Abstract
Blockade of angiogenesis is an important approach for cancer treatment and prevention. VEGF is one of the most critical factors that induce angiogenesis and has thus become an attractive target for anti-angiogenesis treatment. However, most of current anti-VEGF agents often cause some side effects when given chronically. Identification of naturally occurring VEGF inhibitors derived from diet would be one alternative approach with an advantage of known safety. Grape seed extract (GSE), a widely used dietary supplement, is known to have anti-tumor activity. In this study, we have explored the activity of GSE on VEGF receptor and angiogenesis. We found that GSE could directly inhibit kinase activity of purified VEGFR2, a novel activity of GSE that has not been characterized. GSE could also inhibit VEGFR/MAPK mediated signaling pathway in endothelial cells. As a result, GSE could inhibit VEGF induced endothelial cell proliferation and migration as well as sprouts formation from aorta ring. In vivo assay further showed that GSE could inhibit tumor growth and tumor angiogenesis of MDA-MB-231 breast cancer cells in mice. Consistent with the in vitro data, GSE treatment of tumor bearing mice led to concomitant reduction of blood vessel density and phosphorylation of MAP kinase. Depletion of polyphenol with polyvinylpyrrolidone (PVPP) abolished the anti-angiogenesis activity of GSE, suggesting a water soluble fraction of polyphenol in GSE is responsible for the anti-angiogenesis activity. Taken together, this study indicates that GSE is a well tolerated and inexpensive natural VEGF inhibitor and could potentially be useful in cancer prevention or treatment.
Introduction
Angiogenesis, the formation of new blood vessels, plays a critical role in tumor progression. There are multiple steps involved in tumor angiogenesis. Each step provides an opportunity for therapeutic intervention. Although the cellular and molecular mechanisms that govern angiogenesis are only beginning to be understood, it is clear that a balance of pro-angiogenic and anti-angiogenic factors control the formation of new blood vessels (1). Amongst these factors, vascular endothelial growth factor (VEGF) is one of the most critical and specific angiogenesis factors (2). The biological function of VEGF on endothelial cells is mainly mediated through binding to receptor tyrosine kinase, VEGF receptor 1 (flt1/VEGFR1) and VEGF receptor 2 (KDR/flk1/VEGFR2), both are crucial for normal vascular development (2). Binding of VEGF to VEGFR induces conformational changes in the receptor, followed by dimerization and autophosphorylation of the tyrosine residues of the receptor (3). Inhibiting VEGF activity by neutralizing antibodies or introduction of dominant negative VEGF receptors into endothelial cells often results in inhibition of tumor growth (2). In fact, a humanized monoclonal antibody against VEGF, Avastin, is the first angiogenesis inhibitor that was approved by U.S. Food and Drug Administration to treat cancer (4).
While many of the inhibitors that efficiently suppress angiogenesis are currently being tested at various stages of clinical development, diet-based approaches to limit angiogenesis are being actively explored (5). This latter approach has a major merit due to the proven safety for human use. Several safe chemopreventive phytochemicals, such as curcumin, resveratrol and catechins are known to have anti-angiogenesis activity as one of the mechanisms to suppress tumor growth (6, 7). Epidemiological studies indicate that diet and nutrition influence the development of cancer (8, 9). The highest rate of breast cancer is observed in populations with western life styles that include relatively high fat, meat-based, low fiber diets, whereas the lowest rates are typically observed in Asian populations with mainly plant-based diets. The high content of phytochemicals in these plant-based diets has been proposed as the underlying factor responsible for the low breast cancer incidence in Asian women but the mechanisms are relatively unexplored (10).
One of the plants that have high contents of phytochemicals is grape. Grape and red wine are consumed world-widely and have been reported to be associated with reduced risk of cancer. Grapes are rich in polyphenols, of which approximately 60-70% is found in grape seeds. Commercial preparations of grape seed extract (GSE) contain 75 to 95% procyanidins. GSE is marketed as a dietary supplement in the United States, owing to their powerful protective properties against free radicals and oxidative stress.
GSE has been linked to cancer prevention and therapy. Increased consumption of grapes was reported to be associated with reduced cancer risk (11). Studies in carcinogen-induced and genetically engineered cancer models (12-14) have revealed a chemopreventive role of proanthocyanidins in GSE. GSE was also shown to inhibit the growth of a number of cancer cells in vitro (15) and tumor growth in mice (14, 16-21).
Despite the known anti-cancer activity, the mechanisms of the effect of GSE are not fully understood. Understanding such mechanisms is important for exploring the full potential of GSE in chemoprevention and treatment of cancer. Several studies have shown that GSE could negatively regulate a number of cellular functions or signaling molecules in tumor cells, including aromatase activity (20) (16), cell cycle progression (15), EGF-induced mitogenic signaling (22), and NF-κB signaling (23), or could induce caspase activity (24). Recently, GSE was also reported to inhibit endothelial cell proliferation and tube formation on matrigel (25) and reduce vessel density in human prostate tumor (26). These observations suggest that GSE is likely a natural inhibitor of VEGFR and anti-angiogenesis may be another mechanism of its anti-tumor activity. In this study, we have performed further in vitro and in vivo experiments to characterize the anti-angiogenesis and anti-tumor activity of GSE, and explored its possible molecular mechanism. We present evidence that GSE inhibits endothelial cell function, at least in part, via inhibition of VEGFR-MAPK signaling pathway.
Material and Methods
GSE
GSE standardized preparation, constituting of at least 85% (w/w) procyanidins, was provided by San Joaquin Valley Concentrates (Fresno, California) and dissolved in water for all the experiments. The GSE preparation contains approximately 19,36,8, and 22 ng/μg of procyanidins B1, B2, B3, and B4.
