Abstract
During the last few decades, cancer research has focused on the idea that cancer is caused by genetic alterations and that this disease can be treated by reversing or targeting these alterations. The small variations in cancer mortality observed during the previous 30 years indicate, however, that the clinical applications of this approach have been very limited so far. The development of future gene-based therapies that may have a major impact on cancer mortality may be compromised by the high number and variability of genetic alterations recently found in human tumors. This article reviews evidence that tumor cells, in addition to acquiring a complex array of genetic changes, develop an alteration in the metabolism of oxygen. Although both changes play an essential role in carcinogenesis, the altered oxygen metabolism of cancer cells is not subject to the high genetic variability of tumors and may therefore be a more reliable target for cancer therapy. The utility of this novel approach for the development of therapies that selectively target tumor cells is discussed.
CANCER AS A GENETIC DISEASE
The genetic basis of cancer was discovered more than a century ago, when David von Hansemann observed that cells from various carcinoma samples had chromosomal alterations. At the beginning of the 20th century Theothor Bovery suggested that cancer might result as a consequence of these chromosomal aberrations, laying the foundations for viewing cancer as a genetic disease (1). But the idea of cancer as a genetic disease was not widely accepted at that time. If cancer was caused by a genetic mutation, it was not clear why there was a delay of many years between the exposure to a mutagenic agent and the onset of cancer, or why the incidence of cancer increased so dramatically with age. Over time, the observations that no single gene defect causes cancer and that several mutations could be required for cancer to develop explained the long latent period of cancer and the age distribution of this disease (2–4). In the second half of the 20th century, the idea that the development of cancer required the acquisition of several mutations began to be widely accepted, and efforts were made to identify which genes were involved in carcinogenesis (5,6). In the 1980s the first human oncogenes and tumor-suppressor genes were discovered, and the idea that cancer was caused by mutations in these two types of genes was gaining firm ground (7–11). Mutations in oncogenes would increase the synthesis of proteins that stimulate cell proliferation, and mutations in tumor-suppressor genes would result in the loss of proteins that restrain cell proliferation and induce apoptosis. The accumulation of several mutations in these two types of genes would allow cells to proliferate in an uncontrolled fashion and would lead to cancer. It was believed at that time that cancer would be explained by a relatively low number of mutations in these genes, and that cancer would eventually be treatable by reversing or exploiting these genetic changes.
Molecular analyses of human tumors carried out in the last decade have revealed that the genetic alterations of cancer cells are much more numerous and unstable than previously thought (12). For instance, by sampling colorectal premalignant polyp and carcinoma cell genomes, Stoler et al. (13) found that the mean number of genomic changes per carcinoma cell was approximately 11,000. In addition, much evidence has accumulated stating that mutations (changes in the DNA sequence) are not the only cause of the altered gene expression of cancer cells. Epigenetic alterations (heritable and reversible changes other than the DNA sequence) and aneuploidy (numerical and structural abnormalities in chromosomes) are common alterations in tumor cells, which modify gene expression and may also play a crucial role in carcinogenesis (14–18). Such is the genetic complexity and variability of tumor cells, that the idea of understanding cancer in terms of changes in specific genes is losing ground in favor of proposals that seek to rationalize cancer in terms of a limited number of acquired phenotypes (the so-called hallmarks of cancer) and altered cellular pathways (19–21). We are beginning to realize that the high genetic variability of tumor cells is a serious obstacle for the design of gene-based therapies that may have a major impact on cancer mortality (12,21).
Despite the complexity and variability of the cancer genome, much research is devoted to characterizing the genetic profile of tumors to rationalize and personalize cancer therapy (22–24). The recent approval of several cancer-targeted therapies (therapies that are intended to target the molecular defects of cancer cells specifically) indicate that the altered genome of cancer cells can be exploited therapeutically (25). The landmark event in this new field was the development of imatinib mesylate (Gleevec) for the treatment of chronic myeloid leukemia (CML). This drug was developed as a selective inhibitor of the kinase BCR-ABL, the fusion protein product of a chromosomal translocation that is involved in the pathogenesis of CML. Clinical trials revealed that imatinib mesylate induced complete hematological remission in a very high percentage of patients with chronic-phase CML (26). Although this drug has become the standard of care for CML, it is important to note that imatinib mesilate does not cure the disease (it just keeps it under control for many patients for as long as they take the drug) and that some patients treated with this drug develop resistance to treatment (27,28). Other targeted therapies have been approved for cancer treatment in recent years; however, none of these therapies have led to major improvements in the survival of patients with the most common types of cancer. It is important to be reminded that cancer mortality has not changed much during the last three decades (29), and that the small declines observed in recent years in some types of cancer (that is, lung, colorectal, breast and prostate cancer) are not attributable only to better therapies but also to the implementation of prevention and early detection campaigns (30,31).
Although the genetic alterations of tumor cells are extremely numerous and unstable ((12,13,21)), we are moving toward an era of personalized treatments based on the genetic profile of each tumor (22–24). It is expected that much time and effort and many resources will be necessary to develop therapies that will be useful in a small percentage of patients with cancer. In addition to building up a complex array of genetic changes, tumor cells acquire an alteration in the metabolism of oxygen, a process that plays an important role in carcinogenesis and could be exploited to develop therapies for a broad range of patients with cancer.
