Abstract
Histopathological studies and animal models suggest that different physiological and pathophysiological processes exert different subfield specific effects on the hippocampus. High-resolution images at 4T depict details of the internal structure of the hippocampus allowing for in vivo volumetry of hippocampal subfields. The aims of this study were (1) to determine patterns of hippocampal subfield volume loss due to normal aging and Apo e4 carrier state, (2) to determine subfield specific volume losses due to preclinical (MCI) and clinical Alzheimer’s disease (AD) and their modification due to age and Apo e4 carrier state. One hundred fifty seven subjects (119 cognitively healthy elderly controls, 20 MCI and 18 AD) were studied with a high resolution T2 weighted imaging sequence obtained at 4T aimed at the hippocampus. Apo e4 carrier state was known in 95 subjects (66 controls, 14 MCI, 15 AD). Subiculum (SUB), CA1, CA1–CA2 transition zone (CA1–2 transition), CA3- dentate gyrus (CA3&DG) were manually marked. Multiple linear regression analysis was used to test for effects of age, Apo e4 carrier state and effects of MCI and AD on different hippocampal subfields. Age had a significant negative effect on CA1 and CA3&DG volumes in controls (P < 0.05). AD had significantly smaller volumes of SUB, CA1, CA1–2 transition, and MCI had smaller CA1–2 transition volumes than controls (P < 0.05). Apo e4 carrier state was associated with volume loss in CA3&DG compared to non-Apo e4 carriers in healthy controls and AD. Based on these findings, we conclude that subfield volumetry provides regional selective information that allows to distinguish between different normal and pathological processes affecting the hippocampus and thus for an improved differential diagnosis of neurodegenerative diseases affecting the hippocampus.
Keywords: CA1, dentate, volumetry, neurodegeneration, MCI
INTRODUCTION
Its neurochemical properties, for example, high concentrations of glutamate and glutamate receptors and of adrenal steroid receptors, its neuroplasticity and lifelong ability for neurogenesis render the hippocampus particularly vulnerable to various kinds of insults (Schmidt-Kastner and Freund, 1991; McEwen, 2002). As a consequence, hippocampal neuronal dysfunction or neuron loss are not only features of so different brain disorders such as Alzheimer’s disease (AD), multiple sclerosis, epilepsy, schizophrenia, post-traumatic stress syndrome, or traumatic brain injury (McEwen and Magarinos, 1997; Bluemcke et al., 1999; Harrison, 2004; West et al., 2004; Swartz et al., 2006; Papadopoulos et al., 2009), but also of non-brain disorders, for example, hypertension or diabetes (Petrovitch et al., 2000; Korf et al., 2006). However, the hippocampus is not a homogeneous structure but consists of several histologically and functionally specialized but tightly interconnected subfields: the subiculum (SUB), the cornu ammonis sectors (CA) 1–3, and the dentate gyrus (DG) (Duvernoy, 2005). Animal and histopathological studies have shown that different pathological conditions exert regional selective effects on hippocampal subfields, for example, AD and hypoxia damage preferentially CA1 (West et al., 2004), schizophrenia CA2 (Harrison, 2004), and post-traumatic stress syndrome CA3 (McEwen and Magarinos, 1997), and so forth. These findings suggest that neuroimaging methods able to obtain subfield specific information might allow for a better differentiation of different disease processes than total hippocampal volume that is currently the gold standard for the in vivo assessment of hippocampal structural damage.