Cell proliferation
Endothelial cell proliferation in the presence and absence of growth factors was evaluated in both HUVEC (Human Umbilical Vascular Endothelial Cells, Clonetics, Lonza) and BCE (Bovine Capillary Endothelial Cells, a generous gift from Catherine Butterfield and Judah Folkman).
Endothelial cells were plated onto gelatinized 24-well culture plate in 0.5ml DMEM medium (from GIBCO for BCE) containing 10% BCS or serum free endothelial basal medium (EBM-2, from Lonza for HUVEC) containing 10% FCS and incubated for 24 hrs. The medium was replaced with 0.25ml of DMEM + 5% BCS + 1% GPS (for BCE) or 0.25ml of EBM-2 + 2%FCS + 1%GPS (for HUVEC) containing various concentrations of GSE. After 30mins of incubation, additional 0.25 ml of medium was added to achieve a final volume of 0.5ml of medium with or without bFGF (1ng/ml), VEGF(100ng/ml, PeproTech Inc), or endothelial cell growth factor supplements (SingleQuo® kit, Lonza). After 48 hrs, cells were dispersed in trypsin and counted using a Coulter counter.
Cell Migration
Endothelial cell migration was assessed using a modified Boyden chamber assay (27). HUVEC cells (1.5×105) were plated in EBM-2 medium containing 0.05% FCS in the upper chamber of the transwell (8-μm pore, Costar), which were pre-coated with 200μg/ml matrigel (Becton-Dickinson). Cells were then treated with various concentration of GSE for 30 mins at 37°C. EBM-2 medium containing 0.05% FCS and VEGF (50ng/ml) were then added to the lower chamber. After 8 hours, non-migrated cells were removed by cotton swap and migrated cells were stained and examined under microscope. The number of migrated cells was quantified by counting the cells at 40x objectives. Migration was normalized to percent migration, with migration in the presence of VEGF representing the scale of 100%.
Immunoblot
HUVEC cells were cultured in EBM-2 medium containing 2% FCS for 24 hrs and then incubated with various concentrations of GSE for 30 mins before VEGF (100ng/ml) stimulation for 5 mins. Total cells extracts were prepared in Laemmli sample buffer. Proteins were separated by electrophoresis on SDS gels, transferred to PVDF (Polyvinylidence Fluoride) membranes and incubated with the following primary antibody (Cell Signaling): anti-VEGFR2, anti-phospho-VEGFR2, anti-MAKP42/44, and anti-phospho-MAPK42/44. Binding of the primary antibody was detected using a horseradish peroxidase (HRP)-conjugated secondary antibody and chemiluminescent substrate (Pierce). Densitometric analysis was performed using the AlphaEase FC imaging system (Alpha Innotech Corporation, USA).
In vitro kinase assay
An ELISA assay kit (Sigma) was used to determine the ability of GSE to inhibit VEGFR2 tyrosine kinase activity. Briefly, GSE were incubated with VEGFR2 (Upstate) in HEPES buffer solution containing Mn2+ and Mg2+ and ATP in 96-well plates coated with a poly-Glu-Tyr substrate. Phosphorylated tyrosine was then detected by sequential incubation with a mouse IgG anti-phosphotyrosine antibody and a HRP-linked sheep anti-mouse immunoglobulin antibody. Color is developed with HRP chromogenic substrate and quantified by an ELISA reader at wavelength 492. The results were expressed as percent kinase activity. IC50 values were defined as the drug concentration that resulted in 50% inhibition of enzyme activity.
Chick aortic ring assay
The chick aortic ring assay was modified from a rat aortic ring assay as described previously (28, 29). Briefly, aortic arch was dissected from day 12-14 chick embryos and cut into rings and embedded into Matrigel in 4-well plate (NUNC). After incubation for 10 mins at 37°C, the aortic rings were fed with MCDB-131 serum free medium (GIBCO, Invitrogen) containing various concentrations of GSE with or without VEGF (100ng/ml) or ECGS (endothelial cell growth supplement, Sigma) (50μg/ml). Sprouts formed within 24−72 hrs. Images were photographed at 4x objective of Olympus inverted IX81 at 40x magnification. The extent of sprouts formation from chick aortic ring was quantified using image-pro software as described (30).
Tumor formation
6-8 weeks old SCID mice were used for tumor xenografts. Mice were housed in specific pathogen-free conditions, according to the guidelines of the association for Assessment and Accreditation of Laboratory Animal Care. All studies were carried out under approved institutional experimental animal care and use protocols.
Mice were individually gavage fed with 100μl of water control (n=5) or GSE (50mg/kg) (n=6) for 3 weeks. Mice were then given s. c. injection of MDA-MB-231(5×106 cells/mice) and continued to be gavaged daily with GSE or water control. Tumor volume was assessed twice weekly by caliber. Volumes were determined using the formula (Width)2 ×Length ×0.52. Body weight was monitored weekly as an indicator of overall health of the animals.
Immunohistochemistry
Representative tumor tissues (four of each group) were harvested and fixed in 10% neutral buffered formalin at 4°C for 12 hours. All tissues were paraffin embedded. Sections (5 μm) were first stained with hematoxylin-eosin to evaluate tissue viability and quality. Sections were then microwaved in citrate buffer and were co-stained with antibody against MECA (BD Bioscience) to visualize vessels and anti-phospho-MAPK (Cell Signaling) to visualize the phosphorylation status of MAPK. For CD31 staining, sections were permeabilized with 36 μg/mL proteinase K (Roche Diagnostics Corp., Indianapolis, IN) and stained with antibody against CD31 (PECAM) (BD Bioscience). Tyramide signal amplification direct and indirect kits (NEN Life Science Products Inc., Boston, MA) were used to amplify staining signals. Sections were photographed at ×200 magnification using an Olympus AX70 microscope (Melville, NY). Vessel density (average 5 fields) was determined by image-pro software.