KEY ROLE OF ALTERED OXYGEN METABOLISM IN CANCER
Nonmalignant cells use oxygen (O2) to generate energy in the form of ATP through the process of oxidative phosphorylation (oxphos). Accumulating evidence indicates that, instead of fully coupling the metabolism of O2 with the generation of energy, cancer cells activate glycolysis to meet their energy demands and use O2 to generate excessive levels of the reactive oxygen species (ROS) superoxide anion (O2•−) and hydrogen peroxide (H2O2). This alteration in the metabolism of O2 (dysoxic metabolism) is a common feature of cancer cells and plays an important role in carcinogenesis (32–38).
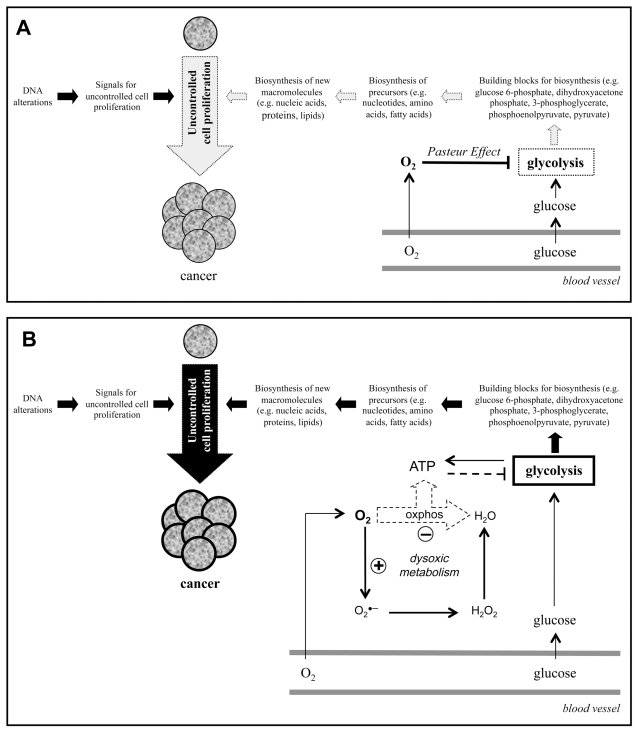
It is well known that uncontrolled cell proliferation is the most relevant feature of cancer. It is also recognized that the genetic defects of cancer cells result in an altered gene expression and in the production of signals that make these cells proliferate in an uncontrolled fashion. Equally important for cell proliferation is that the dividing cell duplicates all its cellular components to create two daughter cells (Figure 1A). To do this, proliferating cells must take nutrients from the blood and use them to synthesize all the macromolecules and cellular components required for the formation of a new cell. The cellular uptake of glucose from the blood and the activation of glycolysis are essential processes for cell proliferation, because the activation of glycolysis provides most of the building blocks required for the synthesis of these macromolecules and cellular components (37). Several glycolytic enzymes are over-expressed in cancer cells and have been shown to play an important role in cancer (39–41). The increased cellular uptake of glucose and the upregulation of glycolysis of cancer cells can indeed be observed with clinical tumor imaging (fluorodeoxyglucose positron-emission tomography) and is currently used for early diagnosis and better management of oncology patients (42).
Figure 1.
Cancer development requires both the acquisition of DNA alterations and a change in the metabolism of oxygen (dysoxic metabolism). (A) The uncontrolled cell proliferation that characterizes cancer requires signals for cell proliferation and the synthesis of new macromolecules (for example, nucleic acids, lipids, proteins). Glycolysis provides building blocks (for example, glucose 6-phosphate, dihydroxyacetone phosphate, 3-phosphoglycerate, phosphoenolpyruvate, pyruvate) that participate in the synthesis of these macromolecules. The presence of O2 can inhibit glycolysis (Pasteur effect) and, therefore, the biosynthesis of new macromolecules required for the uncontrolled cell proliferation that characterizes cancer. (B) A change in the metabolism of O2 (dysoxic metabolism) would allow the activation of glycolysis in the presence of O2 and, therefore, cell proliferation and cancer development.
The fact that the blood vessels deliver both glucose and O2 is a problem for the activation of glycolysis, because the presence of O2 is known to cause glycolysis inhibition (Pasteur effect). This situation suggests that to proliferate cancer cells must develop the capacity to activate glycolysis in the presence of O2 (Figure 1A), a characteristic that was first observed several decades ago by the Nobel laureate Otto Warburg. Warburg also proposed that the high glycolytic rates he observed in cancer cells despite the presence of O2 were caused by a defect in respiration (oxidative phosphorylation) and that this defect was the origin of cancer (43).
Although the activation of glycolysis in the presence of O2 (aerobic glycolysis or the Warburg effect) has repeatedly been observed in cancer cells ((37,42,44)), it is not clear why and how this phenomenon occurs. I first proposed that to proliferate cancer cells (and normal proliferating cells) must activate glycolysis despite the presence of O2 (33,37). As shown in Figure 1A, cell proliferation would be compromised if glycolysis were always inhibited in the presence of O2 (33,37). The same proposal was later made by others (45). As to how this phenomenon occurs, evidence suggests that the change in the metabolism of O2 (dysoxic metabolism) (Figure 1B) is crucial for the activation of glycolysis in the presence of O2 (37). Briefly, high ATP levels repress glycolysis via allosteric inhibition of phosphofructokinase, a key enzyme in the regulation of glycolysis. The possible basis of the Pasteur effect is that the presence of O2 allows ATP synthesis through oxphos, which causes an allosteric inhibition of phosphofructokinase resulting in the inhibition of glycolysis. This proposed mechanism suggests that glycolysis is not directly inhibited by O2, but by ATP, and that the presence of O2 will not cause the inhibition of glycolysis when O2 is not used to generate enough ATP (37). The metabolic switch from oxphos to glycolysis commonly observed in cancer cells ((43,46–48)), along with the increased production of O2•− and H2O2 found in these cells (49–53), supports the idea that cancer cells have this alteration in the metabolism of O2. This alteration may play a crucial role in carcinogenesis by allowing the activation of glycolysis in the presence of O2 and, therefore, the uncontrolled cell proliferation that characterizes cancer (Figure 1B).
