Abstract
Rat umbilical cord matrix (RUCM) cells are stem-cell-like cells and have been shown to reduce neuronal loss in the selectively vulnerable brain regions after cardiac arrest (CA). Here, we investigate whether this protection is mediated by the RUCM cells’ modulation of the post-ischemia inflammation responses, which have long been implicated as a secondary mechanism of injury following ischemia. Brain sections were examined immunohistochemically for GFAP, vimentin, and nestin as markers for astroglia and reactive astrogliosis, Ricinus Communis Agglutinin-1 (RCA-1) as a marker for microglia, and Ki67 as a marker for cell proliferation. Rats were randomly assigned to six experimental groups: (1) 8-min CA without treatment, (2) 8-min CA pretreated with culture medium injection, (3) 8-min CA pretreated with RUCM cells, (4) sham-operated CA, (5) medium injection without CA, and (6) RUCM cell transplantation without CA. Groups 1–3 have significantly higher Ki67+ cell counts and higher GFAP+ immunoreactivity in the hippocampal CA1 region compared to groups 4–6, irrespective of treatment. Groups 1 and 2 have highly elevated GFAP+, vimentin+, and nestin+ immunoreactivity, indicating reactive astrogliosis. Strikingly, RUCM cell treatment nearly completely inhibited the appearance of vimentin+ and greatly reduced nestin+ reactive astrocytes. RUCM cell treatment also greatly reduced RCA-1 expression, which is found to strongly correlate with the neuronal loss in the CA1 region. Our study indicates that treatment with stem-cell-like RUCM cells modulates the inflammatory response to global ischemia and renders neuronal protection by preventing permanent damage to the selectively vulnerable astrocytes in the CA1 region.
INTRODUCTION
Cerebral global ischemia secondary to cardiac arrest (CA) causes oxygen and glucose deprivation in the brain, leading to brain damage or death unless cerebral blood flow is restored immediately. Ischemic brain injury is characterized by a delayed selective loss of vulnerable CA1 pyramidal neurons in the hippocampus. Post ischemic inflammatory response is thought to be a major contributor to the delayed neuronal loss following ischemia 1, 2 and involves both reactive astrocytosis and microgliosis in and around the damaged area of the brain 3, 4.
The process of reactive astrocytosis consists of hyperplasia and hypertrophy of astrocytes along with the concomitant up-regulation of intermediate filaments such as glial fibrillary acidic protein (GFAP), vimentin (VIM), and the re-expression of nestin (NES) 5. Acutely reactive astrocytosis appears to be neuroprotective 6. At later time points, however, the reactive astrocytosis and the resulting glial scarring have been shown to cause a physical and biochemical barrier to axonal regeneration and synaptogenesis, hindering neuronal repair and recovery 7.
The process of microgliosis involves the activation of resident microglia along with the recruitment of peripheral macrophages in and around the ischemia-damaged areas where these cells serve as scavengers for clearing the cellular debris. They can also secrete a variety of cytotoxic and protective chemicals 8. A number of studies, with treatment strategies aimed at reducing microglial activation, have shown to reduce neuronal damages after the treatment 9–12. On the contrary, others have shown that microglia may actually play a protective role after ischemia 13–15.
We have recently demonstrated that transplantation of rat umbilical-cord matrix (RUCM) cells can provide partial protection against neuronal injury after global ischemia 16. These cells, derived from the Wharton’s jelly of the umbilical cord, are primitive stem-cell-like cells 17. They are positive for several important stem cell markers, particularly Oct-4, can be expanded far beyond the Hayflick limit in culture, and can be induced to differentiate into several cell types 17, 18. Rats treated with RUCM cells three days prior to an 8-min CA had only 25–32% neuronal loss in the hippocampal CA1 region compared to the typical 50–68% neuronal loss observed in the untreated or the vehicle-treated animals. Transdifferentiation and stem cell fusion with the host cells were ruled out as the predominating mechanisms for the protection. Other mechanisms, including extracellular signaling, were suggested. Given the fact that inflammatory responses may play a defining role in the delayed neuronal damage, this study aims at examining the mitigating effects of the stem-cell-like RUCM cells on the post-ischemia inflammatory response as a possible mechanism of neuroprotection.
MATERIAL AND METHODS
Animal Groups
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Paraffin-embedded brain tissues, randomly selected from four experimental groups in the previously reported studies 16 and two additional groups added for this investigation, were combined and examined. The six experimental groups are: (1) 8-min cardiac arrest alone (CA only, n = 6); (2) microinjection of the defined media (DM) at four unilateral locations, as detailed previously 16, followed three days later by an 8-min cardiac arrest (DM + CA, n = 7); (3) transplantation of stem-cell-like RUCM cells at the same four unilateral locations followed three days later by an 8-min cardiac arrest (RUCM + CA, n = 6); (4) sham-operated cardiac arrest without pretreatment (sham, n = 4), (5) microinjection of defined media unilaterally at the same four locations without cardiac arrest (DM only, n = 4), and (6) transplantation of RUCM unilaterally at the same four locations without cardiac arrest (RUCM only, n = 5). The DM is composed of the Dulbecco modified Eagle's medium (Invitrogen, Carlsbad, CA) and MCDB-201 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 1× insulin-transferrin-selenium (Invitrogen), 2% fetal bovine serum (BD Biosciences, San Jose, CA), 0.1 nM dexamethasone (Sigma-Aldrich), 0.15% lipid-rich bovine serum albumin (Albumax from Invitrogen), 10 µM ascorbic acid-2-phosphate (Sigma-Aldrich), 1× penicillin/streptomycin (Thermo-Fisher, Suwanee, GA).
