Abstract
Background
Mandated reduction of exposure to nicotine and other cigarette toxins has been proposed as a possible national regulatory strategy. However, tapering using lower yield commercial cigarettes may not be effective in reducing nicotine or tar exposure due to compensatory smoking behavior. We examined the effects of gradual reduction of nicotine yield in commercial cigarettes on smoking behavior, with an assessment of nicotine intake and exposure to tobacco smoke toxins.
Methods
This 10-week longitudinal study of 20 smokers involved smoking the usual brand followed by different brands with progressively lower machine-determined yields, ranging from 0.9 to 0.1 mg nicotine, each smoked for 1 week. Subjects were followed for 4 weeks after returning to smoking the usual brand (or quitting). Smoking behaviors, biomarkers of tobacco smoke exposure, and cardiovascular effects were measured.
Findings
Cotinine and other biomarkers of smoke exposure remained unchanged comparing the usual brand with the 0.4 mg nicotine brands. A 30% to 40% decrease in nicotine, carbon monoxide, and carcinogen exposure comparing 0.1 mg nicotine cigarettes with baseline was observed. Self-efficacy was significantly increased and dependence decreased after tapering.
Implications
We confirm prior cross-sectional population and experimental studies showing complete compensation for cigarettes down to the 0.4 mg nicotine range. Nicotine and tobacco toxin exposure were substantially reduced while smoking 0.1 mg nicotine cigarettes. Our data suggest that the degree of nicotine dependence of smokers may be lowered with progressive yield tapering. Gradual tapering of smokers from regular to ultralow nicotine yield commercial cigarettes might facilitate smoking cessation and warrants future research.
Introduction
Federal regulation of cigarettes and other tobacco products has been recommended as an element of a broad-based approach to reduce the disease burden of tobacco use (1, 2). One component of federal regulation is likely to be the power to mandate limits of emissions of tobacco smoke toxins. Nicotine is responsible for tobacco-induced disease in that it sustains tobacco use. It has been suggested that reduction of nicotine availability would make cigarettes less addicting and might facilitate quitting, thereby reducing smoking-related disease (1, 3, 4). Reduction of delivery of other tobacco smoke toxins would also be part of such a regulatory strategy.
We have previously published research on the progressive tapering of cigarettes with reduced nicotine content (RNC), suggesting that the level of nicotine intake by the smoker could be substantially reduced, with the possibility of reducing the level of addiction (5). However, RNC cigarettes are not widely available. Quest cigarettes are manufactured in two RNC varieties, but these cigarettes are not widely available. Commercial low yield cigarettes have not, in general, been promising with respect to lowering exposure to nicotine and other tobacco smoke toxins, owing to changes in smoking behavior to sustain desired levels of nicotine intake (6). However, there is evidence that nicotine and other toxic exposures are reduced to some extent in smokers of ultralow yield cigarettes in the 0.1 and 0.2 mg nicotine classes (7, 8).
Gradual reduction of nicotine exposure was suggested many years ago as a way to aid smoking cessation (9–12). This was done by having smokers switch to a sequence of cigarette brands with progressively lower nicotine yields as determined by smoking machine testing. Although initial trials were promising, the results of larger trials of “nicotine fading,” as they were called, have, in general, not been encouraging (11, 12). This is most likely because commercial low yield cigarettes contain as much nicotine as do higher yield cigarettes, and it is possible for the smoker to compensate for most low yield cigarettes by smoking their cigarettes more intensively (more frequent or larger puffs; ref. 6). Furthermore, compensation for low yield cigarettes might lead to greater exposure to tobacco smoke toxins, such as carbon monoxide and various carcinogens.
We report here the results of a study of commercial cigarette yield reduction with a fairly comprehensive biochemical assessment of nicotine and carcinogen exposure, as well as measurements of cardiovascular biomarkers of smoking-related effects, and smoking behaviors. To the best of our knowledge, this is the first reduction study with extensive characterization of exposure and effects of different yields of commercially available cigarettes. In this study, we find evidence that exposure to and dependence on nicotine can be reduced with progressive commercial cigarette yield reduction, although nicotine reduction does not occur until smokers switch to ultralow yield cigarettes. Furthermore, carcinogen exposure is significantly lower while smoking ultralow yield cigarettes.
Materials and Methods
Overview of Study Design
This was a 10-week, unblinded study in which smokers smoked their usual brand of cigarette and then five different types of commercial cigarettes of progressively lower nicotine yields, as determined by U.S. Federal Trade Commission (FTC) testing procedures, each for 1 week. Smokers were then followed for an additional 4 weeks after returning to their usual cigarette brand.
Subjects
Twenty healthy smokers were recruited by newspaper advertisements. Subjects were determined not to be interested in quitting smoking in the next 6 months. Subjects included 10 men and 10 women with an average age of 37 years (range, 18–56). Subjects smoked an average of 18.0 cigarettes per day [95% confidence interval (95% CI), 14.5–21.6], had smoked for an average of 20.3 years (14.7–25.9), had an average Fagerström Test for Nicotine Dependence (13) score of 3.9 (2.8–5.0), had an average of 14.9 years of education (14.2–15.6), and had an average screening plasma cotinine concentration of 177 ng/mL (39–264). Subjects were compensated financially for participation in the study, receiving $500 for completion of all study procedures.
Written informed consent was obtained from each subject. The study was approved by the Institutional Review Board at the University of California at San Francisco.