A deviation of the metabolism of O2 from the pathway that generates ATP to the pathway that generates ROS may be necessary for cell proliferation and tumor growth (Figure 1B) (33,37). Evidence suggests that this metabolic switch may also play an important role in tumor metastasis (34). Hypoxia-inducible factor 1 (HIF-1) is a key regulator of O2 homeostasis, and the activation of HIF-1 is known to play a vital role in the most relevant aspects of carcinogenesis, including cell survival, angiogenesis, invasion, metastasis, cellular immortalization and metabolic reprogramming (54–56). Because an increased production of H2O2 (57) and the accumulation of glycolytic metabolites (58) are known to activate HIF-1, it has been proposed that the dysoxic metabolism represented in Figure 1B may play an important role in the activation of HIF-1 (32). This dysoxic metabolism results in increased production of O2•− and H2O2, and evidence suggests that the accumulation of these ROS causes oxidative stress and plays an important role in carcinogenesis ((35,36), (59), (60)). The key role of ROS in carcinogenesis is supported by experimental data showing that cancer cells commonly have increased levels of ROS (49–53), that ROS can induce cell malignant transformation (61,62) and that the malignant phenotype of cancer cells can be reversed by reducing the cellular levels of ROS (63–68). Overall, evidence suggests that the alteration in the metabolism of O2 represented in Figure 1B is a common feature of cancer cells and may play a key role in carcinogenesis (32–38).
A NEW MODEL OF CARCINOGENESIS
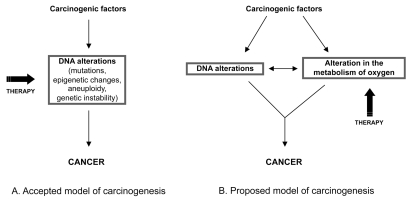
The most accepted model of carcinogenesis postulates that tumorigenesis is caused by DNA alterations and that cancer can be treated by reversing or exploiting these alterations (Figure 2A). A new model of carcinogenesis is proposed in Figure 2B. According to this new model, the development of any cancer requires that the future tumor cell both acquires a complex set of DNA alterations and develops an alteration in the metabolism of O2. It is widely acknowledged that the altered genome of tumor cells plays a key role in carcinogenesis. Evidence suggests that an alteration in the metabolism of O2 from the pathway that generates energy to the pathway that produces ROS may also play an important role in the development of cancer (discussed in this review). This new model considers that both alterations must cooperate for the formation of a cancer; the acquisition of DNA alterations leads to an alteration in the metabolism of O2 and vice versa. Indeed, there is evidence that the transition from a normal to a malignant phenotype brought about by cancer-causing genes is associated with a progressive energy switch from oxphos to glycolysis (69). Alterations in p53, one of the most frequently mutated tumor-suppressor genes in cancer, have also been proposed to participate in the metabolic switch from oxphos to glycolysis (48). Mitochondrial mutations may reduce the activity of oxphos and have been associated with an increase in the cellular production of ROS (70). The activation of several oncogenes is also known to increase the cellular production of O2•− and H2O2 (71–74). Conversely, an alteration in the metabolism of O2 (from the pathway that generates ATP to the pathway that generates O2•− and H2O2) can lead to the acquisition of DNA alterations. H2O2 is indeed well known to induce DNA alterations, including DNA damage, mutations and genetic instability (75–78).
Figure 2.
Models of carcinogenesis. In addition to the acquisition a complex array of DNA alterations proposed in the accepted model of carcinogenesis (A), the model discussed in this review (B) proposes that cancer develops an alteration in the metabolism of oxygen. Although both changes must interact for the development of cancer, the altered oxygen metabolism of tumor cells is not subject to the high genetic complexity and variability of tumors and may therefore be a more reliable target for cancer therapy.
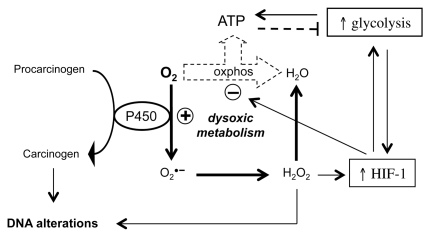
Most carcinogenic agents have been shown to induce DNA alterations. Most carcinogenic agents also induce oxidative stress (38,79), and a deviation in the metabolism of O2 toward the pathway that generates ROS is a key feature of oxidative stress ((38,60,79)). An example of how a carcinogenic agent can induce both DNA alterations and an alteration in the metabolism of O2 is shown in Figure 3. Most chemical carcinogens need to be enzymatically activated to become genotoxic, and the cytochrome P450 (P450) enzymes are the most prominent enzymes involved in such activation (80). The activity of P450 enzymes is associated with the generation of O2•− and H2O2 (81), and H2O2 is well known to induce DNA alterations (75–78). An increase in the generation of H2O2 is associated with the activation of HIF-1 (32,57), which can lead to the repression of oxphos and the activation of glycolysis (82,83). The accumulation of glycolytic intermediates caused by the activation of glycolysis can also increase the activity of HIF-1 (58) (Figure 3). Carcinogenic agents can induce DNA alterations and an altered O2 metabolism independently of P450. For instance, the increase in the intracellular pH induced by some carcinogenic agents seems to be crucial for the development of cancer (84,85). A rise in the intracellular pH can increase the production of O2•− (86) and lead to DNA alterations and a dysoxic metabolism through the pathways represented in Figure 3. Because exposure to many other carcinogenic factors has been associated with an increased production of ROS ((38,59,79)), these carcinogenic factors may also lead to DNA alterations and a dysoxic metabolism through the pathways represented in Figure 3.
