Abstract
Objectives
Our series of studies using transplantation of single hematopoietic stem cells (HSCs) demonstrated that mouse fibroblasts/myofibroblasts are derived from HSCs. In order to determine the origin of human fibroblasts, we established a method for culturing fibroblasts from human peripheral blood (PB) mononuclear cells and studied fibroblasts from gender mismatch HSC transplant recipients and patients with untreated Philadelphia chromosome-positive chronic myelogenous leukemia (CML).
Methods
We cultured PB cells from three female subjects who showed near complete hematopoietic reconstitution from transplantation of granulocyte-colony stimulating factor (G-CSF)-mobilized male PB cells and examined the resulting fibroblasts using fluorescent in situ hybridization (FISH) for Y chromosome. Because the mobilized PB cells may contain mesenchymal stem cells (MSCs), we could not determine the HSC or MSC origin of the fibroblasts seen in culture. To further document the HSC origin of human fibroblasts, we next examined fibroblasts from two patients with untreated CML, a known clonal disorder of HSCs.
Results
All cultured fibroblasts from female recipients of male cells showed the presence of Y-chromosome, indicating the donor origin of fibroblasts. Cultured fibroblasts from the CML patients revealed the presence of BCR-ABL translocation. This demonstration provided strong evidence for the HSC origin of human fibroblasts, because CML is a clonal disorder of the HSC.
Conclusions
These studies strongly suggest that human fibroblasts are derived from HSCs. In addition, the results suggest that fibrosis seen in patients with CML may be a part of the clonal process.
Introduction
Fibroblasts are a major constituent of connective tissue. Not only do they maintain the integrity of connective tissues by producing extracellular matrix, but are also a key regulator of the microenvironment by controlling cell differentiation, proliferation, and migration through cytokine and chemokine production. Therefore, fibroblasts play many roles both in the maintenance of homeostasis and in the development of pathological conditions. Having contractile ability, fibroblasts are particularly important in the normal repair processes of tissue injury and inflammation. However, excessive fibrosis can result in a wide variety of diseases, including atherosclerosis, liver cirrhosis, pulmonary fibrosis, nephrosclerosis and scleroderma.
It is generally believed that fibroblasts, together with adipocytes, osteocytes and chondrocytes, are derived from mesenchymal stem cells (MSCs) in the bone marrow. Recently, our laboratory discovered that fibroblasts/myofibroblasts in many tissues and organs of mice are derived from hematopoietic stem cells (HSCs) [1]. Specifically, we used single HSC transplantation and found that mouse HSCs give rise to glomerular mesangial cells [2], brain microglial cells [3], inner ear fibrocytes [4], fibroblasts in heart valves [5] and tumor-associated fibroblasts [6]. We also documented the HSC origin of fibroblasts grown in culture using the bone marrow cells of mice having received single HSC transplantation [7]. Subsequently, investigators in other laboratories also using single HSC transplantation, described that hepatic stellate cells, a type of myofibroblast [8], and the myofibroblasts seen at the site of cardiac infarction [9] are derived from HSCs. These studies indicated that most, if not all, fibroblasts/myofibroblasts in mice are derived from HSCs and prompted the study of the origin of human fibroblasts described in the present study.
In our previous culture studies of fibroblasts from single HSC transplantation [7], we demonstrated that two known circulating fibroblast progenitors, i.e. fibroblast colony-forming units (CFU-F) [10] and fibrocytes [11] are derived from HSCs. By using a slight modification of the culture method for human fibroblasts from peripheral blood (PB) cells described by Bucala et al. [11], we investigated the fibroblasts cultured from PB from three female recipients of gender-mismatch transplantation. All fibroblasts examined revealed the presence of Y-chromosome, indicating that fibroblasts/myofibroblasts in these patients are of male donor origin. We then studied fibroblasts cultured from PB of untreated patients with Philadelphia chromosome (Ph1)-positive chronic myelogenous leukemia (CML). The (9;22) chromosomal translocation results in the fusion of BCR and C-ABL genes. Since this translocation is found in all hematopoietic lineages, CML has been classified as a stem cell disorder. Therefore, demonstration of the presence of the BCR-ABL fusion gene in all cultured fibroblasts from the patients unequivocally establishes the HSC origin of human fibroblasts.