Study Protocol
Subjects were studied in a community research clinic. Subjects were asked to come to the clinic weekly, at which time cigarettes were dispensed, blood and urine samples were collected, and a battery of questionnaires was administered. Subjects were instructed to smoke their cigarettes as desired but not to smoke any other type of cigarette and not to use other forms of tobacco or nicotine medications. Subjects were told that this was a study of exposure to various tobacco toxins in people switching to cigarettes of progressively lower nicotine and tar yields. They were also told that the question of whether switching to lower yield cigarettes might help some smokers quit smoking would be examined. Subjects were provided with cigarettes at no cost. They were given their usual number of packs smoked per week plus two more packs. Subjects were asked to contact the research staff for additional cigarettes if needed. At each clinic visit (weeks 1–6), the plasma nicotine concentration boost from smoking one cigarette was measured. This cigarette was smoked under observation and plasma nicotine concentrations were measured before and 2 min after smoking the cigarette. Subjects typically attended the clinic in the late afternoon or early evening.
At each assessment, subjects were asked to report their cigarette consumption over the past 7 days. Subjects also completed the Profile of Mood Scale (14), Minnesota Nicotine Withdrawal Scale (15), Center for Epidemiological Studies Depression scale (16), and a cigarette acceptance questionnaire (17). The cigarette acceptance questionnaire includes 7 items, asking about satisfaction, similarity to usual brand, psychologic reward, aversion, respiratory sensations, craving, and perceived strength, which are rated on a Likert scale. On study entry, at the end of the nicotine reduction phase, and at study termination, subjects also completed the Fagerström Test for Nicotine Dependence (13) and a self-efficacy questionnaire (18). The self-efficacy questionnaire is a 14-item instrument that asks smokers to rate their confidence in their ability to resist smoking in various high-risk situations. A higher self-efficacy rating before a quit attempt is associated with a higher probability of quitting smoking and lower risk of relapse after quitting (19).
Plasma samples were assayed for concentrations of nicotine and cotinine (the major proximate metabolite of nicotine); blood was assayed for carboxyhemoglobin and various cardiovascular biomarkers. These cardiovascular biomarkers, which are thought to be predictors of coronary heart disease risk, included WBC count, hemoglobin, C-reactive protein, and fibrinogen. Urine samples were assayed for concentrations of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a carcinogen itself and metabolite of the carcinogenic tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, as well as metabolites of several polycyclic aromatic hydrocarbons (PAH) found in tobacco smoke. NNAL and the PAH metabolites are biomarkers of exposure to common tobacco smoke carcinogens (20).
Cigarettes
The cigarettes were commercially available cigarettes of progressively lower FTC method smoking machine-determined nicotine yields as described in Table 1. These cigarettes also had progressively lower tar and carbon monoxide deliveries by machine testing. The tapering schedule was selected to allow for ~50% reduction in nicotine yield in each of the last three cigarette stages (assuming no compensation).
Table 1.
FTC yields of study cigarettes
| Smoke constituent | Commercial cigarette brand |
|||||
|---|---|---|---|---|---|---|
| Usual brand | Pall Mall 100 SP | Merit King SP | True King SP | Now 100 SP | Carlton 100 SP | |
| Nicotine (mg) | 1.05 (0.89–1.19)* | 0.9 | 0.6 | 0.4 | 0.2 | 0.1 |
| Tar (mg) | 12.1 (10.8–13.3)* | 12 | 8 | 4 | 2 | 1 |
| Carbon monoxide (mg) | 12.8 (11.8–13.7)* | 12 | 10 | 5 | 3 | 1 |
| Ventilation (%)† | NA | 10 | 70 | 59 | 73 | 79 |
NOTE: Based on FTC machine testing 2001.
Mean (95% CI).
From Massachusetts Department of Public Health Document 105 CMR 666.000. Cigarettes and smokeless tobacco products: reports of added constituents and nicotine ratings.
Analytic Chemistry
Plasma nicotine and cotinine were measured by gas chromatography with nitrogen-phosphorous detection modified for simultaneous determination of nicotine and cotinine using a capillary column (21, 22). Urine concentrations of NNAL (free plus conjugated) were measured by liquid chromatography-tandem mass spectrometry as described previously (5). PAH metabolites, including 2-naphthol, 1,2 and 3+4 hydroxyphenanthrenes, 1-hydroxpyrene, and 2-hydroxfluorene, were measured by liquid chromatography-tandem mass spectrometry (23). Cardiovascular biomarkers were assayed by enzyme immunoassay using commercial kits.
Statistical Analysis
Because measurements for each individual were correlated over time, a repeated-measures model was constructed for each of the major variables. A mixed-model regression analysis was done using PROC MIXED in SAS (version 9.1). The primary outcomes were changes from baseline to the end of tapering and to the end of follow-up, so data from weeks 1, 6, and 10 were included in the analyses. Mean (95% CI) were computed at each of the three time points. Percent differences in means were computed for each pair of time points, with P values and 95% CI for the differences constructed using the Tukey-Kramer adjustment for multiple comparisons. Variable values for total NNAL, PAH metabolites, and several of the cardiovascular biomarkers were log transformed to achieve approximate normality, and the analyses were done on the natural logarithm of the values. Geometric mean and corresponding percent difference are reported for log-transformed variables.
All data for the 20 participants who completed the study were included in the primary analysis. Two subjects stopped smoking by week 10, and the analyses were repeated omitting those subjects. Omitting the quitters had, in general, little effects on the statistical analyses. When omitting quitters did affect the analyses, this is reported in the appropriate section of the results. If not mentioned, there was no effect of omitting subjects on the analysis. In a secondary analysis, a week-by-week comparison of cigarette consumption and exposure biomarkers across all time points was done using the Tukey-Kramer test with significance at P < 0.05.
Results
All 20 subjects completed the study. As noted previously, two subjects quit smoking by week 10.
