Abstract
The trivalent inactivated vaccine (TIV) is used to prevent seasonal influenza virus infection in humans, however, the immunogenicity of this vaccine may be influenced by the priming effect of previous influenza vaccinations or exposure to antigenically-related influenza viruses. The current study examines the immunogenicity of a clinically licensed TIV in rabbits naïve to influenza antigens. Animals were immunized with either the licensed TIV, a bivalent (H1 and H3) HA DNA vaccine or the combination of both. Temporal and peak level serum anti-influenza virus IgG responses were determined by ELISA. Functional antibody responses were measured by hemagglutination inhibition and microneutralization against either A/NewCaledonia//20/99 (H1N1) or A/Panama/2007/99 (H3N2) influenza viruses. Our results demonstrate that the immunogenicity of the TIV is low in sero-negative animals. More significantly, the heterologous DNA prime-TIV boost regimen was more immunogenic than the homologous prime-boost using either TIV or DNA vaccines alone. This finding justifies further investigation of HA DNA vaccines as a priming immunogen for the next generation of vaccines against seasonal or pandemic influenza virus infections.
Keywords: Influenza virus, HA protein, DNA vaccine, inactivated flu vaccine, prime-boost
1. INTRODUCTION
Influenza A virus infection continues to be a major public health threat. Seasonal influenza virus infections can produce high morbidity and the emerging threat of pandemic influenza, particularly from an avian source, has become a new concern to the health of the worldwide human population [1–3]. The best option for reducing the impact of influenza virus infection in humans is vaccination [4].
Currently, the main form of licensed human influenza virus vaccines is the traditional trivalent inactivated influenza vaccines (TIV). This vaccine modality incorporates circulating human viral strains: an H1 subtype and an H3 subtype of the influenza A virus plus an influenza B virus. Another type of influenza vaccine is the cold-adapted live influenza virus vaccine (CAIV) which has been shown to be more immunogenic than TIV in inducing protective immunity and may be associated with a longer-lasting and more cross-protective immune response than is elicited by TIV [5]. A new technology termed ‘reverse genetics’ has been developed to generate high growth reassortants [6–11] and combines viral genes from the high growth yield laboratory strain of influenza A virus A/PR/8/34 (H1N1) with genes encoding protective antigens of the target viral strains [12]. However, this technology does not change the subsequent manufacturing process needed to produce large stocks of vaccine viruses to make the final TIV or CAIV formulations. For TIV, additional steps of inactivation and purification of the protective HA antigens are needed. Due to safety concerns, CAIV is not indicated for very young children, the elderly or people with a compromised immune system. Therefore, TIV continues to be the main vaccine used annually to prevent seasonal influenza. Furthermore, the recently licensed pandemic vaccines against H5N1 influenza viruses are also of the inactivated form [13].
Inactivated vaccines are known to have weak immunogenicity but have been used effectively in preventing seasonal influenza virus infection [14]. Usually one injection with TIV can induce satisfactory levels of protective antibody responses [15], as required by regulatory approval for licensing. However, during the testing of inactivated avian influenza virus vaccines to prevent pandemic influenza, it is clear that at least two immunizations are needed and/or a strong adjuvant is required, in order to elicit the same magnitude of protective antibody responses as are seen with TIV for seasonal influenza [16, 17]. One possible reason for this difference in immunogenicity between seasonal TIV and an inactivated avian influenza virus vaccine is the pre-existing immunity against the human virus strains versus a lack of such immunity for the avian virus strains. Human populations, in general, have been exposed to early circulating H1 and H3 serotype influenza viruses and some people have received previous TIV immunizations. These events serve a “priming” effect to the host immune system and thus, one shot of TIV can easily “boost” antibody responses against seasonal influenza viruses. On the other hand, human populations, at least at this point, are still naïve to avian influenza virus strains (such as H5, H7 and H9 serotypes), and one immunization of an inactivated avian influenza vaccine may not be immunogenic to the point where they are able to elicit high level protective antibody responses in subjects naïve to avian influenza virus antigens.
In the current study, we tested the immunogenicity of the licensed, split virus TIV in a naïve rabbit model to study the HA-specific binding and functional antibody responses. The immunogenicity of TIV was compared to a DNA vaccine expressing HA antigens from the same or closely related H1 and H3 viruses. In addition, the relative immunogenicity between the homologous and heterologous prime-boost approaches was determined. Our results indicate that DNA prime-TIV boost was, in fact, the most immunogenic immunization regimen. This finding suggests a new option in the effort to develop the next generation influenza vaccines.
2. MATERIALS AND METHODS
2.1 Inactivated influenza vaccine
The inactivated trivalent influenza vaccine (TIV) used in this study was the licensed split virus TIV, Fluzone, used during the 2004–2005 influenza season. This particular formulation consists of 15 µg of each HA protein from A/NewCaledonia/20/99 (H1N1), A/Wyoming/03/2003 (H3N2) and B/Jiansu/10/2003 in 500 µl of vaccine (one adult human dose). The product was stored at UMMS clinical pharmacy according to manufacturer’s recommendation and the product was used before its expiration date.
2.2 Codon optimized HA DNA vaccines
The codon optimized HA DNA vaccines used in this study were H1 and H3 DNA vaccines expressing the full length wild type HA proteins from A/NewCaledonia/20/99 (H1N1) and A/Panama/2007/99 (H3N2), respectively, as described in a previous study [18]. A/Panama/2007/99 (H3N2) is closely related to A/Wyoming/03/2003 (H3N2).
