Abstract
To evaluate the biocompatibility of subretinal implanted parylene-based Ti/Pt microelectrode arrays (MEA). Eyes were enucleated 3 months after MEAs were implanted into the subretinal space of rabbits. Morphological changes of the retinas were investigated by H&E staining. Immunohistochemical staining for glial fibrillary acidic protein and opsin were performed to evaluate changes in Muller cells and photoreceptors in the retinas. Retina tissue around the array remained intact. Photoreceptor degeneration and glial cell activation were observed in the retina overlaying the MEA implant. However, the cells in the inner retinal layers were preserved. Photoreceptor degeneration and glial cell activation at the MEA–retina interface are expected to be a normal reaction to implantation. Material used in this experiment has good biocompatibility within the subretinal environment and is expected to be promising in the further retinal prosthesis studies.
Keywords: Subretinal electrode array, Biocompatibility
Introduction
Retinitis pigmentosa and age-related macular degeneration are the leading retinal degeneration diseases that cause legal blindness in the world. The common characteristic of these degeneration diseases is degeneration of photoreceptors while leaving the inner retina intact. The intact inner retina provides opportunities for retinal prosthetics to restore vision through stimulating ganglion cells.
A nontoxic, stable and biocompatible substrate of microelectrode arrays (MEAs) is required for long-term usage. Compared with other widely used microfabrication materials, such as PDMS (polydimethylsiloxane), polyimide and silicon, parylene has good biocompatibility, outstanding moisture barrier properties, low Young’s modulus, excellent chemical inertia and extremely high dielectric strength [1]. Parylene coating has good conformability and can be easily patterned by O2 enrolled etching process [2]. In summary, parylene is a good candidate in the applications of implantable biomedical microdevices.
There are generally two ways to introduce the array to the eye, one is through subretinal implantation, while the other is epi-retinal implantation. There are advantages and disadvantages to the different surgeries. Epiretinal devices can be relatively large and incorporate sophisticated powering and data transmission schemes [3], while the subretinal implant has the theoretical advantage of being placed closer to the nearest layer of surviving neurons, the bipolar cells [4].
In this study, a flexible microelectrode array on parylene has been developed and introduced for subretinal stimulation. Flexible microelectrodes are more suitable for intimate attachment to the overlying retina as compared to rigid microelectrodes and epiretinal implants. We evaluated the long-term biocompatibility of a Ti/Pt retinal prosthetic device 3 months after insertion into the subretinal space of rabbits.
Materials and methods
Surgery procedure
All animal procedures were approved by the Hospital Animal Care and Use Committee and followed the ARVO Resolution on the Use of Animals in Ophthalmic and Vision use. Flexible parylene-based MEAs were developed and provided by Peking University. In the animal experiments, the MEAs were implanted into the subretinal space. Three rabbits were used in this study. The experiment was conducted as follows: After anesthesia, inferior conjunctiva was incised and inferior rectus was dissected to expose inferior sclera. A scleratomy was made 8 mm from the limbus. Intraocular pressure was decreased via paracentesis. A retina detachment inferior to the optic disc was made with viscoelastic through a pars plana incision. A careful cut was made through the choroid and through the sclerotomy until viscoelastic came out. Then, the 1-mm width MEA chip was inserted into the subretinal space 3 mm inferior to the optic papilla and fixed to the sclera by suture. The tail of MEA strip was scrolled and embedded under the conjunctiva.
Tissue preparation
After 3 months of the MEA implantation, the eyes were enucleated under deep anesthesia to prevent artificial post-mortem ischemic changes. A mark was made at the sclera side under which the implant was located. The eyes were placed in 10% neutral formaldehyde for 24 h. To avoid disruption artifacts when sectioning the electrode array with conventional microtomes, the implant was gently removed from the retina. The eyes were prepared, serially dehydrated, embedded in paraffin, and cut into 5-μm specimens for hematoxylin–eosin staining (H&E staining) and immunohistochemistry studies.