Cigarette Consumption
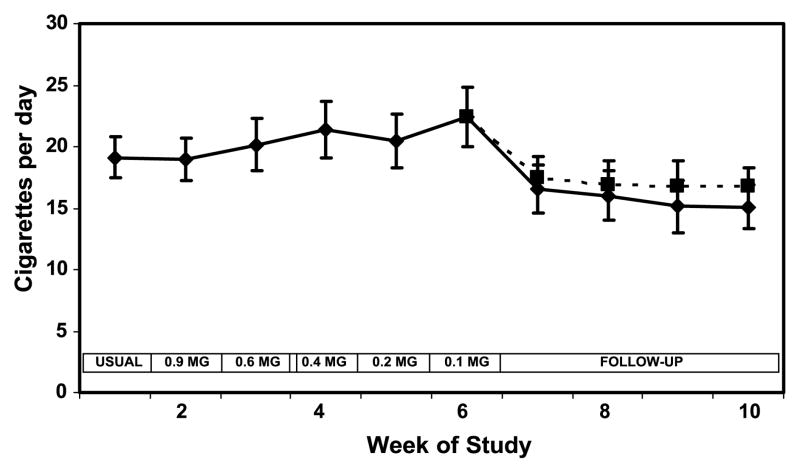
Mean cigarette consumption increased during weeks 3 to 6, increasing from 19.1 cigarettes per day at baseline to 22.4 cigarettes per day at week 6 (Fig. 1; Table 2). The changes in number of cigarettes smoked per day over the 6 weeks were not statistically significant. Cigarettes smoked per day were significantly lower at week 10 (the end of the 4-week follow-up period; 15 cigarettes per day) compared with the end of week 6.
Figure 1.
Cigarette consumption over weeks of the study during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE.
Table 2.
Smoking behavior and chemical exposures while smoking reduced nicotine yield cigarettes
| Week 1, mean (95% CI) | Week 6, mean (95% CI) | Week 10, mean (95% CI) | Week 6-week 1, mean % difference (95% CI) | Week 10-week 1, mean % difference (95% CI) | Week 10-week 6, mean % difference (95% CI) | |
|---|---|---|---|---|---|---|
| Cigarettes per day | 19.1 (15.6–22.6) | 22.4 (17.5–27.3) | 15.1 (11.4–18.7) | 17 (−4 to 39) | −21 (−52 to 9) | −33 (−57 to −8) |
| Pre-smoking plasma nicotine (ng/mL) | 14.1 (8.5–19.7) | 7.4 (4.8–10.0) | 8.8 (4.6–13.0) | −48 (−81 to −15) | −38 (−68 to −7) | 19 (−27 to 66) |
| Nicotine boost (ng/mL) | 9.6 (7.4–11.8) | 3.8 (2.7–4.9) | Not measured | −61 (−81 to −40) | Not measured | Not measured |
| Blood carboxyhemoglobin (%) | 3.3 (2.4–4.2) | 2.5 (1.8–3.1) | 1.9 (1.1–2.8) | −26 (−43 to −8) | −41 (−71 to −12) | −21 (−56 to 14) |
| Plasma cotinine (ng/mL) | 180 (122–237) | 107 (73–142) | 140 (78–201) | −40 (−61 to −20) | −22 (−48 to 4) | 30 (−11 to 71) |
| Total NNAL* (pmol/mg creatinine) | 1.08 (0.70–1.67) | 0.69 (0.47–1.02) | 0.61 (0.36–1.01) | −36 (−49 to −20) | −44 (−64 to −13) | −13 (−42 to 31) |
| 2-Naphthol* (pmol/mg creatinine) | 75.3 (55.7–101.8) | 52.88 (42.2–66.1) | 49.98 (35.7–69.9) | −30 (−42 to −15) | −34 (−55 to −2) | −5 (−34 to 35) |
| 1-Hydroxyphenanthrene* (pmol/mg creatinine) | 1.09 (0.85–1.41) | 0.77 (0.58–1.03) | 1.11 (0.79–1.54) | −29 (−56 to 13) | 1 (−34 to 55) | 43 (−6 to 117) |
| 2-Hydroxyphenanthrene* (pmol/mg creatinine) | 0.58 (0.44–0.76) | 0.52 (0.39–0.69) | 0.52 (0.36–0.75) | −10 (−38 to 30) | −11 (−39 to 30) | −1 (−33 to 46) |
| 3+4-Hydroxyphenanthrene * (pmol/mg creatinine) | 1.45 (1.09–1.92) | 1.21 (0.92–1.59) | 1.27 (0.89–1.82) | −16 (−31 to 2) | −12 (−39 to 27) | 5 (−23 to 42) |
| Total hydroxyphenanthrene* (pmol/mg creatinine) | 3.23 (2.57–4.06) | 2.64 (2.09–3.34) | 3.01 (2.21–4.11) | −18 (−37 to 6) | −7 (−32 to 29) | 14 (−15 to 53) |
| 1-Hydroxypyrene * (pmol/mg creatinine) | 1.63 (1.23–.16) | 1.04 (0.76–1.43) | 1.30 (0.82–2.07) | −36 (−52 to −15) | −20 (−49 to 25) | 25 (−16 to 85) |
| 2-Hydroxyfluorene * (pmol/mg creatinine) | 8.19 (5.50–12.19) | 6.25 (4.44–8.79) | 6.58 (4.53–9.56) | −24 (−45 to 7) | −20 (−51 to 32) | 5 (−28 to 54) |
NOTE: Week 1 is while smoking usual brand. Week 6 is while smoking the lowest yield commercial cigarette (0.1 mg nicotine). Week 10 is 4 weeks after the end of smoking reduced nicotine yield cigarettes. Analysis based on 20 subjects, including 2 subjects who quit smoking at week 10. Significant differences in bold (P < 0.05).
Geometric mean.