Materials and Methods
Cell preparation and culture of fibroblasts
Cell culture of human fibroblasts from circulating fibrocytes was carried out using a modification of the method described by Bucala et al. [11] in that non-adherent mononuclear cells (MNCs) rather than the adherent cell fraction of PB cells were cultured. Ten to twenty milliliters of PB was obtained from healthy adult volunteers, three female patients transplanted with granulocyte-colony stimulating factor (G-CSF)-mobilized HSCs from male donors and two patients with untreated Ph1-positive CML. Table 1 describes the patients who provided the blood samples. MNCs were isolated from PB cells by density gradient centrifugation using Lympholyte H (Cedarlane Laboratories Limited, Ontario, Canada). The samples were suspended at a concentration of 2.5–5.0 × 105 cells/mL in media consisting of -modification of Eagle's medium (ICN Biomedicals, Aurora, OH, USA) and either 20% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA, USA) or a combination of 10% FBS and 10% human serum from AB type male (NABI, Miami, FL, USA) and then incubated in fibronectin-coated 4-well culture slides (Becton-Dickinson Biosciences, San Jose, CA, USA) at 37°C in a humidified atmosphere with 5% CO2 in air for 7–14 days.
Table 1.
Patient information
| BMT | Primary Diagnosis |
Day of sample collection after BMT |
% chimerism after BMT |
*WBC (/mL) |
*Hgb (gm/dL) |
*Plt (x103/mL) |
|---|---|---|---|---|---|---|
| 1 | MDS | day 39 | 100% (days 62 and 130) | 16,830 | 11.0 | 328 |
| 2 | MDS | day 271 | 99% (days 179 and 368) | 11,310 | 13.9 | 289 |
| 3 | AML | day 59 | 99.9% (day 41) 99.4% (day 100) | 8,310 | 13.6 | 163 |
| CML | ||||||
| 1 | CML | 27,430 | 11.9 | 210 | ||
| 2 | CML | 41,840 | 12.8 | 1,103 |
Values on the same day of fibroblast culture. BMT, bone marrow transplantation; MDS, myelodysplastic syndrome; AML, acute myelogenous leukemia; CML, chronic myelogenous eukemia; WBC, white blood cell count; Hgb, hemoglobin; Plt, platelet count.
Immunohistochemical analyses
Cultured cells were fixed with 4% paraformaldehyde, permeabilized with 0.02% Triton X-100/Ca2+-, Mg2+-free phosphate buffered saline (PBS) and blocked in 5% normal donkey serum (DS; Jackson ImmunoResearch Laboratories, West Grove, PA, USA)/3% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA)/PBS for 30 minutes. Cells were then incubated with either mouse monoclonal antibody to vimentin (Abcam, Cambridge, MA, USA), rabbit polyclonal antibody to -smooth muscle actin (SMA) (Abcam) or mouse monoclonal antibody to pro-collagen type I (Millipore, Bedford, MA) diluted in 5% DS/3% BSA/PBS for 45 minutes followed by Cyanine (Cy3)-conjugated anti-mouse or anti-rabbit IgG antibodies for 30 minutes (Jackson ImmunoResearch Laboratories) and stained with Hoechst 33342 (Sigma-Aldrich, St Louis, MO). For staining with mouse monoclonal antibody to fibroblast (5B5, against human prolyl-4-hydroxylase; Abcam), cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100/PBS and blocked in 8%BSA/PBS for 1 hour. Cells were then incubated with the primary antibody in PBS overnight followed by Cy3-conjugated anti-mouse antibody in PBS for 1 hour and finally stained with Hoechst 33342. Epifluorescence and DIC imaging was conducted using a Leica DMR microscope. Images were processed using Adobe Photoshop CS2 software (Adobe Systems, Inc., San Jose, CA, USA).
Flow Cytometric Analysis
Freshly isolated, uncultured PB MNCs and day-14 cultured fibroblasts grown from non-adherent PB MNCs were fixed with 4% paraformaldehyde for 15 minutes and washed with PBS/0.1%BSA. They were stained with fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD45, allophycocyanin (APC)-conjugated mouse anti-human CD11b/Mac-1or APC-conjugated mouse anti-human CD56 for 30 minutes at 4°C. All antibodies were purchased from BD Biosciences. Cells were washed twice and analyzed by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). To stain for pro-collagen-I, cells were prepared using the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) according to the manufacturer’s protocol. The cells were then stained with mouse anti-human pro-collagen-I monoclonal antibody (Millipore) for 30 minutes at 4°C, washed and stained with phycoerythrin (PE)-conjugated goat anti-mouse IgG (R & D Systems, Minneapolis, MN, USA) for 30 minutes at 4°C. The cells were then washed and analyzed by FACSCalibur.