Morphological evaluation and immunohistochemistry staining
All incubation steps were performed at room temperature. Paraffin was removed by immersing the slides in xylene. Tissue was rehydrated through a graded series of alcohol. Sections were rinsed with 10 mM phosphate-buffered saline (PBS), pH 7.2, blocked by 5% normal goat serum for 30 min, and then put in 0.1% Triton X-100 in 10 mM PBS for 10 min. Sections were incubated with different primary antibodies for 1 h. Primary antibodies used in the present studies are mouse anti-rabbit monoclonal anti-glial fibrillary acidic protein (GFAP) (Abcam, 1:400 dilution) and mouse anti-rabbit monoclonal anti-Opsin (Sigma, 1:1,000 dilution). A secondary antibody, FITC-conjugated goat anti-mouse immunoglobulins (Invitrogen, 1:100 dilution), was applied to each section for 30 min. A Hoechst stain (1:1,000 dilution) was applied to the sections for 1 min to counterstain nuclei.
Results
H&E staining showed that the outer retina layer directly overlying the implant changed to a disorganized structure. Disorganization and breaks in the RPE layer were also observed. The inner retina and underlying choroid remained intact (Fig. 1).
Fig. 1.
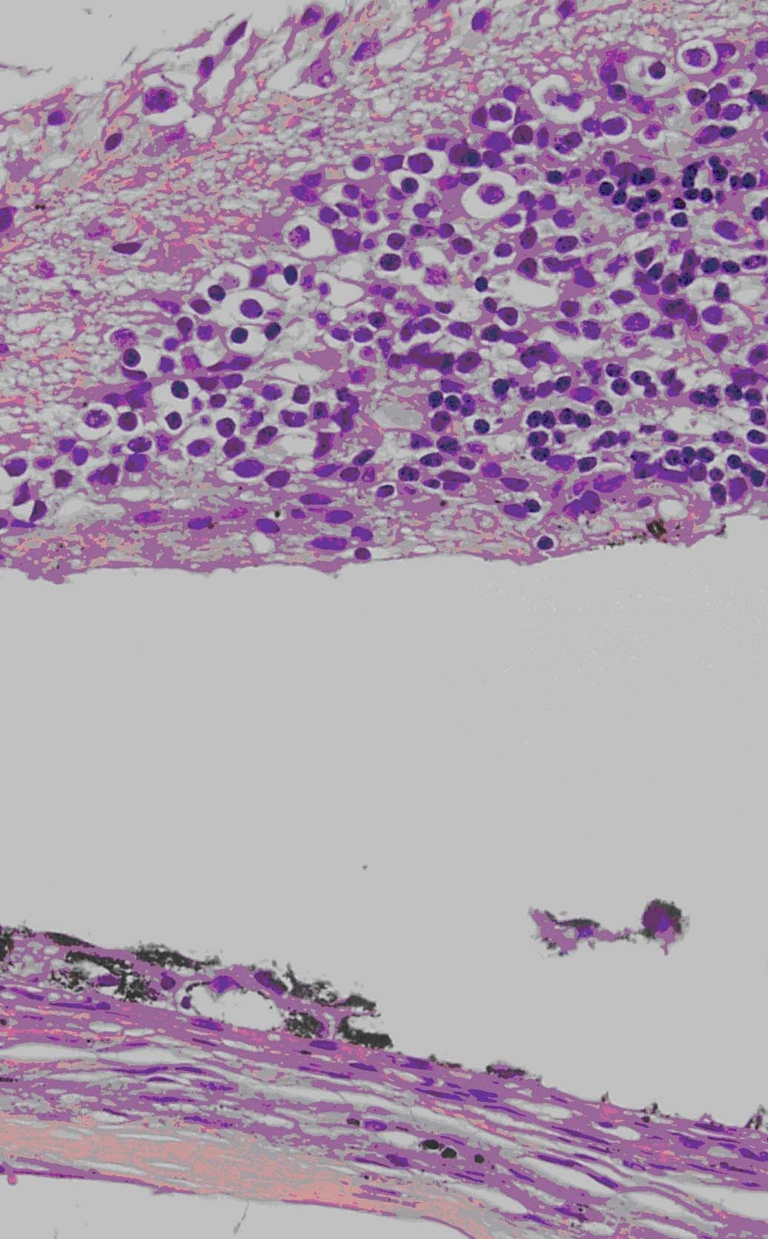
Hematoxylin and eosin staining of retina overlying MEA. MEA location is indicated by asterisk in the subretinal space. Retina overlying the implant is disorganized. Disorganization and discontinuities in the RPE were observed (arrow). Final magnification, ×100
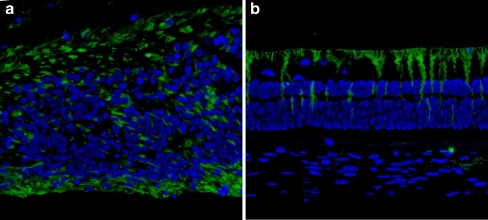
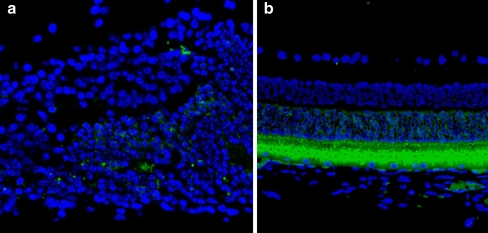
To further investigate the changes on different retinal cell types caused by the MEA implantation, immunofluorescence staining was performed. GFAP-labeled cells were found overlying the MEA with very strong staining (Fig. 2a) compared to normal distant retina (Fig. 2b), suggesting occurrence of a glial cell activation related to inflammatory reactions to MEA. Opsin-positive-labeled cells were distributed sporadically above the GFAP-labeled cells in the retinal area above the MEA implantation (Fig. 3a), while in the retinal area distant to the MEA they appeared normal (Fig. 3b), suggesting degeneration of photoreceptors overlying the MEA.
Fig. 2.
a GFAP-labeled cells in retina overlaying the implant (arrows). Asterisk indicates the implantation location. b GFAP immunofluorescence labeling of retina distant to MEA. GFAP (green); nuclei (blue). Final magnification, ×100
Fig. 3.
a Opsin immunofluorescence labeling of retina overlaying the implant. Opsin is a characteristic protein in photoreceptors. The image shows a dispersed distribution of the Opsin labeled cells (arrows), with a decreased density. Asterisk denotes the implantation location. b Opsin immunofluorescence labeling of retina distant to MEA. Opsin is labeled by FITC: fluorescence green; nuclei is stained by Hochest: fluorescence blue. Final magnification, ×100