The overall goal of this study is to demonstrate how subfield specific information from high resolution magnetic resonance imaging (MRI) images can be used to differentiate between physiological and pathological processes, and thus improve the diagnosis of AD. AD is the most common form of dementia as it is estimated that the majority of the 24 million people in world diagnosed with dementia suffer from AD. The cause of the most common form, that is, sporadic, late onset AD, is still unknown. Age is the most consistent epidemiological risk factor with the prevalence of the disease increasing from 1% at age 65 to 40–50% by the age of 95 (Wang and Ding, 2008). The best documented genetic risk factor for sporadic AD is apolipoprotein E4 (Apo e4), although it is insufficient to explain all cases of sporadic late onset AD as only 40–80% of all AD patients carry the Apo e4 allele (Waring and Rosenberg, 2008). There is increasing evidence that the molecular pathomechanisms leading to AD are active several years before the patient becomes cognitively impaired (DeKosky and Marek, 2003). The concept of mild cognitive impairment (MCI) is an attempt to clinically define this transition from normal aging to possible AD. Subjects diagnosed with MCI are not demented, but have significant deficits in one or several cognitive domains and an increased risk to develop dementia. The most relevant subtype for AD is amnestic MCI, which is characterized by subjective and objective memory deficits but otherwise intact cognitive functions.
In this study, high resolution hippocampal images obtained at 4T and a manual marking scheme for hippocampal subfield volumetry were used to address the following questions: (1) Can a subfield specific effect of Apo e4 and age be detected in cognitively intact subjects. (2) Are there subfield specific effects for MCI and AD, and how are they influenced by age and Apo e4.
METHODS
Study Populations
The subjects described in this study participated in different research projects undertaken by Center for Imaging of Neurodegenerative Diseases together with clinical collaborators at the University of California, San Francisco, the Veterans Administration Center, San Francisco and the California Pacific Medical Center, San Francisco. The findings in a subset of them have been reported previously (Mueller et al., 2007, 2008). Each of these projects had been reviewed and approved by the committees of Human Research of each of the involved institutions. Informed consent had been obtained by all participants or their legal representatives according to the Declaration of Helsinki. The effects of normal aging on subfields were studied in 119 cognitively intact, healthy subjects [female/male (f/m): 64/55, mean age: 53.4 ± 17.2, age range: f 22–85 yrs] who had been recruited as controls for various projects. The effects of Apo E4 were studied in a subset of 66 subjects of this population (f/m: 29/37, Apo e4 pos., i.e., at least one Apo e4 allele: 23; Apo e4 neg., i.e., no Apo e4 allele: 43, mean age: 61.0 ± 13.9), who agreed to genetic testing. Eighteen subjects (f/m: 6/12 mean age 69.1 ± 9.6, f/m: 6/12, mean MMSE 21.6 ± 5.1) diagnosed with AD according to the criteria of the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA), and 20 subjects (f/m 6/14, mean age: 73.5 ± 7.1, f, mean MMSE 28.0 ± 2.1) meeting the criteria (Petersen et al., 1999) for amnestic MCI were used to study the effect of preclinical and clinical AD on hippocampal subfields. The information regarding Apo E genotype was available for 15 AD subjects (mean age 67.5 ± 9.3, Apo e4 pos: 10, Apo e4 neg. 5) and 14 MCI (mean age 74.5 ± 6.5, Apo e4 pos: 7 Apo e4 neg.: 7).
MRI Acquisition
The following sequences were acquired on a Bruker Med-Spec 4T system controlled by a Siemens Trio™ console and equipped with a USA instruments eight channel array coil that consisted of a separate transmit coil enclosing the eight receiver coils: (1) High resolution T2 weighted fast spin echo sequence (TR/TE: 3,500/19 μs, 0.4 × 0.4 mm in plane resolution, 2-mm slice thickness, 24 interleaved slices without gap for subfield volumetry. (2) T2 weighted turbospin echo sequence (TR/TE 8,390/70 μs, 150° flip angle, 0.9 × 0.9 × 3 mm nominal resolution, 54 slices) for the determination of the intracranial volume (ICV).