Cardiac Arrest and Resuscitation
CA and resuscitation were performed as described before 16, 19–22. Briefly, male Sprague-Dawley rats weighing 234 ± 27 g (Harlan Sprague Dawley, Inc., Indianapolis, IN) were subjected to a clinically relevant outcome model of CA. Under isoflurane anesthesia, rats were intubated orotracheally, mechanically ventilated, and paralyzed with pancuronium bromide (2 mg/kg). CA was induced by an i.v. bolus injection of an ultra-short-acting β1-blocker, esmolol (6.25 mg), along with the stoppage of mechanical ventilation. Resuscitation was started either immediately (sham) or after 8 minutes by 100% O2 ventilation along with a retrograde infusion of oxygenated blood mixed with resuscitation mixture containing heparin (5 U/ml), sodium bicarbonate (0.05 mEq/ml), and epinephrine (8 µg/ml).
RUCM cell transplantation
As characterized in detail previously 16, RUCM cells were cultured and confirmed to be Oct-4 positive. Multiple lines of experimental evidence, including immunohistochemistry, flow cytometry, transcript analysis, and karotype stability beyond Hayflick limit, support the notion that these cells are stem-cell-like cells (meaning that they show some characteristics of stem cells). Three days prior to CA, approximately 4 × 104 RUCM cells in 10 µL (2.5 µL at each site) were injected at the following four sites in the left hemisphere: dorsal thalamic nucleus, dorsal hippocampus, corpus callosum (CC), and dorsal cortex. As controls, defined medium was injected in the exact same manner at the same four locations.
Tissue Preparation
Ten days after RUCM cell or DM injection and seven days after CA and resuscitation, rats were anesthetized with isoflurane and perfused with buffered 10% formalin phosphate. The brain was extracted from the skull and stored in buffered 10% formalin for 48 hours. The brain section containing the dorsal hippocampal region was embedded in paraffin and sliced into 6-µm-thick coronal sections using an American Optical model 820 microtome. Approximently 40 sections were collected from the region between −3.5 and −3.75 mm from the bregma. Two sections spaced apart by a section were randomly selected for each type of staining. Another two sections, approximately 15 sections posterior to the first two, were also selected for each staining.
Immunostaining
Sections were deparaffinized, rehydrated, and then boiled in 10 mM sodium citrate (pH = 6.0) for antigen retrieval. Standard immunohistochemistry techniques were used. The primary antibodies were incubated overnight at 4°C using mouse anti-GFAP (1:200) from Neuromics (Edina, MN); rabbit anti-vimentin (1:200), rabbit anti-Ki67 (1:100), and goat anti-Iba1 (1:50) from Abcam (Cambridge, MA); and biotinylated RCA-1 (1:2000) from Vector Laboratories (Burlingame, CA). Secondary antibodies used in the study were Alexa Fluor 594 goat anti-mouse IgG2a (1:500), Alexa Fluor 488 goat anti-mouse IgG1 (1:500), and Alexa Fluor 488 goat anti-rabbit IgG (1:500) from Invitrogen; Cy3 goat anti-rabbit (1:250) from Jackson Immunoresearch (West Grove, PA); and biotinylated goat anti-rabbit (1:500) from Dako (Glostrup, Denmark). For diaminobenzidine (DAB) staining, sections were incubated with ExtrAvidin peroxidase (HRP) conjugate (1:1,000; Sigma-Aldrich). Tissues labeled with HRP were developed with a solution of 0.67 mg/ml DAB (Sigma-Aldrich) and 0.13 µl of 30% H2O2 per mL of PBS 23. DAB-stained slides were then dehydrated and cleared in xylene and coverslipped with Eukitt mounting medium (EMS, Hatfield, PA). For fluorescence imaging, nuclear DNA was stained with 4’-6-diamidino-2-phenylindole (DAPI) 1 µg/mL in PBS (Sigma-Aldrich) and coverslipped using Fluoromount-G (Southern Biotech, Birmingham, AL).
Images were acquired using the In Vivo 3.2 software (Media Cybernetics, Bethesda, MD) driving an Olympus IX81 microscope (Tokyo, Japan) with a Prior motorized stage, a Sutter Lambda xenon exciter light source, an Olympus disc-scanning unit (DSU), and captured with an ORCA-ER (Hamamatsu, Japan) digital camera. All images were post-processed and analyzed in a blinded manner (i.e., investigators were unaware of the group identities) using Image-Pro AMS (Media Cybernetics). Percent positive area stained for GFAP, NES, and RCA-1 in the CA1 regions were analyzed by thresholding segmentation. The CA1 regions of interest were defined by closely outlining the cell layer as determined by using the DAPI nuclear staining (see online supplementary materials). The same regions used for determining the percent positive areas were used for cell counts. GFAP+ cell density was also calculated by counting all GFAP+ cell soma (defined as an area of GFAP+ immunoreactivity clearly in association with a nuclear stain, see online supplementary materials) in the image plane of the CA1 pyramidal cell layer, normalized by the area within which the counting was performed. Vimentin was analyzed by counting VIM+ cells within the CA1 region of the hippocampus. Ki67 was analyzed by counting Ki67+cells in four pre-determined, non-overlapping, equally spaced, 0.15 mm2 circles on each side of the hippocampus.
Data Analysis
Statistical analysis was performed using SigmaStat software (Systat Software, Inc, San Jose, CA) and GraphPad PRISM (GraphPad Software, Inc., San Diego, CA). Three-way ANOVA was performed using brain side (injected and contralateral), cardiac arrest (with and without), and treatment type (none, DM, or RUCM) as group identifiers (categorical factors). When a significant result was detected with ANOVA, a post hoc Holm-Sidak test was used for pair-wise comparisons. The results are reported as mean ± SEM, and a p value of < 0.05 was considered significant.