Figure 3.
Carcinogenic agents can induce DNA alterations and an alteration in the metabolism of oxygen. This figure represents an example of how a carcinogenic agent can induce both DNA alterations and a dysoxic metabolism via P450. See text for references and further details.
According to the most accepted model of carcinogenesis, the DNA alterations of tumor cells are a target for cancer therapy (Figure 2A). However, as discussed in above, the high number and variability of these DNA alterations is an obstacle for the design of gene-based therapies that may have a major impact on cancer mortality (12,21). The importance of the new model of carcinogenesis represented in Figure 2B is that it offers an alternative target for the treatment of cancer, which is not subject to the high genetic variability of tumor cells and may therefore be more easily targeted. How the altered O2 metabolism of cancer could be used therapeutically to kill tumor cells selectively is discussed in the following section.
TARGETING THE ALTERED OXYGEN METABOLISM OF TUMOR CELLS FOR THE TREATMENT OF CANCER
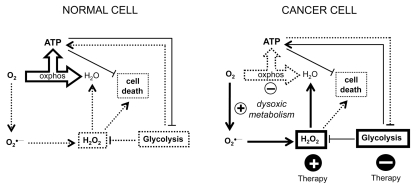
Tumor cells and normal cells metabolize O2 differently; this difference could be exploited to target tumor cells selectively (Figure 4). Normal cells have full oxphos capacity, low production of H2O2 and glycolysis inhibition in the presence of O2. Normal cells do not need to maintain high glycolytic activity to ensure their survival. Cancer cells have an alteration in the metabolism of O2 (dysoxic metabolism), which is associated with oxphos repression, increased production of H2O2 and increased glycolytic activity. The high glycolytic activity of cancer cells is essential for their survival, because it prevents cell death induced by ATP depletion and H2O2 accumulation. The dysoxic metabolism of cancer cells can be exploited to kill cancer cells selectively by increasing the cellular levels of H2O2 and/or by attenuating glycolysis. These effects could be achieved by the use of prooxidant agents and glycolysis inhibitors, alone or in combination.
Figure 4.
Utility of the altered oxygen metabolism of cancer cells to selectively kill them. Cancer cells and normal cells metabolize oxygen differently. Because the basal levels of H2O2 are higher in cancer cells than in normal cells, a specific increase in the concentrations of H2O2 may lead to cytotoxic concentrations in cancer cells but not in normal cells. In addition, because the activation of glycolysis in cancer cells is essential to prevent cell death induced by ATP depletion and H2O2 accumulation, the attenuation of glycolysis in cancer cells can induce their death. Normal cells would be less affected by this strategy, because they do not need to have increased glycolytic rates to ensure their survival. See text for further details. Dotted lines indicate that the pathway or process is repressed. Bolded lines indicate that the process is activated or that the levels of the molecule are increased.
Selective Killing of Cancer Cells by H2O2 and Prooxidant Agents
Recent data suggest that oxidative stress may play a role in the anticancer activity of many chemotherapeutic agents commonly used in cancer treatment, including paclitaxel, cisplatin, doxorubicin, arsenic trioxide, bortezomib, procarbazine and etoposide (87–101). For instance, although it has been known for many years that the microtubule protein tubulin is the therapeutic target for paclitaxel (taxol), recent experiments have shown that H2O2 plays an important role in paclitaxel-induced cancer cell death (87,89). The role of ROS in the activity of many anticancer agents is increasingly being acknowledged, and the induction of oxidative stress by prooxidant agents is emerging as an attractive anticancer strategy ((36,94,99,102–106)).
H2O2 seems to be a key player in oxidative stress–induced cancer cell death. Many anticancer agents, such as paclitaxel, doxorubicin and arsenic trioxide, produce H2O2 ((87,90,92)), and H2O2 is known to be an efficient inducer of cell death in cancer cells ((36,93,107)). Interestingly, cancer cells seem more susceptible to H2O2-induced cell death than nonmalignant cells (108–110). Investigating several cancer and normal cell lines, Chen et al. (108) observed that high concentrations of ascorbic acid selectively killed cancer cells and that this effect was mediated by H2O2. They showed, for instance, that a concentration of 50 μmol/L H2O2 induced a higher percentage of cell death in Burkitt lymphoma cells than 250 μmol/L in normal lymphocytes and normal monocytes (108). In vitro and in vivo data indicate that tumor cells produce higher concentrations of H2O2 than their normal counterparts (49–53). These data, and the fact that there is a threshold of H2O2 above which cells cannot survive, may explain why specific concentrations of H2O2 induce selective killing of cancer cells (36). Overall, evidence suggests that increasing the levels of H2O2 in cancer cells by using prooxidant agents may be an important therapeutic strategy. The concentration of a prooxidant agent required to generate levels of H2O2 that kill cancer cells but not normal cells could be determined in cell culture experiments. Then, by using an appropriate route of administration, such concentrations should be achieved in vivo to observe a selective antitumor effect.