PVPP depletion
GSE was incubated with polyvinylpyrrolidone (PVPP) at room temperature for 30mins, and then centrifuged at 13,000rpm for 10mins to remove PVPP. The absorbance of supernatant at wavelength of 280 was measured to ensure that polyphenol is removed.
Statistical analysis
Data were expressed as mean ± S.D. Student t test is used to determine statistical significance between control and test group. P < 0.05 is considered to be statistically significant.
Results
Effect of GSE on VEGFR2 signaling
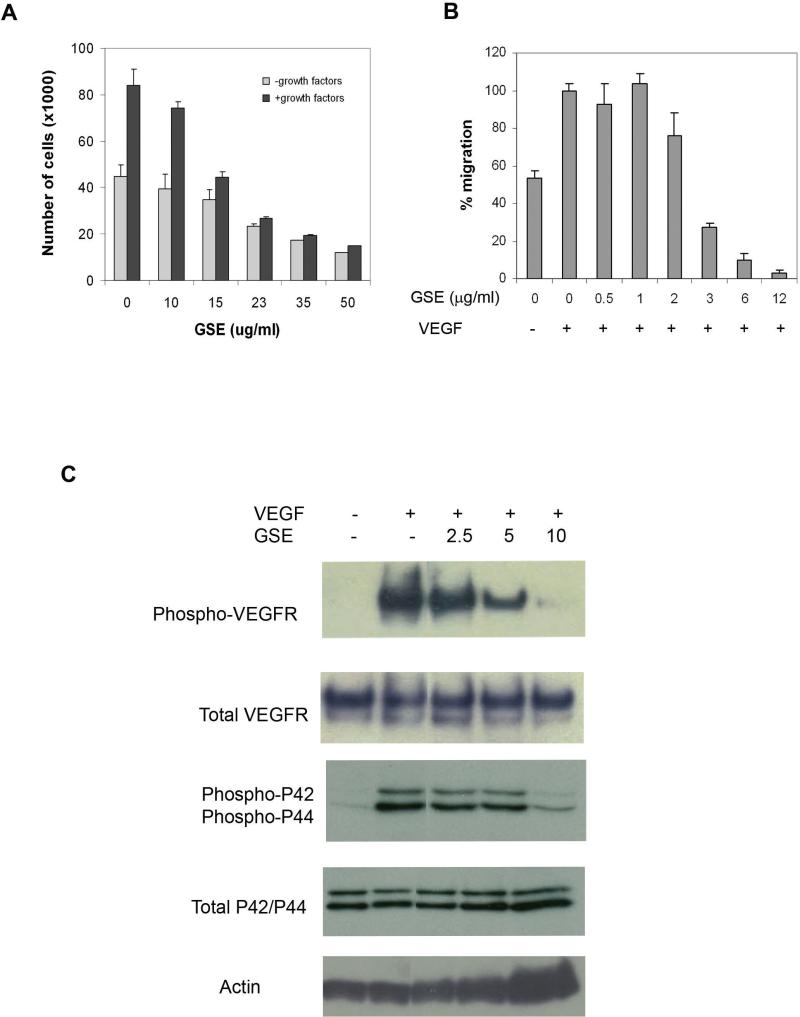
To characterize the activity of GSE on VEGFR and angiogenesis, we started by comparing the effect of GSE on proliferation of endothelial cells cultured in the presence and absence of growth factors, as growth stimulation (such as VEGF) plays a critical role in triggering normal angiogenesis and tumor angiogenesis. HUVEC (Human Umbilical Vascular Endothelial Cells) and BCE (Bovine Capillary Endothelial Cells) were incubated with various concentrations of GSE for 30mins prior to the addition of VEGF (100ng/ml), bFGF (1ng/ml) or growth medium containing various growth factors. The treated cells were then counted after 48 hours. Shown in Figure 1A, a greater inhibitory activity of GSE was observed in cells treated with growth factors containing VEGF than in cells cultured in the absence of growth stimulation. Similar result was also obtained with BCE cells (data not shown). This result suggests that GSE may regulate angiogenesis by interfering with the growth factor induced signaling pathway.
Figure 1.
GSE inhibits VEGFR2 signaling in endothelial cells. (A) GSE inhibits endothelial cell proliferation. HUVECs were plated onto gelatinized 24-well culture plates and treated with various concentrations of GSE for 30 mins. The cells were then stimulated with endothelial cell growth factor supplements. After 48 hrs, cells were dispersed in trypsin and counted using a Coulter Counter. (B) GSE inhibits endothelial cell migration. Endotheliall cell migration was assessed using a modified Boyden chamber assay. HUVEC cells were plated in the upper chamber of the transwell and treated with various concentration of GSE for 30 mins. VEGF (50ng/ml) were then added to the lower chamber. After 8 hours, non-migrated cells were removed by cotton swap and migrated cells were stained and examined under microscope. The data were represented as a percentage of control without GSE treatment in the presence of VEGF. Each bar represents the mean ± SD. (C) GSE inhibits VEGFR2 signaling. Quiescent HUVECs were incubated in serum free medium in the presence or absence of GSE for 30 mins, followed by stimulation with 100ng/ml VEGF for 5 mins. Phosphorylation of VEGFR-2 or MAPK was assessed by western blot using anti-phospho-VEGFR antibody or anti-phospho-MAPK antibody. Total VEGFR or MAPK was analyzed using anti-VEGFR antibody or anti-MAPK antibody. The results showed that GSE inhibited phosphorylation of VEGFR2 and MAPK in HUVEC.