Biochemical Exposures
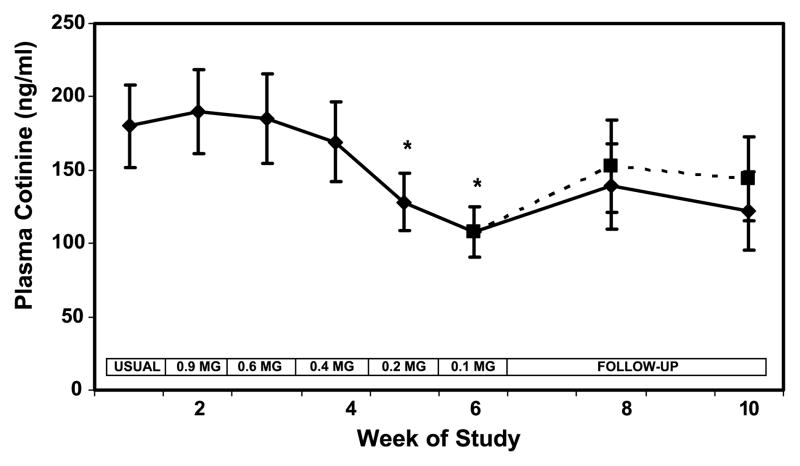
Plasma cotinine concentrations were similar from weeks 1 to 4 but decreased significantly when subjects smoked the 0.2 and 0.1 mg nicotine yield cigarettes (Fig. 2). Plasma cotinine levels were 40% lower at week 6 compared with baseline (Table 2). During follow-up, cotinine levels increased (30%) compared with week 6, but still remained lower (22%) than baseline, although these differences were not significant. Inclusion of quitters resulted in a significant lowering of serum cotinine at week 10 compared with baseline.
Figure 2.
Plasma cotinine concentration over weeks of the study during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE. *P < 0.05 compared to viral brand.
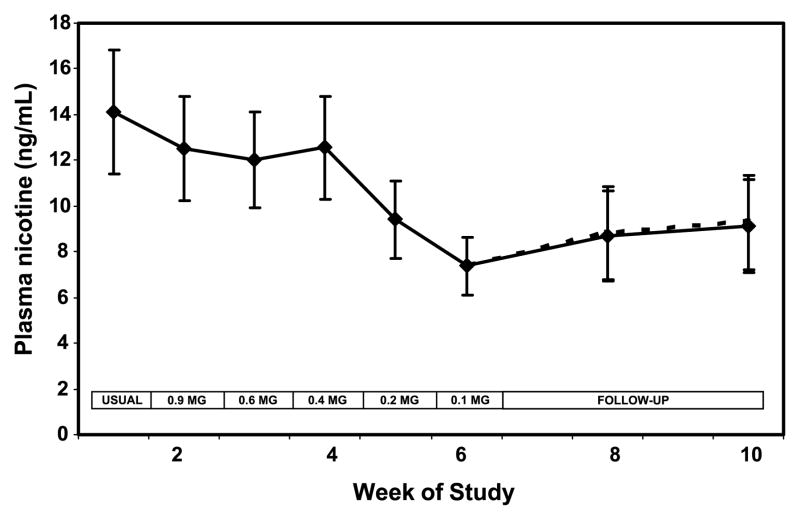
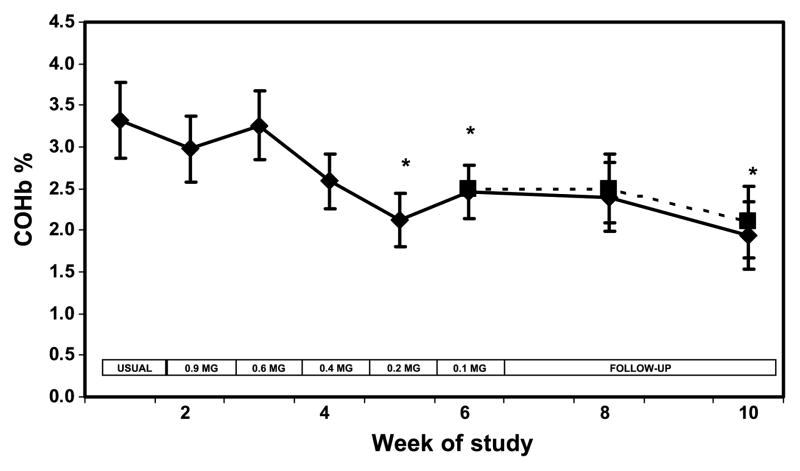
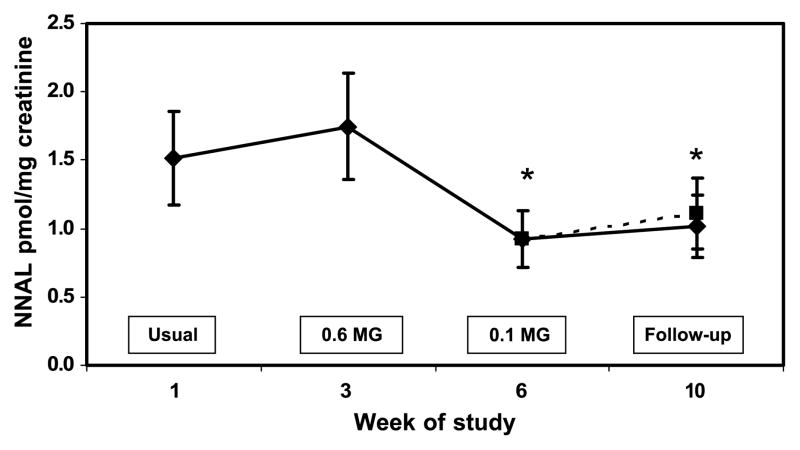
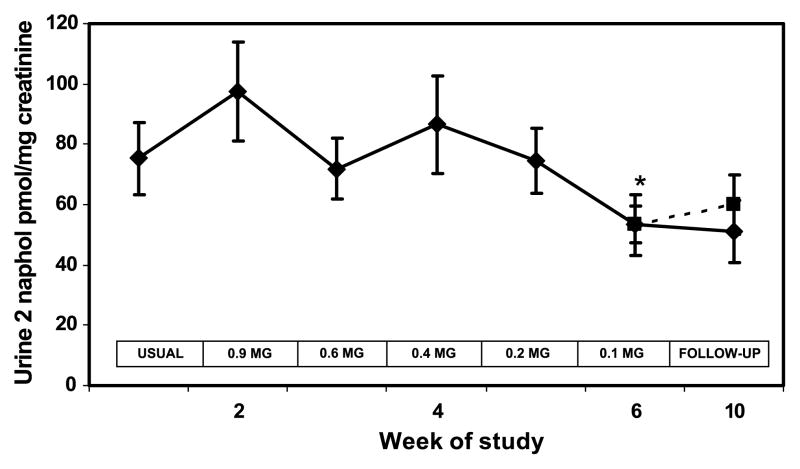
Plasma nicotine concentrations followed a pattern similar to cotinine over time (Fig. 3). The boost in plasma nicotine before and after smoking a single cigarette decreased significantly at week 6 compared with baseline (Table 2). Blood carboxyhemoglobin was significantly lower when smoking the 0.2 and 0.1 mg nicotine cigarette brands and at follow-up (Fig. 4). Urine NNAL excretion was unchanged at week 3 but was significantly lower at week 6 and at follow-up compared with baseline (Fig. 5). Urine excretion of the PAH metabolite 2-naphthol was significantly lower at weeks 6 and 10 compared with baseline (Fig. 6; Table 2). Excretion of 2-naphthol was significantly lower at weeks 3, 6, and 10 compared with week 2. Excretion of 1-hydroxypyrene was significantly lower at week 6 compared with baseline and week 3. All of the other PAH metabolites were also, on average, lower at week 6 compared with baseline, but the difference was not significant.
Figure 3.
Plasma nicotine concentration over weeks of the study during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE.
Figure 4.
Blood carboxyhemoglobin concentration over weeks of the study during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE. * P < 0.05 compared to viral brand.
Figure 5.
Urine NNAL concentration during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE. * P < 0.05 compared to viral brand.
Figure 6.
