Abstract
The Anaphase-Promoting Complex (APC) is an E3 ubiquitin ligase that regulates mitosis and G1 by sequentially targeting cell-cycle regulators for ubiquitination and proteasomal degradation. The mechanism of ubiquitin chain formation by APC and the resultant chain topology remains controversial. By using a single-lysine APC substrate to dissect the topology of ubiquitinated substrates, we find that APC-catalyzed ubiquitination has an intrinsic preference for the K11 linkage of ubiquitin that is essential for substrate degradation. K11 specificity is determined by an E2 enzyme, UBE2S/E2-EPF, that elongates ubiquitin chains after the substrates are pre-ubiquitinated by UbcH10 or UbcH5. UBE2S copurifies with APC; dominant-negative Ube2S slows down APC substrate degradation in functional cell-cycle extracts. We propose that Ube2S is a critical, unique component of the APC ubiquitination pathway.
Keywords: E2 enzyme, E3 ligase, proteasome, cell cycle, mitosis
During protein ubiquitination, a lysine residue on a target protein is conjugated to ubiquitin through an enzymatic cascade involving E1 activating enzymes, E2 conjugating enzymes, and E3 ligases (1). Chain formation can proceed in the same manner by repetitively adding ubiquitins to one or more of seven lysines on conjugated ubiquitin, potentially generating ubiquitin chains of several different topologies (2, 3). The linkage, topology, and length of the ubiquitin chains are thought to determine the fate of the tagged protein: K48-linked ubiquitin for degradation and K63 for signaling DNA damage and inflammation (4).
We have studied the linkage and topology of ubiquitin chains generated by the APC, a multisubunit E3 enzyme that controls cell-cycle progression (5). Recently, two interesting observations have been made about chain topology: First, different E2s might be required in concert to form long ubiquitin conjugates on APC substrates (6). Second, the density of ubiquitin, rather than the length of the chains, may qualify substrates for degradation (7).
Like most E3 ligases, APC modifies multiple lysine residues on each substrate, generating a diverse mixture of products. Poor resolution obtained in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) hinders analysis. Even when distinct bands are observed, they may not correspond to single products. A band of a discrete molecular weight may reflect ubiquitination on multiple lysine residues or, alternatively, the formation of ubiquitin chains on single-lysine sites.
Dissecting the topology of the ubiquitin chains requires a careful simplification of the ubiquitination reaction under meaningful conditions. To achieve that, following earlier work of Petroski and Deshaies (8), we have modified the well-characterized APC substrate, securin, and generated a model substrate that has only a single-lysine residue available for ubiquitination, thereby limiting chain formation to a single chain. By using this model substrate, we discovered that the ubiquitin conjugates formed on APC substrates are preferentially linked through ubiquitin lysine 11. Similar results have recently been shown by Jin et al. (9). We also found that another E2, UBE2S/E2-EPF, assembles K11-linked ubiquitin chains on APC substrates that have been pre-ubiquitinated by UbcH10 or UbcH5. During the preparation of this manuscript, two reports came out and confirmed an essential role of UBE2S in APC mediated cell cycle regulation (10, 11).
Results
Securin With a Single Lysine Can Support Efficient APC-Dependent Degradation.
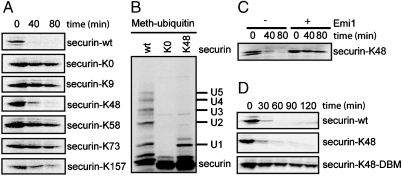
Sites of ubiquitination can be studied most easily by substituting methylated ubiquitin for ubiquitin, thus limiting the reactions to single steps of modification. By using methylubiquitin, Cyclin B1, and securin, each reveal a minimum of five sites of modification (12, 13). To simplify our studies, we made constructs that allow only a single ubiquitin chain to be formed. A lysine-less version (securin-K0), made by mutating all 20 lysines to arginines, is stable in G1 phase extracts (Fig. 1 A second from top) and unable to be ubiquitinated in an in vitro ubiquitination reaction by using purified APC (Fig. 1 B Lane 2 vs. Lane 1). Because the Degradation box (D-box) sequence is known to play an important role in securin degradation (14), we examined lysine residues around the D-box. Starting with securin-K0. We reverted one of the three of the most highly conserved residues around the D-box (residues 61–68) back to lysine, producing K48, K58, and K73 forms of securin (14). We also reverted to lysine residues at the N terminus (K9) and the C terminus (K157) that were far from the D-box. As shown in Fig. 1 A, among the mutants, securin-K48 was degraded with kinetics most similar to that of the wild-type securin. Degradation of securin-K48 was blocked by the APC inhibitor, Emi1 (Fig. 1 C), thus excluding nonspecific proteolysis in the extract system used. In in vitro ubiquitination reactions using purified APC and methylated ubiquitin, only one ubiquitin was added to securin-K48, whereas as least five ubiquitins were added to the wild-type protein (Fig. 1 B). It is noteworthy that methylated ubiquitin suppresses but does not eliminate ubiquitin chains completely, probably due to residual wild-type ubiquitin in the reaction mix (Fig. 1 B Lane 3). We conclude that ubiquitination of only a single site on securin can lead to efficient APC-dependent ubiquitination and subsequent proteasomal degradation. The D-box mutant (DBM) of securin-K48 (securin-K48-DBM) was stable in the cell extracts (Fig. 1 D), as has been shown for the DBM of wild-type securin, suggesting the single-lysine securin behaves like a prototypical APC substrate (13).