RT-PCR
Total RNA was extracted from day-14 cultured fibroblasts, PB MNCs and passage 2 human foreskin fibroblast cell line (HFF-1, ATCC, Manassas, VA, USA) using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Two micrograms of total RNA were reverse transcribed using Superscript III single strand synthesis RT system (Invitrogen). Aliquots from this reaction were used for real-time PCR assays using the indicated gene-specific primers (Table 2) and Super Script platinum SYBER green mix (Invitrogen). PCR was performed using the LightCycler 2.0 (Roche, Basel, Switzerland). The basic PCR reaction conditions were: 94°C for 2 minutes then 39 cycles of 95° C for 10 seconds, 57°C for 20 seconds, 72° C for 40 seconds. Hypoxanthine phosphoribosyl transferase served as the normalization control. Relative expression analysis was conducted using the program LinRegPCR according to the suggested specifications [12].
Table 2.
PCR primer sequences and GeneBank accession numbers
| Primer Name |
Sequence 5`-3` | Sequence 3`-5` | GeneBank Accession # |
Amplicon Size |
Ta °C |
|---|---|---|---|---|---|
| hDDR | CTTTCCCGCCACGAGC | ACCCCAAAGGCCCACA | NM_001014796 | 329 | 59 |
| pro-Col Iα1 | AGTTCGAGTATGGCGG | CAGTGACGCTGTAGGT | NM_000088 | 231 | 60 |
| Vimentin | GACAAAGCCCGCGTCG | GGCGTCATTGTTCCGGT | NM_212482 | 426 | 60 |
| Fibronectin | ACAGGACGGACATCTT | GGTCGGCATCATAGTTCT | NM_212482 | 230 | 58 |
| HPRT | CTTGCTCGAGATGTGATG | GTCTGCATTGTTTTGCCAG | NM_000194.1 | 290 | 60 |
Ta, annealing temperature; hDDR, human discoidin domain receptor; pro-Col Iα1, pro-collagen 1 alpha 1; HPRT, hypoxanthine phosphoribosyl transferase.
Fluorescence in situ hybridization (FISH) studies
FISH was performed on the fibroblasts isolated from female recipients of gender mismatch transplantation and the fibroblasts from Ph1-positive CML patients for identification of Y-chromosome and BCR/ABL translocation, respectively. Prior to hybridization, the cultured fibroblast samples from each source underwent rapid aging (2 minutes at 73° C in 2x sodium saline citrate (SSC) with 0.4% NP-40), followed with a digest stage (20 minutes at 37° C in 500ug/ml proteinase I). After a 5-minute wash in PBS, the slides were dehydrated with ethanol (2 minutes in 70%, 80% and 100%). The CEP X/Y DNA probe kit (Abbott Molecular Inc., Des Plaines, IL, USA) was used for X and Y chromosome analysis. This probe is a mixture of a SpectrumOrange labeled CEP X probe and a SpectrumGreen labeled CEP Y probe specific for the alpha satellite centromeric region of chromosome X and the satellite 111 (Yq12) region of chromosome Y. Vysis LSI BCR/ABL Dual Translocation probe (Abbott Molecular, Inc.) was used for detection of the Ph1 translocation. This probe is a mixture of LSI ABL probe labeled with Spectrum Orange and LSI BCR labeled with SpectrumGreen. Each culture sample well received 5ul of specific probe solution. The samples were then cover-slipped, sealed and then denatured per protocol. After 12 hours of denaturing, the slides were washed in SCC and 1% NP-40. 5µl DAP-II counterstain was applied to each. The slides were coverslipped and then evaluated using the CytoVision NT Probe Capture and Analysis program (Genetix Ltd., UK).