RESULTS
Glial fibrillary acidic protein immunostaining
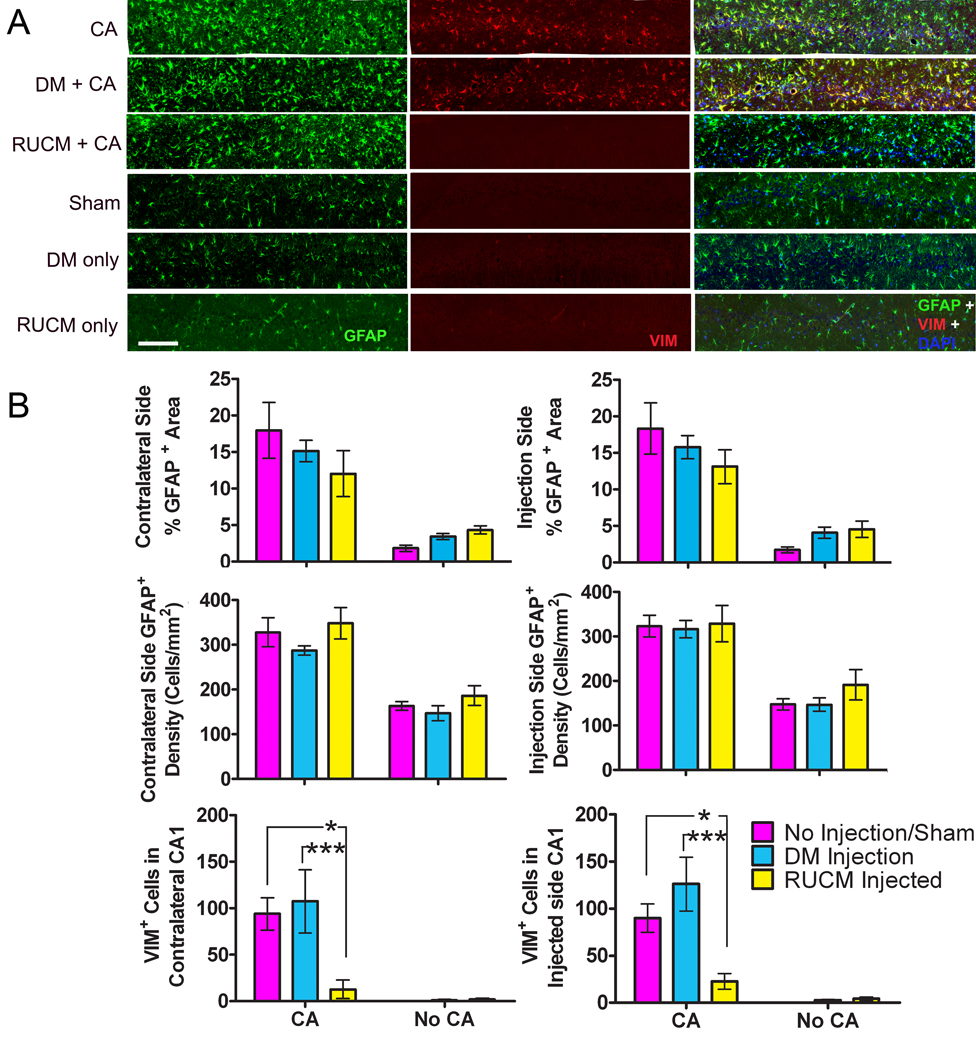
Upregulation of the type 3 intermediate filament protein GFAP along with hyperplasia and hypertrophy of astrocytes are the hallmarks of astrocytosis in response to insults on the central nervous system 24. Fluorescent immunostaining for GFAP was used to examine if pretreatment with stem-cell-like RUCM cells had any effect on astrocytosis in response to an 8-min cardiac arrest. Immunostaining revealed a significant increase in GFAP staining in the CA1 region of the hippocampus for all animals subjected to 8 min of cardiac arrest (Figure 1). At higher magnification, this increase appeared to be due to an increase both in the number of astrocytes and in the thickness as well as the number of GFAP+ processes, commonly seen with reactive astrogliosis (Figure 2). Semi-quantitative analysis of the percent GFAP+ area and semi-quantitative cell counting in the CA1 region supported our initial observation, revealing that the contralateral and ipsilateral (injection) sides of the CA1 regions had, respectively, 17.9 ± 3.8 % and 18.3 ± 3.5 %, and 328 ± 33 and 323 ± 24 cells/mm2 for Group 1 (CA only); 15.2 ± 1.5 % and 15.8 ± 1.6 %, and 287 ± 10 and 317 ± 19 cells/mm2 for Group 2 (DM +CA); 12.0 ± 3.2 % and 13.1 ± 2.3 %, and 348 ± 35 and 329 ± 41 cells/mm2 for Group 3 (RUCM + CA); 1.8 ± 0.4 % and 1.7 ± 0.4 %, and 163 ± 10 and 147 ± 13 cells/mm2 for Group 4 (sham); 3.4 ± 0.4 % and 4.1 ± 0.8 %, 147 ± 17 and 147 ± 15 cells/mm2 for Group 5 (DM only); and 4.3 ± 0.6 % and 4.5 ± 1.1 %, and 186 ± 22 and 192 ± 34 cells/mm2 for Group 6 (RUCM only). On average, the percent GFAP+ areas are about 3–4 times higher in the CA groups than in the non-CA groups, whereas the GFAP+ cell densities increased only by a factor of ~2 in the CA groups compared to the non-CA groups (Figure 1). This suggests that the additional percentage increases in the positive GFAP staining areas are due to thicker and more numerous astrocytic processes.
Figure 1.