Postprocessing and Manual Marking of Hippocampal Subfields
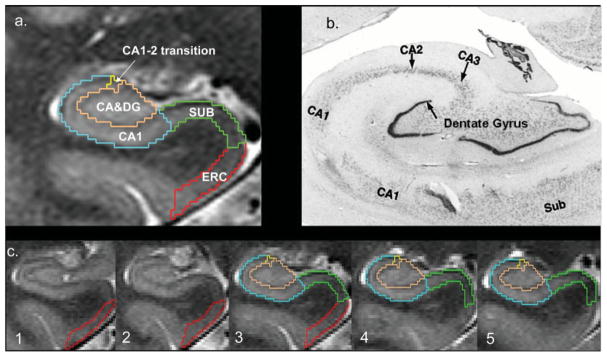
The method used for subfield marking including assessment of measurement reliability has been described in detail previously (Mueller et al., 2007, 2008). The marking scheme depends on anatomical landmarks, particularly, on a hypointense line representing myelinated fibers in the stratum moleculare/lacunosum that can be reliably visualized on these high resolution images. The distance between a point on this hypointense line and the outer boundary of the hippocampus provides a measure of the thickness of the hippocampal allocortex at this point. External and internal hippocampal landmarks are used to further subdivide the hippocampus into SUB, CA1, CA1–2 transition zone, and CA3 dentate gyrus. To summarize the procedure briefly: The marking starts on the first slice on which the head of the hippocampus is no longer visible (cf. Fig. 1). On this slice, the hippocampal subfields [CA1–CA3, dentate (DG), SUB and entorhinal cortex (EC) (ERC)] are marked manually. In addition, ERC is marked on the two slices anterior to this starting slice and SUB and CA1–3, including DG are marked on the two slices posterior to the starting slice. The most medial point of the temporal cortex is chosen as the medial border of ERC and the medial end of the collateral sulcus is chosen as its lateral border. The CA1/SUB border is determined by drawing a line perpendicular to the edge of SUB touching the medial border of the hippocampus. The CA1/CA2 border is determined by dividing the line along the longest diameter of the hippocampus by two and drawing a line perpendicular to this line. A region supposedly representing mainly CA2 was marked in a square-like manner, that is, its height at the CA1/CA2 boundary also determined its length, while its overall shape was determined by the course of the outer boundary of the hippocampus and the hypointense line representing myelinated tissue in the strata moleculare/lacunosum. Using this definition, CA2 is influenced by the thickness of the medial dorsal part of CA1. To reflect this, the region was named CA1–2 transition zone (CA1–2 transition) rather than CA2. The remainder of the hippocampal formation consisting of CA3 and dentate gyrus is marked as one region (CA3&DG). The ICV was calculated from the skull-stripped T2 image.
FIGURE 1.
(a) Parcellation scheme used for manual marking of subfields. As it is not possible to identify individual hippocampal layers at 4 T, the scheme was based on reliably recognizable anatomical landmarks even though this resulted in a part of the prosubiculum and subiculum proper being counted toward the CA1 sector. ERC, entorhinal cortex; CA1–2, CA1–CA2 transition zone (cf. methods in text); CA3&DG, CA3 and dentate gyrus. (b) Histological preparation of hippocampal subfields, arrow, dentate gyrus. (c) Typical example of hippocampal subfield markings. No. 1 is the most anterior slice, No. 5 the most posterior slice. No. 3 is referred to in the text as “starting” slice. Red, ERC; green, subiculum; blue, CA1; yellow, CA1–2 transition; maroon, CA3&DG.
Statistical Analysis
After excluding the presence of left/right differences, the sum of left and right subfield volumes were used for the analyses of the effects of aging, Apo E4, and AD on subfields. Multiple linear regression analyses were used to test for group differences, age, or Apo E4 effects if appropriate. Posthoc analyses were done with Duncan’s tests or Mann–Whitney tests (Apo e4 effect in AD and MCI). ICV in ccm was used as a covariate in all these analyses to account for volumetric differences due to different head sizes. In the results section and in the graphical representation, subfield volumes are reported as dimensionless units normalized to the head size using the following formula: volumenorm = volumeraw × 1,000/ICV in ccm.