Results
Development of a cell culture method for fibroblasts from PB
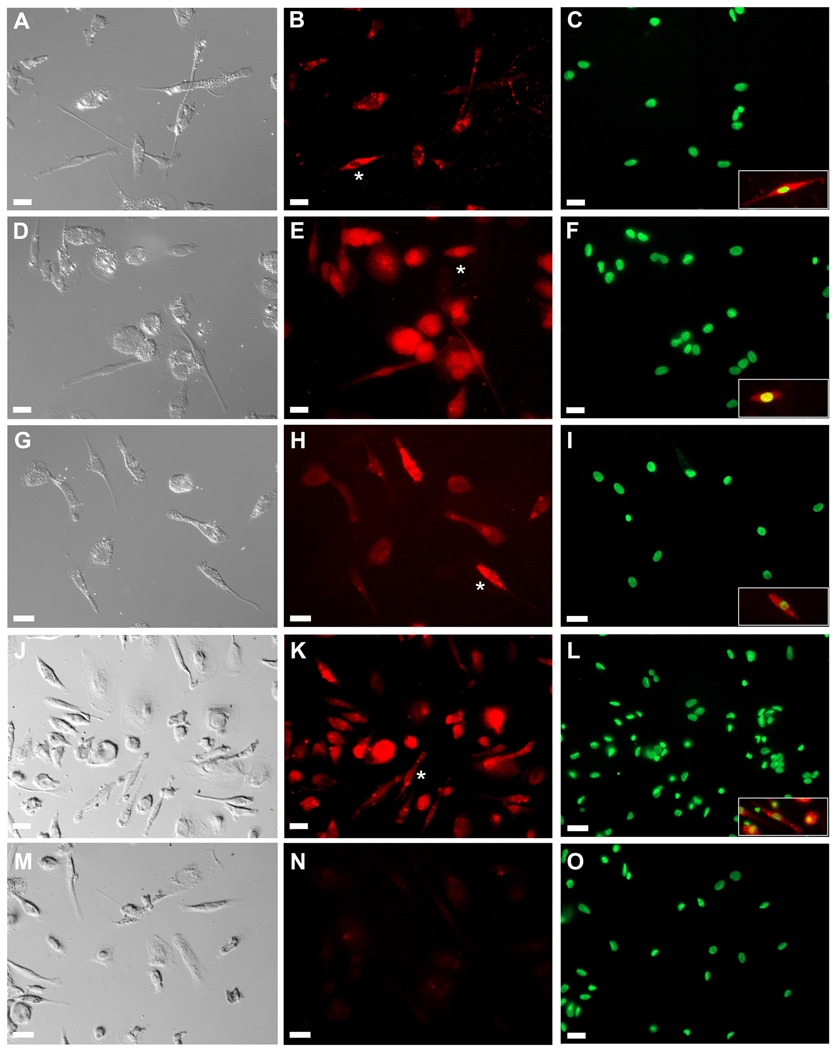
PB MNCs cultured in fibronectin-coated 4-well culture slides were examined immunohistochemically. As shown in Figure 1, analysis of cultured cells using DIC microscopy showed adherent cells with the classic morphology of fibroblasts including an elongated, polygonal or spindle-shape with a clear, ovoid nucleus. Immunohistochemical analysis showed that all cells stained positive for αSMA, pro-collagen type I, vimentin and 5B5 (a marker of prolyl-4-hydroxylase), markers associated with fibroblasts. Flow cytometric comparison of MNCs before and after culture showed reduction in the percentages of cells expressing CD11b or CD56 and increase in the percentage of cells expressing pro-collagen-I from 4.28% to 33.10% (Figure 2). In addition, the relative loss of intensity of CD45 expression after culture was evident in all analyses. Quantitative PCR revealed very high expression of mRNA for pro-collagen Iα1, vimentin and fibronectin by the cultured cells relative to those by MNCs, at levels higher than or similar to those of a human foreskin fibroblast cell line, HFF-1 (Figure 3).
Figure 1. Morphological and immunohistochemical analysis of fibroblasts cultured from PB.
Morphological identification of fibroblasts was based on DIC images (Panels A, D, G, J and M). Cells were then stained with antibodies to SMA (Panel B), collagen type I (COL-1) (Panel E), vimentin (Panel H) or 5B5 (prolyl 4-hydroxylase; Panel K). Analysis showed that all cultured cells with morphological characteristics of fibroblasts expressed markers associated with this cell type. Panel N shows control staining with secondary antibodies only. Panels C, F, I, L and O show nuclear staining with Hoechst dye. Insets in Panels C, F, I, L and O show superimposition of antibody staining (red) and nuclear staining (green) for cells indicated by asterisks in Panels B, E, H, and K. Bar = 25 m.
Figure 2. Flow cytometric analysis of PB MNCs and cultured fibroblasts.
