci. 2004;66:1395–1401. doi: 10.1292/jvms.66.1395. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Kato T, Kanamasa S, Park EY. Molecular chaperone-assisted production of human α-1, 4-N-acetylglucosaminyltransferase in silkworm larvae using recombinant BmNPV bacmid. Mol Biotechnol. 2009;43:67–75. doi: 10.1007/s12033-009-9174-8. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, RV Bruggner, J Costas, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O’leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;318:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Murata T, Murakami K, Suzuki T, I-PJ HK, Suzuki Y, Usui T. Chemoenzymatic synthesis of artificial glycopolypeptides containing multivalent sialyl oligosaccharides with a γ-polyglutamic acid backbone and their effect on inhibition of infection by influenza viruses. Bioorg Med Chem. 2007;15:1383–1393. doi: 10.1016/j.bmc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ogata M, I-PJ HK, Murata T, Shimada S, Kozaki W, Park EY, Suzuki T, Usui T. Chemoenzymatic synthesis of sialoglycopolypeptides as glycomimetics to block infection by avian and human influenza viruses. Bioconjugate Chem. 2009;20:539–549. doi: 10.1021/bc800460p. [DOI] [PubMed] [Google Scholar]
- Ogata M, Nakajima M, Kato T, Obara T, Yagi H, Kato K, Usui T, Park EY. Synthesis of sialoglycopolypeptide for potentially blocking influenza virus infection using a rat α2,6-sialyltransferase expressed in BmNPV bacmid-injected silkworm larvae. BMC Biotechnol. 2009b;9:54. doi: 10.1186/1472-6750-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palhan VB, Sumathy S, Gopinathan KP. Baculovirus mediated high-level expression of luciferase in silkworm cells and larvae. Biotechniques. 1995;19(100):102–104. [PubMed] [Google Scholar]
- Park EY, Kageshima A, Kwon MS, Kato T. Enhanced production of secretory Bombyx mori larvae using recombinant BmNPV bacmid integrated signal sequence. J Biotechnol. 2007;129:681–688. doi: 10.1016/j.jbiotec.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Park EY, Abe T, Kato T. Improved expression of fusion protein using cysteine-protease-and chitinase-deficient Bombyx mori (silkworm) multiple nucleopolyhedrovirus bacmid in silkworm larvae. Biotechnol Appl Biochem. 2008;49:135–140. doi: 10.1042/BA20070098. [DOI] [PubMed] [Google Scholar]
- Park EY, Ishikiriyama M, Nishina T, Kato T, Yagi H, Kato K, Ueda H. Human IgG1 expression in silkworm larval hemolymph using BmNPV bacmid and its N-linked glycan structure. J Biotechnol. 2009;139:108–114. doi: 10.1016/j.jbiotec.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Pérez-Filgueira DM, Resino-Talaván P, Cubillos C, Angulo I, Barderas MG, Barcena J, Escribano JM. Development of a low-cost, insect larvae-derived recombinant subunit vaccine against RHDV. Virol. 2007;364:422–430. doi: 10.1016/j.virol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Qiu P, Ding Y, Qin J, Han KK, Zhu D. Expression of biologically active monomeric form of human M-CSF in baculovirus infected silkworm, Bombyx mori. Biol Chem Hoppe Seyler. 1994;375:413–418. doi: 10.1515/bchm3.1994.375.6.413. [DOI] [PubMed] [Google Scholar]
- Sarramegna V, Talmont F, Demange P, Milon A. Heterologous expression of G-protein-coupled receptors: comparison of expression systems from the standpoint of large-scale production and purification. Cell Mol Life Sci. 2003;60:1529–1546. doi: 10.1007/s00018-003-3168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Kajikawa M, Kuroki K, Motohashi T, Shimojima T, Park EY, Kondo S, Yagi H, Kato K, Maenaka K. Silkworm expression and sugar profiling of human immune cell surface receptor, KIR2DL1. Biochem Biophys Res Commun. 2009;387:575–380. doi: 10.1016/j.bbrc.2009.07.065. [DOI] [PubMed] [Google Scholar]
- Schengrund C-L. “Multivalent” saccharides: development of new approaches for inhibiting the effects of glycosphingolipid-binding pathogens. Biochem Pharmacol. 2003;65:699–707. doi: 10.1016/S0006-2952(02)01553-8. [DOI] [PubMed] [Google Scholar]
- Shivappa RB, McAllister PE, Edwards GH, Santi N, Evensen Ø, Vakharia VN. Development of a subunit vaccine for infectious pancreatic necrosis virus using a baculovirus insect/larvae system. Dev Biol Basel Karger. 2005;121:165–174. [PubMed] [Google Scholar]
- Sugiura T, Sugita S, Imagawa H, Kanaya T, Ishiyama S, Saeki N, Uchiyama A, Tanigawa M, Kuwano A. Serological diagnosis of equine influenza using the hemagglutinin protein produced in a baculovirus expression system. J Virol Methods. 2001;98:1–8. doi: 10.1016/S0166-0934(01)00332-9. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagano Y, Kato H, Matsumoto M, Nerome K, Nakajima K, Nobusawa E. Human influenza A virus hemagglutinin distinguishes sialyl oligosaccharides in membrane-associated gangliosides as its receptor which mediates the adsorption and fusion processes of virus infection. J Biol Chem. 1986;261:17057–17061. [PubMed] [Google Scholar]