RESULTS
Subfield Specific Effects of Age and Apo e4 on Cognitively Intact Subjects
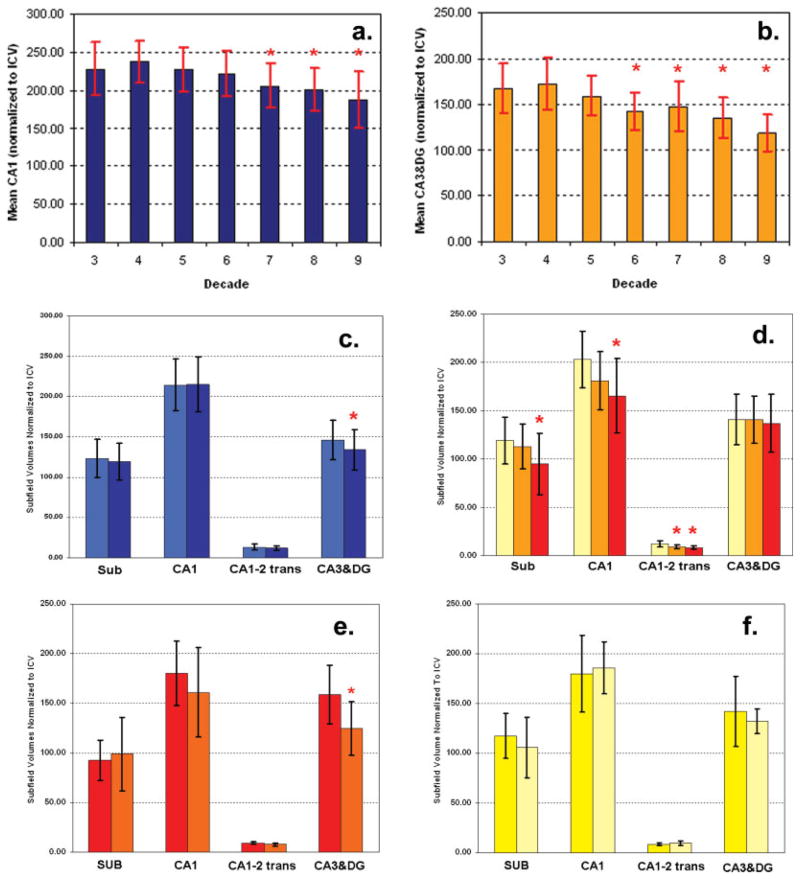
A significant negative age effect was found for CA1 (β = −1.02, P < 0.0001) and CA3&DG (β = −1.15, P < 0.001). In the next step, the subjects were grouped into age decades (3rd–9th decade) in order to test if CA1, CA3&DG, and total hippocampus volume change disproportionately over the age range (analysis of variance, Duncan posthoc tests, P < 0.05). CA1 volumes were stable until the 6th decade, after 60 yrs of age; CA1 volumes started getting increasingly smaller. CA3&DG volumes stayed stable up to the 5th decade and then became increasingly smaller (cf. Figs. 2a,b).
FIGURE 2.
(a) Age effect on CA1 in healthy controls. *, smaller (P < 0.05) compared to CA1 volume 3rd–5th decade; (b) Age effect on CA3-dentate in healthy controls. *, smaller (P < 0.05) compared to CA3&DG volume 3rd–4th decade. (c) Apo e4 effects in healthy controls (age range 22–85 yrs of age). Mean volumes and standard deviation (SD) of subjects without Apo e4 (bright blue), dark blue bars, mean volumes and SD of subjects with at least one Apo e4 allele (dark blue). *, smaller (P < 0.05) in Apo e4 carriers compared to non-Apo e4 carriers. (d) Mean volumes and SD of healthy, elderly controls (yellow), MCI (orange), and AD (red). *, smaller (P < 0.05) compared to corresponding volume in controls. (e) Apo E4 effect in AD. Mean volumes and SD of AD without Apo e4 (red) and with at least one Apo e4 allele (orange). *, smaller (P < 0.05) in Apo e4 carriers compared to non-Apo e4 carriers. (f) Apo E4 effect in MCI. Mean volumes and SD of MCI without Apo e4 (yellow) and MCI with at least one Apo e4 allele (bright yellow).