Urine 2-naphthol concentration during progressive yield reduction of cigarettes (weeks 1–6) and after return to usual cigarettes or quitting (weeks 7–10). Yields in the boxes refer to FTC machine-determined nicotine yield. Solid line includes all subjects; dashed line excluded two quitters at week 10. Points, mean for 20 subjects; bars, SE. * P < 0.05 compared to viral brand.
Cardiovascular Measurements and Biomarkers
Body weight, blood pressure, and heart rate were unchanged over the course of the study (Table 3). No significant changes were noted comparing baseline and the end of tapering for WBC count, hemoglobin, high-density lipoprotein cholesterol, and C-reactive protein. Fibrinogen tended to be decreased at week 6 but was significantly lower at week 10 compared with baseline. The change in fibrinogen was no longer significant when quitters were omitted.
Table 3.
Cardiovascular biomarkers while smoking reduced nicotine yield cigarettes
| Week 1, mean (95% CI) | Week 6, mean (95% CI) | Week 10, mean (95% CI) | Week 6-week 1, mean (95% CI) | Week 10-week 1, mean (95% CI) | Week 10-week 6, mean (95% CI) | |
|---|---|---|---|---|---|---|
| Body weight (kg) | 77.0 (70.7–83.3) | 77.5 (71.0–84.0) | 77.4 (71.1–83.7) | 1 (−1 to 2) | 1 (−1 to 2) | 0 (−1 to 1) |
| Systolic blood pressure (mm Hg) | 126.3 (119.4–133.2) | 124.8 (118.0–131.6) | 126.6 (120.8–132.4) | −1 (−7 to 5) | 0 (−6 to 6) | 1 (−4 to 7) |
| Diastolic blood pressure (mm Hg) | 80.5 (75.8–85.3) | 79.2 (74.8–83.6) | 80.0 (76.4–83.5) | −2 (−7 to 4) | −1 (−9 to 8) | 1 (−7 to 9) |
| Heart rate | 73.9 (69.1–78.7) | 76.1 (69.3–83.0) | 75.1 (68.9–81.3) | 3 (−7 to 13) | 2 (−7 to 11) | −1 (−10 to 7) |
| WBC count (1,000) | 6.5 (5.8–7.3) | 6.4 (5.7–7.1) | 6.4 (5.8–6.9) | −2 (−12 to 7) | −3 (−16 to 10) | −1 (−14 to 12) |
| Hemoglobin (%) | 14.4 (13.6–15.1) | 14.2 (13.4–14.9) | 14.3 (13.5–15.1) | −1 (−4 to 2) | 0 (−3 to 2) | 1 (−1 to 3) |
| High-density lipoprotein cholesterol (mg/dL) | 57.6 (49.1–66.0) | 58.3 (48.0–68.7) | 59.1 (49.0–69.2) | 1 (−8 to 11) | 3 (−6 to 11) | 1 (−7 to 10) |
| C-reactive protein* (Ag/mL) | 0.87 (0.52–1.45) | 0.87 (0.48–1.57) | 0.92 (0.53–1.60) | 0 (−36 to 57) | 6 (−32 to 67) | 6 (−32 to 65) |
| Fibrinogen (mg/dL) | 251.8 (216.4–287.1) | 223.2 (189.8–256.6) | 189.9 (157.4–222.4) | −11 (−25 to 2) | −25 (−48 to −1) | −15 (−36 to 6) |
NOTE: Week 1 is while smoking usual brand. Week 6 is while smoking the lowest yield commercial cigarette (0.1 mg nicotine). Week 10 is 4 weeks after the end of reduced nicotine commercial cigarette smoking. Significant differences in bold.