(A) Representative hippocampal CA1 sections immunochemically stained for both GFAP and VIM immunoactivities. Horizontal bar represents 150 µm. (B) Bar graphs summarizing the percent GFAP+ areas, GFAP+ cells densities, and VIM+ cell counts in the contralateral and injection sides, averaged for each of the six experimental groups (see text for details). Pink, cyan, and yellow bars represent no pretreatment (or sham), DM injection, and RUCM cell transplantation, respectively. Notice the pronounced reduction in the appearance of VIM+ astrocytes after 8-min cardiac arrest and 7-day recovery in the animals treated with stem-cell-like RUCM cells. *, p < 0.05; **, p < 0.01.
Figure 2.
GFAP (Ggreen) staining at the 20× magnification showing increased staining within the hippocampal CA1 regions for all experimental groups with 8-min cardiac arrest. Notice the thicker and more numerous astrocytic processes. Blue color shows the nuclear stain for DAPI.
Vimentin immunostaining
Vimentin is another type 3 intermediate filament protein that is usually upregulated during reactive astrocytosis 24. Vimentin is also expressed in radial glia and neural precursor cells. In our study, animals not subjected to cardiac arrest (Groups 4–6) appear to have little VIM staining; the trace amount appears to be associated with the injection needle tract in the CA1 region on the injection side. Three-way ANOVA showed that cardiac arrest (P < 0.001), and different injection treatments (no injection, DM injection, and RUCM cell transplantation; P = 0.002) as categorical factors were significant sources of variation. Brain side was found not to be a significant source of variation (P = 0.683). A significant interaction between cardiac arrest and injection treatment was detected (P = 0.001). Holm-Sidak post hoc comparisons revealed that among the animals subjected to 8-min CA, Group 1 (CA only) was significantly different from Group 3 (RUCM + CA) (P < 0.05), and Group 2 (DM + CA) was also significantly different from Group 3 (P < 0.001). No differences were detected between Groups 1 and 2, or among Groups 4 (Sham), 5 (DM only), and 6 (RUCM only). In Group 1 (CA only), the VIM+ cell counts are 90 ± 15 and 94 ± 17 per CA1 section on the injection side and contralateral side, respectively. In Group 2 (DM + CA), the range of VIM+ cell counts is not significantly different from Group 1, being 126 ± 28 and 107 ± 34 on the injection side and contralateral side, respectively. While the total number of VIM+ cells is significantly less than the number GFAP+ cells in the same CA1 area, all VIM+ cells in the CA1 region seem to always co-localize with GFAP+ cells (Figure 1). The most striking and interesting finding is that the animals in Group 3, which received stem-cell-like RUCM cell transplantation 3 days before cardiac arrest, show a greatly reduced amount of VIM+ cells in the hippocampal CA1 regions. On the injection side, CA1 VIM+ cell counts for Group 3 ranged from 7 to 62 (23 ± 8). Most of these cells appeared to associate with the damage along the needle tract. On the contralateral side, two animals in Group 3 has no detectable VIM+ cells in the CA1 region, three had counts ranging from 2 to 6, and only one animal had a count of 13 ±10 on the different sections analyzed. All VIM+ cells had the typical star shape associated with astrocytes (data not shown).
Nestin immunostaining
Nestin is a type 4 intermediate filament that is usually expressed in neural precursors and can also be reactivated in the reactive astrocytes. We observed from our immunostaining that Group 1 (CA only) and Group 2 (DM + CA) had high levels of NES+ immunoreactivity on both the contralateral (Figure 3) and the injection sides in the hippocampal CA1 regions. Again, cardiac arrest and injection treatment as categorical factors were found to be a significant sources of variation (P < 0.001, and P = 0.012 respectively). A significant interaction between CA and injection treatment was detected (P < 0.01). Brain side was not a source of significant variation (P = 0.93). Post hoc analysis revealed significant differences between Groups 2 (DM + CA) and 3 (RUCM + CA) (P = 0.003). The percentage of positive NES staining area in the CA1 region in Group 1 (CA only) is 3.8 ± 1.0 % and 4.4 ± 1.1 % on the contralateral and injection side, respectively, and 6.7 ± 2.11 % and 7.1 ± 1.8 %, respectively, in Group 2 (DM + CA). In Group 3 with RUCM cell treatment, animals had significantly less NES+ immunoreactivity (2.1 ± 0.8 % on the contralateral side and 1.5 ± 0.7 % on the injection side). The NES+ cells have the typical star-like morphology (data not shown).
Figure 3.
(A) Representative CA1 sections stained for Nestin. Astrocytes show Nestin+ expression in rats that were subjected to 8-min of cardiac arrest. Photomicrographs were obtained at 10× magnification. Bar represents 150 µm. (B) Bar graphs summarizing the percent Nestin+ areas in the contralateral and injection sides averaged for each of the six experimental groups. Pink, cyan, and yellow bars represent no pretreatment (or sham), DM injection, and RUCM cell transplantation, respectively. Treatment with stem-cell-like RUCM cells 3 days before cardiac arrest greatly reduced the amount of nestin+ astrocytes in the CA1 region of the hippocampus. ***, P = 0.003.