2.3 Immunization of New Zealand White (NZW) rabbits
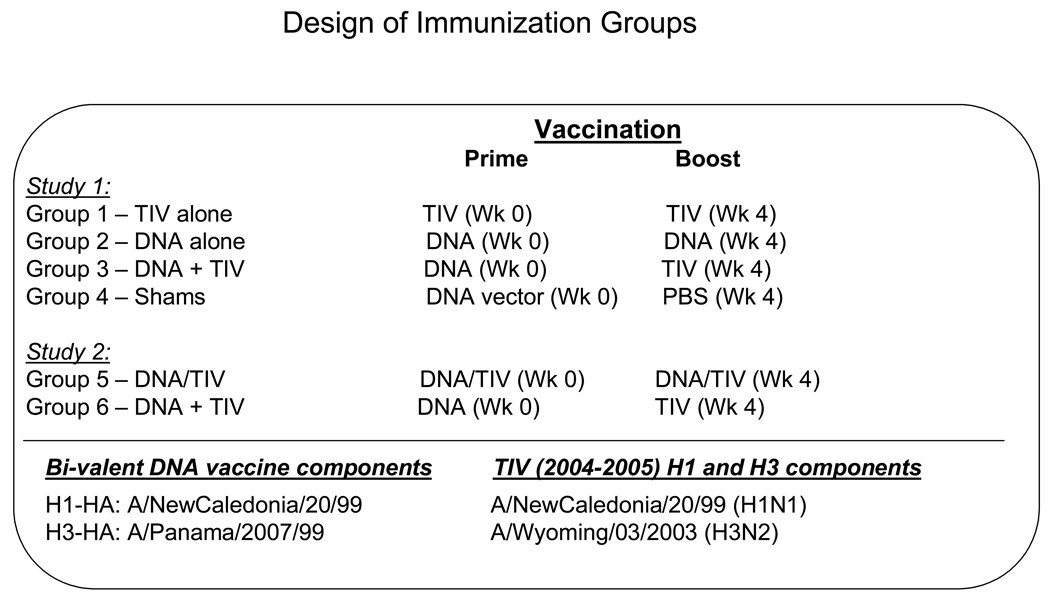
NZW rabbits (~ 2 kg body weight) were purchased from Millbrook Breeding Labs (Amherst, MA) for immunogenicity studies. Rabbits were housed in the Department of Animal Medicine at the University of Massachusetts Medical School in accordance with IACUC approved protocol. The rabbits (5 rabbits/group) received two immunizations at Weeks 0 and 4 with different combination immunizations (Fig 1). Half of a human dose (0.25 ml) of TIV was administered by intramuscular (IM) injection at each time point as indicated for Groups 1, 3, 5 and 6. The bivalent H1 and H3 HA DNA vaccines were delivered by a Helios gene gun (Bio-Rad) at the shaved abdominal skin, as previously reported [18]. For each immunization, a total of 36 µg of H1 and H3 HA DNA vaccine (18 µg of each HA DNA vaccine) was delivered to animals in Groups 2, 3, 5 and 6 at each immunization as indicated (Fig. 1). Animals in Group 4 served as a control, receiving 36 µg of empty DNA vector by gene gun at Week 0 and 0.25 ml of PBS by IM at Week 4. Animals in Group 5 received both the TIV and HA DNA vaccines concurrently. Serum samples were taken prior to the first immunization and 2 weeks after each immunization for the study of HA-specific antibody responses.
Fig. 1.
Design of immunization studies in New Zealand White Rabbits.
2.4 ELISA (Enzyme-linked immunosorbent assay)
ELISA was conducted to measure the HA-specific antibody (IgG) responses in immunized rabbits, as previously described [18]. The 96-well flat-bottom plates were coated with 100 µl of ConA (50 µg/ml) for 1 hour at room temperature, and washed 5 times with PBS containing 0.1% Triton X-100. Subsequently, the plates were incubated overnight at 4°C with 100 µl of transiently expressed HA antigen at 1 µg/ml from 293T cells transfected with the H1 or H3 HA DNA vaccine plasmids. After being washed 5 times as described above, the plates were then blocked with 200 µl/well of blocking buffer (5% non-fat dry milk, 4% Whey, 0.5% Tween-20 in PBS at pH7.2) for 1 hour. After five washes, 100 µl of serially diluted rabbit or mouse serum was added in duplicate wells and incubated for 1 hour. After another set of washes, the plates were incubated for 1 hour at 37°C with 100 µl of biotinylated anti-rabbit or anti-mouse IgG (Vector Laboratories, Burlingame, CA) diluted at 1:1000 in Whey dilution buffer (4% Whey, 0.5% Tween-20 in PBS). Then, 100 µl of horseradish peroxidase-conjugated streptavidin (Vector Laboratories) diluted at 1:2000 in Whey buffer was added to each well and incubated for 1 hour. After the final washing, the plates were developed with 3,3’,5,5’ Tetramethylbenzidine (TMB) solution at 100 µl per well (Sigma, St. Louis, MO) for 3.5 minutes. The reactions were stopped by adding 25 µl of 2 M H2SO4, and the plates were read at OD 450 nm. The end titration titer was determined as the highest serum dilution that has an OD reading above twice of that from the negative control serum.
2.5 Preparation of influenza A virus stocks
The human influenza A viruses A/NewCaledonia/20/99 (H1N1), and A/Panama/2007/99 (H3N2) were obtained from the Center for Disease Control (CDC, Atlanta, GA). The A/NewCaledonia/20/99 virus represents the H1N1 component of the licensed human TIV and the A/Panama/2007/99 (H3N2) virus closely matches the H3N2 components of the licensed human TIV used in the current study. These viruses were cultured in the allantoic cavities of 10-day old embryonated hen eggs and incubated for 2 days at 37°C. The allantoic fluid was collected and stored at −80°C. The viruses were titrated in Madin-Darby Canine Kidney (MDCK) cell cultures to determine the plaque forming units per ml (pfu/ml).
2.6 Assays for protective antibodies
Both hemagglutination inhibition (HI) and microneutralization antibody (MN) responses were measured, as previously reported [18]. Sera were treated with receptor-destroying enzyme (RDE; Sigma-Aldrich) as described [19]. The lyophilized bacterial neuramindase product was reconstituted with 5 ml sterile distilled water, diluted with 95 ml calcium saline (pH 7.2), aliquoted and stored at −20°C (working RDE). Working RDE was combined with each sera sample in a 4:1 ratio (0.2 ml RDE: 0.05 ml serum) and incubated overnight at 37°C. Following overnight incubation, 0.15 ml of 2.5% sodium citrate was added to each sample and incubated for 30 minutes at 56°C to inactivate the remaining RDE. Finally, we added 0.1ml of PBS to raise the starting serum dilution to 1:10.