Fig. 1.
Securin with a single lysine can support efficient APC-dependent degradation. (A, C, and D) Degradation of securin and its mutants in cell extracts. 35S-labeled proteins were degraded in cell extracts prepared from Hela S3 cells synchronized in G1 phase. APC inhibitor Emi1 was added as indicated. (B) Mono-ubiquitination of securin and its mutants. 35S -labeled proteins were ubiquitinated in the presence of methylated ubiquitin. The reactions were analyzed by autoradiography.
A Preference for K11 Linkages.
Lysine 48-linked ubiquitin chains (not to be confused with single-lysine 48 on securin) are thought to be the major targeting signals for proteasomal degradation (4). However, in a recent study, ubiquitin conjugates formed on APC substrates were shown to utilize multiple linkages (7). Because, in these experiments, the substrate was both multi-ubiquitinated (ubiquitination on multiple lysine residues) and poly-ubiquitinated (formation of ubiquitin chains), it remains unclear which linkage has been used for a specific ubiquitin chain; hence we examined ubiquitin conjugates on a substrate where only a single ubiquitin chain can be formed.
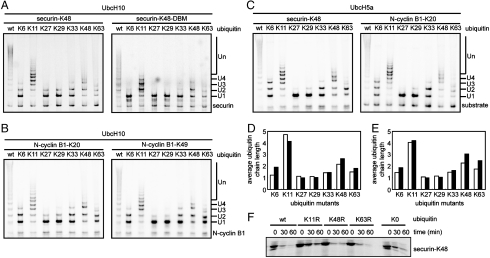
We examined ubiquitination products generated with purified APC, E1, E2s (UbcH10 or UbcH5a), purified securin-K48, and a series of ubiquitin mutants, each containing all but one of its lysine residues converted to arginine. We denote these ubiquitin mutants by their single, intact lysine, as in ubiquitin K63 that refers to a ubiquitin containing six lysines mutated to arginine, with only lysine 63 available for conjugation. Results in Fig. 2 A clearly show that ubiquitin K11 generates the longest ubiquitin chain (> 6 ubiquitin molecules) on securin-K48 substrate. Wild-type ubiquitin appeared to give somewhat longer chains, but, of course, the wild-type ubiquitin can make branched chains of unknowable complexity, whereas the single-lysine ubiquitin mutants can only assemble linear chains. Reactions with linkages made through the K6, K27, K29, or K33 of ubiquitin formed short di- or tri-ubiquitinated products, with the mono-ubiquitinated species being the predominant product. Reactions with linkages made through K48 and K63 of ubiquitin formed products with slightly longer ubiquitin chains. When securin-K48-DBM was used, the linkage preferences were almost the same, but the products had comparatively shorter ubiquitin chains (Fig. 2 A right), as have been observed for DBM of wild-type securin (13). These observations were not specific to securin. Similar results were obtained by using single-lysine mutants of the N-terminal fragment of cyclin B1 (N-cyclin B1) (Fig. 2), suggesting that linkage preference does not depend on the position of the lysine residues in the substrates, nor on the identity of the substrates.
Fig. 2.