Microglia Response in the CA1
Microglia are the key regulators of the inflammatory response in the central nervous system. In response to insults and injuries, microglia become activated and change their morphology and function from a resting ramified state to an activated phagocytic state. We used a lectin stain (Ricinus Communis Agglutinin 1, RCA-1) to visualize the microglia response in the CA1 region of the hippocampus in our study. The animals not subjected to 8-min cardiac arrest (Groups 4–6) were virtually devoid of RCA-1+ staining (Figure 4). Group 1 (CA only) and Group 2 (DM + CA) had numerous RCA-1+ cells dispersed throughout the CA1 regions with the morphology of the activated, phagocytic type. Subjecting the animals to 8-min CA was a significant source of variation (P < 0.001), so were the different injection treatments (P < 0.001). Brain side was again not a significant source of variation (P = 0.883). The interaction between the injection treatment and CA was highly significant (P < 0.001). Semi-quantitative analysis revealed that Group 1 had 17.8 ± 0.9 % and 16.0 ± 1.2 % RCA-1+ areas for the injection and contralateral CA1 regions, respectively. Group 2 had 14.0 ± 2.1 % and 16.1 ± 1.8 % RCA-1+ areas, respectively. Group 3 appeared to have less RCA-1+ immunoactivity that was distributed in tight clusters in the CA1 region (7.1 ± 2.0 % and 6.1 ± 1.5 % RCA-1+ areas in the injection and contralateral CA1 regions, respectively) and are significantly different from both Groups 1 and 2 (P < 0.001, Holm-Sidak post hoc test between Groups 1 and 3 or Groups 2 and 3). Direct counting of microglia proves to be difficult because even at high magnifications it is nearly impossible to determine where one cell ends and another begins especially where they form compound granular corpuscles.
Figure 4.
(A) Representative CA1 sections stained for microglia using RCA-1. Microglia appear as darkly stained cells while neurons appear as white spots. All rats subjected to 8-min cardiac arrest show the appearance of microglia. Photomicrographs were obtained at 10× magnification. Bar represents 150 µm. (B) Bar graphs summarizing the percent RCA-1+ areas in the contralateral and injection sides, averaged for each of the six experimental groups. Pink, cyan, and yellow bars represent no pretreatment (or sham), DM injection, and RUCM cell transplantation, respectively. Pretreatment with stem-cell-like RUCM cells reduces the RCA-1+ area and changes their distribution. ***, P < 0.001.
Cell Proliferation
Proliferation of microglia and astrocytes is another hallmark of the inflammatory response to ischemia in the brain. Ki67 is a nuclear protein found in cells that are in all phases of the active cell cycle. We used Ki67 immunohistochemistry to examine if stem-cell-like RUCM cell treatment has a measurable effect on the increase in cell proliferation associated with the inflammatory response to ischemia. We observed an increase in the number of Ki67+ cells in the CA1 region for all 8-min CA groups (Figure 5). Having or not having a cardiac arrest was found to be the major source of variation (P < 0.001). There were no other significant sources of variation or interactions detected..
Figure 5.
(A) Representative sections in the CA1 region of the hippocampus stained for Ki67. Photomicrographs were obtained at 10× magnification. The scale bar represents 150 µm. (B) Bar graphs summarizing the Ki67+ cell counts in the contralateral and injection sides, averaged for each of the six experimental groups. Pink, cyan, and yellow bars represent no pretreatment (or sham), DM injection, and RUCM cell transplantation, respectively. Eight minute cardiac arrest, irrespective of treatment, increases the number of Ki67+ cells.
To determine what cell types were involved in proliferation, we performed double immunolabeling studies for Ki67 along with GFAP or the microglia marker Iba-1 in Group 2 (DM + CA) and Group 3 (RUCM + CA). High-magnification DSU confocal microscopy revealed that both microglia (Figure 6A) and astrocytes (Figure 6B) were involved in the proliferation. Semi-quantitative analysis showed no significant differences between Groups 2 and 3 for the proportions of Ki67+cells that were microglia or astrocytes (data not shown).
Figure 6.
High-magnification (60×), maximum-intensity projection of 6-µm-thick image stacks showing co-localization of (A) IBA-1+ microglia (red) expressing Ki67 (green) and (B) GFAP+ astrocytes (green) expressing Ki67 (red) in the CA1 region of the rat hippocampus after 8-min cardiac arrest and 7-day reperfusion.
DISCUSSION
Our study used a pretreatment strategy to examine possible mechanisms of RUCM cell-mediated neuronal protection against global cerebral ischemia, allowing us to test the hypothesis that the transplanted cells can become activated by acute ischemia and improve the global histological outcome without evoking transdifferentiation or cell fusion as the predominant modes of protection. Although posttreatment is currently the most desirable modality in many clinical situations, pretreatment strategies are also highly clinically relevant, especially considering the rapid advancement in early diagnosis and preventative medicine and the development of state-of-the-art surgical interventions. For example, in a number of advanced surgical procedures, such as implantation of automatic internal defibrillators, pediatric surgeries for repairing complex congenital malformations25, adult cardiac surgeries involving the aortic arch 26, 27, and neurosurgeries for the repair of intracranial aneurysms 23, 28, controlled circulatory arrests resulting in cerebral global ischemia are often necessary. Developing pretreatment strategies aimed at reducing neurological complications is essential for the future advancement of these and other novel medical procedures.
The 8-min global cerebral ischemia performed in this experiment resulted in a 51.7 ± 8.7% (injection side) and 50.2 ± 7.8 % (contralateral side) loss of CA1 neurons in Group 1 (CA only), and a 70.4 ± 6.9% (injection side) and 62.1 ± 8.2 % (contralateral side) loss of CA1 neurons in Group 2 (DM + CA) 16. Pretreatment with stem-cell-like RUCM cells (Group 3) led to significant protection, cutting neuronal loss by at least 50% to 33.8 ± 2.7 % (injection side) and 24.3 ± 4.2 % (contralateral side).