2.7 Hemagglutination inhibition (HI) assay
HI assays were performed using standard methods [20]. Briefly, 25 µl of each influenza virus strain with an HA titer of 8 HA units, was mixed with 25 µl of 2-fold dilutions of the specific RDE-treated serum in PBS in V-bottom 96-well plates. After 30 minutes incubation at room temperature, 50 µl of 0.5% chicken erythrocytes was added to each mixture. The plates were kept at 4°C until a positive hemagglutination was developed in non-serum containing control wells. The HI titer was defined as the highest dilution of the serum able to inhibit hemagglutination.
2.8 Microneutralization (MN) assay
Titers of neutralizing antibodies (NAbs) were determined, as previously described [21]. In brief, 50 µl of influenza virus containing 100 pfu was incubated with 50 µl of 2-fold dilutions of the specific RDE-treated serum for 1 hour at room temperature in a 96-well plate containing an MDCK cell monolayer. After incubation, the virus-serum mixtures were removed from the wells. The cells were incubated at 37°C for 2 days, in MEM/BA supplemented with 1 µg/ml of TPCK trypsin in the presence of 2-fold dilutions of the specific RDE-treated serum. The MN titer was defined as the highest dilution of serum that neutralized 100 plaque-forming units of virus in MDCK cell cultures (as detected by negative hemagglutination).
2.9 Statistical analysis
Student’s t test was used to analyze the differences in antibody responses between animal immunization groups, as measured by ELISA, HI and MN antibody titers. A p value of less than 0.05 was considered significant.
3. RESULTS
3.1 Design of immunization studies in NZW rabbits
Two immunogenicity studies were organized in the current report (Fig. 1). For the first study, three groups of NZW rabbits were immunized twice with one of the following immunization regimens. Animals in Group 1 received ½ of the normal human dose of a clinically licensed TIV influenza vaccine for the 2004–2005 flu season by intramuscular (IM) injection. This TIV includes HA antigens from influenza virus isolates A/NewCaledonia/20/99 (H1N1) and A/Wyoming/03/2003 (H3N2). Group 2 received a bivalent DNA vaccine that expresses one HA antigen from the same H1 serotype virus (A/NewCaledonia/20/99) as in TIV and another HA antigen from an H3 serotype virus A/Panama/2007/99 (H3N2) which was included as part of the TIV vaccine for the 2003–2004 season. Both of these HA genes were codon optimized which does not change the original amino acid sequences of the HA antigens. The immunogenicity of these two HA-expressing DNA vaccines has been previously reported in both rabbits and mice models [18]. Each rabbit received a total of 36 µg of the bivalent HA DNA vaccine, delivered by a gene gun. In the third group, rabbits were first immunized with the bivalent HA DNA vaccine, followed by a second immunization using TIV as the boost. Group 4 is the negative control group, receiving vector DNA at Week 0 and PBS Week 4.
Based on the results of the first study (see below), a second study (Groups 5 and 6) was conducted to compare the immunogenicity of co-delivery of the DNA and TIV vaccine formulations at both immunizations (Weeks 0 and 4) vs. a sequential DNA prime-TIV boost regimen, as described above (Fig. 1).
3.2 Immunogenicity of homologous vs. heterologous prime-boost regimens
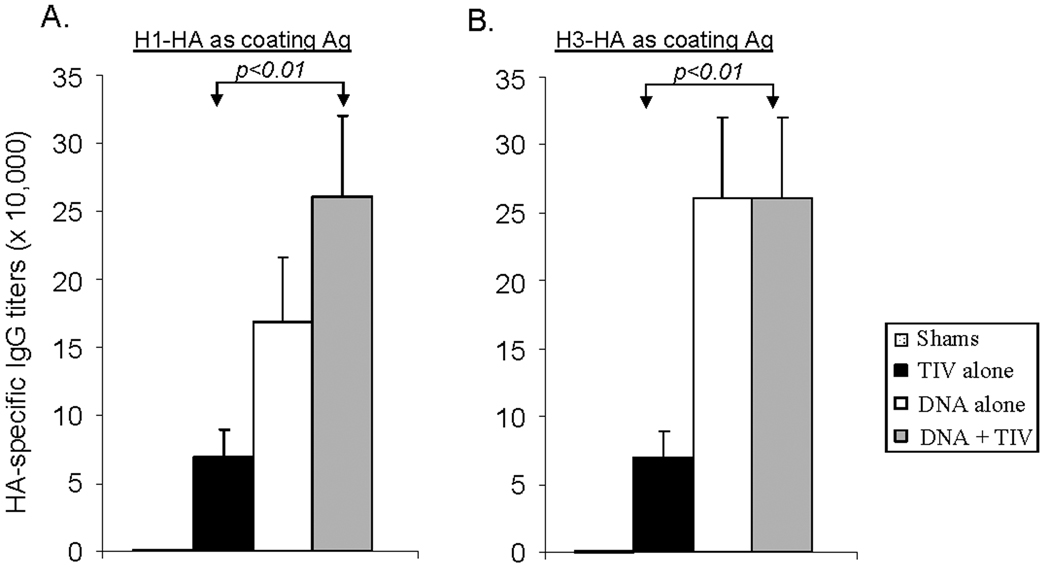
Levels of HA-specific IgG responses in immunized rabbit sera were first measured by ELISA (Fig. 2). At the end of two immunizations, TIV was able to elicit high titer anti-HA IgG up to ~1:70,000. In contrast, DNA immunization was able to elicit even higher titers of anti-HA IgG than TIV: approximately twice that against HA of the H1 serotype (Fig. 2-A) and 3–4 fold higher against HA of the H3 serotype (Fig. 2-B). Results from the current study indicate that the DNA prime and TIV boost was also able to elicit either similar or higher serum IgG responses against HA of both the H1 and H3 serotypes when compared with the DNA alone group (Fig. 2). The difference in the levels of anti-HA IgG between DNA prime-TIV boost and TIV prime-TIV boost was statistically significant (p < 0.01) (Fig. 2). Control rabbits in Group 4 did not show detectable HA-specific IgG responses.