The effect of GSE on VEGF-driven endothelial cell migration was also evaluated by using a modified Boyden chamber assay (27). Shown in Figure 1B, at a concentration of 3μg/ml, GSE abolished VEGF induced migration without inhibiting the attachment of endothelial cells to the membrane.
We next evaluated the effect of GSE on VEGF signaling, one of the most potent and specific angiogenesis factors. Strong evidence shows that blocking activity of VEGF receptor 2 (VEGF-R2 or KDR) can limit the ability of most tumors to stimulate the formation of blood vessels (2). Thus, we tested the effect of GSE on VEGF-induced tyrosine phosphorylation of VEGFR-2. Serum starved HUVECs were treated with GSE or control buffer for 30 mins, followed by stimulation with VEGF for 5 mins. The phosphorylation state of VEGFR-2 was assessed by western blot using anti-phospho-VEGFR2 antibody. Shown in Figure 1C, GSE inhibited VEGF-induced tyrosine phosphorylation of VEGFR2 in a dose dependent manner. The total amount of VEGFR protein in each sample of cells remained comparable, suggesting that inhibition of phosphorylation of VEGFR2 by GSE was not due to reduced VEGFR2 expression. These results suggest that GSE are potent inhibitors of VEGF-induced tyrosine phosphorylation of VEGFR-2.
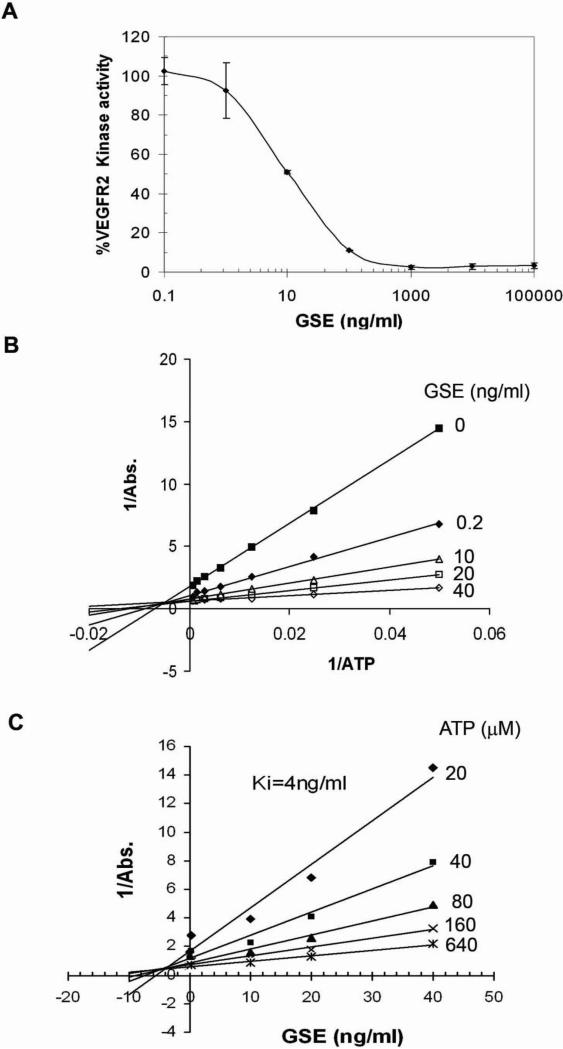
To determine whether the inhibition of tyrosine phosphorylation of VEGFR2 by GSE is caused by inhibition of the kinase activity of VEGFR, we performed an ELISA-based in vitro tyrosine kinase assay. As a positive control, an inhibitor of VEGFR, SU5416, showed inhibition of kinase activity with an IC50 of 1μM (data not shown), in agreement with a previous report (31). GSE also showed a strong inhibition of VEGFR kinase activity, with an IC50 of about 10ng/ml (Figure 2A).
Figure 2.
GSE inhibits VEGFR2 kinase activity. (A) GSE inhibited VEGFR2 kinase activity. VEGFR2 and various concentrations of GSE were incubated in kinase reaction buffer containing ATP in 96-well plates coated with a poly-Glu-Tyr substrate. Phosphorylation of the substrate was monitored with a purified phosphotyrosine specific monoclonal antibody conjugated to HRP followed by chromogenic reaction with HRP substrate. Data were represented as percentage of control that was not treated with GSE. (B) Lineweaver-Burk plot and (C) Dixon plot of the inhibition of VEGFR with GSE. Increased concentration of ATP was incubated with VEGFR2 and various concentration of GSE and monitored for their effect on kinase activity.
To define the biochemical mechanism for the inhibition of VEGFR2 by GSE, we examined the effect of increasing concentrations of ATP on the inhibitory activity of GSE (Figure 2B &2C). The Lineweaver-Burk plot showed that increasing concentrations of GSE had strong effect on the apparent 1/Vmax (y-axis intersection), indicating GSE behaves mainly as a noncompetitive inhibitor for ATP binding. The Dixon plot revealed a Ki of 4ng/ml for the inhibition of GSE.
To further dissect the downstream signaling pathway of VEGFR that might be affected by GSE, we examined the effect of GSE on VEGF-induced activation of MAP kinase in HUVEC cells. MAP kinase (32) is one of the key components involved in the signaling pathways which support endothelial cell proliferation. Shown in Figure 1C, VEGF caused a significant up-regulation of phosphorylation of both MAPKp44 and MAPKp42 isoforms in HUVEC cells. Treating HUVEC cells with GSE inhibited the VEGF-induced phophorylation in both of the MAPK proteins, while the total amount of MAPK was unaffected.