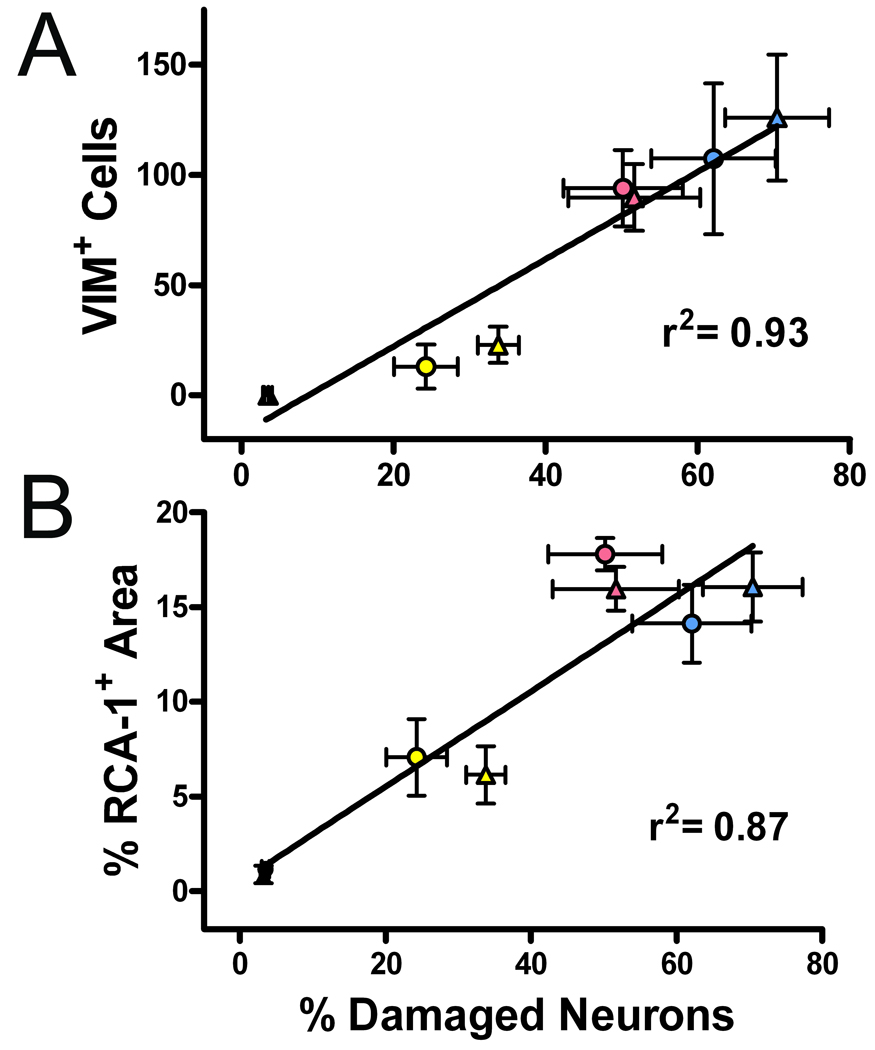
Examination of the brain tissues revealed similar results for the degree of GFAP+ reactive astrocytes and the amount of cell proliferation (as determined by Ki67 staining) for all 8-min CA groups (Groups 1–3). Although the neuronal loss from the global cerebral ischemia is not completely prevented, our study indicates that treatment with stem-cell-like RUCM cells greatly reduces the appearance of the activated microglia and the VIM+/NES+ reactive astrocytes in the hippocampal CA1 region in these rats. These reductions are strongly correlated with the reduction of neuronal loss in the same region. As shown in Figure 7, both VIM+ cell counts and percent RCA-1+ area are correlated with the percentage of damaged pyramidal neurons in the CA1 region, with correlation coefficients of 0.93 and 0.87, respectively.
Figure 7.
Correlations between percentage of the hippocampal CA1 neuronal loss and (A) VIM+ cells or (B) activated microglia. Data are taken from the within-group averages from the contralateral (●) and injection (▲) sides of the six experimental groups as detailed in the text.
Previous reports have shown that increases in GFAP correlates well with neuronal death following injury 29, 30. More recent studies have suggested the possibility that the increases in GFAP+ astrocytic hypertrophy might be an astroglia reaction to ischemia and can actually have neuroprotective functions 31–33. Astrocytes can play a number of protective roles after ischemia, including the uptake and transport of glutamate and glucose, repair of the blood brain barrier, and the production and release of neurotrophic factors 34, 35. Several studies demonstrating protection against ischemia-induced injuries by compounds targeting the inflammatory response to ischemia also show increases in GFAP immunostaining along with reductions in neuronal damage and microglia activation 11, 12, 36, 37. We observed similar GFAP immunoreactivity increases associated with the 8-min cardiac arrest. A lack of the concomitant increases in VIM and NES in animals pretreated with stem-cell-like RUCM cells could in fact suggest that RUCM cells might have immunomodulatory properties, thereby altering the resultant astrogliotic response to CA. Anti-inflammatory effects due to intravenous infusion of umbilical-cord-blood-derived cells after ischemia have been demonstrated recently 38. It is possible that umbilical-cord-matrix-derived cells have similar properties.
The lack of astroglial expression of VIM and significant reduction of NES in the RUCM cell-treated group may just be a sign of no or little permanent astroglial damage in these animals. It has been suggested 3 that the VIM+ astroglia, but not GFAP+ ones, correlate with permanent injury after ischemia. The re-expression of VIM and NES in reactive astrocytes is most commonly associated with a wide variety of brain injuries and always seen in the injured areas 3, 5, 29, 30, 39, 40. The protection of astrocytes from permanent damage by stem-cell-like RUCM cells is consistent with and in support of the recent proposal 41 that the selective vulnerability of CA1 neurons is due to the selective vulnerability of the CA1 astrocytes. Stem-cell-like-cell protection of astroglial cells leads to the reduced long-term neuronal loss in the CA1 region.
Despite the strong correlations seen in Figure 7, the causal relationship between the presence of VIM re-expression and neural injury is, however, not clear. It has been suggested that VIM+ astrocytes found in and around the injury are associated with hyperplasia and are representative of the newly formed immature astrocytes. Our data do not support this hypothesis because in the RUCM + CA group, while VIM+ cells are almost absent, we do observe many GFAP+cells co-expressing Ki67 (Figure 6B), suggesting that these cells are either newly divided or in the process of division.