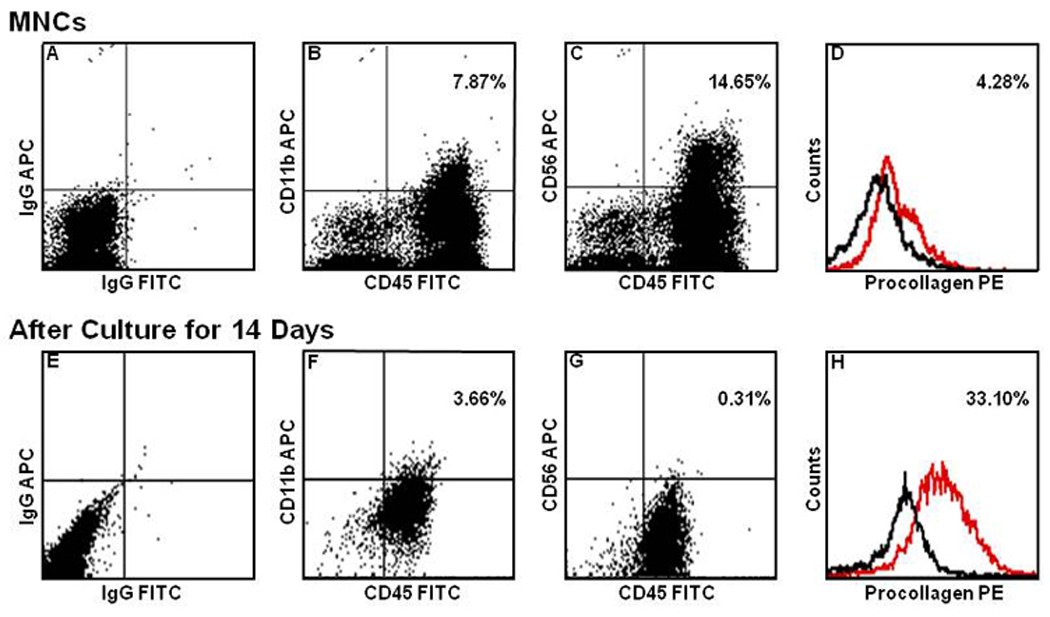
Scales in the abscissa of all Panels were adjusted to isotype controls (Panels A, E and black lines in Panels D and H) and the relative fluorescence intensity of the data is expressed in logarithmic scales. The number of cells expressing CD11b (Panels B and F) and CD56 (Panels C and G) decreased and the intensity of CD45 expression weakened following culture. In contrast, the number of cells expressing pro-collagen-I (red lines in Panels D and H) increased with culture.
Figure 3. RT-PCR analysis of fibroblasts cultured from PB.
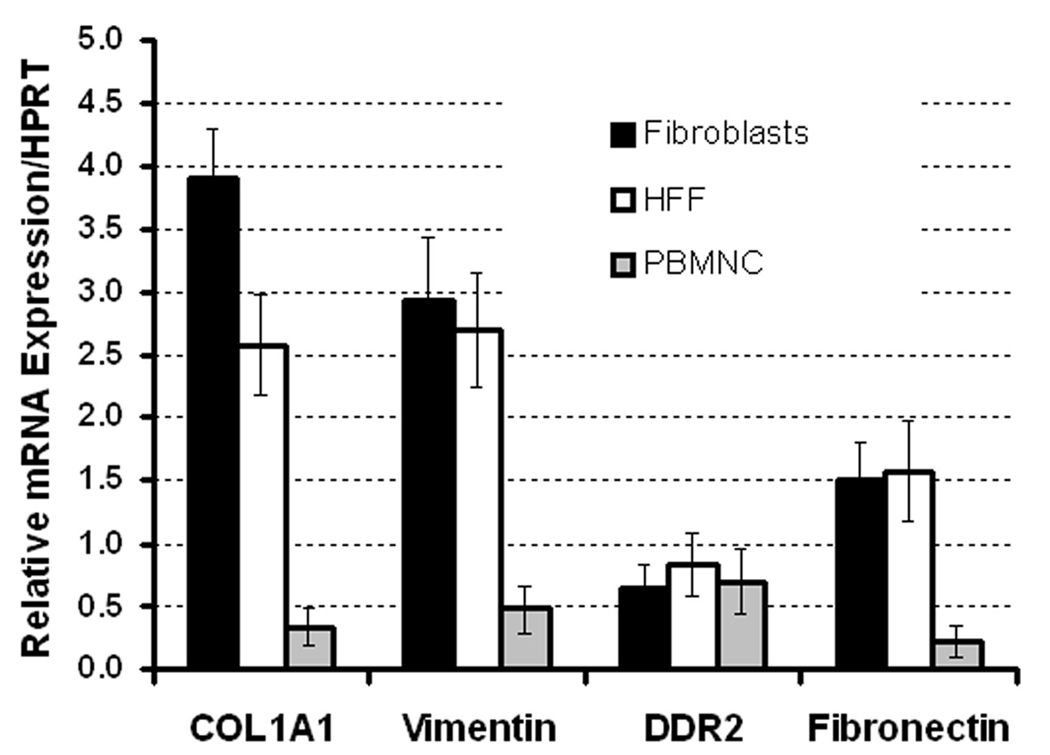
Real-time RT-PCR was conducted on total RNA extracted from PB MNCs, cultured fibroblasts and a human foreskin fibroblast cell line (HFF-1). Analysis showed that cultured human fibroblasts expressed mRNA for pro-collagen Iα1, vimentin and fibronectin at levels similar to a HFF-1 and far higher than those by PB MNCs. These findings provide biochemical support for the identity of the cultured cells as fibroblasts.