Selective Killing of Cancer Cells by Glycolysis Inhibition
The increased glycolytic activity of cancer cells seems to be important for keeping adequate energy levels in these cells. Xu et al. (111) observed that the inhibition of glycolysis severely depleted ATP in cancer cells and induced cell death, especially in cancer cells with mitochondrial respiration defects. The dependence of cancer cells on glycolytic energy seems to increase as malignant transformation occurs (69). It has been proposed that this increased dependence on glycolysis for energy generation is an important metabolic difference between normal and malignant cells that may serve for developing therapeutic strategies to preferentially kill cancer cells ((44,112,113)). Several glycolysis inhibitors have shown anticancer effects (for example, 2-deoxy-D-glucose, lonidamine, 3-bromopyruvate and dichloroacetate) and some of them have entered the clinical trial stage of investigation ((37,44,112), (114)). For example, it has been shown that dichloroacetate, a known glycolysis inhibitor that has been used in humans for decades in the treatment of lactic acidosis and inherited mitochondrial diseases, induced marked anticancer effects in mice (115). The authors found that dichloroacetate in the drinking water at clinically relevant doses for up to 3 months prevented and reversed tumor growth in vivo, without apparent toxicity and without affecting hemoglobin, transaminase or creatinine levels. They concluded that the ease of delivery, selectivity and effectiveness of dichloroacetate make this agent an attractive candidate for cancer therapy, one that can be rapidly translated into phase II–III clinical trials (115). Other strategies could be used to inhibit or exploit the increased glycolytic activity of cancer cells. Because an increase in the activity of the Na+/K+-ATPase pump is associated with the activation of glycolysis (116,117), the inhibition of this pump (for example, by cardiac glycosides) may result in the inhibition of glycolysis and the selective killing of cancer cells (113,118). The activation of glycolysis is known to increase the concentration of protons in the cytosol. These protons must be extruded to prevent acid-induced cell death. The inhibition of the cellular systems involved in the extrusion of protons in cancer cells may also lead to the selective killing of cancer cells (119).
Combination of Prooxidant Agents with Glycolysis Inhibitors for Anticancer Therapy
Although ROS can induce cancer cell death, tumor cells are known to develop mechanisms that prevent ROS from reaching cytotoxic levels. The glutathione and thioredoxin antioxidant systems are crucial for detoxifying ROS. These antioxidant systems are activated in cancer cells and play an important role in the development of resistance to many anticancer agents (120–126). Likewise, although the inhibition of glycolysis is an attractive anticancer strategy, in vivo experiments suggest that the inhibition of glycolysis may not be sufficient to induce potent anticancer effects. Accordingly, although the glycolysis inhibitor 2-deoxy-D-glucose is an efficient inducer of cell death in vitro (127), its anticancer in vivo activity is not very high when it is used as a single agent. The anticancer activity of 2-deoxy-D-glucose has been explored in combination with chemotherapeutic drugs and radiation, and some of these combinations have entered clinical trials ((44,112,128–132)).
Prooxidant agents could be combined with glycolysis inhibitors to maximize their anticancer activity. Evidence indicates that prooxidant agents can increase the cellular levels of H2O2 and that glycolysis inhibitors can reduce the capacity of cells to detoxify H2O2. Experimental data have shown that malignant cells are more susceptible to glucose deprivation than nontransformed cells, and that an increase in the levels of H2O2 may mediate the cytotoxic effect induced by glucose deprivation ((53,133–135)). Two possible mechanisms may explain why the activation of glycolysis performs an important function in protecting tumor cells from H2O2-induced cell death. First, the activation of glycolysis increases the formation of pyruvate, which is an efficient scavenger of H2O2 (136–139). Second, glucose metabolism through the pentose phosphate pathway regenerates NADPH from NADP+ in a reaction in which glucose-6-phosphate is converted into 6-phosphogluconolactone by the enzyme glucose-6-phosphate dehydrogenase. The regeneration of NADPH is required for H2O2 detoxification through the glutathione peroxidase/glutathione reductase system and through the thioredoxin peroxidase/thioredoxin reductase system ((134,140,141)) (Figure 5).
Figure 5.
Key role of glycolysis in the detoxification of H2O2. Increased glucose metabolism helps detoxify H2O2 by increasing the levels of the H2O2 scavenger pyruvate and by regenerating NADPH. Glutathione reductase (GR) and thioredoxin reductase (TrxR) need NADPH to regenerate glutathione (GSH) and thioredoxin [Trx(SH)2], which are used by glutathione peroxidase (GPx) and thioredoxin peroxidase (TPx) to detoxify H2O2. Thiol (SH)-reactive agents can react with the SH groups of GSH and Trx(SH)2 and induce a prooxidant effect by disrupting the GR/GPx and TrxR/TPx antioxidant systems.