A recent study 4 suggested that VIM+ astrocytes appeared to be correlated more with the cell migration than with cell proliferation. Reactive astrocytes expressing nestin have also been implicated in migration42. Re-expression of VIM in mature astrocytes may impart similar functions to them as radial glia 43. Radial glia not only function as neural progenitors but also offer a scaffold to guide and support migrating neuroblasts. Supporting this is the observation 44 that the newly divided (BrdU+) progenitor cells were always found to be in close association with VIM+ astrocytes, but not co-localized with them. The VIM expression observed in Group 1 (CA only) and Group 2 (DM + CA) may be guiding the migration of neural precursors or microglia and macrophages to the site of lesion, and the stem-cell-like RUCM cell treatment (Group 3) prevents the damage or inflammation, thereby suppressing the signals for the cell migration. Our RCA-1 staining seems to support this conclusion.
Another possible explanation for the lack of VIM+ and reduction in NES+ reactive astrocytes is that the stem-cell-like RUCM cells release messenger molecules that inhibit the formation of glial scars. The Wharton’s jelly, from which RUCM cells are isolated, has been shown to contain high concentrations of a wide variety of growth factors and cytokines 45. VIM+ and NES+ reactive astrocytes are almost always reported to be in and around areas of glial scarring, and it has been demonstrated that glial scarring is an impediment to axonal regeneration post-injury 7, 46, 47. The glial scar formation has been shown to be reduced or ablated when the expression of VIM is reduced by anti-sense expression or VIM and GFAP are eliminated in knock-out mice 48, 49. It has also been shown 50 that the glial scar formation is modulated through the endothelin B receptor on reactive astrocytes. The expression of endotherlin B receptor in astrocytes has been shown to be dependent on both VIM and GFAP expression 51. Therefore, it is possible that preventing VIM expression can result in the inhibition of glial scar formation, leading to the regeneration of axons for the survival of the CA1 neurons.
CONCLUSION
It is clear from our GFAP immunostaining that treatment with stem-cell-like RUCM cells prior to cardiac arrest does not inhibit the reactive astrocytosis normally associated with cerebral ischemia. It does, however, prevent the appearance of VIM+ reactive astrocytes and reduce NES+ reactive astrocytes in the selectively vulnerable CA1 regions, suggesting the possibility that the stem-cell-like RUCM cells prevent permanent astroglial damage. The large reduction in microglial activation and altered distribution suggests that RUCM cell transplantation has immunomodulatory effects. Combined, stem-cell-like RUCM cells offer protection against neuronal injury after global cerebral ischemia by enhancing the survivability of the astroglia in the selectively vulnerable regions.
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to thank Dr. Kathy Mitchell for providing the RUCM cells and Mr. Marc Uy for early participation in the stem cell transplantation experiments. This work was supported in part by a grant from NINDS and NHLBI (R01NS/HL036124).
Footnotes
Author Contributions:
Aaron Hirko: Data collection, analysis, and intepretation; manuscript/figure preparation Renee Dallasen: Data collection; tissue preparation for histology; stem cell culturing Sachiko Jomura: Data collection; cardiac arrest and resuscutation; stem cell culturing Yan Xu: Conception and design; financial support; provision of study material; data interpretation; manuscript writing; final approval of manuscript
SUPPLEMENTAL INFORMATION
A supplemental figure is available online free of charge at http://stemcells.alphamedpress.org.
REFERENCES
- 1.Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke. Curr Opin Neurol. 2001;14:349–353. doi: 10.1097/00019052-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Petito CK, Morgello S, Felix JC, et al. The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metab. 1990;10:850–859. doi: 10.1038/jcbfm.1990.141. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 6.Pekny M, Johansson CB, Eliasson C, et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies SJ, Goucher DR, Doller C, et al. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood PL. Microglia as a unique cellular target in the treatment of stroke: potential neurotoxic mediators produced by activated microglia. Neurol Res. 1995;17:242–248. doi: 10.1080/01616412.1995.11740321. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Lin S, Fan LW, et al. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N, Komine-Kobayashi M, Tanaka R, et al. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36:2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- 11.Yrjanheikki J, Tikka T, Keinanen R, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe H, Abe H, Takeuchi S, et al. Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neuroscience letters. 2000;289:53–56. doi: 10.1016/s0304-3940(00)01252-0. [DOI] [PubMed] [Google Scholar]
- 14.Capone C, Frigerio S, Fumagalli S, et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE. 2007;2:e373. doi: 10.1371/journal.pone.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi Y, Tomimatsu Y, Suzuki H, et al. The intra-arterial injection of microglia protects hippocampal CA1 neurons against global ischemia-induced functional deficits in rats. Neuroscience. 2006;142:87–96. doi: 10.1016/j.neuroscience.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Jomura S, Uy M, Mitchell K, et al. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- 17.Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 19.Liachenko S, Tang P, Hamilton RL, et al. A reproducible model of circulatory arrest and remote resuscitation in rats for NMR investigation. Stroke. 1998;29:1229–1238. doi: 10.1161/01.str.29.6.1229. discussion 1238-1229. [DOI] [PubMed] [Google Scholar]