Identification of Y-chromosome in the fibroblasts cultured from female recipients of gender mismatch stem cell transplantation
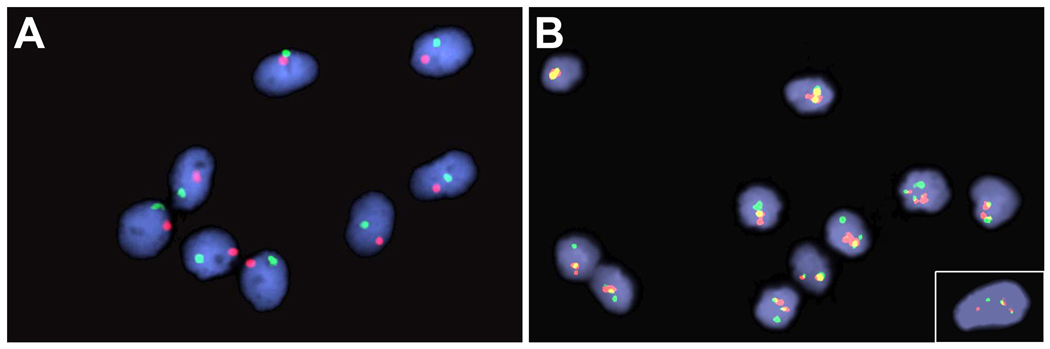
Next, we cultured PB fibroblasts from three female recipients of gender mismatch stem cell transplantation using the culture method we established and analyzed their sex chromosomes with FISH. These patients showed almost complete hematopoietic reconstitution by male cells as described in Table 1. Figure 4, Panel A depicts a high magnification image of the FISH analysis of the cultured fibroblasts from one patient. All fibroblasts exhibited both X and Y chromosome indicating the male donor origin of the circulating fibroblast precursors.
Figure 4. FISH analysis of Y-chromosome in cultured fibroblasts from a male-to-female bone marrow transplant patient and BCR/ABL translocation in cultured fibroblasts from two untreated CML patients.
Fibroblasts cultured from a transplant recipient were stained with SpectrumOrange labeled CEP X probe and a SpectrumGreen labeled CEP Y probe (Panel A). Panel A shows clear presence of Y-chromosomes in all fibroblasts. Presented in panel B are cultured cells from two patients with Ph1 positive CML that were processed with Vysis LSI BCR/ABL Dual Translocation probe (a cell from the second patient is shown in the inset). The yellow color indicates the site of BCR/ABL translocation.
Identification of BCR-ABL translocation in cultured fibroblasts from untreated CML patients
In a subsequent study, we cultured fibroblasts from the PB of two untreated patients with CML whose blood exhibited Ph1 chromosome and examined BCR-ABL translocation in the cultured fibroblasts by FISH. As shown in Figure 4, Panel B, all fibroblasts derived from the first patient showed BCR-ABL translocation (yellow). Many, but not all, of the fibroblasts derived from the second patient showed BCR-ABL translocation. An example of a positive fibroblast is shown in the inset of Figure 4. Since CML is a clonal disorder of hematopoietic progenitors or stem cells, this observation strongly suggests the hematopoietic stem cell origin of human fibroblasts.