Fig. 2. Antigen specific IgG titers.
Peak level serum anti-HA IgG antibody responses induced by vaccination with the DNA vector/PBS (shame), TIV alone, DNA alone or DNA prime + TIV boost regimens in NZW rabbits as measured by ELISA with (A) H1-HA as the coating antigen or (B) H3-HA as the coating antigen. Both HA antigens were produced from supernatant of 293T cells transfected with the HA DNA vaccine plasmids. Data shown are the geometric mean titers of each group (5 rabbits/group), with standard deviation bars (error bars). The statistical difference between each group was determined and groups with p < 0.01 are indicated.
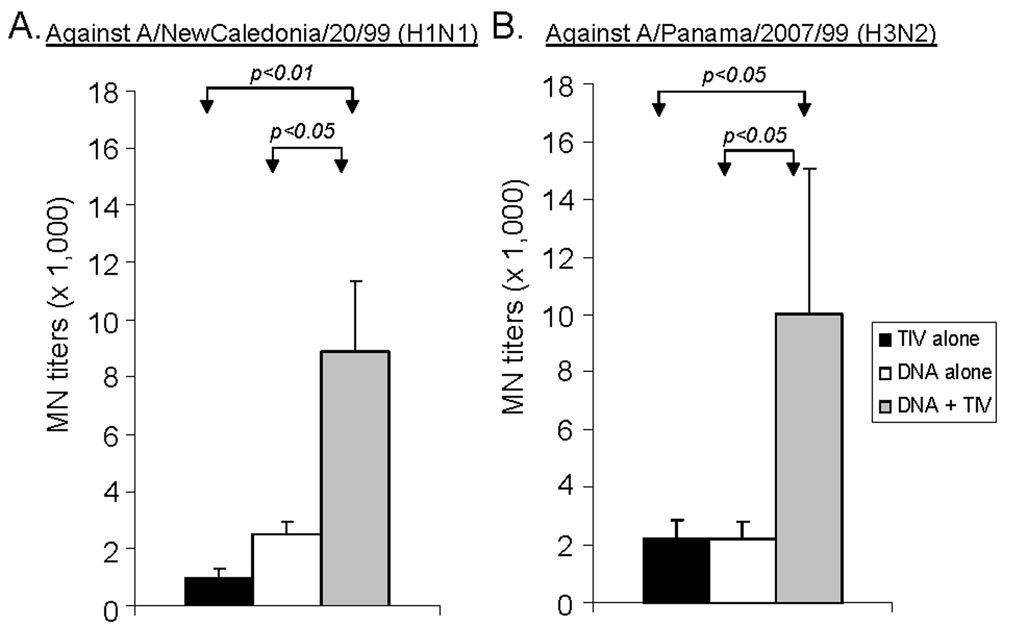
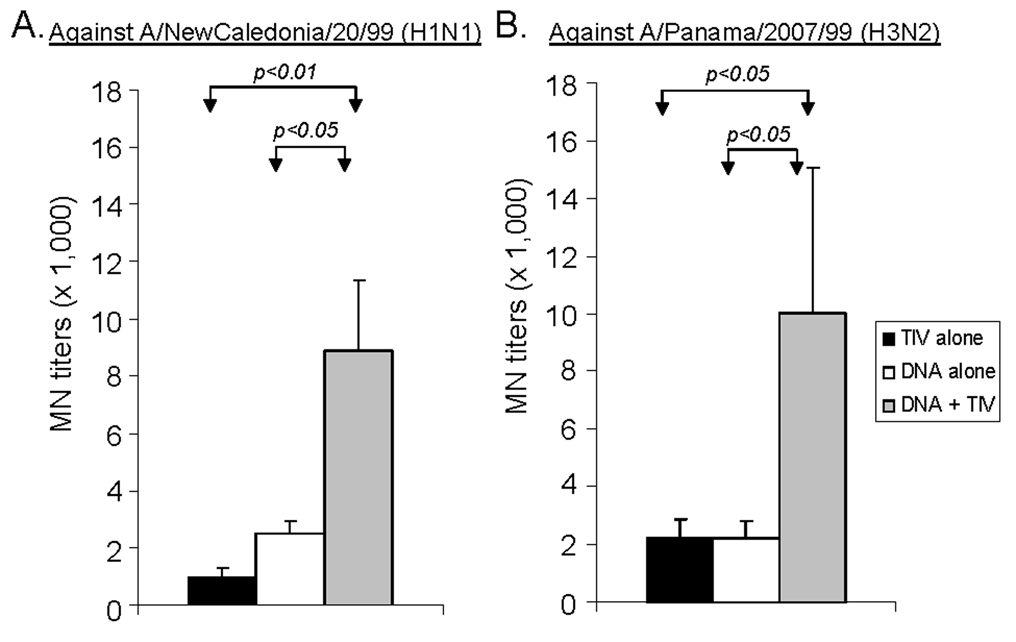
The levels of functional antibodies in immunized rabbit sera were measured using hemagglutination inhibition (HI) and microneutralization (MN) assays (Fig. 3–Fig. 4). For antibodies against H1 serotype virus, a matched A/NewCaledonia/20/99 (H1N1) was used. The difference between homologous (DNA alone or TIV alone) and heterologous (DNA-TIV) prime-boost regimens was highly significant. DNA-TIV consistently elicited higher levels of HI antibodies than was observed following DNA alone or TIV alone (p<0.01, in both cases). For MN antibodies, the DNA-TIV regimen elicited higher levels of antibodies when compared to the DNA alone or TIV alone regimens (p<0.05 and p<0.01, respectively). For antibodies against H3 serotype viruses, both A/Panama/2007/99 (H3/N2), matching the HA used for DNA prime and A/Wyoming/03/2003 (H3N2), matching the HA used for TIV boost, were tested. A similar pattern of functional antibodies were observed for both viruses and only data for A/Panama/2007/99 are included here. The difference between homologous and heterologous prime-boost regimens was also significant (Fig. 4). The HI and MN antibody titers in pre-bleed and vector control immune rabbit sera in the above assays were < 1:40 (data not shown).