A preference for K11 linkage in APC-catalyzed ubiquitin chain assembly. (A, B, and C) Ubiquitination of single-lysine substrates in the presence of different ubiquitin mutants. 35S-labeled substrates were ubiquitinated in the presence of UbcH10 (A and B) or UbcH5a (C). (D and E) Quantitation of the weighed average length of the ubiquitin chains formed with UbcH10 (empty column) and UbcH5a (filled column) in the presence of different ubiquitin mutants on securin-K48 (D) and N-cyclin B1-K20 (E) as shown in Fig. 2 A –C. (F) Degradation of securin-K48 in cell extracts is suppressed by K11R ubiquitin mutant. 35S labeled securin-K48 was degraded in G1 phase cell extracts that were supplemented with excess amount of indicated ubiquitin mutants. The reactions were analyzed by autoradiography.
As APC-dependent ubiquitination can be mediated by both UbcH10 and UbcH5 (15, 16), we examined reactions with different E2 enzymes. Both of the reactions with UbcH10 and UbcH5a yield similar products on securin-K48 (Fig. 2 A left and C left) and cyclin B1-K20 (Fig. 2 B left and C right). However, quantitative examination revealed a slight preference for K6, K48, and K63 linkages in reactions with UbcH5a than those with UbcH10 (Fig. 2 C, D, and E).
We used dominant-negative ubiquitin mutants in which lysine 11 (K11R), 48 (K48R), or lysine 63 (K63R) were substituted with arginine to evaluate the importance of K11-linked ubiquitin conjugates in substrate degradation. Each mutant had six available lysines with only one mutated to arginine. K48R or K63R ubiquitin mutants had no effect on securin-K48 degradation, compared with the wild-type ubiquitin (Fig. 2 F). By contrast, adding K11R ubiquitin significantly inhibited securin-K48 degradation to the same extent as lysine-free ubiquitin (K0 ubiquitin). The incomplete inhibition is almost certainly due to competing wild-type ubiquitin in the assay. Thus, lysine-11-linked ubiquitin conjugates are not only the preferred product of APC but are essential for degradation. These observations are consistent with the results that Jin and colleagues reported recently (9).
The Role of E2-25K.
The E2-25K conjugating enzyme (also know as UbcH1) mediates the assembly of long ubiquitin chains on APC substrates in conjunction with Ubc4 in yeast (6). Although this model was extended to human cells, we could not confirm this (Fig. 2 A). We were concerned that their conditions for human cells were not physiological, particularly because E2-25K has a high propensity to synthesize ubiquitin chains in the absence of any E3 (17).
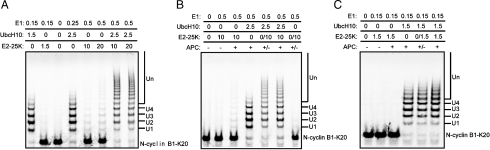
We have reexamined the role of E2-25K by using N-cyclin B1-K20 as a single-lysine substrate for ubiquitination under conditions that we believe are more realistic, specifically, concentrations of E2s at 1.5 μM instead of 10–20 μM, lower concentrations of E1 (0.15 μM instead of 0.5 μM). The following experiments we performed at 37 °C. As shown in Fig. 3 A, when our revised conditions were used, reactions with UbcH10 went to completion and formed long ubiquitin chains on the single-lysine substrate (Lane 1), and addition of polymers of ubiquitin with E2-25K alone was not detectable (Lane 2). However, when we used the conditions employed in Morgan’s experiment, a small amount of the substrate was converted to ubiquitinated products with very long ubiquitin chains (Lanes 5 and 6) with E2-25K alone. These reactions were very processive (very long chains, few intermediates) but inefficient compared to reactions with UbcH10 alone at a lower concentration (Lane 4). When high concentrations of UbcH10 and E2-25K were combined in the same reaction, we, too, observed a complete conversion of the substrate and formation of products with very long ubiquitin chains (Lanes 7 and 8).
Fig. 3.
The cooperativity between UbcH10 and E2-25K in vitro is concentration dependent and APC independent. (A) Ubiquitination of N-cyclin B-K20 with purified APC in the presence of UbcH10, E2-25K and both of the E2s. The concentrations of the enzymes are in μM. (B and C) Ubiquitination of N-cyclin B-K20 in the presence (+) or absence (-) of APC with E2-25K, UbcH10, or both. The lane (+/-) designates a reaction, where substrate was ubiquitinated first with UbcH10 for 1 h, then the products were separated from APC and ubiquitinated with E2-25K for another 1 h. The concentrations of the enzymes are in μM. The reactions were analyzed by autoradiography.