The analysis was repeated in the control population with known Apo e state and Apo e state included in the model. There was significant effect for Apo e4 (β = 9.45, P = 0.042) on CA3&DG, indicating that subjects without Apo e4 had larger CA3&DG volumes (146 ± 24.1) than subjects with Apo e4 (134.06 ± 25.1). Similarly as in the total population, there was also a significant effect for age on CA3&DG (β = −0.78, P = 0.02); however, the interaction between Apo e4 and age was not significant. CA1 volume was not influenced by Apo e4 carrier state (P = 0.81), but showed a significant age effect (β = −1.69; P = 0.0002) as in the previous analysis (cf. Fig. 2c).
Subfield Specific Effects of AD and MCI and Their Modification by Age and Apo e4
AD and MCI were analyzed together with an age- and gender-matched group of 53 cognitively intact controls. Multiple regression analysis showed significant effects for disease group (defined as control, MCI, and AD) for SUB (P = 0.003), CA1 (P = 0.0001), and CA1–2 transition (P < 0.0001) but not for CA3&DG. There was again a significant negative effect of age on CA1 (P = 0.0067) as in the previous analyses. Post-hoc analyses showed that AD had significantly reduced SUB (94.9 ± 31.4 vs. 119.2 ± 23.96), CA1 (165.6 ± 38.6 vs. 203.3 ± 29.3), and CA1–2 transition volumes (8.5 ± 2.0 vs.12.1 ± 3.3) compared to controls whereas CA3&DG volumes were not different (137.1 ± 29.9 vs. 141.0 ± 26.2). In contrast, the atrophy was more restricted in MCI who showed significant volume losses in CA1–2 transition (9.02 ± 1.64) compared to controls, but not in SUB (112.9 ± 23.4), CA1 (181.2 ± 30.3), or CA3&DG (140.9 ± 24.4). There were no significant differences between AD and MCI (cf. Fig. 2d).
The analysis was repeated in a subgroup with known Apo e4 carrier state (AD: Apo e4 pos/neg: 10/5; MCI: Apo e4 pos/neg: 7/7: 7; Controls: Apo e4 pos/neg 14/24, Apo e4 neg: 24). The results regarding group effect remained unchanged, that is, AD had smaller SUB, CA1, CA1–2 transition volumes and MCI had smaller CA1–2 transition volumes than controls. CA3&DG had a significant age effect (β = −1.58, P = 0.024) and Apo e4 effect (Apo e4 negative β = 15.7, P = 0.0031). Posthoc tests showed that AD with Apo e4 had smaller CA3&DG than those without (124.8 ± 26.8 vs. 159.0 ± 29.6) as did the group of elderly (60–85 yrs) controls (127.0 ± 24.7 vs. 144.0 ± 24.8). MCI with the Apo e4 also had smaller CA3&DG than those without (132.0 ± 12.5 vs. 141.9 ± 35.3); however, the difference was not significant (Figs. 2e,f).
DISCUSSION
The most important finding of this study was that in vivo subfield volumetry obtained using high resolution MR images and a manual parcellation scheme was able to identify regional selective effects of age and Apo e4 and AD on the hippocampus.
Influence of Age and Apo e4 on Hippocampal Subfields
Consistent with the findings of a previous study from this laboratory (Mueller et al., 2007), age was associated with volume loss in CA1. In addition to this, the current study also found a significant age effect for CA3&DG. The analysis by decade suggests that both CA1 and CA3&DG volumes remain relatively stable up to the 5th–6th decade, after which a continuous volume loss begins. There are two possible explanations for the discrepant finding regarding CA3&DG in the two studies. One is that compared to the current study, the sample size of the previous study was considerably smaller (n = 42) and thus had not sufficient power to detect the age effect on CA3&DG. The alternative explanation is that the two populations differ regarding another, unknown factor influencing CA3&DG. For example, based on the findings in the subgroup with known Apo e4 carrier state, it cannot be excluded that the distribution of Apo e4 carriers in the current study is such that it favors a volume loss of CA3&DG with increasing age (e.g., higher percentage of Apo e4 carriers in the group >50 yrs of age than in group < 50 yrs).