Fig. 3.
HI antibody responses in NZW rabbit sera immunized with different vaccination regimens (i.e., TIV alone, DNA alone or DNA prime + TIV boost). The HI antibody titers at Week 6 (2 weeks after the 2nd immunization) are shown as geometric means for each group (5 rabbits/group), with standard deviations (error bars) against (A) A/NewCaledonia/20/99 (H1N1) and (B) A/Panama/2007/99 (H3N2) influenza viruses. The statistical difference between each group was determined and groups with p < 0.05 or p < 0.01 are indicated.
Fig. 4.
The MN responses in NZW rabbit sera immunized with different vaccination regimens (i.e., TIV alone, DNA alone or DNA prime + TIV boost). NAb titers at Week 6 (2 weeks after the 2nd immunization) against (A) A/NewCaledonia/20/99 (H1N1) and (B) A/Panama/2007/99 (H3N2) virus infection for MDCK cells are shown as the geometric means from each group (5 rabbits/group) with standard deviations (error bars). The statistical difference between each group was determined and groups with p < 0.05 or p < 0.01 are indicated.
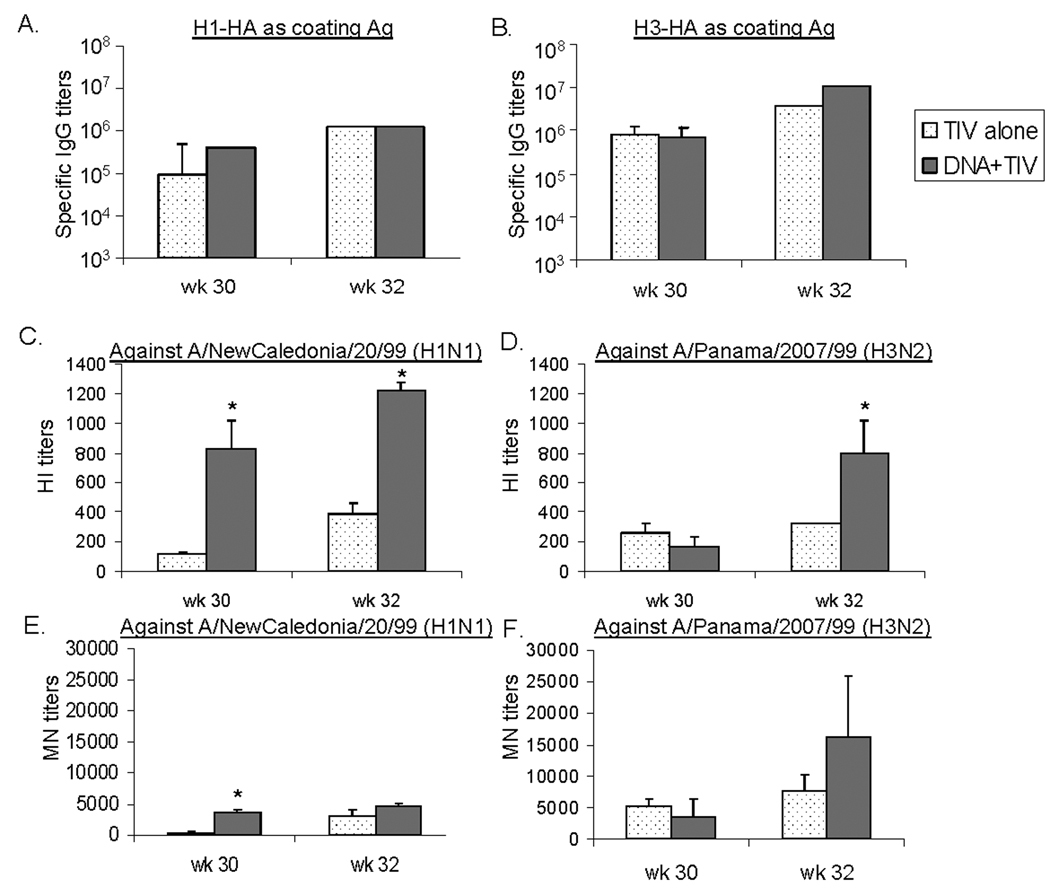
3.3 Long-term antibody responses in immunized animals
In order to determine long-term antibody responses in immunized rabbits, animals in Group 1 (TIV-TIV) and Group 3 (DNA-TIV) were followed for more than 30 weeks. At that time, the overall serum HA-specific antibodies against either H1-HA or H3-HA antigens, according to ELISA, were similar between these two groups (Fig. 5-A and Fig. 5B, Week 30). However, functional antibodies (HI and MN) against A/NewCaledonia/20/99 were significantly higher in the DNA-TIV (Group 3) when compared to the group that received TIV-TIV alone (Group 1) (p < 0.05) (Fig. 5-C and 5-E, Week 30). On the other hand, HI and MN antibodies against the H3 serotype virus showed that there was no significant difference between rabbits which received TIV-TIV (Group 1) and those that received DNA-TIV (Group 3) (Fig. 5-D and 5-F, Week 30). Because the H3 serotype HA antigens used in TIV prime and DNA boost are not completely matched, the functional antibodies may not be properly boosted against the key protective epitopes. This may explain the difference of functional antibodies between H1 and H3 serotypes observed in the above experiment.
Fig. 5.