Inhibition of proliferation and VEGFR activation in endothelial cells appeared to require higher doses of GSE than inhibition of VEGFR kinase activity in vitro (Figure 1 vs. Figure 2). This may be due to that the active components in GSE are not stable in culture or not able to penetrate well into the cells to exert effect. Nevertheless, these results collectively suggest that GSE might block angiogenesis and tumor growth via a novel activity --- direct inhibition of a key receptor tyrosine kinase of the endothelial cells and its downstream signaling cascade.
Effect of GSE on sprout formation of aortic ring
Compared to in vitro proliferation and migration assays, organ culture methods, such as rat aorta ring assay (28) are thought to more closely mimic multiple stages of in vivo angiogenesis, including endothelial cell proliferation, migration, and tube formation. To further characterize the anti-angiogenesis activity of GSE, we next performed aorta ring assay.
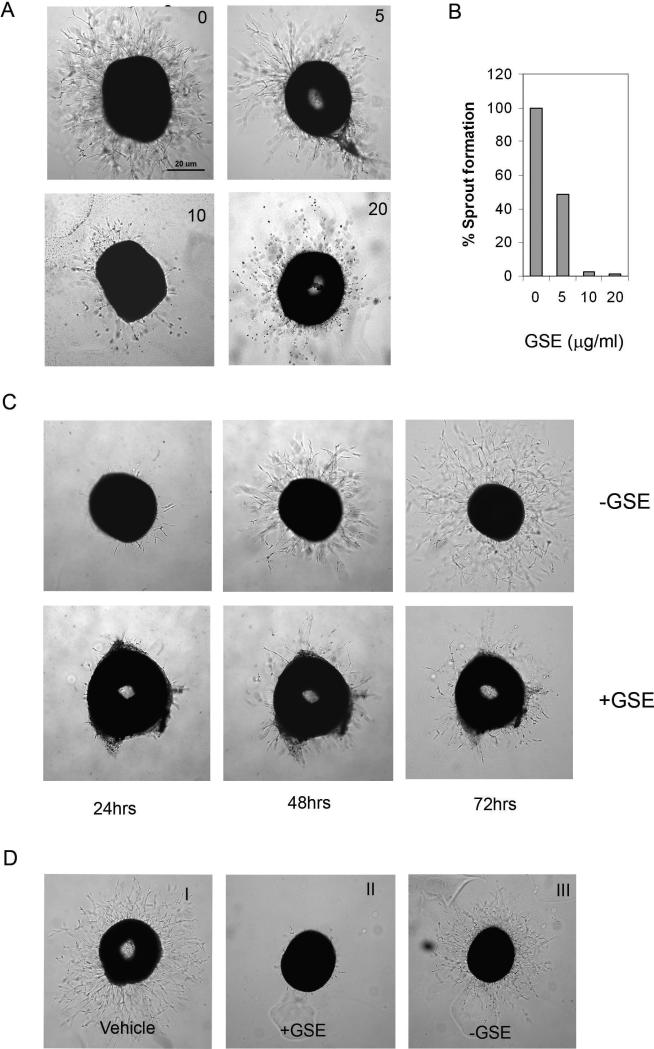
To study the effect of GSE on microvessel sprout formation, we used a modified aortic ring sprout formation assay, chick aortic arch assay (29). In this assay, chick aortic rings were embedded in the matrigel and fed with medium containing different concentrations of GSE. The rings were then stimulated with VEGF or ECGS (endothelial cell growth supplement). Sprout formation was examined under microscope. Microvessels were initially noticed in VEGF or ECGS-containing samples. Treatment with GSE resulted in a dose dependent decrease in capillary sprouting --- the growing sprouts were shorter and fewer cells migrated into the matrix (Figure 3A and 3B), indicating that GSE could inhibit VEGF and ECGS-induced microvessel sprouting.
Figure 3.
GSE inhibit sprouts formation from chicken aorta. (A) Chick aortic rings were placed in Matrigel gels and fed with MCDB131 serum free medium containing various concentration of GSE in the presence or absence of ECGS (50μg/ml). The effect of GSE on endothelial cell sprouts formation from various aorta samples were examined on day 3. (B) Quantification of sprouts formation. Results were represented as percentage of control that was not treated with GSE. (C) Chicken aortic rings were incubated with or without GSE (10μg/ml) in presence of ECGS. The sprouts formation was examined on day 1, day 2 and day 3. (D) Inhibition of GSE on vessel sprout formation is reversed once GSE is removed from the medium. Aorta rings were first incubated with (panel II) or without GSE (10μg/ml) (panel I) for 48 hrs. The culture was then replaced with fresh medium without GSE and further incubated in the presence of ECGS for 72 hours (panel III).
To test whether the inhibition by GSE was reversible, we performed chicken aorta ring assay with the following modification --- aortic ring was cultured first with both ECGS and GSE for the indicated incubation time (2, 24, and 48hrs), then GSE was removed from the culture and aorta ring was continually cultured with ECGS. Shown in Figure 3C, inhibition of vessel sprouts was released when GSE was removed, suggesting that inhibition of vessel sprouts by GSE is reversible and not likely due to general cytotoxicity.