- Suzuki T, Kanaya T, Okazaki H, Ogawa K, Usami A, Watanabe H, Kadono-Okuda K, Yamakawa M, Sato H, Mori H, Takahashi S, Oda K. Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J Gen Virol. 1997;78:3073–3080. doi: 10.1099/0022-1317-78-12-3073. [DOI] [PubMed] [Google Scholar]
- Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/S0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme JC, Couble P. Germ line transformation of the silkworm Bombyx mori L. using piggyBac transposon-derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Tateno M, Toyooka M, Shikano Y, Takeda S, Kuwabara N, Sezutsu H, Tamura T. Production and characterization of the recombinant human μ-opioid receptor from transgenic silkworms. J Biochem. 2009;145:37–42. doi: 10.1093/jb/mvn147. [DOI] [PubMed] [Google Scholar]
- Tomita M, Munetsuna H, Sato T, Adachi T, Hino R, Hayashi M, Shimizu NN, Tamura T, Yoshizato K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol. 2003;21:52–56. doi: 10.1038/nbt771. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu X, Liu G, Cao C, Huang H, Xu Z, Liu J. Expression and porcine lactoferrin by using recombinant baculovirus in silkworm, Bombyx mori L., and its purification and characterization. Appl Microbiol Biotechnol. 2005;69:385–389. doi: 10.1007/s00253-005-1998-y. [DOI] [PubMed] [Google Scholar]
- Wei WL, Qin JC, Sun MJ. High-level expression of human butyrylcholinesterase gene in Bombyx mori and biochemical–pharmacological characteristic study of its product. Biochem Pharmacol. 2000;60:121–126. doi: 10.1016/S0006-2952(00)00238-0. [DOI] [PubMed] [Google Scholar]
- Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Xia Q, Zhou Z, Lu C, Cheng T, Dai F, Li B, Zhao P, Zha X, Cheng T, Chai C, Pan G, Xu J, Liu C, Lin Y, Qian J, Hou Y, Wu Z, Li G, Pan M, Li C, Shen Y, Lan X, Yuan L, Li T, Xu H, Yang G, Wan Y, Zhu Y, Yu M, Shen W, Wu D, Xiang Z, Yu J, Wang J, Li R, Shi J, Li H, Li G, Su J, Wang X, Li G, Zhang Z, Wu Q, Li J, Zhang Q, Wei N, Xu J, Sun H, Dong L, Liu D, Zhao S, Zhao X, Meng Q, Lan F, Huang X, Li Y, Fang L, Li C, Li D, Sun Y, Zhang Z, Yang Z, Huang Y, Xi Y, Qi Q, He D, Huang H, Zhang X, Wang Z, Li W, Cao Y, Yu Y, Yu H, Li J, Ye J, Chen H, Zhou Y, Liu B, Wang J, Ye J, Ji H, Li S, Ni P, Zhang J, Zhang Y, Zheng H, Mao B, Wang W, Ye C, Li S, Wang J, Wong GKS, Yang H. A draft sequence for the genome of the domesticated silkworm (Bombyx mori) Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- Xia Q, Cheng D, Duan J, Wang G, Cheng T, Zha X, Liu C, Zhao P, Dai F, Zhang Z, He N, Zhang L, Xiang Z. Microarray-based gene expression profile in multiple tissue of the domesticated silkworm Bombyx mori. Genome Biol. 2007;8:R162. doi: 10.1186/gb-2007-8-8-r162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Suzuki Y, Suzuki T, Li MQ, Nidom CA, Sakai-Tagawa Y, Muramoto Y, Ito M, Kiso M, Hiromoto T, Shinya K, Sawada T, Kiso M, Usui T, Murata T, Lin Y, Hay A, Haire LF, Stevens DJ, Russell RJ, Gamblin SJ, Skehel JJ, Kawaoka Y. Hemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature. 2006;444:378–382. doi: 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamao M, Nishiyama H, Sugihara S, Nagaoka S, Tomita M, Yoshizato K, Tamura T, Mori H. New and highly efficient method for silkworm transgenesis using Autographa californica nucleopolyhedrovirus and piggyBac transposable elements. Biotechnol Bioeng. 2004;88:849–853. doi: 10.1002/bit.20296. [DOI] [PubMed] [Google Scholar]
- Yamao M, Katayama N, Nakazawa H, Yamakawa M, Hayashi Y, Hara S, Kamei K, Mori H. Gene targeting in the silkworm by use of a baculovirus. Genes Dev. 1999;13:511–516. doi: 10.1101/gad.13.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Miao Y, Li X, Wu X, Zhao A, Nakagaki M. Cloning and expression of manganese superoxide dismutase of the silkworm, Bombyx mori by Bac-to-Bac/BmNPV baculovirus expression system. Appl Microbiol Biotechnol. 2006;73:181–186. doi: 10.1007/s00253-006-0462-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hu J, Miao Y, Zhao A, Zhao T, Wu D, Liang L, Miikura A, Shiomi K, Kajiura Z, Nakagaki M. Expression of EGFP-spider dragline silk fusion protein in BmN cells and larvae of silkworm showed the solubility is primary limit for dragline protein yield. Mol Biol Rep. 2008;35:329–335. doi: 10.1007/s11033-007-9090-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xia Q, Xu J, Chen J, Nie Z, Wang D, Zhang W, Chen J, Zheng Q, Chen Q, Kong L, Ren X, Wang J, Lv Z, Yu Wei, Jiang C, Liu, L, Sheng Q, Jin Y, Wu X (2009) Aligning the proteome and genome of the silkworm, Bombyx mori. Funct Inter Genomics. doi:10.1007/s10142-009-0127-x [DOI] [PubMed]