Geometric mean.
Subjective Responses
No significant changes were observed in the POMS score over the course of the study. The Center for Epidemiological Studies Depression rating score tended to be higher at week 10 (mean, 11.4; 95% CI, 5.9–16.9) compared with baseline (mean, 8.4; 95% CI, 4.1–12.7) and week 6 (mean, 8.9; 95% CI, 3.8–14.0), but the difference was not significant. Average MNWS withdrawal scores were higher at week 6 (mean, 15.8; 95% CI, 8.5–23.1) and week 10 (mean, 16.8; 95% CI, 8.9–24.7) compared with baseline (mean, 13.4; 95% CI, 7.1–19.6), but these changes were not significant. The only significant change in the individual withdrawal symptom scores was in eating, which was higher at week 10 (mean, 3.0; 95% CI, 1.6–4.5) compared with baseline (mean, 1.4; 95% CI, 0.2–2.6). The cigarette acceptance questionnaire analysis showed that the 0.1 mg nicotine cigarette was rated as being less strong, less flavorful, of less quality, and less satisfying than the usual brand, but these differences were not statistically significant.
Quitting/Dependence/Self-Efficacy during Follow-up
Subjects did not intend to quit smoking at entry into the study, and all were still smoking at the end of the cigarette yield taper (at week 6). Four weeks after tapering, two subjects quit smoking, which was confirmed by cotinine measurement.
The self-efficacy rating, reflecting confidence in resisting smoking, was significantly higher at week 6 (mean, 67.9; 95% CI, 56.0–79.8) and week 10 (mean, 70.9; 95% CI, 59.3–82.6) compared with baseline (mean, 56.6; 95% CI, 46.7–66.8). The Fagerström Test for Nicotine Dependence was lower at week 10 (mean, 3.25; 95% CI, 2.12–4.38) compared with week 6 (mean, 4.10; 95% CI, 2.91–5.29) and baseline (mean, 3.90; 95% CI, 2.65–5.15), the latter two of which were similar.
Discussion
To our knowledge, this is the first study to characterize nicotine and carcinogen exposures and cardiovascular responses to forced switching of smokers to progressively lower nicotine yield commercial cigarettes. Numerous other studies, both cross-sectional and experimental forced switching studies, have found that smokers compensate completely or nearly completely for commercial low yield cigarettes with nicotine yields of ≥0.6 mg (6, 24). Correspondingly, we found that plasma cotinine concentrations, blood carbon monoxide, NNAL, and PAH metabolite excretion changed very little from baseline to the 0.4 mg nicotine yield level (study week 4).
While smoking the 0.2 and 0.1 mg nicotine yield cigarettes, commonly described as ultralow yield cigarettes, there was a significant reduction in nicotine intake as well as in carbon monoxide, NNAL, and PAH exposure. There was also a 17% average increase in cigarettes smoked per day when smoking the lowest yield cigarette, although this change was not statistically significant. The reduction in nicotine intake despite smoking more cigarettes per day has been reported previously in both short-term switching studies and cross-sectional studies of smokes of ultralow yield cigarettes in the 0.1 to 0.2 nicotine category as well (7, 8). One short-term switching study showed a reduction in carcinogen exposure as indicated by significantly reduced mutagenic activity of the urine after switching from regular to 0.1 mg nicotine cigarettes (8). A recent cross-sectional study of nicotine and carcinogen exposure with different yield cigarettes by Hecht et al. did not find lower exposure with ultralight cigarettes (25). However, in the Hecht et al. study, the ultralight cigarette category included brands with tar yields of ≤6.5 mg tar, which overlaps with the 0.4 and 0.6 mg nicotine cigarettes in our study, for which there was full compensation. It is unclear in the Hecht et al. study how many subjects, if any, smoked cigarettes with 0.1 or 0.2 mg nicotine yields.
We observed that after completing a commercial low yield tapering regimen that includes ultralow yield cigarettes, 4 weeks later, smokers were, on average, smoking fewer cigarettes per day and had lower dependence ratings, suggesting that the level of dependence had decreased compared with entering into the study. The findings that self-efficacy ratings were higher at the end of tapering and that the Fagerström Test for Nicotine Dependence was lower at week 10 compared with baseline also supports the idea that the level of dependence had decreased during the course of the study. The decrease in dependence observed in our study might be due to reduced nicotine exposure but could also be due to participation in an intensive research study. Furthermore, it is unknown whether a change in dependence measured in the present short-term tapering study would persist after the end of the study.
Comparison of the data from this study with that of our previously published study of progressive reduction in nicotine content of cigarettes is informative. The RNC cigarettes used in our previously published study are not commercially available but were manufactured specifically for research purposes (5). RNC yield cigarettes are low yield because nicotine has been extracted from the tobacco, but other characteristics of the different yield cigarettes, including tar and carbon monoxide emissions, are similar for all yield levels. In contrast, the commercial low yield cigarettes used in the present study are low yield because of engineering characteristics, particularly paper and filter ventilation (26). The nicotine content of the tobacco in commercial low yield cigarettes is comparable with that of higher yield cigarettes (27). For the most popular low yield cigarette brands (yields of ≥0.6 mg nicotine), it is quite easy for the smoker to obtain plenty of nicotine. By puffing more intensively and/or blocking ventilation holes with fingers or lips and/or by taking more puffs per cigarette, nicotine and tar intake is substantially increased above standard machine test values (6). For ultralow yield cigarettes in the 0.1 to 0.2 mg nicotine range, there is considerable compensation, but ventilation is so extensive that, on average, smokers are not able to fully compensate. The present and prior studies indicate that nicotine and carcinogen exposure is reduced by ~30% to 40% while smoking 0.1 mg nicotine cigarettes compared with higher yield cigarettes (7, 8). It should be noted that the market share of ultralow yield cigarettes with nicotine yields of 0.1 or 0.2 mg nicotine is very small (<1%), presumably reflecting that fact that nicotine delivery from these cigarette is less than desired for most smokers.