In vivo anti-tumor angiogenesis activity of GSE
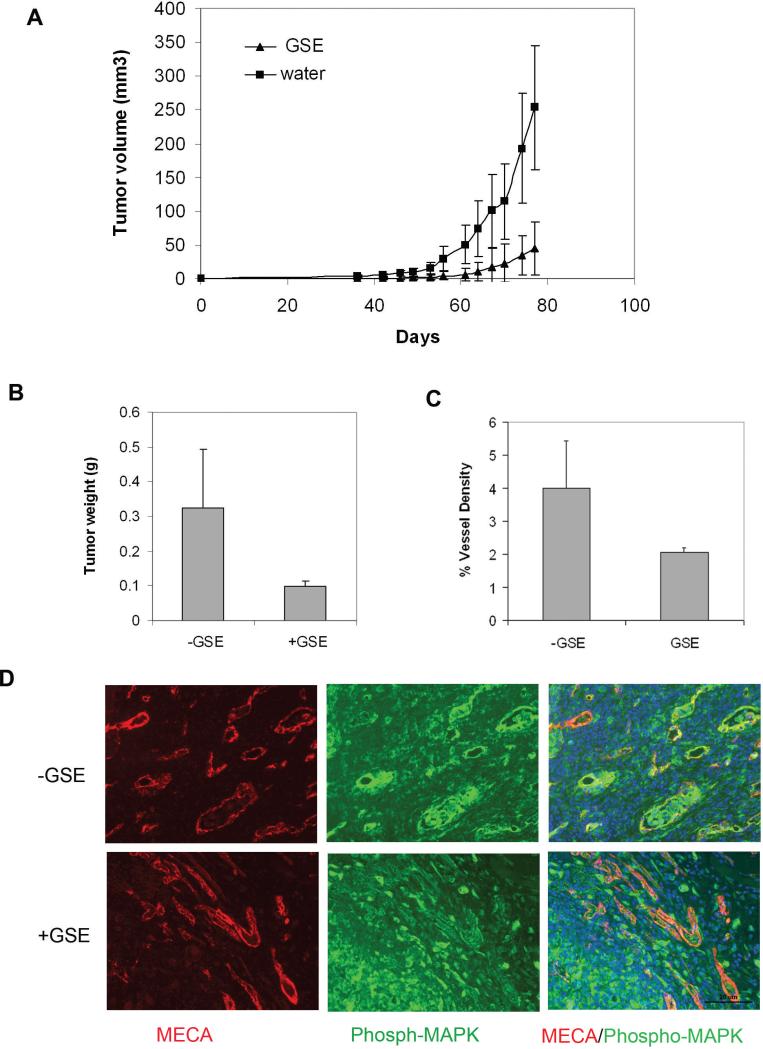
To study the potential in vivo anti-tumor angiogenesis activity of GSE, we tested the effect of GSE in a tumor model in mice. The experimental mice were pre-fed with GSE (+GSE) or water only without GSE (-GSE) by oral administration. The mice were then implanted with MD-MBA-231 breast cancer cells. Treatment of mice with GSE markedly inhibited MDA-MB-231 tumor growth compared to the control group treated with water (Figure 4A and 4B). GSE treatment appeared to have no obvious toxicity and showed no detectable effect on body weight and behavior of mice bearing MDA-MB-231 (data not shown).
Figure 4.
GSE inhibits in vivo angiogenesis. (A) GSE inhibits MDA-MB-231 tumor growth and tumor angiogenesis in vivo. MDA-MB-231 cells were subcutaneously injected into the back of SCID mice. GSE (50mg/kg/day) ( +GSE) or water control (-GSE) were orally given to each mouse before and after the tumor implantation. Tumor volumes were compared between water control and the GSE-treated as a function of time. (B) Tumors were excised and weighed after the treatment. (C) Quantification of vessel density in the tumors based on CD31 staining. (D) Blood vessels in the tumors were co-stained with MECA-32 antibody against panendothelial cell antigen (red) and anti-phosphorylated MAPK (green) to assess tumor angiogenesis.
To further evaluate the status of tumor angiogenesis, we examined vessel formation in tumors treated with GSE. Tumor tissues were co-stained with MECA-32 antibody against pan-endothelial cell antigen and anti-phosphorylated MAPK. These two antibodies detect the vessels and the phosphorylation status of MAPK (33), respectively. Compared to the control group treated with water, GSE treatment led to a significant decrease in tumor vessel counts (Figure 4C) and a decrease in the level of MAPK phosphorylation (Figure 4D). These results further indicate that GSE is an angiogenesis inhibitor likely via its action in interfering MAP kinase signaling pathway.
