Abstract
The ribonuclease inhibitor (RI) is a cytosolic protein and a potent inhibitor of bovine pancreatic ribonuclease (RNase A). Amphibian homologues and variants of RNase A that evade RI are cytotoxic. Here, we employ RNA interference along with amphibian and mammalian ribonucleases to demonstrate that RI protects cells against exogenous ribonucleases. These data indicate an imperative for the molecular evolution of RI and suggest a means to enhance the cytotoxicity of mammalian ribonucleases.
Cells have evolved means to control the catalytic activities of enzymes that would otherwise be toxic. The enzymes that degrade proteins and nucleic acids—the conveyors of biochemical information—are especially worrisome in this regard. Consequently, many proteases are synthesized as zymogens, which are activated in an appropriate spatial and temporal manner. Some proteases also have cognate inhibitor proteins that protect cellular proteins against deleterious degradation. Although no natural zymogens of ribonucleases are known (1), cognate inhibitor proteins do exist. One—the ribonuclease inhibitor protein (RI1)—is especially notable.
RI is a cytosolic protein that has been detected in all examined mammalian cell types (2). RI binds with femtomolar affinity to bovine pancreatic ribonuclease (RNase A), as well as mammalian homologues (Figure 1A) (3–6). Although these ribonucleases are secretory enzymes, they are able to invade mammalian cells and degrade cellular RNA, including siRNA (7). The binding of ribonucleases to RI prevents the manifestation of their ribonucleolytic activity in the cytosol, disarming them as cytotoxins (8).
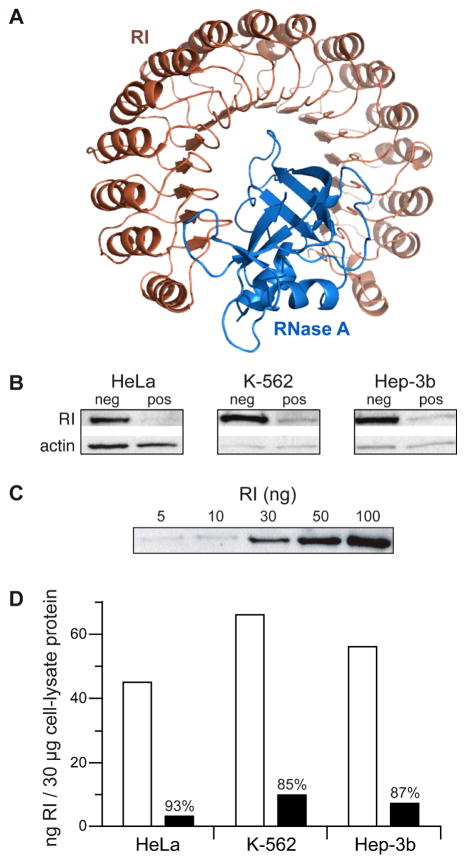
Figure 1.
(A) Structure of the porcine RI·RNase A complex (PDB entry 1dfj (9)). (B) Immunoblot of a lysate (30 μg total protein) from HeLa, K-562, and Hep-3B cells transfected with pGE-neg or pGE-pos, and probed with anti-RI or anti-actin antibodies. (C) Immunoblot of RI (5–100 ng) probed with an anti-RI antibody. (D) Bar graphs showing quantitation of the data in panels A and B. Open bars, pGE-neg; filled bars, pGE-pos. Values indicate the extent of knockdown.
Onconase® (ONC) and other amphibian homologues of RNase A do not bind to RI under physiological conditions (10, 11). These amphibian ribonucleases demonstrate potent toxicity towards tumor cells, in particular (12, 13), and ONC is on the verge of approval as a second-line chemotherapeutic agent for malignant mesothelioma. Like ONC, engineered variants of both RNase A (14, 15) and its human homologue (16, 5) that evade RI are cytotoxic (17). Their cytotoxic activity correlates strongly with their catalytic activity in the presence of RI (18, 19, 15). These self-consistent observations were confounded by a recent publication, which concluded that the role of RI is only to neutralize those ribonucleases that are intrinsically cytotoxic (20). In other words, RI might not be a guardian against ribonucleases, despite its extraordinary affinity for these enzymes (3–6). Herein, we have examined this conclusion, which is critical to the understanding of the biological role of both ribonucleases and RI.