Because E2-25K can synthesize ubiquitin chains in the absence of E3s, we were concerned that reactions with E2-25K might have been E3 independent (17). As shown in Fig. 3 B, 0.5 μM E1 and 10 μM E2-25K did not generate ubiquitinated products in the absence of APC, whereas a small amount of the substrate was ubiquitinated in the presence of APC (compare Lane 2 with Lane 3). These results indicate that the APC is required for the nucleation of ubiquitin conjugates. However, it was unclear if elongation was also APC dependent. To test the role of E2-25K in the elongation reaction, the substrate was first ubiquitinated with UbcH10 (Lane 4); APC was then removed by immuno-depletion and E2-25K was added to the reaction for another 1 h at 37 °C (Lane 5). Omitting UbcH10 in the first step but adding E-25K in the second step showed that the depletion of APC was complete (Lane 7). The final products of this reaction were almost identical to those formed in a reaction where APC was present throughout the reaction (Lane 6) and, hence, E2-25K mediated elongation does not require APC.
E2-25K alone, under more physiological conditions, does not drive ubiquitination in the presence or absence of APC (Fig. 3 C Lanes 2 and 3); most importantly, it does not act synergistically with UbcH10 (Fig. 3 C Lanes 5 and 6). These results call into question the physiological significance of reactions that require 10–20 μM E2-25K hat are many times higher than the reported in vivo concentration of E2-25K of about 0.03 μM (18). Therefore, while the Morgan data were convincing that E2-25K was a specific elongation factor for APC in yeast, we conclude that the human homolog is unlikely to share that function.
UBE2S/E2-EPF Assembles K11-Linked Ubiquitin Chains.
Human APC might use a similar mechanism to that in yeast but with different E2s. UBE2S/E2-EPF was cloned in a screen of human keratinocyte cDNAs in the skin disease, endemic pemphigus foliaceus (EPF) (19). The protein catalyzes E3-independent, auto-ubiquitination and supports E3-dependent ubiquitin conjugation and model substrate degradation in reticulocyte fractions (20). The ubiquitin chains formed in UBE2S-catalyzed reactions were exclusively linked through lysine 11 of ubiquitin, and were able to bind the subunit 5 of the 26S proteasome (21). Recently, UBE2S was found to be overexpressed in breast tumors and its expression correlates with genes that function in the G2/M phases of the cell cycle, including CYCLIN B2, CDC20, and UBE2C (UbcH10). Moreover, UBE2S protein oscillates through the cell cycle and reaches peak level at late mitosis (22).
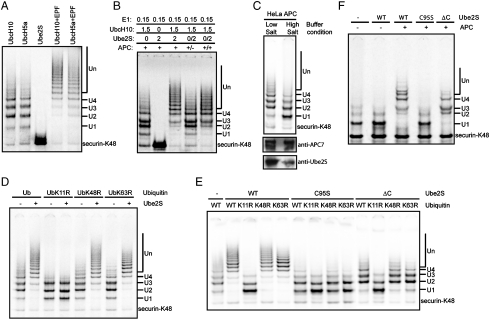
These interesting properties led us to test whether it could support APC-catalyzed ubiquitination. As shown in Fig. 4 A, purified recombinant UBE2S, unlike UbcH10 and UbcH5a, could not support APC-catalyzed ubiquitination on securin-K48 but when added in combination with UbcH10 or UbcH5, it supported much longer ubiquitin chains. Whereas UbcH10 or UbcH5a alone only makes ubiquitin chains of 2–4 ubiquitins long in an APC-catalyzed reaction, combination with UBE2S extends the chain length to over 10–15 ubiquitins. To test whether this activity is dependent on APC, we used the same assay described in Fig. 3 C. Securin-K48 was ubiquitinated with APC and UbcH10 first, then APC was removed from the reaction mixture and UBE2S was added (Fig. 4 B). Longer ubiquitin chains were produced when APC was present through the entire course of the reaction (Lane 3). When APC was present only at early stages, the chains were shorter (Lane 4). But when APC was added back after the immuno-depletion, the chains are longer again, confirming the lack of ubiquitin-chain extension in the second stage was not due to nonspecific damage to the reaction mixture during the immuno-depletion process (Lane 5). This suggests that the activity of UBE2S in these reactions is APC-dependent, unlike that of E2-25K. APC is activated by CDC20 in mitosis and by Cdh1 in G1 phase (23). Ubiquitination reactions, with either APC/CDC20 purified from mitotic extracts or with APC/Cdh1 purified from G1 extracts, were indistinguishable.