CONCLUSIONS
Cancer kills more than six million people worldwide every year (142). The mortality rate of this disease has not changed much in the past few decades even in developed countries such as the United States (29). The small decreases observed in recent years in some types of cancer (29) are not attributable only to better therapies, but also to the implementation of prevention and early detection campaigns. The goal of these campaigns is to make people aware that many cancers can be prevented by following several guidelines, and that cancer therapy is effective when this disease is detected early (30,31). Despite these campaigns, many cancers are still diagnosed when cells from a primary tumor have already metastasized to other parts of the body. At this stage of disease, tumor cells are no longer localized and can no longer be eliminated by surgery or radiotherapy. The main form of treatment at this point is chemotherapy, which consists of delivering drugs systemically so that they can reach and kill the tumor cells. But most of these drugs are toxic to both tumor and normal cells, cause severe side effects in patients and, therefore, need to be used at suboptimal levels. The low efficacy of chemotherapy in patients with advanced cancers is reflected in the low 5-year survival rates observed in these patients (29). For instance, cancer statistics show that the most commonly diagnosed cancer in the world is lung cancer (142), that approximately 50% of patients diagnosed with lung cancer have distant metastasis (29) and that only 3% of these patients manage to survive more than 5 years (29). The low efficacy of cancer therapy for the treatment of patients with metastasis makes the development of novel therapeutic approaches necessary.
A novel therapeutic approach has emerged strongly in recent years. This approach seeks to attack the tumor cells selectively and is based on understanding of the differences between tumor cells and nonmalignant cells. It has been known for many years that tumor cells have genetic alterations and much research has been done to identify these alterations. Recent analyses of human cancers have revealed, however, that the genetic defects of tumor cells are much more numerous and unstable than expected (12,13). In addition, the genetic alterations of tumor cells are not the same in different types of cancer or even in different people with the same type of cancer. Given these circumstances it will probably be very difficult to develop future gene-based therapies that may be useful in a wide range of patients with cancer ((12,20,21)). Despite the complexity of the cancer genome, much research is devoted to characterizing the genetic profile of tumors to rationalize and personalize cancer therapy (22–24).
An alternative approach has been discussed in this review. In addition to building up a complex set of DNA changes, evidence suggests that the development of any cancer requires that tumor cells acquire an alteration in the metabolism of oxygen. Interestingly, this alteration in the metabolism of oxygen can make cancer cells vulnerable to therapeutic intervention. Their increased basal levels of H2O2 and their higher dependence on glycolysis for their survival make cancer cells more susceptible than normal cells to treatment with prooxidant agents and glycolysis inhibitors. Because this alteration in the metabolism of oxygen seems to be a common feature of tumor cells, this therapeutic approach could be used for the treatment of a wide range of patients with cancer. Future research will reveal whether this alternative approach will be sufficient to increase the survival of patients with advanced cancers, or whether it will be necessary to use it in combination with traditional chemotherapy and/or novel targeted therapies.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Nature milestones in cancer. Nat Rev Cancer. 2006;6:S8–9. [Google Scholar]
- 2.Nordling CO. A new theory on cancer-inducing mechanism. Br J Cancer. 1953;7:68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fardon JC. A reconsideration of the somatic mutation theory of cancer in the light of some recent developments. Science. 1953;117:441–5. doi: 10.1126/science.117.3043.441. [DOI] [PubMed] [Google Scholar]
- 4.Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954;8:1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Reddy EP, Reynolds RK, Santos E, Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982;300:149–52. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- 8.Parada LF, Tabin CJ, Shih C, Weinberg RA. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297:474–8. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb M, Shimizu K, Perucho M, Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982;296:404–9. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- 10.Bishop JM. The molecular genetics of cancer. Science. 1987;235:305–11. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- 11.Stanbridge EJ. Identifying tumor suppressor genes in human colorectal cancer. Science. 1990;247:12–3. doi: 10.1126/science.2403692. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J, Hahnfeldt P, Hlatky L. Cancer: looking outside the genome. Nat Rev Mol Cell Biol. 2000;1:76–9. doi: 10.1038/35036100. [DOI] [PubMed] [Google Scholar]
- 13.Stoler DL, et al. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci U S A. 1999;96:15121–6. doi: 10.1073/pnas.96.26.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobuzio-Donahue CA. Epigenetic changes in cancer. Annu Rev Pathol. 2009;4:229–49. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 15.Prehn RT. Cancers beget mutations versus mutations beget cancers. Cancer Res. 1994;54:5296–300. [PubMed] [Google Scholar]
- 16.Jaffe LF. Epigenetic theories of cancer initiation. Adv Cancer Res. 2003;90:209–30. doi: 10.1016/s0065-230x(03)90007-8. [DOI] [PubMed] [Google Scholar]
- 17.Rasnick D, Duesberg PH. How aneuploidy affects metabolic control and causes cancer. Biochem J. 1999;340:621–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–41. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 21.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 22.Garman KS, Nevins JR, Potti A. Genomic strategies for personalized cancer therapy. Hum Mol Genet. 2007;16:R226–32. doi: 10.1093/hmg/ddm184. [DOI] [PubMed] [Google Scholar]
- 23.van’t Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. 2008;452:564–70. doi: 10.1038/nature06915. [DOI] [PubMed] [Google Scholar]
- 24.Hayden EC. Personalized cancer therapy gets closer. Nature. 2009;458:131–2. doi: 10.1038/458131a. [DOI] [PubMed] [Google Scholar]