Discussion
The origin of fibroblasts/myofibroblasts has been debated for a long time. Friedenstein and his colleagues [13,14] first presented in vitro evidence for a bone marrow origin over thirty years ago. They demonstrated the presence of fibroblast precursors called colony-forming units-fibroblasts (CFU-F) in the bone marrow. Subsequently, however, the concept of epithelial-mesenchymal transition became more widely accepted. This was modeled after embryonic development and was based on the finding that the major population of fibroblasts during renal fibrosis is derived from tubular epithelium [15]. Recently, however, a number of mouse and human bone marrow transplantation studies have revived the notion of the bone marrow as the source of fibroblasts/myofibroblasts. Using either Y-chromosome or green fluorescent protein (GFP) as a marker of donor cells, investigators have presented evidence that hepatic stellate cells [16], pericryptal myofibroblasts in the intestine and colon [17], myofibroblasts in wounded skin [18] and the fibroblasts in pulmonary fibrosis [19] are derived from bone marrow. Transplantation of bone marrow cells was also shown to reduce the magnitude of liver fibrosis that had been induced with carbon tetrachloride [20].
Bone marrow cells are thought to contain two types of stem cells, HSCs and MSCs. In order to determine which type of stem cell is the source of fibroblasts/myofibroblasts, we have carried out a series of studies of tissue reconstitution by single HSCs in mice and found that fibroblasts/myofibroblasts in many organs and tissues, such as glomerular mesangial cells of the kidney [2], brain microglial cells and perivascular cells [3], tumor-associated fibroblasts [6], inner ear fibrocytes [4] and heart valve fibroblasts [5], are derived from HSCs. These findings were summarized in our review [1]. Subsequently, investigators in other laboratories, using single HSC transplantation, presented evidence that hepatic stellate cells [8] and myofibroblasts generated by cardiac infarction [9] are also derived from HSCs. In addition to this in vivo evidence for an HSC origin of tissue fibroblasts/myofibroblasts, we have succeeded in culture of fibroblasts from bone marrow cells of mice with single HSC transplantation [7]. We also documented, in these clonally engrafted mice, the presence of two known types of fibroblast precursors, i.e. CFU-F [10] and PB fibrocytes [11], of donor single HSC origin. This cell culture study was consistent with the results of the transplantation studies and strongly suggested that most, if not all, mouse fibroblasts/myofibroblasts are derived from HSCs.
Using a modification of the culture method for circulating fibrocytes described by Bucala et al. [11], we established a method for culturing human fibroblasts from PB cells. The cultured cells displayed typical polygonal or spindle shape and stained positive for collagen type I [11], αSMA, vimentin, and 5B5 (prolyl-4 hydroxylase). Quantitative PCR of the cultured cells confirmed the expression of mRNA for pro-collagen Iα 1, vimentin and fibronectin at levels higher higher than those by MNCs and similar to those by a human skin fibroblast cell line. Flow cytometric comparison of the MNCs and the cultured cells also supported the fibroblastic nature of the cultured cells. Using this culture assay we then demonstrated the donor male cell origin of fibroblasts in three female transplant recipients who show almost full hematopoietic reconstitution by male cells. Since fibroblasts are an important component of the stromal cell population, this finding may conflict with the general belief in clinical bone marrow transplantation that bone marrow stromal cells are of host origin. Although the majority of publications support this dogma [21–28], some studies described chimerism of marrow stromal cells [29–33]. There would be a number of possible explanations for the apparent discrepancy among these results, such as methods for identification of donor stromal cells (i.e., cell culture vs. PCR) and different underlying diseases. Regardless, our finding is consistent with the documentation of bone marrow-derived tissue myofibroblasts in the intestine [17], liver [16] and heart valve [34] of recipients of gender-mismatch bone marrow or organ transplantation.
Although our studies of PB cells from transplant subjects clearly established the donor origin of fibroblasts, they did not unequivocally establish the HSC origin of human fibroblasts. Therefore, we next studied fibroblasts grown from two patients with Ph1-positive CML and found BCR-ABL translocation in the cultured fibroblasts. The significance of our finding is two-fold. First, since CML is a clonal disorder of hematopoietic progenitors/stem cells, identification of BCR-ABL translocation in fibroblasts supports the concept of an HSC origin of human fibroblasts. It also has significant implication in understanding the nature of bone marrow fibrosis seen in patients with myelo-proliferative disorders. While bone marrow fibrosis is the major pathological feature of idiopathic myelofibrosis, varying degrees of fibrosis is also seen in the bone marrow of patients with CML, essential thrombocytosis and polycythemia vera. Earlier, two studies established the concept that bone marrow fibrosis associated with myelo-proliferative disorders is reactive fibrosis and not of the malignant clone. Jacobson et al. [35] carried out a culture study of a female patient with idiopathic myelofibrosis who also was heterozygous for glucose-6-phosophate dehydrogenase (G6PD), X-linked isoenzyme. They discovered both B-and A isoenzymes in skin biopsies and fibroblasts cultured from bone marrow, but only type A isoenzyme in the blood cells. A few years later, Castro-Malaspina et al [36] studied fibroblasts cultured from six patients with Ph1-positive CML with or without myelofibrosis. They found only normal karyotype in the cultured fibroblasts. In contrast to these, a study of karyotypes of MSCs cultured from patients with myelodysplastic syndrome, another form of clonal hematopoietic disorder, revealed the presence cytogenetic abnormalities in many cases [37]. We have no ready answers for the apparent discrepancies among these reports, including ours, other than possible variations in the size of the clone in individual patients. Further studies are needed to clarify the nature of fibroblasts and fibrosis seen in patients with clonal hematopoietic disorders, including myeloproliferative disorders.