Fig. 4.
UBE2S assembles K11 linked ubiquitin chains on APC substrates. (A) Cooperativity between UbcH10 or UbcH5a and UBE2S. 35S-labeled securin-K48 were ubiquitinated in the presence of different combinations and concentrations of E2s, as indicated. (B) UBE2S’s ubiquitin-chain extension activity is APC-dependent. 35S labeled securin-K48 was ubiquitinated in the presence of different combinations and concentrations of E2s as indicated. The Lane (+/-) designates a reaction, where substrate was ubiquitinated first with UbcH10 for 1 h, then the products were separated from APC and ubiquitinated with UBE2S for another 1 h. (C) UBE2S is required for synthesizing long ubiquitin chains. 35S-labeled securin-K48 were ubiquitinated with UbcH10 and APC that were purified under different conditions. Purified APC samples were immuno-blotted with anti-APC7 and anti-UBE2S antibodies. (D) UBE2S makes K11 specific ubiquitin chains. 35S labeled securin-K48 were ubiquitinated in the absence (-) or presence (+) of UBE2S and UbcH10 with different ubiquitin mutants. (E) UBE2S’s ubiquitin-chain extension activity requires its catalytic residue and the C-terminal domain. 35S-labeled securin-K48 were ubiquitinated in the absence (-) or presence of UBE2S mutants and UbcH10 with different ubiquitin mutants. (F) UBE2S extends ubiquitin chains on pre-ubiquitinated substrate. 35S-labeled securin-K48 were first ubiquitinated with GST-UbcH5a. After removing APC and GST-UbcH5a by immunodepletion, the products were used as substrate for a second ubiquitination reaction with (+) or without (-) APC in presence of a different UBE2S mutant. All the ubiquitination reactions were analyzed by autoradiography.
If UBE2S were essential, why was its requirement was not identified earlier in previous biochemical experiments that identified UbcH10? As shown in Fig. 4 C, we found that UBE2S efficiently copurifies with APC during immunoaffinity purification from HeLa cells extracted under normal conditions by using washes with low-salt buffers (13). However, under high-salt wash conditions that were employed in the purification of APC from Xenopus eggs (7), very little UBE2S copurified with APC. Whereas APC purified under the low-salt conditions forms long ubiquitin chains (> 4 ubiquitins), APC purified under the high-salt condition makes mostly mono-ubiquitinated products. All of the previous ubiquitination studies by using APC and UbcH10 (or UbcH5) alone would have had endogenous UBE2S in the reactions and this may account for the relatively long ubiquitin chains formed under these conditions. It is especially noteworthy that Xenopus laevis APC purified under high-salt wash conditions only makes short biquitin chains with average chain length less than two and this has been interpreted to be the normal state (7). However, reactions using Xenopus APC purified under high-salt condition and supplemented with UBE2S, or by using Xenopus APC purified under low-salt condition, formed longer ubiquitin chains. Thus, we believe that UBE2S is required for making long ubiquitin chains with both Xenopus and human APC, and the variable length of ubiquitin chains is a direct consequence to the variable levels of UBE2S.
Because UBE2S builds ubiquitin chains exclusively through the lysine-11 linkage, we asked whether the complete APC/UbcH10/UBE2S reaction generated ubiquitin chains exclusively through K11 linkage. As shown in Fig. 4 D, in reactions where UBE2S was present, long ubiquitin chains were formed when wild-type ubiquitin or ubiquitin mutants other than the ubiquitin K11R were present. However, in the reactions with UbK11R, only short ubiquitin chains were produced and addition of UBE2S did not change their length (Fig. 4 D Lanes 3 and 4). Thus the ubiquitin chains formed in APC/UbcH10/UBE2S-catalyzed reactions are linked through K11.
To test whether UBE2S functions as a second E2 in a sequential reaction, like E2-25K, we prepared a point mutant of the predicted active site cysteine mutated to serine (C95S). As shown in Fig. 4 E, reactions with wild-type UBE2S make long ubiquitin chains with a K11 linkage specificity. However, reactions with the C95S mutant of UBE2S not only produce short ubiquitin chains, but also lose the linkage specificity, as three different K/R ubiquitin mutants generate similar products. More importantly, when compared with the reaction with UbcH10 alone (Lane 1), the reactions with C95S mutant of UBE2S make shorter ubiquitin chains with mainly mono- and di-ubiquitinated products (Lane 6–9). In addition to its ubiquitin-conjugating domain, UBE2s has a C-terminal extension that is required to extend ubiquitin chains. Reactions including the UBE2SΔC mutant (UBE2S with the C-terminal domain truncated) generated products similar to those of reaction with UbcH10 alone and the linkage specificity of the ubiquitin chains was maintained (Fig. 4 E). Therefore, the enzymatic activity of UBE2S is required to form long ubiquitin chains formed by APC in the presence of UbcH10.