- 25.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–37. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 26.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 27.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 28.Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–53. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 30.Kushi LH, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–81. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 31.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Lazaro M. HIF-1: Hypoxia-inducible factor or dysoxia-inducible factor? FASEB J. 2006;20:828–32. doi: 10.1096/fj.05-5168hyp. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Lazaro M. Does hypoxia really control tumor growth? Cell Oncol. 2006;28:327–9. doi: 10.1155/2006/251376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Lazaro M. Why do tumors metastasize? Cancer Biol Ther. 2007;6:141–4. doi: 10.4161/cbt.6.2.3950. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Lazaro M. Excessive superoxide anion generation plays a key role in carcinogenesis. Int J Cancer. 2007;120:1378–80. doi: 10.1002/ijc.22493. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Lazaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Lazaro M. The Warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem. 2008;8:305–12. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Lazaro M. Role of oxygen in cancer: looking beyond hypoxia. Anticancer Agents Med Chem. 2009;9:517–25. doi: 10.2174/187152009788451806. [DOI] [PubMed] [Google Scholar]
- 39.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chesney J, et al. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci U S A. 1999;96:3047–52. doi: 10.1073/pnas.96.6.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 42.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 43.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–74. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 45.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isidoro A, et al. Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J. 2004;378:17–20. doi: 10.1042/BJ20031541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unwin RD, et al. Proteomic changes in renal cancer and co-ordinate demonstration of both the glycolytic and mitochondrial aspects of the Warburg effect. Proteomics. 2003;3:1620–32. doi: 10.1002/pmic.200300464. [DOI] [PubMed] [Google Scholar]
- 48.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–3. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 49.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8. [PubMed] [Google Scholar]
- 50.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 51.Zieba M, et al. Comparison of hydrogen peroxide generation and the content of lipid peroxidation products in lung cancer tissue and pulmonary parenchyma. Respir Med. 2000;94:800–5. doi: 10.1053/rmed.2000.0825. [DOI] [PubMed] [Google Scholar]
- 52.Lim SD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–7. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 53.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–9. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Lazaro M. Hypoxia-inducible factor 1 as a possible target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:2332–5. doi: 10.1158/1055-9965.EPI-06-0369. [DOI] [PubMed] [Google Scholar]
- 57.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 58.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 59.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–81. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 60.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto M, Kawai K, Reznikoff CA, Oyasu R. Transformation in vitro of a nontumorigenic rat urothelial cell line by hydrogen peroxide. Cancer Res. 1996;56:4649–53. [PubMed] [Google Scholar]
- 62.Suh YA, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 63.Arnold RS, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Church SL, et al. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993;90:3113–7. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Safford SE, Oberley TD, Urano M, St Clair DK. Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase. Cancer Res. 1994;54:4261–5. [PubMed] [Google Scholar]
- 66.Yan T, Oberley LW, Zhong W, St Clair DK. Manganese-containing superoxide dismutase overexpression causes phenotypic reversion in SV40-transformed human lung fibroblasts. Cancer Res. 1996;56:2864–71. [PubMed] [Google Scholar]
- 67.Zhang Y, Zhao W, Zhang HJ, Domann FE, Oberley LW. Overexpression of copper zinc superoxide dismutase suppresses human glioma cell growth. Cancer Res. 2002;62:1205–12. [PubMed] [Google Scholar]
- 68.Hyoudou K, et al. Inhibition of metastatic tumor growth in mouse lung by repeated administration of polyethylene glycol-conjugated catalase: quantitative analysis with firefly luciferase-expressing melanoma cells. Clin Cancer Res. 2004;10:7685–91. doi: 10.1158/1078-0432.CCR-04-1020. [DOI] [PubMed] [Google Scholar]
- 69.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A. 2005;102:5992–7. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishikawa K, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 71.Irani K, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 72.Vafa O, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–44. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 73.Sattler M, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–8. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 74.Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–8. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx-mutants of Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:9317–22. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dayal D, Martin SM, Limoli CL, Spitz DR. Hydrogen peroxide mediates the radiation-induced mutator phenotype in mammalian cells. Biochem J. 2008;413:185–91. doi: 10.1042/BJ20071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson AL, Loeb LA. Microsatellite instability induced by hydrogen peroxide in Escherichia coli. Mutat Res. 2000;447:187–98. doi: 10.1016/s0027-5107(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 79.Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–96. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 80.Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400:201–13. doi: 10.1016/s0027-5107(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 81.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–31. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 82.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 83.Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–4. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 84.Reshkin SJ, et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185–97. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- 85.Harguindey S, Orive G, Luis PJ, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na(+)/H(+) antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin-one single nature. Biochim Biophys Acta. 2005;1756:1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Simchowitz L. Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J Clin Invest. 1985;76:1079–89. doi: 10.1172/JCI112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alexandre J, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–8. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
- 88.Alexandre J, et al. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98:236–44. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- 89.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–7. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 90.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11. [PubMed] [Google Scholar]