- 20.Liachenko S, Tang P, Hamilton RL, et al. Regional dependence of cerebral reperfusion after circulatory arrest in rats. J Cereb Blood Flow Metab. 2001;21:1320–1329. doi: 10.1097/00004647-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Liachenko S, Tang P, Xu Y. Deferoxamine improves early postresuscitation reperfusion after prolonged cardiac arrest in rats. J Cereb Blood Flow Metab. 2003;23:574–581. doi: 10.1097/01.WCB.0000057742.00152.3F. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Liachenko S, Tang P. Dependence of early cerebral reperfusion and long-term outcome on resuscitation efficiency after cardiac arrest in rats. Stroke. 2002;33:837–843. doi: 10.1161/hs0302.104198. [DOI] [PubMed] [Google Scholar]
- 23.Young WL, Lawton MT, Gupta DK, et al. Anesthetic management of deep hypothermic circulatory arrest for cerebral aneurysm clipping. Anesthesiology. 2002;96:497–503. doi: 10.1097/00000542-200202000-00038. [DOI] [PubMed] [Google Scholar]
- 24.Pekny M, Wilhelmsson U, Bogestal YR, et al. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- 25.Amir G, Ramamoorthy C, Riemer RK, et al. Neonatal brain protection and deep hypothermic circulatory arrest: pathophysiology of ischemic neuronal injury and protective strategies. Ann Thorac Surg. 2005;80:1955–1964. doi: 10.1016/j.athoracsur.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Augoustides JG, Pochettino A, Ochroch EA, et al. Clinical predictors for prolonged intensive care unit stay in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2006;20:8–13. doi: 10.1053/j.jvca.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Kleisli T, Raissi SS, Nissen NN, et al. Cavo-atrial tumor resection under total circulatory arrest without a sternotomy. Ann Thorac Surg. 2006;81:1887–1888. doi: 10.1016/j.athoracsur.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 28.Strebel S, Mendelowitsch A, Kindler C. Rupture of a giant intracranial aneurysm while starting cardiopulmonary bypass for hypothermic circulatory arrest. J Neurosurg Anesthesiol. 2004;16:263–265. doi: 10.1097/00008506-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Petito CK, Halaby IA. Relationship between ischemia and ischemic neuronal necrosis to astrocyte expression of glial fibrillary acidic protein. Int J Dev Neurosci. 1993;11:239–247. doi: 10.1016/0736-5748(93)90082-o. [DOI] [PubMed] [Google Scholar]
- 30.Oblinger MM, Singh LD. Reactive astrocytes in neonate brain upregulate intermediate filament gene expression in response to axonal injury. Int J Dev Neurosci. 1993;11:149–156. doi: 10.1016/0736-5748(93)90075-o. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara T, Lewen A, Noshita N, et al. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 32.Briones TL, Woods J, Wadowska M, et al. Astrocytic changes in the hippocampus and functional recovery after cerebral ischemia are facilitated by rehabilitation training. Behav Brain Res. 2006;171:17–25. doi: 10.1016/j.bbr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Beamer CA, Brooks DM, Lurie DI. Motheaten (me/me) mice deficient in SHP-1 are less susceptible to focal cerebral ischemia. J Neurosci Res. 2006;83:1220–1230. doi: 10.1002/jnr.20825. [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Cheng FC, Lu YZ, et al. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Exp Neurol. 2006;201:225–233. doi: 10.1016/j.expneurol.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 36.Pei Z, Cheung RT. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J Pineal Res. 2004;37:85–91. doi: 10.1111/j.1600-079X.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 37.Weng YC, Kriz J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp Neurol. 2007;204:433–442. doi: 10.1016/j.expneurol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Vendrame M, Gemma C, de Mesquita D, et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 39.Kitamura O. Immunohistochemical investigation of hypoxic/ischemic brain damage in forensic autopsy cases. Int J Legal Med. 1994;107:69–76. doi: 10.1007/BF01225492. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin SA, Scheff SW. Intermediate filament change in astrocytes following mild cortical contusion. Glia. 1996;16:266–275. doi: 10.1002/(SICI)1098-1136(199603)16:3<266::AID-GLIA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang YB, Voloboueva LA, Xu LJ, et al. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi H, Matsumoto H, Kumon Y, et al. Expression of heparanase in nestin-positive reactive astrocytes in ischemic lesions of rat brain after transient middle cerebral artery occlusion. Neuroscience letters. 2007;417:250–254. doi: 10.1016/j.neulet.2007.02.075. [DOI] [PubMed] [Google Scholar]
- 43.Leavitt BR, Hernit-Grant CS, Macklis JD. Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons. Experimental Neurology. 1999;157:43–57. doi: 10.1006/exnr.1999.6982. [DOI] [PubMed] [Google Scholar]
- 44.Alonso G. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia. 2001;34:253–266. doi: 10.1002/glia.1059. [DOI] [PubMed] [Google Scholar]
- 45.Sobolewski K, Malkowski A, Bankowski E, et al. Wharton's jelly as a reservoir of peptide growth factors. Placenta. 2005;26:747–752. doi: 10.1016/j.placenta.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Alonso G, Privat A. Reactive astrocytes involved in the formation of lesional scars differ in the mediobasal hypothalamus and in other forebrain regions. J Neurosci Res. 1993;34:523–538. doi: 10.1002/jnr.490340505. [DOI] [PubMed] [Google Scholar]
- 47.Cho KS, Yang L, Lu B, et al. Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci. 2005;118:863–872. doi: 10.1242/jcs.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin J, Cai W. Effect of vimentin on reactive gliosis: in vitro and in vivo analysis. J Neurotrauma. 2004;21:1671–1682. doi: 10.1089/neu.2004.21.1671. [DOI] [PubMed] [Google Scholar]
- 49.Ribotta MG, Menet V, Privat A. Glial scar and axonal regeneration in the CNS: lessons from GFAP and vimentin transgenic mice. Acta Neurochir Suppl. 2004;89:87–92. doi: 10.1007/978-3-7091-0603-7_12. [DOI] [PubMed] [Google Scholar]
- 50.Rogers SD, Peters CM, Pomonis JD, et al. Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia. 2003;41:180–190. doi: 10.1002/glia.10173. [DOI] [PubMed] [Google Scholar]
- 51.Wilhelmsson U, Li L, Pekna M, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.