We tested whether UBE2S could extend ubiquitin chains that are pre-formed on APC substrates by using the same assay as we had previously, but, here, purifying the pre-formed products. When both APC and UBE2S were added for the second ubiquitination reaction, the mono-ubiquitinated substrate was converted to products with long ubiquitin chains (Fig. 4 F Lane 3). However, no additional ubiquitin chains were produced in the presence of APC and UBE2S C95S (Fig. 4 F Lane 4).
The Role of UBE2S In Substrate Degradation.
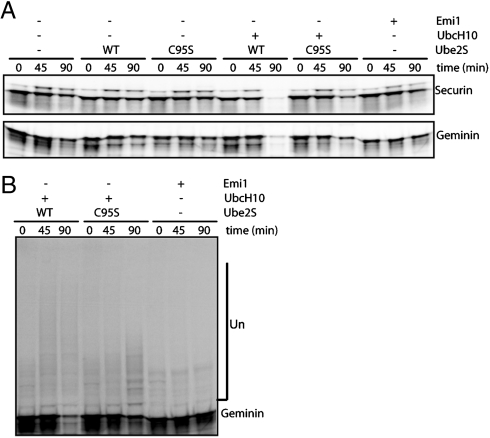
As shown in Fig. 5 A, both securin and geminin remain stable in extracts made from nocodazole-arrested cells that maintain an active spindle-assembly checkpoint (24, 25). The addition of UBE2S or UBE2S C95S to this extract system had no effect on the degradation of APC substrates, unlike the addition of UbcH10 that drives the system into anaphase and activates APC (25, 26). We used the ability of UbcH10 to override the checkpoint in these extracts to ask whether UBE2S is important for mitotic exit. The addition of UBE2S C95S partially blocked the degradation of securin and geminin, compared with the addition of UBE2S. However, the addition of Emi1 completely inhibited the degradation. Longer ubiquitin chains were formed on substrates that were incubated in extracts supplemented with wild-type UBE2S compared to the C95S mutant, whereas Emi1 blocked the formation of ubiquitin chains on substrates (Fig. 5 B).
Fig. 5.
UBE2S mutant perturbs APC activity in functional cell extracts. (A) 35S-labeled securin and geminin were assayed for degradation in mitotic phase HeLa cell extracts that have been supplemented with wild-type or dominant-negative UbcH10 and UBE2S, or Emi1, as indicated. The reactions were analyzed by autoradiography. (B) Overexposure of the degradation experiment shown in A enables detection of ubiquitination of geminin in the extracts.
Discussion
To dissect the complex topology of ubiquitin conjugates formed on APC substrates, we simplified the ubiquitination reaction by restricting modification to a single site. Although all known APC substrates are ubiquitinated on multiple lysine residues, we showed here that a single-lysine site was sufficient to promote efficient and APC specific degradation. By using the single-lysine securin as a model system, we found that UBE2S acts in concert with UbcH10 or UbcH5 to make K11-linked ubiquitin chains on APC substrates.
K11-Linked Ubiquitin Chain as a Signal for Proteasomal Degradation.
It has been well established that ubiquitin chains assembled through K48 act as a signal for proteolysis by the proteasomes and that K63-linked chains signal DNA damage tolerance, inflammatory response, protein trafficking, and ribosomal protein synthesis (2, 4). In yeast, all seven possible ubiquitin linkages have been identified, though the functions of linkages other than K48 and K63 have not been elucidated (3). Recently, ubiquitin conjugates formed by Xenopus laevis APC in an in vitro reaction were shown to have three different linkages (K11, K48, and K63) (7). However, the functional relevance of each linkage was not specified.