The comparison of biochemical changes with the two different types of cigarettes illustrates these differences. Cotinine levels fell progressively in relation to the decline in nicotine content with RNC cigarette tapering (16) but declined only for the lowest two yields for the commercial low yield cigarettes. Carbon monoxide and PAH exposures and cardiovascular biomarkers did not change over the course of tapering of RNC cigarettes despite a decline in nicotine intake, consistent with the characteristics that carbon monoxide and tar emissions are similar for cigarettes across all nicotine levels. In contrast, for commercial low yield cigarettes, nicotine, carbon monoxide, and PAH exposures declined together at the lowest yield levels, consistent with the effects of extensive ventilation affecting all smoke constituents similarly.
Although RNC cigarettes have not been available in the past, the progressive reduction of nicotine yield of commercial cigarettes was studied as a possible approach to smoking cessation treatment many years ago (9–12). The gradual and progressive reduction of yields was termed “nicotine fading.” Typical nicotine fading schedules involved reducing the nicotine yield of commercial cigarette brands by 30%, 60%, and 90%, on a weekly basis, and subjects were asked to monitor their “daily nicotine intake,” computed as the product of nicotine yield and cigarettes smoked that day (9). The early trials of nicotine fading with small numbers of subjects seemed promising (9, 10), but later larger clinical trials found no benefit of brand fading in promoting quitting (11, 12).
The present study would be equivalent to reducing machine-determined nicotine yields, on average, by 10%, 40%, 60%, 80%, and 90% at weekly intervals. Measurement of nicotine intake using plasma cotinine concentrations indicated that actual daily nicotine intake was unchanged from baseline to the 60% reduction stage but decreased, on average, 40% when nominal nicotine yields were reduced by 90%. Thus, the 30% and 60% reduction stages described in the published nicotine fading trials would not be expected to be associated with a significant decline in nicotine exposure, whereas the 90% reduction levels would be expected to be associated with a significant reduction in nicotine intake. The present study, which included tapering by 80% and 90% of initial nicotine yield in the last 2 weeks, did seem to lower the level of nicotine dependence. Whether a more gradual reduction and the inclusion of more ultralow yield cigarette yield levels might translate into a better quitting outcome in nicotine fading procedures is unknown.
RNC cigarettes deliver less nicotine than regular cigarettes, but other components of the smoke are the same; therefore, much of the sensory sensations of smoking regular cigarettes are still present. In contrast, commercial ultralow yield cigarettes are highly ventilated, so the smoke is more dilute and sensory effects are quite different from those of regular cigarettes. Whether the lesser sensory stimulation associated with highly ventilated ultralow yield cigarettes would be as effective as that of RNC cigarettes in reducing tobacco withdrawal symptoms and in aiding smoking cessation is unknown.
As was the case with RNC cigarette tapering, we found no evidence of adverse changes in the cardiovascular biomarkers that we measured (primarily markers of inflammation) to suggest an increased cardiovascular risk with commercial low yield cigarette tapering.
Our study has several limitations. The number of subjects was small, so the power to detect modest changes is limited. Our subjects were more highly educated than the average smoker, limiting the generalizability of our findings with respect to the typical smoker. Compliance with smoking of low yield cigarettes could not be proven, but the decline in biochemical exposure when smoking the lowest yield cigarette brands suggests that subjects were for the most part compliant. It is possible that the demand characteristics of participating in a research study produced different smoking behavior with respect to the ultralow yield cigarettes than would have been observed without the implicit demands imposed by participating in a research study. That is, subjects may have felt that they could not engage in strategies to increase the nicotine yield of the ultralow yield cigarettes (such as squeezing the filter to block ventilation) without biasing the results of the study. Subjects received free cigarettes during this trial, and the period of tapering was relatively brief. It is unclear how smokers would respond to having to pay for cigarettes that were not as satisfying as the usual brand. Thus, further work will be needed to determine whether the results of this study generalize to smoking in the natural environment.
Another methodologic concern is the lack of a control group of smokers smoking cigarettes of yields similar to their usual brands who are exposed to the same experimental procedures. A control group was not included because the aim of the study was primarily to simulate a regulatory policy that might mandate progressive reductions in cigarette yields. It was not intended to be a study of low yield cigarettes as an intervention to promote smoking cessation. However, there are several reasons to believe that our results were not due to the study procedures per se. (a) Cigarette consumption remained stable or increased over the course of yield tapering. Despite relatively stable cigarette consumption, actual exposures to various tobacco smoke toxins decreased substantially. If the study procedures affected smoking behavior, one would expect cigarette consumption to decrease (reflecting health concerns) and one would not expect a marked decrease in chemical exposures per cigarette smoked. (b) We recently published another study with a similar research design, in which subjects smoked cigarettes with progressive lower nicotine content but with unchanged yields of other tobacco smoke toxins. In that study, there was the expected decrease in nicotine levels, but no change in carbon monoxide or PAH levels, indicating that the study procedure did not affect the intensity that each cigarette was smoked. (c) Our data are entirely consistent with cross-sectional population studies and experimental switching studies of commercial cigarettes of differing yields as mentioned previously. These prior studies provide external validity to our findings.