Discussion
Maintenance of the integrity of the inner retina in the presence of the implants and their electrical activity are important for the effect of subretinal implantation with electrode arrays in individuals with photoreceptor degeneration. In our study, the retina overlying the implant showed disorganization of architecture and photoreceptor degeneration. These changes also observed in previous subretina implanting studies [5, 6]. In the study of cat subretina microphotodiode array implantation [1], intensity of several selected metabolic indicators was also changed concomitantly. RPE disorganization and discontinuities could be caused by almost unavoidable physical damage to RPE during the surgery process especially when pushing the array forward under the focal detached retina. RPE damage demonstrated the breakdown of the blood–retina barrier. However, the underlying array damage does not significantly affect signal transportation in this device.
Glial cells play an important role in response to various retina insults, including injury, retina detachment, and photoreceptor degeneration [7–12]. In our study, the glial cell processes surrounding the implant in the subretinal space was detected by immunofluorescence. Increased GFAP labeling suggests the activation of glial cells overlaying the implant following the implantation surgery and this may be an inflammatory reaction to MEA.
According to our results, the disorganization, photoreceptor degeneration, and the activation of glial cells could be caused by retina insults including direct operation injury, retina detachment, and blockage of choroidal nutrients to the outer retina. Artificial retinal detachment caused by electrode assay implanting is inevitable in surgical manipulations. Persistent retina detachment leads to a loss of photoreceptors, a decrease in the thickness of retina in cats and widespread, multilayered degeneration in rabbits [13, 14]. Choroidal nutrients are the major resource to the outer retina nutrition. Defects of nutrients for overlying photoreceptors and inflammation reaction to MEA lead to glial cell activation, photoreceptor degeneration and result in a disruption of normal retina structure. However, these changes only occur in the area immediately overlying MEA, leaving areas distant to the MEA intact. In retinitis pigmentosa patients, the degeneration of photoreceptors occurs while the inner retina preserved. We suggest that retinitis pigmentosa patients are good candidates for MEA implantation.