We have found that APC has a strong preference for making K11-linked ubiquitin chains. This linkage specificity is determined by a previously unrecognized E2 of the reaction, UBE2S. substrates modified with K11-linked ubiquitin chains bind the proteasome recruiting factors efficiently (9). More importantly, when a ubiquitin mutant that lacks K11 was added to a cell cycle extracts, it inhibited the degradation of APC substrates, showing that the K11 linkage is essential for degradation. Injection of ubiquitin K11R into frog embryo stopped the early embryonic cell cycles, confirming the K11 linkage is important for cell cycle progression in vivo (9).
K11 linked ubiquitin chains are not unique to APC substrates. While yeast APC synthesizes K48 linked ubiquitin chains, K11 chains are the third most abundant species in yeast (after K48 and K63) (3). K11 linked ubiquitin chains are prominent in pull-downs of UBA-UBX proteins that are the cofactors of p97, a chaperone involved in endoplasmic reticulum-associated degradation (27). K11 linked ubiquitin chains also accumulate in tissues of animal model for neurodegenerative disorder, like Huntington’s disease (28).
The Role of Sequential E2 Activity.
In ubiquitin conjugates formed with Xenopus laevis by using Ubc4 or UbcH10 as the E2, King and colleagues found that the ubiquitin chains were linked through K11, K48 and K63 of ubiquitin with an average chain length of less than two and that these were competent for degradation (7). These results were contradict to the previous thought that ubiquitin chains of greater than four are a signal for degradation (29). However, in a cellular context, where UBE2S is present, APC substrates should be modified with long ubiquitin chains.
A recent report by Rape and colleagues found that a set of surface residues surrounding lysine 11 of ubiquitin were important for ubiquitin chain synthesis by APC in vitro and similar sequence motifs could also be found in certain APC substrates (9). It was proposed that these structural features of the substrate and ubiquitin were recognized by APC and UbcH10 to promote ubiquitin-chain initiation and elongation, respectively. Whereas this model elegantly explains how a single E2/E3 pair could coordinate the different steps of ubiquitin-chain assembly, our results show that, for APC, the ubiquitin chain is actually made in a two-step reaction by two different E2s. Whereas the set of residues around lysine 11 of ubiquitin may, indeed, determine the K11 linkage, specificity of the chain elongation reaction, we doubt whether a similar structural motif is a prerequisite for conjugating the first ubiquitin. Securin K48 is not near such a motif; but lysine-48 is fully competent for ubiquitin-chain initiation.
Although Rodrigo-Brenni and Morgan extended their observations of E2-25K from yeast to mammalian cells, we question a role of E2-25K and its associated K48 ubiquitin linkages in mammalian cells. The important linkage for degradation of APC substrates in HeLa cells appears to be K11 and not K48. The synergy between UbcH10 and E2-25K Rodrigo-Brenni and Morgan observed in HeLa cells is most likely due to the fact that the E2s were present at exceedingly high concentrations. At lower concentrations, closer to the physiological level, there was no observable effect of E2-25K and the cooperative behavior with UbcH10 was also not observed.
However, we do, indeed, think the favored process for both yeast and human appears to be that APC assembles ubiquitin chains on its substrates in two steps. First, UbcH10 or UbcH5 puts on the first ubiquitin of a chain and may actually mono-ubiquitinate multiple lysine residues of the substrate. Second, UBE2S elongates the ubiquitin chain specifically along the K11 linkage. When there is little UBE2S associated with APC, it makes mostly mono-ubiquitinated products. UBE2S only works on substrates that have been pre-ubiquitinated and a single ubiquitin is enough to initiate a long ubiquitin chain. Under all the conditions we tested, UBE2S’s activity is dependent on APC, unlike E2-25K, which can elongate chains in an E3 independent fashion at high concentrations (17). A C-terminal-domain-deletion mutant of UBE2S maintains the same K11 linkage specificity but has lower processivity, suggesting the linkage specificity is determined by the core domain of the E2 and that the C-terminal domain determines the processivity of ubiquitin-chain extension. Similar observations have been made on Ubc1 (6).
A similar mode of ubiquitin chain assembly through sequential E2s actions has been proposed recently for different Really Interesting New Gene (RING) and U-box E3 ligases, suggesting this may be a general way of assembling ubiquitin chains of a specific topology on substrates by E3 ligases (30, 31). It was proposed that the first conjugation reaction may lack specificity for a particular lysine residue on a substrate as long as the lysine is accessible to the E2–E3 complex. In contrast, ubiquitin chain elongation occurs through a specific lysine residue on ubiquitin. Specificity, therefore, would reside in the elongation step. This is reminiscent of the ubiquitin chain assembly process by the Skp1-Cullin-F-box (SCF)-CDC34 system (32). UBE2S, E2-25K, and Ubc13/Mms2 have been found to support K11-, K48, and K63-linked ubiquitin-chain extension in a two-step reaction, resp. We would predict that the specificity of other ubiquitin chain linkages and topologies would also be achieved in a similar manner.