Long-term antibody responses in NZW rabbit sera immunized with different vaccination regimens (i.e., TIV alone or DNA prime + TIV boost). Rabbits received a 3rd immunization with TIV at Week 30 (26 weeks after the last injection). Serum samples were collected at Weeks 30 (prior to the 3rd immunization) and 32. Binding and functional antibodies against A/NewCaledonia/20/99 (H1N1) and A/Panama/2007/99 (H3N2) were measured. Peak level serum (A) anti-H1-HA IgG and (B) anti-H3-HA antibody responses, as measured by ELISA; HI antibody responses against (C) A/NewCaledonia/20/99 (H1N1) and (D) A/Panama/2007/99 (H3N2); and MN antibody responses against (E) A/NewCaledonia/20/99 (H1N1) and (F) A/Panama/2007/99 (H3N2), shown as geometric means from each group (5 rabbits/group) with standard deviations (error bars), if applicable. * indicates a p < 0.05 between the two immunization regimens at a particular time point.
These rabbits received a 3rd immunization with TIV on the same day following blood collection, at 26 weeks after the previous 2nd immunization. Two weeks later (Week 32), both binding and functional antibodies were measured. ELISA showed further increases of HA-specific serum IgG in these animals but the levels after the boost are similar for the TIV-TIV and DNA-TIV groups (Fig. 5-A and 5-B, Week 32). Levels of functional antibodies (HI and MN) against both the H1 and H3 serotypes were also increased after the boost in both groups (Fig. 5-C to Fig. 5-F, Week 32). Final functional antibody titers were again higher in the DNA-TIV group than in the TIV-TIV group. The difference was significant for the HI titers (p < 0.05).
3.4 Immunogenicity of co-delivery vs. sequential prime-boost regimens
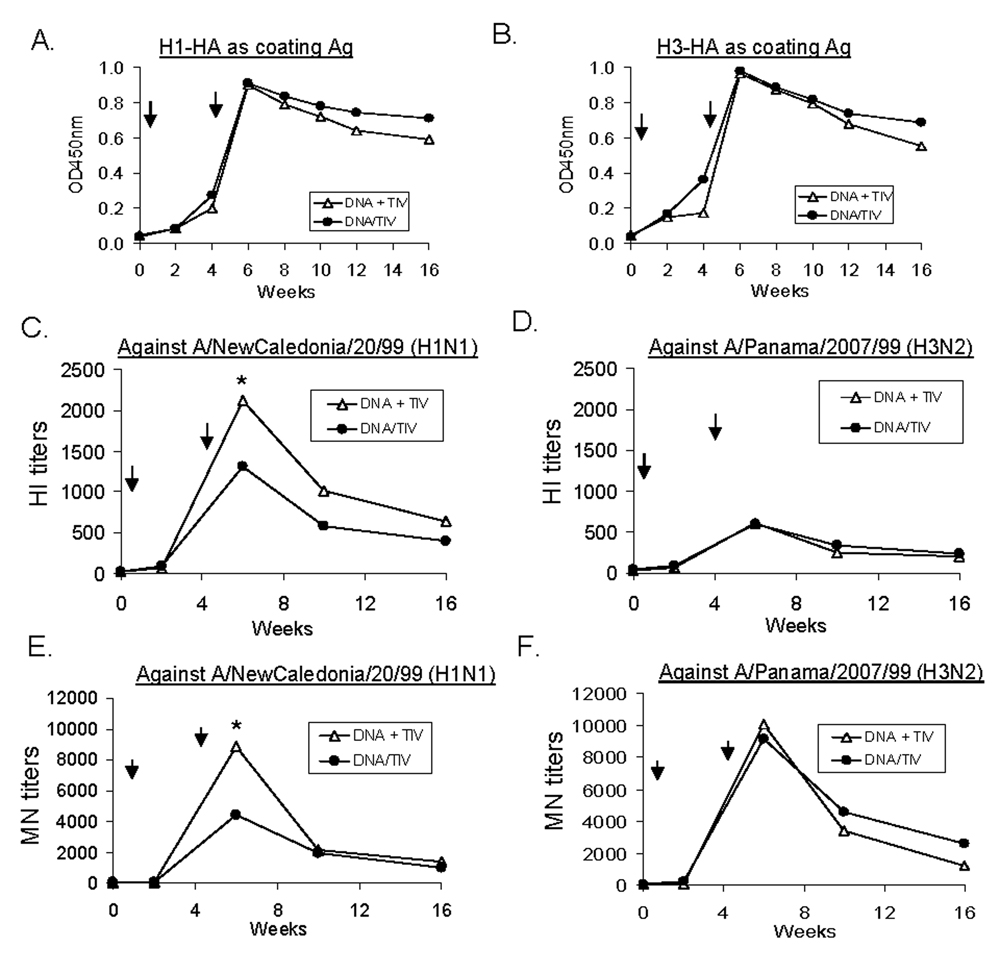
Results from the above study demonstrated that a combination of HA DNA vaccines and TIV vaccines is more immunogenic than either vaccine alone in eliciting high level antibody responses, including long-term functional antibody responses. Next, we wanted to determine whether the same high levels of immune responses can be observed if both types of vaccines are delivered simultaneously with only one immunization as compared to the sequential delivery regimen that requires two immunizations. A second immunogenicity study was conducted (Fig. 1). Group 5 (DNA/TIV × 2) received both the HA DNA vaccine and TIV at the same time at Weeks 0, followed with a boost with the same combination DNA/TIV at Week 4. Rabbits in Group 6 (DNA-TIV) received the HA DNA vaccine at Week 0, and the TIV at Week 4, as described above for Group 3. The dosing for the HA DNA vaccine and TIV was the same at each immunization which essentially doubled the total amount of DNA and TIV for the study period in Group 5 when compared to Group 6.
After one immunization, antibody responses (both binding and functional antibodies) elicited by the co-delivery of DNA and TIV (Group 5) were the same as observed in Group 6, which received either DNA or TIV alone once (Fig. 6) and both were at barely detectable levels. This indicates that co-administration of DNA and TIV in one immunization did not achieve any boosting effect (even when they are delivered via different methodologies, i.e., IM and gene gun) when compared to using either component alone.
Fig. 6.