Parylene has been suggested as a suitable encapsulation material for retinal prostheses [6, 15]. In our study, incited tissue reactions and fibrosis did not occur around the implant by the end of observation period, indicating a stable biocompatibility of parylene-based Ti/Pt microelectrode arrays. These results show that the material used in the experiment has good biocompatibility within the subretinal environment in the rabbit eye and are promising tools for use in retinal prosthesis studies in future.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program) under Award Number 2006AA04Z352.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kroschwitz JI. Kirk-Othmer encyclopedia of chemical technology. New York: Wiley; 1998. [Google Scholar]
- 2.Meng E, Tai Y-C. Parylene etching techniques for microfluidics and biomems 18th IEEE Int. Conf. on Micro Electro Mechanical Systems. Piscataway: IEEE; 2005. pp. 568–571. [Google Scholar]
- 3.Yanai D, Lakhanpal RR, Weiland JD, Mahadevappa M, Van Boemel G, Fujii GY, Greenberg R, Caffey S, de Juan E, Jr, Humayun MS. The value of preoperative test in the selection of blind patients for a permanent microelectronic implant. Trans Am Ophthalmol Soc. 2003;101:223–230. [PMC free article] [PubMed] [Google Scholar]
- 4.Chow AY, Pardue MT, Perlman JI, Ball SL, Chow VY, Hetling JR, Peyman GA, Liang C, Stubbs EB, Jr, Peachey NS. Subretinal implantation of semiconductor-based phosodiodes: durability of novel implant designs. J Rehabil Res Dev. 2002;39:313–321. [PubMed] [Google Scholar]
- 5.Pardue MT, Stubbs EB, et al. Immunohistochemical studies of the retina following long-term implantation with subretinal microphotodiode arrays. Exp Eye Res. 2001;73:333–343. doi: 10.1006/exer.2001.1041. [DOI] [PubMed] [Google Scholar]
- 6.Montezuma SR, Lowenstein J, Scholz C, Rizzo JF. Biocompatibility of materials implanted into the subretinal space of Yucatan pigs. Invest Ophthalmol Vis Sci. 2006;47:3514–3522. doi: 10.1167/iovs.06-0106. [DOI] [PubMed] [Google Scholar]
- 7.Miller B, Miller H, Patterson R, Ryan SJ. Retinal wound healing. Cellular activity at the vitreoretinal interface. Arch Ophthalmol. 1986;104:281–285. doi: 10.1001/archopht.1986.01050140139037. [DOI] [PubMed] [Google Scholar]
- 8.Funata M, Wendel RT, de la Cruz Z, Green WR. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992;12:289–298. doi: 10.1097/00006982-199212040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez-Chona F, Song BK, Geisert EE. Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–2746. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamana T, Kita M, Ozaki S, Negi A, Honda Y. The process of closure of experimental retinal holes in rabbit eyes. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:81–87. doi: 10.1007/s004170050014. [DOI] [PubMed] [Google Scholar]
- 11.Erickson PA, Fisher SK, Guérin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Invest Ophthalmol Vis Sci. 1987;30:191–211. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 12.Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:1321–1328. [PubMed] [Google Scholar]
- 13.Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–942. [PubMed] [Google Scholar]
- 14.Faude F, Francke M, Makarov F, Schuck J, Gärtner U, Reichelt W, Wiedemann P, Wolburg H, Reichenbach A. Experimental retinal detachment causes widespread and multilayered degeneration in rabbit retina. J Neurocytol. 2001;30:379–390. doi: 10.1023/A:1015061525353. [DOI] [PubMed] [Google Scholar]
- 15.Schanze T, Hesse L, Lau C, Greve N, Haberer W, Kammer S, Doerge T, Rentzos A, Stieglitz T. An optically powered single-channel stimulation implant as test system for chronic biocompatibility and biostability of miniaturized retinal vision prostheses. IEEE Trans Biomed Eng. 2007;54(6 Pt 1):983–992. doi: 10.1109/TBME.2007.895866. [DOI] [PubMed] [Google Scholar]