Acknowledgements
This work was supported by National Institutes of Health grants R01 DK077821 (M.O.), P01 CA78582 (D.K.W.) and by the office of Research and Development, Medical Research Services, Department of Veterans Affairs (A.C.L.). The authors would like to acknowledge the Hollings Cancer Center Flow Cytometry Core and specifically thank Dr. Haiqun Zeng.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 2.Masuya M, Drake CJ, Fleming PA, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101:2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 3.Hess DC, Abe T, Hill WD, et al. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Lang H, Ebihara Y, Schmiedt RA, et al. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: mesenchymal cells and fibrocytes. J Comp Neurol. 2006;496:187–201. doi: 10.1002/cne.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visconti RP, Ebihara Y, LaRue AC, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 6.LaRue AC, Masuya M, Ebihara Y, et al. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Exp Hematol. 2006;34:208–218. doi: 10.1016/j.exphem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Ebihara Y, Masuya M, Larue AC, et al. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006;34:219–229. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Miyata E, Masuya M, Yoshida S, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008;111:2427–2435. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
- 9.Fujita J, Mori M, Kawada H, et al. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750–2759. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 11.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 13.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 14.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11:345–349. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes SJ, Russo FP, Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Brittan M, Hunt T, Jeffery R, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 21.Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 22.Laver J, Jhanwar SC, O'Reilly RJ, Castro-Malaspina H. Host origin of the human hematopoietic microenvironment following allogeneic bone marrow transplantation. Blood. 1987;70:1966–1968. [PubMed] [Google Scholar]
- 23.Raskind WH, Singer JW, Morgan CA, Fialkow PJ. Host origin of marrow stromal cells obtained from marrow transplant recipients and transformed in vitro by simian virus-40. Exp Hematol. 1988;16:827–830. [PubMed] [Google Scholar]
- 24.Agematsu K, Nakahori Y. Recipient origin of bone marrow-derived fibroblastic stromal cells during all periods following bone marrow transplantation in humans. Br J Haematol. 1991;79:359–365. doi: 10.1111/j.1365-2141.1991.tb08041.x. [DOI] [PubMed] [Google Scholar]
- 25.Koc ON, Peters C, Aubourg P, et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 1999;27:1675–1681. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 26.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27:1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 27.Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30:937–942. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 28.Rieger K, Marinets O, Fietz T, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33:605–611. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Keating A, Singer JW, Killen PD, et al. Donor origin of the in vitro haematopoietic microenvironment after marrow transplantation in man. Nature. 1982;298:280–283. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka J, Kasai M, Imamura M, et al. Evaluation of mixed chimaerism and origin of bone marrow derived fibroblastoid cells after allogeneic bone marrow transplantation. Br J Haematol. 1994;86:436–438. doi: 10.1111/j.1365-2141.1994.tb04764.x. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 32.Cilloni D, Carlo-Stella C, Falzetti F, et al. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood. 2000;96:3637–3643. [PubMed] [Google Scholar]
- 33.Sanchez-Guijo FM, Sanchez-Abarca LI, Villaron E, et al. Posttransplant hematopoiesis in patients undergoing sibling allogeneic stem cell transplantation reflects that of their respective donors although with a lower functional capability. Exp Hematol. 2005;33:935–943. doi: 10.1016/j.exphem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Deb A, Wang SH, Skelding K, et al. Agnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood. 1978;51:189–194. [PubMed] [Google Scholar]
- 36.Castro-Malaspina H, Gay RE, Jhanwar SC, et al. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood. 1982;59:1046–1054. [PubMed] [Google Scholar]
- 37.Flores-Figueroa E, Arana-Trejo RM, Gutierrez-Espindola G, Perez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29:215–224. doi: 10.1016/j.leukres.2004.06.011. [DOI] [PubMed] [Google Scholar]