The conclusions of our study are as follows. As has been shown in many other studies, we found that forced switching from regular cigarettes to popular low yield cigarettes with machine-determined yields of ≥0.6 mg nicotine is associated with complete or nearly complete compensation, such that there is no reduction in exposure to nicotine or tobacco smoke toxins. When switching to ultralow yield cigarettes in the 0.1 to 0.2 mg nicotine yield range, exposure to nicotine and tobacco smoke toxins are substantially decreased. It is unknown whether this extent of reduction of tobacco smoke toxins would have any beneficial effect on health. Epidemiologic studies of low yield cigarettes, in general, have shown no reduction in health risk compared with higher yield cigarettes, but these studies included very few smokers of cigarettes that correspond with our two lowest yield brands (28). We also found that tapering down to ultralow yield cigarettes may have reduced the level of nicotine dependence. Our experimental trial with a relatively small group of smokers does not establish that ultralow yield cigarettes are less addictive and less hazardous or that switching to ultralow yield cigarettes leads to a meaningful lower level of toxicant exposure. However, the possibility that gradual tapering of smokers from regular to ultralow nicotine yield commercial cigarettes might facilitate smoking cessation is suggested by our research and warrants consideration for future research, particularly larger clinical trials of smokers, especially those who are not interested in standard smoking cessation treatments, in more real-world situations.
Acknowledgments
Grant support: National Cancer Institute, USPHS grant CA78603, National Institute on Drug Abuse, NIH grants DA02277, DA12393, and DA016752, and California Tobacco Research Program grant 10RT-0215. Carried out in part at the General Clinical Research Center at San Francisco General Hospital Medical Center with support of the Division of Research Resources, NIH grant RR-00083.
We thank Dr. Faith Allen for data management, Patricia Buley and Diane Geraldizo for coordinating the clinical study, Lita Ramos for performing the nicotine and cotinine analyses, the U.S. Centers for Disease Control and Prevention for cigarette smoke analyses, and Marc Olmsted for editorial assistance.
Footnotes
Disclosure of Potential Conflicts of Interest
Benowitz has served as a paid expert witness in litigation against tobacco companies, including issues related to low yield cigarettes.
References
- 1.Gray N, Henningfield JE, Benowitz NL, et al. Toward a comprehensive long term nicotine policy. Tob Control. 2005;14:161–5. doi: 10.1136/tc.2004.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine. A blueprint for the nation. Washington (DC): National Academy Press; 2007. Ending the tobacco problem. [Google Scholar]
- 3.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 4.Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Tob Control. 1998;7:281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL. Compensatory smoking of low yield cigarettes. In: Shopland DR, Burns DM, Benowitz NL, Amacher RH, editors. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. NCI Smoking and Tobacco Control Monograph No. 13. Bethesda (MD): U.S. NIH, National Cancer Institute; 2001. Oct, pp. 39–64. NIH Publication No. 02-5074. [Google Scholar]
- 7.Gori G", Lynch CJ. Smoker intake from cigarettes in the 1-mg Federal Trade Commission tar class. Regul Toxicol Pharmacol. 1983;3:110–20. doi: 10.1016/0273-2300(83)90035-1. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Jacob P, III, Yu L, Talcott R, Hall S, Jones RT. Reduced tar, nicotine, and carbon monoxide exposure while smoking ultralow-but not low-yield cigarettes. JAMA. 1986;256:241–6. [PubMed] [Google Scholar]
- 9.Foxx RM, Brown RA. Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. J Appl Behav Anal. 1979;12:111–25. doi: 10.1901/jaba.1979.12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foxx RM, Axelroth E. Nicotine fading, self-monitoring and cigarette fading to produce cigarette abstinence or controlled smoking. Behav Res Ther. 1983;21:17–27. doi: 10.1016/0005-7967(83)90122-5. [DOI] [PubMed] [Google Scholar]
- 11.Lando HA, McGovern PG. Nicotine fading as a nonaversive alternative in a broad-spectrum treatment for eliminating smoking. Addict Behav. 1985;10:153–61. doi: 10.1016/0306-4603(85)90021-8. [DOI] [PubMed] [Google Scholar]
- 12.Brown RA, Lichtenstein E, McIntyre KO, Harrington-Kostur J. Effects of nicotine fading and relapse prevention on smoking cessation. J Consult Clin Psychol. 1984;52:307–8. doi: 10.1037//0022-006x.52.2.307. [DOI] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.McNair DM, Lorr M, Doppleman LF. Profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 15.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacol Biochem Behav. 1999;62:165–72. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 18.Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. J Consult Clin Psychol. 1988;56:104–10. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- 19.Baer JS, Holt CS, Lichtenstein E. Self-efficacy and smoking reexamined: construct validity and clinical utility. J Consult Clin Psychol. 1986;54:846–52. doi: 10.1037//0022-006x.54.6.846. [DOI] [PubMed] [Google Scholar]
- 20.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 22.Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–52. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 23.Jacob P, III, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL, Hall SM, Herning RI, Jacob P, III, Jones RT, Osman AL. Smokers of low yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309:139–42. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- 25.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693–8. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski LT, O’Connor RJ, Sweeney CT. Cigarette design. In: Shopland DR, Burns DM, Benowitz NL, Amacher RH, editors. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. NCI Smoking and Tobacco Control Monograph No. 13. Bethesda (MD): U.S. NIH, National Cancer Institute; 2001. Oct, pp. 39–64. NIH Publication No. 02-5074. [Google Scholar]
- 27.Kozlowski LT, Mehta NY, Sweeney CT, et al. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–75. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns DR, Major JM, Shanks TG, Thun MJ, Samet JM. Smoking lower yield cigarettes and disease risks. In: Shopland DR, Burns DM, Benowitz NL, Amacher RH, editors. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. NCI Smoking and Tobacco Control Monograph No. 13. Bethesda (MD): U.S. NIH, National Cancer Institute; 2001. Oct, pp. 65–158. NIH Publication No. 02-5074. [Google Scholar]