Binding and functional antibody responses in NZW rabbit sera immunized with different vaccination regimens (i.e., co-administration of DNA + TIV or sequential DNA prime + TIV boost). Rabbits were immunized at Weeks 0 and 4 (as indicated by the arrows) and serum samples were collected 2 weeks for after each immunization. Peak level serum (A) anti-H1-HA IgG and (B) anti-H3-HA IgG responses, as measured by ELISA; HI antibody responses against (C) A/NewCaledonia/20/99 (H1N1) and (D) A/Panama/2007/99 (H3N2); and MN antibody responses against (E) A/NewCaledonia/20/99 (H1N1) and (F) A/Panama/2007/99 (H3N2), shown as geometric means from each group (5 rabbits/group). * indicates a p < 0.05 between the two immunization regimens at a particular time point.
After two immunizations, the overall antibody responses elicited in these two groups were very similar (Fig. 6). Both the temporal pattern and the peak levels of HA-specific IgG responses were almost identical (Fig. 6-A) which was not unexpected given the fact that both groups received the DNA prime-TIV boost. For the functional antibody analyses, similar antibody response kinetics were observed, except for the peak level HI and MN antibody titers against the A/NewCaledonia/20/99 virus, in which levels were higher following the DNA prime-TIV boost approach compared to when these two types of vaccines were co-administered (p<0.05) (Fig. 6-B and 6-C). This result suggests that there is no added benefit when both types of vaccines were co-delivered, which was surprising considering the fact that this co-delivery approach actually doubled the amount of administered vaccines for the entire two-immunization course. However, as indicated by the results from the co-delivery approach after one immunization, this second study confirmed the importance of prime-boost in sequence rather than simply combining two vaccines in one formulation.
4. DISCUSSION
In the current study, we used the licensed influenza vaccine against the H1 and H3 serotypes as a model system to study the immunogenicity of an inactivated influenza virus vaccine in animals that were sero-negative to influenza virus antigens. Then, we compared the relative levels of protective antibodies between homologous (DNA + DNA or TIV + TIV) and heterologous (DNA + TIV) prime-boost vaccination strategies. In this study, New Zealand White rabbits were used to study the immunogenicity of various vaccination regimens. In many previous studies, mouse models were frequently used with the potential benefit to observe the protective effect of vaccines when immunized mice are subject to a lethal influenza virus challenge. On the other hand, achieving protection in mouse models is relatively easy even when the levels of protective antibodies were below detection prior to challenge [22]. At the same time, each mouse can only provide a limited amount of blood which does not allow for the extensive array of antibody assays used to characterize different antibody responses. Rabbit is a widely used animal model for the study of vaccine immunogenicity due to the fact that rabbits are highly immunogenic in that they produce high-titer antigen-specific antibodies and a large amount of blood can be obtained for various assays, as we have recently demonstrated [18]. While there is limited experience in using rabbit model for challenge studies, functional antibody analyses provide surrogate markers for the protection efficacy of influenza vaccines. For example, the levels of increase in HI antibody responses are used as part of the licensing criteria for human influenza vaccines. Using rabbit immune sera in the current study, we were able to conduct various binding and functional antibody assays to compare the relative immunogenicity among different influenza vaccination regimens.
In current report, we have shown that the clinically licensed trivalent inactivated influenza virus vaccine (TIV) has poor immunogenicity in naïve animal hosts. Furthermore, HA-specific antibodies and functional antibodies, as measured by HI and MN assays, were even lower than in HA DNA vaccinated animals when the same two-dose immunization schedule at Weeks 0 and 4 was employed. Interestingly, the heterologous DNA prime and TIV boost elicited significantly higher levels of both binding and protective antibodies when compared to homologous prime-boost immunization regimens that used either the TIV or DNA vaccines alone. The effect of this heterologous prime-boost approach is long-lasting and was still the most effective approach when the immunized hosts received another TIV boost vaccination approximately 6 months later. On the other hand, one time co-delivery of DNA and TIV, with the same amount of two vaccines as that used in the regular sequential DNA-TIV regimen, could not achieve the same levels of antibody responses elicited by the DNA-TIV. Even with two immunizations, the co-delivery approach was not more effective in eliciting antibody responses when compared to the sequential prime-boost regimen despite the fact that the co-delivery approach resulted in the administration of 2 times the dose given to animals in the sequential prime-boost group. Putting these data together, the sequential approach has clear immunologic advantage that can not be replaced by the homologous prime-boost or co-delivery of both types of vaccines.
New mutant strains of seasonal influenza H1 and H3 serotypes continue to emerge and the worldwide spread of the highly pathogenic avian influenza H5 serotype has raised concern over the next influenza pandemic. Administration of the currently licensed TIV has been effective in reducing the morbidity and mortality associated with seasonal influenza virus infections in humans with only one injection [23]. This was accomplished most likely due to a priming effect on the immune system as a result of previous vaccination and/or to previous exposure to related influenza virus serotypes. However, in the event of a pandemic influenza virus of the H5N1 serotype, a pre-existing immunity would be lacking in the majority of the population as would any previous vaccination. This, in conjunction with recent literature suggesting that two immunizations are needed for an inactivated H5 avian influenza vaccine to be effective in eliciting protective levels of antibody responses [13, 16, 17], would require that an unprecedented number of vaccine doses be produced. While a number of strategies have been tested to increase the immunogenicity of inactivated H5 avian influenza vaccines, including higher antigen doses and the inclusion of an adjuvant [16], this H5 inactivated vaccine still requires two immunizations in order to be protective. Furthermore, the need for higher doses and/or additional components (i.e., adjuvants) will add an additional burden to a complicated influenza vaccine production process and will further impact an already insufficient influenza vaccine production capacity throughout the world. Therefore, additional novel strategies are needed to prepare the world against a potential pandemic avian influenza virus infection.