The histopathological correlate of the age-related volume loss is not entirely clear. Gray matter volume loss in neuroimaging studies is usually interpreted as neuron loss. Findings of autopsy and animal studies regarding age-related neuron loss are controversial though. Although some describe age-related loss of pyramidal neurons in CA1 and/or loss of granular cell loss in the dentate (Driscoll et al., 2003) others find only mild, nonsignificant neuron loss even in the oldest old (Keuker et al., 2000). However, there are other age-related structural changes described in CA1 and dentate, for example, age-related loss of astrocytes and oligodendrocytes (Hayakawa et al., 2007), loss of GABA-ergic interneurons (Shi et al., 2004), reduction of dendritic complexity (Geinismann et al., 2004; Davies et al., 2003, von Bohlen et al., 2006), and axonal degeneration (Ypsilanti et al., 2008) and so forth that are likely to contribute to the age-related volume loss in CA1 and CA3&DG found in this study.
The findings of this study suggest a selective effect of Apo e4 on CA3&DG in healthy controls. Apo e is a major lipid transporter of the brain that plays an important role in the regulation of maintenance and repair processes and also has neuroprotective properties. It exists in three isoforms, Apo e2, Apo e3, and Apo e4. Apo e4 is not only the least effective of the three, but can even exert detrimental effects, for example, disrupt the neuronal cytoskeleton, enhance the deposition of amyloid β or form neurotoxic fragments due to its inherent instability (Mahley et al., 2006). CA3&DG contains the dentate gyrus that is characterized not only by a high neuronal plasticity, but also by the capability of lifelong neurogenesis, that is, functions which are likely to be negatively influenced by Apo e4. There is evidence that this is indeed the case. Several studies in transgenic animals and also in autopsy studies in humans, for example, have found that Apo e4 is associated with impaired repair mechanisms and reduced synaptic plasticity in the dentate (Cambon et al., 2000; White et al., 2001; Ji et al., 2003). Furthermore, Levi and Michaelson (2007) found that environmental enrichment, which enhances neurogenesis in transgenic Apo e3 mice, is associated with increased apoptosis in transgenic Apo e4 mice. It is tempting to speculate that the CA3&DG volume loss observed in cognitively intact Apo e4 carriers in this study reflects indeed the loss of synaptic connectivity/neuroplasticity described in those histopathological studies. However, histopathological correlation studies will be necessary to confirm this assumption.
Influence of AD on Hippocampal Subfields
Hippocampal volume loss in AD was characterized by volume loss in SUB, CA1, CA1–2 transition but spared CA3&DG. This finding is consistent with the distribution of hippocampal neuron loss described in histopathological studies of AD (West et al., 2004, Zarow et al., 2005). MCI had a similar distribution of subfield volume loss as AD; however, only the volume loss in CA1–2 transition was severe enough to be significant. The mild, nonsignificant volume loss in SUB, CA1, CA3&DG is in good agreement with the mild, nonsignificant neuronal cell described in a histopathological study of MCI by West et al. (2004). West et al. (2004) however analyzed CA2 together with CA3 and described mild nonsignificant neuron loss in the CA2–3 region that seems to contrast with the severe volume loss in CA1–2 transition found in this study. However, significant neuron loss in CA2 with relative preserved neuron counts in CA3 has been described in AD (Fukutani et al., 2000; Zarow et al., 2005), and thus it cannot be excluded that this discrepancy is simply due to the fact that neither study assessed CA2 alone but combined it with a different neighboring subfield. Moreover, there is evidence from other studies that pathological alterations in CA2 can indeed occur in the early, preclinical stages of AD. For example, Ho et al. (2001) described that the expression of the proinflammatory protein cyclooxygenase 2 is upregulated in CA2 and CA3 but not CA1 in MCI, and Takayama et al. (2002) demonstrated that the accumulation of neurofibrillary tangles starts in CA2 in a subset of AD patients. Nonetheless, histopathological correlation studies will be necessary to confirm that CA1–2 transition or the medial dorsal aspect of the hippocampus is indeed early affected in MCI and to identify the histopathological correlate of this regional selective volume loss.