Water soluble polyphenol of GSE in anti-angiogenesis activity
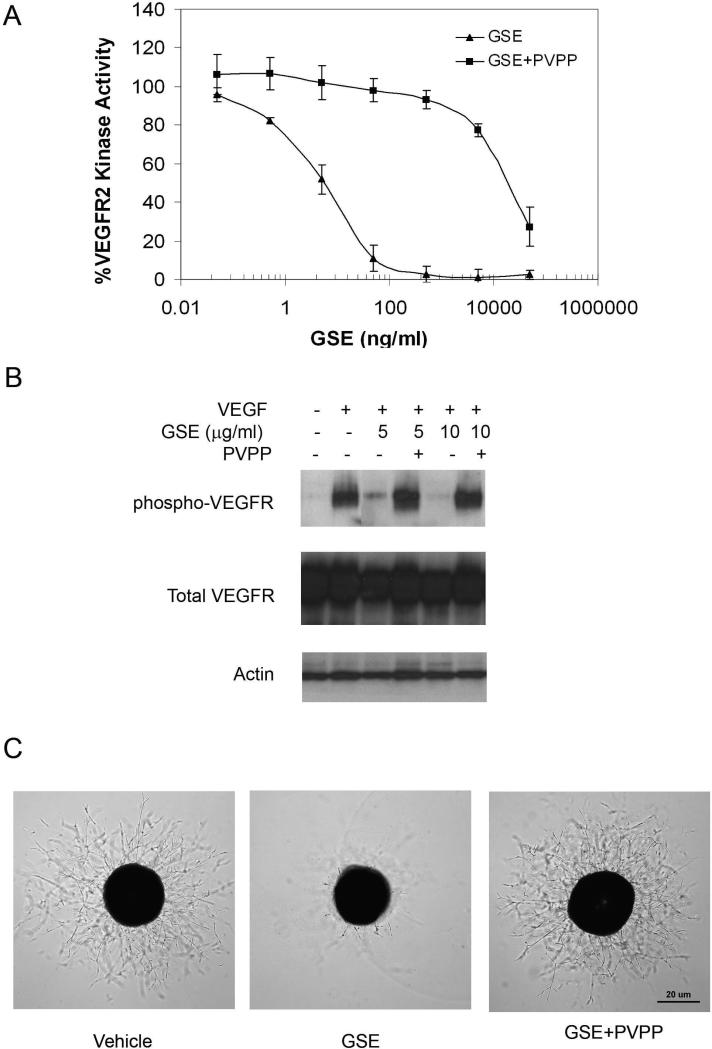
The composition of GSE has been studied extensively and is known to be rich in polyphenols. The polyphenols in GSE are mainly procyanidins. To test whether polyphenol is responsible for the anti-angiogenesis activity of GSE, we used polyvinylpyrrolidone (PVPP) to remove polyphenol from GSE. PVPP forms hydrogen bonds with phenolic compounds, yielding a PVPP-phenolic precipitate which can be removed by centrifugation. PVPP-treated GSE was tested in VEGFR kinase assay, endothelial cell proliferation assay and aorta ring assay. Shown in figure 5, PVPP-treated GSE had little effect on VEGFR2 kinase activity (Figure 5A) and cell proliferation (data not shown) and did not inhibit VEGF-induced phosphorylation of VEGFR in endothelial cells (Figure 5B). Removal of polyphenol also eliminated the inhibitory activity of GSE on aorta ring sprouts formation (Figure 5C). These results suggest that a water soluble polyphenol in GSE, probably one of the procyanidins, is likely responsible for the anti-angiogenesis activity of GSE.
Figure 5.
PVPP depletes anti-angiogenesis activity in GSE. GSE was incubated with PVPP for 30mins and followed by centrifugation to remove PVPP. The supernatant was then tested for the effects on (A) the kinase activity of purified VEGFR2, (B) phosphorylation of VEGFR2 in HUVEC, and (C) sprouts formation from chick aorta.
Discussion
GSE is widely consumed as a dietary supplement and possesses anticancer activity against various cancers (16-19, 21). Previous reports suggested anti-angiogenesis as one possible mechanism for its anti-tumor activity (18, 25). In this study, we have further characterized the anti-angiogenesis and anti-tumor activity of GSE, and started to dissect the possible molecular mechanisms underlying these activities. Our data indicate that GSE can inhibit angiogenesis both in vitro and in vivo via blocking VEGFR-MAPK activation.
Our results showed that GSE could inhibit various aspects of angiogenesis, including growth factor-induced endothelial cell proliferation, migration, tube formation on matrigel (data not shown), sprout formation from aorta and angiogenesis in tumor. The inhibitory activity of GSE on endothelial cell functions is not likely due to cell toxicity, although GSE can induce cell apoptosis at higher concentration (data not shown) (25). This was reflected in our observation that GSE has a rather specific activity for actively proliferating endothelial cells (growth factor-stimulated proliferation) (Figure 1) and was further supported by the results that inhibition of vessel sprouting was reversible upon removal of GSE (Figure 3).
As a first step to study the possible molecular mechanism of the anti-angiogenesis activity of GSE, we have looked into the effect of GSE on the receptor for VEGF, one of the most critical angiogenesis factors. VEGFR2 is a potent target for cancer treatment. Many antibodies and chemical agents that inhibit VEGFR signaling have been developed and tested in the different stages of clinical trials. However, much less is known for the kinase inhibitory activity in natural products. Our results revealed a novel activity of GSE --- inhibition of VEGFR2 kinase activity and suppression of its MAPK downstream signaling.
In addition to study the in vitro anti-angiogenesis activity of GSE using HUVEC cells in various assays, we have also examined the effect of GSE on tumor growth and tumor angiogenesis in mice using a xenograph model. Our results showed that GSE treatment inhibited tumor growth in mice, concomitant with a reduction in tumor vessel counts. This effect of GSE could be the result of the blockage of VEGFR-MAPK signaling, as evidenced by the observation that phosphorylation of MAPK was suppressed in the GSE treated mice, consistent with our in vitro study. Further studies are needed to evaluate the potential of the anti-angiogenesis activity of GSE in tumorigenesis and tumor progression using a carcinogen-induced or genetically engineered tumor model, as such models would more closely mimic the multi-stage tumor progression in human patients.
A water soluble fraction of polyphenol from GSE seems to be responsible for its anti-angiogenesis activity. The major form of polyphenol in commercial preparations of grape seed extract (GSE) is procyanidins, Including procyanidin B1-B5, procyanidin C1, Procyanidin B5-3’-gallate, which comprise 75% to 95% in GSE (16, 17, 34, 35). Future studies will be needed to further define the individual polyphenol component of GSE, alone or in combination, for anti-angiogenesis and anti-tumor activity
Angiogenesis inhibitors have emerged as a new class of drugs that can be used to treat and prevent cancer. The efficacy of anti-angiogenic agents may be improved if they can be used chronically. However, most of the current anti-angiogenesis agents, approved for clinical use or being tested in trial, result in certain side effects such as hypertension, bleeding, fever, diarrhea etc. Our study indicates that GSE, a dietary supplement, is a well tolerated and inexpensive natural angiogenesis inhibitor through suppressing VEGFR kinase activity and its downstream signaling in endothelial cells and could potentially be used chronically to enhance its anti-cancer activity in cancer prevention or treatment.