While the current study was not designed to conduct an in-depth immunological analysis on the mechanism(s) of why a heterologous prime-boost regimen would be more effective than a homologous prime-boost, our results indicate that the DNA prime may be more effective than the TIV in priming the immune system. This hypothesis is partially supported by our current results showing that a prime-boost regimen with the DNA vaccine alone is more immunogenic than the TIV alone prime-boost approach. It is possible that DNA immunization is more effective in eliciting better and potentially longer lasting HA-specific B cell memory. Well established literature on B cell immunology suggests that low dose antigen delivery is more effective in eliciting better antibody responses and B cell memory [24, 25]. If a DNA vaccine is truly effective in priming a longer lasting B cell memory, then this technology can be very useful for the overall strategy of avian influenza vaccine development against a potential pandemic.
A key challenge facing avian influenza vaccine developers is the lack of sufficient manufacturing facilities in the world. With such limited capacity, the ability to produce enough doses of an avian influenza vaccine at the time of outbreak of a pandemic within a narrow window of time would be extremely difficult, if not impossible. Stockpiling of vaccine can relieve some of this pressure but the world’s stockpiling capacity is also limited as the shelf life of past seasonal influenza vaccines has been short, partially due to the need to modify influenza vaccine formulations to correspond to the predicted circulating viruses for that particular season. In the case of avian influenza, it is already known that H5N1 avian influenza viruses already have several different genetic clades based on its evolution in the last 10 years. At this point, nobody can predict which strain of H5 virus will be the source of a potential pandemic which makes stockpiling vaccines very difficult.
Results from the current report provide a more attractive solution. DNA vaccines expressing different H5 HA antigens can be produced and administered to the population in order to prime the immune system before an influenza pandemic. This will allow for a strong recall protective antibody response upon the administration of an inactivated H5 avian influenza vaccine boost which can be produced at the time of pandemic outbreak. Since the currently licensed inactivated avian influenza vaccine requires two doses in order to elicit an immune response, DNA immunization will reduce the number of inactivated avian influenza virus vaccine doses by at least half. Production of a DNA vaccine is simple and has a relatively low cost, particularly if the DNA vaccine can be delivered by a highly effective delivery method as was recently shown using a gene gun device to elicit protective antibody responses in humans [26]. Multiple DNA vaccines expressing several different HA antigens from the H5 serotype can be injected at the same time to serve as a prime for broad protection once boosted with an inactivated influenza virus vaccine matching with the pandemic strain. However, practical issues need to be considered for including a DNA prime. For example, how to clearly label the prime and boost vaccines so there will be no confusion on which one should be used first. However, this should not be a problem for pre-pandemic avian flu immunization since the DNA prime immunization will be given once and the boost is only needed at the time of pandemic outbreak. Future studies should analyze the immunogenicity and protection potential a DNA prime-inactivated vaccine boost strategy against H5N1 viruses in both animal model and human populations.
Acknowledgements
This work was partially supported by NIH/NIAID U01 AI 056536 (S. Lu) and CIVIA, a human immunology center supported by NIH/NIAID U19 grant AI62623 (AG-S). The project also used core facility resources at the University of Massachusetts Medical School supported by NIH grant 5P30DK32520 from the NIDDK. We thank Dr. Jill M. Grimes-Serrano for critical reading of the manuscript and Richard Cadagan for excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palese P. Influenza: old and new threats. Nat Med. 2004 Dec;10(12 Suppl):S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005 Sep 29;353(13):1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 3.Webster RG, Govorkova EA. H5N1 influenza--continuing evolution and spread. N Engl J Med. 2006 Nov 23;355(21):2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 4.Palese P, Garcia-Sastre A. Influenza vaccines: present and future. J Clin Invest. 2002 Jul;110(1):9–13. doi: 10.1172/JCI15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 6.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999 Nov;73(11):9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002 Aug 19;20(25–26):3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann G, Fujii K, Kino Y, Kawaoka Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc Natl Acad Sci (USA) 2005;102(46):6108–6113. doi: 10.1073/pnas.0505587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann G, Watanabe T, Ito H, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci (USA) 1999;96(16):9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schickli JH, Flandorfer A, Nakaya T, Martinez-Sobrido L, García-Sastre A, Palese P. Plasmid-only rescue of influenza A virus vaccine candidates. Philos Trans R Soc Lond B Biol Sci. 2001;356(1416):1965–1973. doi: 10.1098/rstb.2001.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20(25–26):3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 13.FDA. FDA Approves First U.S. Vaccine for Humans Against the Avian Influenza Virus H5N1; 2007. 2007 April;17
- 14.FDA. [cited 2007 2007/11/14];Influenza: Vaccination Still the Best Protection [FDA Consumer magazine] 2006 Available from: http://www.fda.gov/fdac/features/2006/506_influenza.html. [PubMed]
- 15.Cox RJ, Brokstad KA, Ogra P. Influenza Virus: Immunity and Vaccination Strategies. Comparison of the Immune Response to Inactivated and Live, Attenuated Influenza Vaccines. Scandinavian J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 16.Bresson J-L, Perronne C, Launay O, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. The Lancet. 2006;367(9523):1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Zhang J, Dong X, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. The Lancet. 2006;368(9540):991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Taaffe J, Parker C, et al. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J Virol. 2006;80(23):11628–11637. doi: 10.1128/JVI.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendal AP, Skehel JJ, Pereira MS. World Health Organization Collaborating Centers from Reference and Researchon Influenza: concepts and procedures for laboratory-based influenza surveillance. 1982:B17–B35.
- 20.Kendal AP, Skehel JJ, Pereira MS. World Health Organization Collaborating Centers for Reference and Research on Influenza: Concepts and Procedures for Laboratory-Based Influenza Surveillance. 1982:B17–B35.
- 21.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71(6):4347–4355. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Nat Acad Sci USA. 1993;90(24):11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21(16):1769–1775. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Fernandez A, Milstein C. Low antigen dose favours selection of somatic mutants with hallmarks of antibody affinity maturation. Immunology. 1998 Feb;93(2):149–153. doi: 10.1046/j.1365-2567.1998.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bot A, Antohi S, Bona C. Immune response of neonates elicited by somatic transgene vaccination with naked DNA. Front Biosci. 1997 May 1;2:d173–d188. doi: 10.2741/a181. [DOI] [PubMed] [Google Scholar]
- 26.Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006 May 22;24(21):4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]