Similar to controls Apo e4 in AD was associated with a volume loss in CA3&DG, that is, the only subfield not affected by the AD process. CA3&DG was also smaller in MCI with Apo e4 than in those without it but not significantly so, which is most likely due to the small sample size of this group. None of the other subfields showed an Apo e4 effect. But again, the sample size of the AD and MCI group was relatively small and thus it cannot be excluded that we lacked the statistical power to differentiate between the strong AD effects active in CA1, CA1–2, SUB and ERC, and the probably more subtle effect of Apo e4. The findings in this small sample therefore have to be considered preliminary and have to be confirmed in a larger population. Ultimately, histopathological correlation studies will be necessary to verify and identify the histopathological correlate of the Apo e4 induced volume loss in CA3&DG in AD. Studies in transgenic animals and autopsy studies in AD patients however already provide some insight in potential underlying mechanisms of this finding. Jin et al. (2004), for example, showed that similar to findings in cognitively intact Apo e4 carriers, Apo e4 is associated with a reduced dendritic spine density in the dentate of AD. In addition to this, there is also evidence from studies in transgenic animals and autopsy studies of AD patients that AD is associated with an increased neurogenesis in the dentate and appearance of new neurons in CA1, but that these compensatory reactions are blunted by Apo e4 (Ji et al., 2003; Levi et al., 2007; Lopez-Toledano and Shelanski, 2007). Any of these mechanisms or a combination thereof could contribute to the selective CA3&DG volume loss observed in this study.
CONCLUSIONS
This study shows that hippocampal subfield volumetry provides potentially clinically useful information, which cannot be obtained by total hippocampal volumetry that is currently considered to be the gold standard in structural neuroimaging for the assessment of hippocampal damage. Consequently, there has been quite some effort over the last years to develop manual, semiautomated, and automated techniques for direct or indirect subfield volumetry (Zeineh et al., 2001; Wang et al., 2003; Lin et al., 2005; Mueller et al., 2007; Sicotte et al., 2008; Van Leemput et al., 2008; Yushkevich et al., 2009). Each of these methods has its strengths and limitations. Manual methods usually relay on internal hippocampal structures that are only visible on high resolution images acquired at higher field strength (3–7 T). The labeling is time consuming and requires an experienced rater, which limits their usefulness for large studies. Automated methods segment the hippocampus and then either warp the subject image onto a hippocampal subfield atlas or probabilistic subfield model or use a shape analysis approach to make inferences about subfield specific volume losses. Automated methods are more sensitive toward physiological and pathological shape variants and intensity changes, and more likely to be negatively influenced by suboptimal image quality than manual methods. If subfield volumetry, therefore, is to replace total hippocampal volumetry as the gold standard for the assessment of hippocampal pathology in the future, it will be necessary to develop a unified approach to evaluate the performance of the different methods for different clinical questions and to define a unified subfield parcellation scheme, since there are currently as many different parcellation schemes as there are labeling techniques.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: RO1 AG010897, P01 AG12435.