We employed RNA interference (RNAi (21, 22)) to silence cytosolic RI and thereby impair the putative protection afforded by the inhibitor. We examined the effects of RI silencing in three human cell lines: HeLa (cervix), K-562 (bone marrow), Hep-3b (liver). Cells that contained normal or silenced levels of RI were exposed to both RI-evasive and non-evasive ribonucleases.
Plasmid pGE-pos, which directs the transcription of a short hairpin RNA (shRNA) that targets RI, was capable of reducing RI production in all three cell lines. Analysis of the lysates of the cells transfected with pGE-pos or GE-neg (which directs the expression of an shRNA that does not have significant similarity to any sequence in the human genome) indicated that the knockdown of RI was substantial. Still, bands indicative of low levels of RI were present in the lysates of all three cell lines (Figure 1B). Normalizing the intensity of these bands to the intensity of an actin control (Figure 1B) and known amounts of RI (Figure 1C) enabled quantitation of the extent of knockdown to be 85–93% (Figure 1D). These values are typical for RNAi-mediated knockdown (21, 22). Next, we tested the susceptibility of cells transfected with pGE-pos or pGE-neg to RI-evasive and non-evasive ribonucleases. These ribonucleases were ONC, an RNase A variant (G88R RNase A) that has diminished affinity for RI but retains full ribonucleolytic activity (14), and wild-type RNase A.
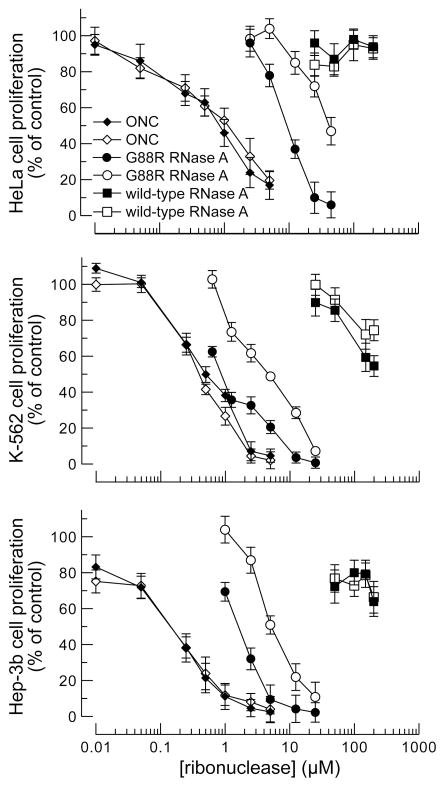
Human cells transfected with pGE-pos or pGE-neg were equally vulnerable to ONC (Figure 2; Table 1). This finding is consistent with the lack of affinity of RI for ONC (10, 11), and demonstrates that RI does not neutralize every foreign ribonuclease that is intrinsically cytotoxic. Importantly, this finding also indicates that any nonspecific silencing by RNAi, which has been observed in other systems (23), is not an issue in our system.
Figure 2.
Graphs showing the effect of ribonucleases on the proliferation of HeLa, K-562, and Hep-3B cells transfected with pGE-pos or pGE-neg. Cell proliferation was measured by monitoring the incorporation of [methyl-3H]thymidine into genomic DNA. Data points indicate the mean (±SE) of three separate experiments carried out in triplicate. Open symbols, pGE-neg; filled symbols, pGE-pos. Values of IC50 are listed in Table 1.
Table 1.
Effect of RI-knockdown on the Inhibition of Cell Proliferation by Ribonucleases (IC50; μM)
| ribonuclease |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | ONC | G88R RNase A | RNase A | |||||||
| HeLa | neg | 0.9 ± 0.2 | 1.1 ± 0.4 | 67 ± 11 | 6.7 ± 1.1 | >200 | — | |||
| pos | 0.8 ± 0.2 | 10 ± 2 | >200 | — | ||||||
| K-562 | neg | 0.49 ± 0.07 | 0.96 ± 0.18 | 4.5 ± 0.6 | 4.6 ± 0.8 | >200 | — | |||
| pos | 0.51 ± 0.06 | 0.97 ± 0.09 | >200 | — | ||||||
| Hep-3b | neg | 0.14 ± 0.03 | 1.1 ± 0.3 | 13 ± 2 | 4.3 ± 1.5 | >200 | — | |||
| pos | 0.13 ± 0.03 | 3.0 ± 0.9 | >200 | — | ||||||
In contrast to their response to ONC, human cells transfected with pGE-pos or pGE-neg were not equally vulnerable to G88R RNase A (Figure 2; Table 1). The proliferation of cells exposed to this variant decreased substantially upon knockdown of RI. Clearly, RI modulates the effect of exogenous ribonucleases on human cells. The increased vulnerability (4.3- to 6.7-fold) is close to that expected for cells that have lost 85–93% of their RI (Figure 1D). The order of the imposed effects correlates as well (HeLa > K-562 ≈ Hep-3b). These data are consistent with the overproduction of RI conferring human cells with additional protection against a ribonuclease (24).