- 91.Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–53. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 92.Ubezio P, Civoli F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic Biol Med. 1994;16:509–16. doi: 10.1016/0891-5849(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 93.Ikeda K, et al. Involvement of hydrogen peroxide and hydroxyl radical in chemically induced apoptosis of HL-60 cells. Biochem Pharmacol. 1999;57:1361–5. doi: 10.1016/s0006-2952(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 94.Fang J, Nakamura H, Iyer AK. Tumor-targeted induction of oxystress for cancer therapy. J Drug Target. 2007;15:475–86. doi: 10.1080/10611860701498286. [DOI] [PubMed] [Google Scholar]
- 95.Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900–7. doi: 10.1074/jbc.273.41.26900. [DOI] [PubMed] [Google Scholar]
- 96.Gorman A, McGowan A, Cotter TG. Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett. 1997;404:27–33. doi: 10.1016/s0014-5793(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 97.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–23. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 98.Perez-Galan P, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 99.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–40. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 100.Oh SY, et al. Selective cell death of oncogenic Akt-transduced brain cancer cells by etoposide through reactive oxygen species mediated damage. Mol Cancer Ther. 2007;6:2178–87. doi: 10.1158/1535-7163.MCT-07-0111. [DOI] [PubMed] [Google Scholar]
- 101.Doroshow JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad Sci U S A. 1986;83:4514–8. doi: 10.1073/pnas.83.12.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 104.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–6. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 105.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–69. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–12. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- 107.Hirpara JL, Clement MV, Pervaiz S. Intra-cellular acidification triggered by mitochondrial-derived hydrogen peroxide is an effector mechanism for drug-induced apoptosis in tumor cells. J Biol Chem. 2001;276:514–21. doi: 10.1074/jbc.M004687200. [DOI] [PubMed] [Google Scholar]
- 108.Chen Q, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102:13604–9. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maeda H, et al. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–46. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 110.Djavaheri-Mergny M, Wietzerbin J, Besancon F. 2-Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene. 2003;22:2558–67. doi: 10.1038/sj.onc.1206356. [DOI] [PubMed] [Google Scholar]
- 111.Xu RH, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–21. [PubMed] [Google Scholar]
- 112.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 113.Lopez-Lazaro M. Digitoxin as an anti-cancer agent with selectivity for cancer cells: possible mechanisms involved. Expert Opin Ther Targets. 2007;11:1043–53. doi: 10.1517/14728222.11.8.1043. [DOI] [PubMed] [Google Scholar]
- 114.Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–66. doi: 10.1016/j.biocel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 115.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 116.Paul RJ, Bauer M, Pease W. Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science. 1979;206:1414–6. doi: 10.1126/science.505014. [DOI] [PubMed] [Google Scholar]
- 117.James JH, et al. Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle: implications for increased muscle lactate production in sepsis. J Clin Invest. 1996;98:2388–97. doi: 10.1172/JCI119052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez-Lazaro M, et al. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J Nat Prod. 2005;68:1642–5. doi: 10.1021/np050226l. [DOI] [PubMed] [Google Scholar]
- 119.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. Proton transport inhibitors as potentially selective anticancer drugs. Anti-cancer Res. 2009;29:2127–36. [PubMed] [Google Scholar]
- 120.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–81. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 121.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–20. [PubMed] [Google Scholar]
- 122.Yang P, Ebbert JO, Sun Z, Weinshilboum RM. Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: a review. J Clin Oncol. 2006;24:1761–9. doi: 10.1200/JCO.2005.02.7110. [DOI] [PubMed] [Google Scholar]
- 123.Zhang K, Mack P, Wong KP. Glutathione-related mechanisms in cellular resistance to anticancer drugs. Int J Oncol. 1998;12:871–82. doi: 10.3892/ijo.12.4.871. [DOI] [PubMed] [Google Scholar]
- 124.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–6. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 125.Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–9. [PubMed] [Google Scholar]
- 126.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29:312–22. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 127.Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87:805–12. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maschek G, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 129.Coleman MC, et al. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322–31. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 130.Lin X, et al. 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–7. [PubMed] [Google Scholar]
- 131.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–70. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hernlund E, et al. Potentiation of chemotherapeutic drugs by energy metabolism inhibitors 2-deoxyglucose and etomoxir. Int J Cancer. 2008;123:476–83. doi: 10.1002/ijc.23525. [DOI] [PubMed] [Google Scholar]
- 133.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells: a fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–62. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 134.Ahmad IM, et al. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–63. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 135.Jelluma N, et al. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4:319–30. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]
- 136.Nath KA, et al. alpha-Ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione-induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:C227–36. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 137.Miwa H, Fujii J, Kanno H, Taniguchi N, Aozasa K. Pyruvate secreted by human lymphoid cell lines protects cells from hydrogen peroxide mediated cell death. Free Radic Res. 2000;33:45–56. doi: 10.1080/10715760000300601. [DOI] [PubMed] [Google Scholar]
- 138.Ramakrishnan N, Chen R, McClain DE, Bunger R. Pyruvate prevents hydrogen peroxide-induced apoptosis. Free Radic Res. 1998;29:283–95. doi: 10.1080/10715769800300321. [DOI] [PubMed] [Google Scholar]
- 139.Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991;88:1886–93. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tuttle SW, Varnes ME, Mitchell JB, Biaglow JE. Sensitivity to chemical oxidants and radiation in CHO cell lines deficient in oxidative pentose cycle activity. Int J Radiat Oncol Biol Phys. 1992;22:671–5. doi: 10.1016/0360-3016(92)90500-h. [DOI] [PubMed] [Google Scholar]
- 141.Averill-Bates DA, Przybytkowski E. The role of glucose in cellular defences against cytotoxicity of hydrogen peroxide in Chinese hamster ovary cells. Arch Biochem Biophys. 1994;312:52–8. doi: 10.1006/abbi.1994.1279. [DOI] [PubMed] [Google Scholar]
- 142.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]