References
- Bluemcke I, Beck H, Lie AA, Wiestler OD. Molecular neuropathology of human mesial temporal lobe epilepsy. Epilepsy Res. 1999;36:205–223. doi: 10.1016/s0920-1211(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of ages human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:685–692. doi: 10.1016/s0306-4522(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Davies HA, Kelly A, Dhanrajan TM, Lynch MA, Rodriquez JJ, Stewart MG. Synaptophysin immunogold labeling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Res. 2003;986:191–195. doi: 10.1016/s0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward to move forward: Early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoullos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. Functional Anatomy, Vascularization and Serial Sections with MRI. 3. Berlin, Heidelberg, New York: Springer; 2005. The Human Hippocamps. [Google Scholar]
- Fukutani Y, Cairns NJ, Shiozawa M, Sasaki K, Sudo S, Isaki K, Lantos PL. Neuron loss and neurofibrillary degeneration in the hippocampal cortex in late-onset sporadic Alzheimer’s disease. Psychiatry Clin Neurosci. 2000;54:523–529. doi: 10.1046/j.1440-1819.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hayakawa N, Kato H, Araki T. Age-related changes of astrocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev. 2007;128:311–316. doi: 10.1016/j.mad.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS, Pasinetti GM. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2001;58:487–492. doi: 10.1001/archneur.58.3.487. [DOI] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform specific regulation of dendtritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XQ, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuker JIH, Luiten PGM, Fuchs E. Capillary changesin hippocampal CA1 and CA3 areas of the aging rhesus monkey. Acta Neuropathol. 2000;100:665–672. doi: 10.1007/s004010000227. [DOI] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old man with type 2 diabetes: The Honolulu Asia Aging Study. Diabetes Care. 2006;10:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurochem. 2007;100:202–210. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- Levi O, Dolev I, Belinson H, Michaelson DM. Intraneuronal amlyloid-β plays a role in mediating the synergistic pathological effects of apoE4 and environmental stimulation. J Neurochem. 2007;103:1031–1040. doi: 10.1111/j.1471-4159.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Dutton RA, Lee AD, Geaga JA, Hayashi KM, Toga AW, Engel J, Thompson PM. Three-dimensional pre-operative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1049–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young mice overexpressing human APP(sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A cuasative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: Allostasis, allosteric load and aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW. Measurements of hippocampal subfields and age related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28:719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4T. Neuroimage. 2008;42:42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment, clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesberry W, Nelson J, Davies DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofirbrillary tangles and brain weight at death: The HAAS Honolulu-Asia Aging study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD 67 immunoractive neurons and boutons in the hippocampus of middle-aged and old Fisher 344xBrown Norway rats. J Comp Neurol. 2004;478:282–291. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Takayama N, Iseki E, Yamamoto T, Kosaka K. Regional quantitative study of formation process of neurofibrillary tangles in the hippocamps of non-demented elderly brains: Comparison with late onset Alzheimer’s disease brains. Neuropathology. 2002;22:147–153. doi: 10.1046/j.1440-1789.2002.t01-1-00433.x. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B. Model-based segmentation of hippocampal subfields in ultra high resolution in vivo MRI. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2008;11:235–243. doi: 10.1007/978-3-540-85988-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado HM, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape analysis across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- Wang XP, Ding HL. Alzheimer’s disease: Epidemiology, genetics, and beyond. Neurosci Bull. 2008;24:105–109. doi: 10.1007/s12264-008-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring SC, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch Neurol. 2008;65:329–334. doi: 10.1001/archneur.65.3.329. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- White F, Nicoll JAR, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8:611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Ypsilanti AR, Girão da Cruz MT, Burgess A, Aubert I. The length of hippocampal cholinergic fibers is reduced in the aging brain. Neurobiol Aging. 2008;29:1666–1679. doi: 10.1016/j.neurobiolaging.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Avants BB, Pluta J, Das S, Minkoff D, Mechanic-Hamilton D, Glynn S, Pickup S, Liu W, Gee JC, Grossman M, Detre JA. A high resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4T. Neuroimage. 2009;44:385–398. doi: 10.1016/j.neuroimage.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Unfolding the human hippocampus with high resolution structural and functional MRI. Anat Rec. 2001;265:111–120. doi: 10.1002/ar.1061. [DOI] [PubMed] [Google Scholar]