Finally, human cells transfected with pGE-pos or pGE-neg were equally vulnerable to wild-type RNase A (Figure 2; Table 1). What is the expectation here? The cytosolic concentration of RI is ~4 μM in a wide variety of cell types (24). In vitro, the value of its Ki is 44 fM for RNase A (3). In cellulo, this value is substantially lower due to the effects of molecular crowding (25, 26). Accordingly, the concentration of RNase A in the cytosol must reach a high level to manifest measurable toxicity. The translocation of ribonucleases to the cytosol is, however, inefficient (27, 28). Thus, retaining 7–15% of its RI (Figure 1D) could certainly afford a cell with adequate defense against RNase A, as was observed herein (Figure 2; Table 1). The absence of an effect cannot be interpreted to mean that RNase A lacks intrinsic cytotoxicity. Indeed, the microinjection of wild-type RNase A to a concentration of only 29 pM was found to be cytotoxic to amphibian cells (29), which lack RI and are defenseless against mammalian ribonucleases. In that study, RNase A was at least as cytotoxic as ricin and diptheria toxin, and 102-fold more cytotoxic than α-sarcin.
Our findings have practical consequences. Gene therapy regimens that employ RNAi are not on the immediate horizon. In contrast, the use of small-molecule antagonists to interfere with protein–protein interactions is achieving notable success (30–32). The interface in the human RI·RNase 1 complex contains 19 hydrogen bonds within its 2800 Å2 of buried surface area (5). Our data indicate that this interface, though unusually large and polar, is an opportune target for antagonists, which could enhance the cytotoxicity of an exogenous or endogenous mammalian ribonuclease. Work to discover such antagonists is on-going in our laboratory.
Supplementary Material
Footnotes
Abbreviations used: ONC, Onconase® (a registered trademark of Alfacell Corp.); PDB, Protein Data Bank; RI, ribonuclease inhibitor protein; RNAi, RNA interference; RNase A, bovine pancreatic ribonuclease; RNase 1, human pancreatic ribonuclease; shRNA, short hairpin RNA; siRNA, small interfering RNA.
This work was supported by Grant CA073808 (NIH). K.A.D. was supported by the Louis and Elsa Thomsen Wisconsin Distinguished Fellowship Award from the College of Agricultural and Life Sciences at the University of Wisconsin–Madison.
Experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Plainkum P, Fuchs SM, Wiyakrutta S, Raines RT. Creation of a zymogen. Nat Struct Biol. 2003;10:115–119. doi: 10.1038/nsb884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson KA, Haigis MC, Raines RT. Ribonuclease inhibitor: Structure and function. Prog Nucleic Acid Res Mol Biol. 2005;80:349–374. doi: 10.1016/S0079-6603(05)80009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee FS, Shapiro R, Vallee BL. Tight-binding inhibition of angiogenin and ribonuclease A by placental ribonuclease inhibitor. Biochemistry. 1989;28:225–230. doi: 10.1021/bi00427a031. [DOI] [PubMed] [Google Scholar]
- 4.Vicentini AM, Kieffer B, Matthies R, Meyhack B, Hemmings BA, Stone SR, Hofsteenge J. Protein chemical and kinetic characterization of recombinant porcine ribonuclease inhibitor expressed in Saccharomyces cerevisiae. Biochemistry. 1990;29:8827–8834. doi: 10.1021/bi00489a046. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, McCoy JG, Bingman CA, Phillips GN, Jr, Raines RT. Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J Mol Biol. 2007;367:434–449. doi: 10.1016/j.jmb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RJ, Lavis LD, Raines RT. Intraspecies regulation of ribonucleolytic activity. Biochemistry. 2007;46:13131–13140. doi: 10.1021/bi701521q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Ardelt B, Ardelt W, Shogen K, Darzynkiewicz Z. Cytotoxic ribonuclease onconase targets RNA interference (siRNA) Cell Cycle. 2008;7:3258–3261. doi: 10.4161/cc.7.20.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Souček J, Matoušek J, Raines RT. Catalytic activity of bovine seminal ribonuclease is essential for its immunosuppressive and other biological activities. Biochem J. 1995;308:547–550. doi: 10.1042/bj3080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186. doi: 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Mikulski SM, Ardelt W, Rybak SM, Youle RJ. A cytotoxic ribonuclease. Study of the mechanism of onconase cytotoxicity. J Biol Chem. 1993;268:10686–10693. [PubMed] [Google Scholar]