Acknowledgements
We thank Dr. Judah Folkman (1933-2008) for his mentorship, inspiration and vision. We thank Dr. Tim Synold and Binxin Xi for analyzing the composition of GSE and for their suggestion, Drs. Susan Kane and Edward Newman for their valuable discussions. This work is supported by NIH grants CA44735 and ES08258 to SC and Stop Cancer Foundation and Concern Foundation grants to WW.
REFERENCE
- 1.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7 doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 6.Dulak J. Nutraceuticals as anti-angiogenic agents: hopes and reality. J Physiol Pharmacol. 2005;56(Suppl 1):51–69. [PubMed] [Google Scholar]
- 7.Albini A, Noonan DM, Ferrari N. Molecular Pathways for Cancer Angioprevention. Clin Cancer Res. 2007;13:4320–4325. doi: 10.1158/1078-0432.CCR-07-0069. [DOI] [PubMed] [Google Scholar]
- 8.Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 9.Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986;58:2363–2371. doi: 10.1002/1097-0142(19861201)58:11<2363::aid-cncr2820581102>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Adlercreutz H, Mousavi Y, Clark J, Hockerstedt K, Hamalainen E, Wahala K, Makela T, Hase T. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol. 1992;41:331–337. doi: 10.1016/0960-0760(92)90359-q. [DOI] [PubMed] [Google Scholar]
- 11.Zheng T, B. P., Willett WC, Hu H, Dan J, Evstifeeva TV, Niu S, MacMahon B. A case-control study of oral cancer in Beijing, People's Republic of China. Associations with nutrient intakes, foods and food groups. Eur J Cancer B Oral Oncol. 1993;29B:45–55. doi: 10.1016/0964-1955(93)90010-c. [DOI] [PubMed] [Google Scholar]
- 12.Singletary KW, M. B. S. K., Meline B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer. 2001;39:252–258. doi: 10.1207/S15327914nc392_15. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Hall P, Smith M, Kirk M, Prasain JK, Barnes S, Grubbs C. Chemoprevention by Grape Seed Extract and Genistein in Carcinogen-induced Mammary Cancer in Rats Is Diet Dependent. J. Nutr. 2004;134:3445S–3452. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]
- 14.Raina K, Singh RP, Agarwal R, Agarwal C. Oral Grape Seed Extract Inhibits Prostate Tumor Growth and Progression in TRAMP Mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal C, Sharma Y, Zhao J, Agarwal R. A Polyphenolic Fraction from Grape Seeds Causes Irreversible Growth Inhibition of Breast Carcinoma MDA-MB468 Cells by Inhibiting Mitogen-activated Protein Kinases Activation and Inducing G1 Arrest and Differentiation. Clin Cancer Res. 2000;6:2921–2930. [PubMed] [Google Scholar]
- 16.Eng ET, Ye J, Williams D, Phung S, Moore RE, Young MK, Gruntmanis U, Braunstein G, Chen S. Suppression of Estrogen Biosynthesis by Procyanidin Dimers in Red Wine and Grape Seeds. Cancer Res. 2003;63:8516–8522. [PubMed] [Google Scholar]
- 17.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 18.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. International Journal of Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 19.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape Seed Extract Inhibits In vitro and In vivo Growth of Human Colorectal Carcinoma Cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 20.Kijima I, Phung S, Hur G, Kwok S-L, Chen S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006;66:5960–5967. doi: 10.1158/0008-5472.CAN-06-0053. [DOI] [PubMed] [Google Scholar]
- 21.Arii M, M. M., Hosoyama R, Ariga H, Yamaji N, Kataoka S. Chemopreventive effect of grape seed extract on intestinal carcinogenesis in the APCMin mouse. . Proc Am Assoc Cancer Res. 1998;39:20. [Google Scholar]
- 22.Tyagi A, A. R., Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 23.Dhanalakshmi S, A. R., Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. Int J Oncol. 2003;23:721–727. [PubMed] [Google Scholar]
- 24.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal C, S. R., Dhanalakshmi S, Agarwal R. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol Rep. 2004;11:681–685. [PubMed] [Google Scholar]
- 26.Rana P, Singh AKTSDRACA. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. International Journal of Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 27.Kisker O, Becker CM, Prox D, Fannon M, D'Amato R, Flynn E, Fogler WE, Sim BKL, Allred EN, Pirie-Shepherd SR, Folkman J. Continuous Administration of Endostatin by Intraperitoneally Implanted Osmotic Pump Improves the Efficacy and Potency of Therapy in a Mouse Xenograft Tumor Model. Cancer Res. 2001;61:7669–7674. [PubMed] [Google Scholar]
- 28.Nicosia RF, O. A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol. 1990;26:119–128. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- 29.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis Assays: A Critical Overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 30.Berger AC, Wang X-Q, Zalatoris A, Cenna J, Watson JC. A murine model of ex vivo angiogenesis using aortic disks grown in fibrin clot[star, open]. Microvascular Research. 2004;68:179–187. doi: 10.1016/j.mvr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Fong TA, S. L., Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 32.Takahashi T, U. H., Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 33.Solorzano CC, Jung YD, Bucana CD, McConkey DJ, Gallick GE, McMahon G, Ellis LM. In Vivo Intracellular Signaling as a Marker of Antiangiogenic Activity. Cancer Res. 2001;61:7048–7051. [PubMed] [Google Scholar]
- 34.Agarwal C, Veluri R, Kaur M, Chou S-C, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3'-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis: bgm045. 2007 doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- 35.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]