Materials and Methods
Detailed materials and methods can be found in the SI Materials and Methods. UBE2S and mutants were purified as His-tagged proteins from E. coli. In vitro degradation assay and ubiquitination assay were performed according to (13) and the APC were purified from extracts of synchronized human HeLa S3 cells.
Supplementary Material
Acknowledgments.
We thank members of the Kirschner lab for many helpful discussions and Nick Larsen for providing important reagents. T.W. is a Leukemia & Lymphoma Society Fellow. J.L.G. was the recipient of an European Molecular Biology Organization fellowship. This work was funded by Grant 5R01GM39023-21 from the National Institutes of General Medical Sciences (to M.W.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912802107/DCSupplemental.
References
- 1.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2001;1685:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 4.Pickart CM, Fushman D. Polyubiquitin chains: Polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Peters JM. The anaphase-promoting complex/cyclosome: Amachine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick DS, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 8.Petroski MD, Deshaies RJ. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol Cell. 2003;11:1435–1444. doi: 10.1016/s1097-2765(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 9.Jin L, , Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnet MJ, et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nature Cell Biol. 2009;11:1363–1369. doi: 10.1038/ncb1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson A, et al. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci USA. 2009;106:18213–18218. doi: 10.1073/pnas.0907887106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 15.Aristarkhov A, et al. E2-C, a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc Natl Acad Sci USA. 1996;93:4294–4299. doi: 10.1073/pnas.93.9.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, King RW, Peters JM, Kirschner MW. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol. 1996;6:455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Pickart CM. A 25-kilodalton ubiquitin carrier protein (E2) catalyzes multiubiquitin chain synthesis via lysine 48 of ubiquitin. J Biol Chem. 1990;265:21835–21842. [PubMed] [Google Scholar]
- 18.Pickart CM, Vella AT. Levels of active ubiquitin carrier proteins decline during Erythroid maturation. J Biol Chem. 1988;263:12028–12035. [PubMed] [Google Scholar]
- 19.Liu Z, Diaz LA, Haas AL, Giudice GJ. cDNA cloning of a novel human ubiquitin carrier protein. An antigenic domain specifically recognized by endemic pemphigus foliaceus autoantibodies is encoded in a secondary reading frame of this human epidermal transcript. J Biol Chem. 1992;267:15829–15835. [PubMed] [Google Scholar]
- 20.Liu Z, Haas AL, Diaz LA, Conrad CA, Gudice GJ. Characterization of a novel keratinocyte ubiquitin carrier protein. J Biol Chem. 1996;271:2817–2822. doi: 10.1074/jbc.271.5.2817. [DOI] [PubMed] [Google Scholar]
- 21.Baboshina OV, Haas AL. Novel multiubiquitin chain linkages catalyzed by the conjugating enzymes E2EPF and RAD6 are recognized by 26 S proteasome subunit 5. J Biol Chem. 1996;271:2823–2831. doi: 10.1074/jbc.271.5.2823. [DOI] [PubMed] [Google Scholar]
- 22.Tedesco D, et al. The ubiquitin-conjugating enzyme E2-EPF is overexpressed in primary breast cancer and modulates sensitivity to topoisomerase II inhibition. Neoplasia. 2007;9:601–613. doi: 10.1593/neo.07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 24.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with anaphase-promoting complex/cylosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104:4870–4875. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy SK, Rape M, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 26.Summers MK, Pan B, Mukhyala K, Jackson PK. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell. 2008;31:544–556. doi: 10.1016/j.molcel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandru G, et al. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134:804–816. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett EJ, et al. Global changes to the ubiquitin system in Huntingtons disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- 29.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen DE, Brzovic PS, Klevit RE. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14:941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- 31.Windheim M, Peggie M, Cohen P. Two different classes of E2 ubiquitin-conjugating enzymes are required for the mono-ubiquitination of proteins and elongation by polyubiquitin chains with a specific topology. Biochem J. 2008;409:723–729. doi: 10.1042/BJ20071338. [DOI] [PubMed] [Google Scholar]
- 32.Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-CDC34. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.