- 11.Turcotte RF, Raines RT. Interaction of onconase with the human ribonuclease inhibitor protein. Biochem Biophys Res Commun. 2008;377:512–514. doi: 10.1016/j.bbrc.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Raines RT. Ribonucleases as novel chemotherapeutics: The ranpirnase example. BioDrugs. 2008;22:53–58. doi: 10.2165/00063030-200822010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardelt W, Shogen K, Darzynkiewicz Z. Onconase and Amphinase, the antitumor ribonucleases from Rana pipiens oocytes. Curr Pharm Biotechnol. 2008;9:215–225. doi: 10.2174/138920108784567245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leland PA, Schultz LW, Kim BM, Raines RT. Ribonuclease A variants with potent cytotoxic activity. Proc Natl Acad Sci USA. 1998;98:10407–10412. doi: 10.1073/pnas.95.18.10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkoski TJ, Kurten EL, Mitchell JC, Raines RT. Disruption of shape-complementarity markers to create cytotoxic variants of ribonuclease A. J Mol Biol. 2005;354:41–54. doi: 10.1016/j.jmb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Leland PA, Staniszewski KE, Kim BM, Raines RT. Endowing human pancreatic ribonuclease with toxicity for cancer cells. J Biol Chem. 2001;276:43095–43102. doi: 10.1074/jbc.M106636200. [DOI] [PubMed] [Google Scholar]
- 17.Rutkoski TJ, Raines RT. Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr Pharm Biotechnol. 2008;9:185–189. doi: 10.2174/138920108784567344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bretscher LE, Abel RL, Raines RT. A ribonuclease A variant with low catalytic activity but high cytotoxicity. J Biol Chem. 2000;275:9893–9896. doi: 10.1074/jbc.275.14.9893. [DOI] [PubMed] [Google Scholar]
- 19.Dickson KA, Dahlberg CL, Raines RT. Compensating effects on the cytotoxicity of ribonuclease A variants. Arch Biochem Biophys. 2003;415:172–177. doi: 10.1016/s0003-9861(03)00214-5. [DOI] [PubMed] [Google Scholar]
- 20.Monti DM, D’Alessio G. Cytosolic RNase inhibitor only affects RNases with intrinsic cytotoxicity. J Biol Chem. 2004;279:39195–39198. doi: 10.1074/jbc.C400311200. [DOI] [PubMed] [Google Scholar]
- 21.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 22.Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98:9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattecharjee A, Meyerson M, Collins FS. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci USA. 2003;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigis MC, Kurten EL, Raines RT. Ribonuclease inhibitor as an intracellular sentry. Nucleic Acids Res. 2003;31:1024–1032. doi: 10.1093/nar/gkg163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis RJ. Macromolecular crowding: Obvious but unappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold U. Aspects of the cytotoxic action of ribonucleases. Curr Pharm Biotechnol. 2008;9:161–168. doi: 10.2174/138920108784567263. [DOI] [PubMed] [Google Scholar]
- 28.Benito A, Vilanova M, Ribó M. Intracellular routing of cytotoxic pancreatic-type ribonucleases. Curr Pharm Biotechnol. 2008;9:169–179. doi: 10.2174/138920108784567281. [DOI] [PubMed] [Google Scholar]
- 29.Saxena SK, Rybak SM, Winkler G, Meade HM, McGray P, Youle RJ, Ackerman EJ. Comparison of RNases and toxins upon injection into Xenopus oocytes. J Biol Chem. 1991;266:21208–21214. [PubMed] [Google Scholar]
- 30.Cochran AG. Protein–protein interfaces: Mimics and inhibitors. Curr Opin Chem Biol. 2001;5:654–659. doi: 10.1016/s1367-5931(01)00262-9. [DOI] [PubMed] [Google Scholar]
- 31.Whitty A, Kumaravel G. Between a rock and a hard place. Nat Chem Biol. 2006;2:112–118. doi: 10.1038/nchembio0306-112. [DOI] [PubMed] [Google Scholar]
- 32.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.