Abstract
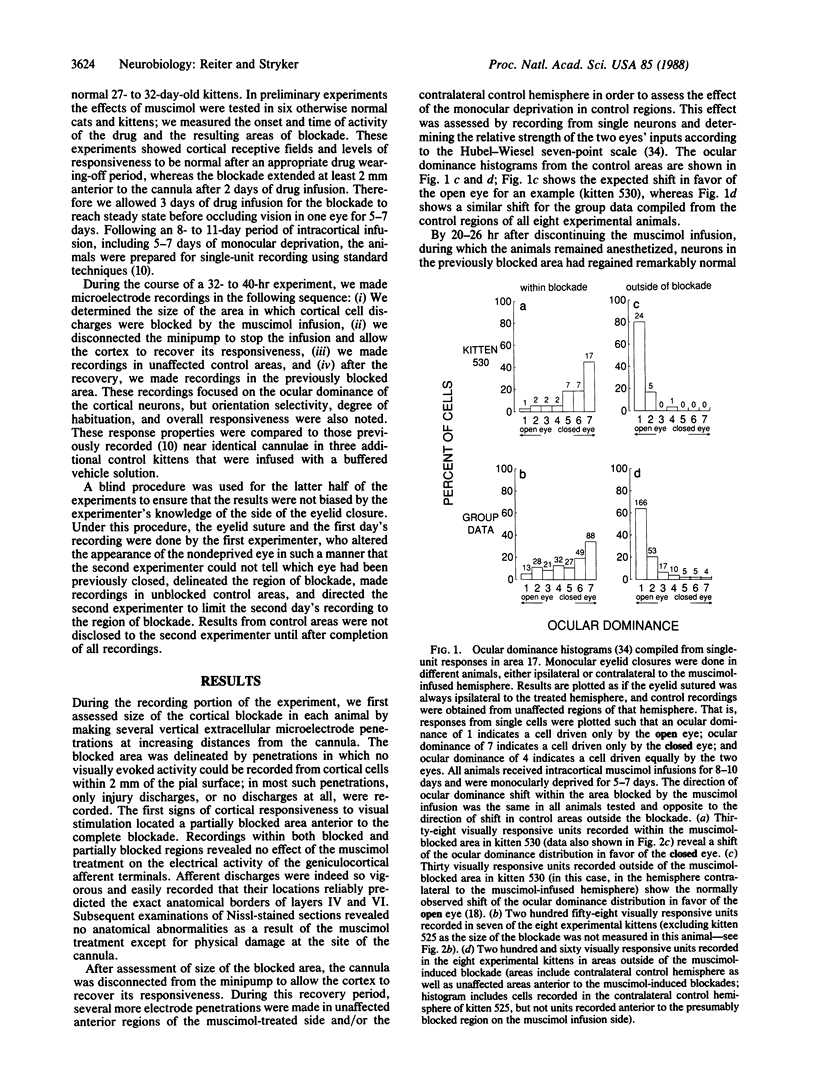
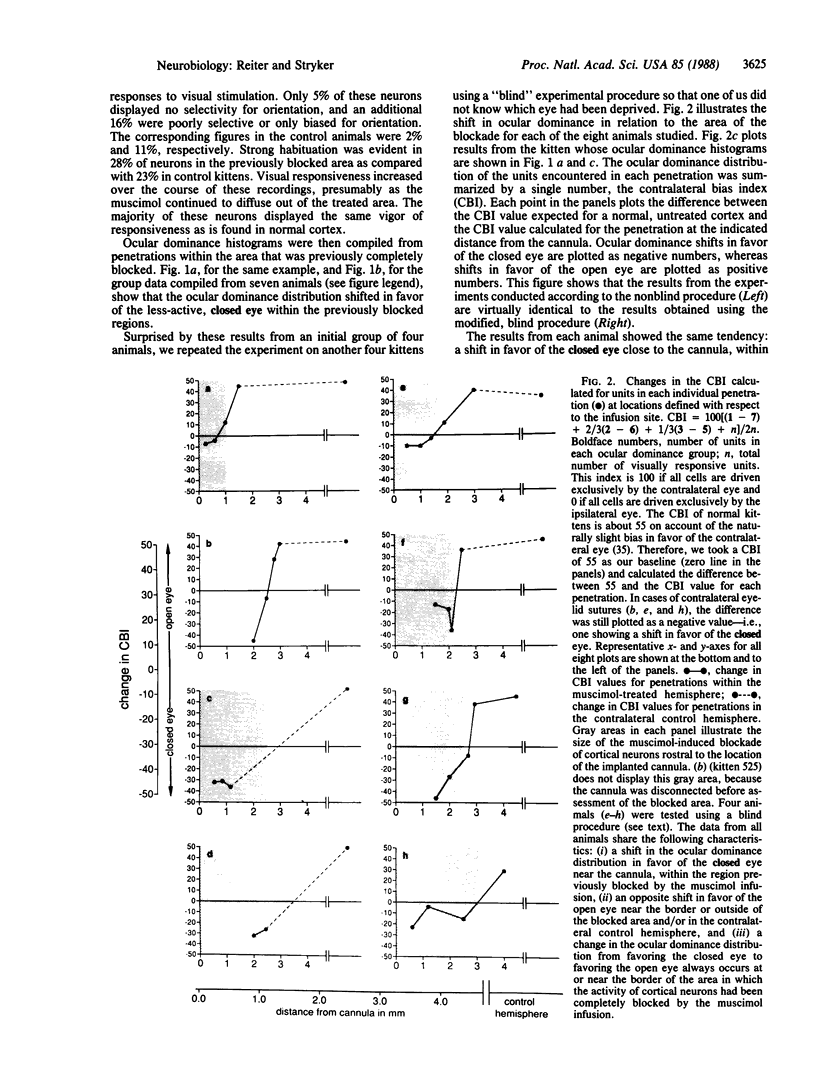
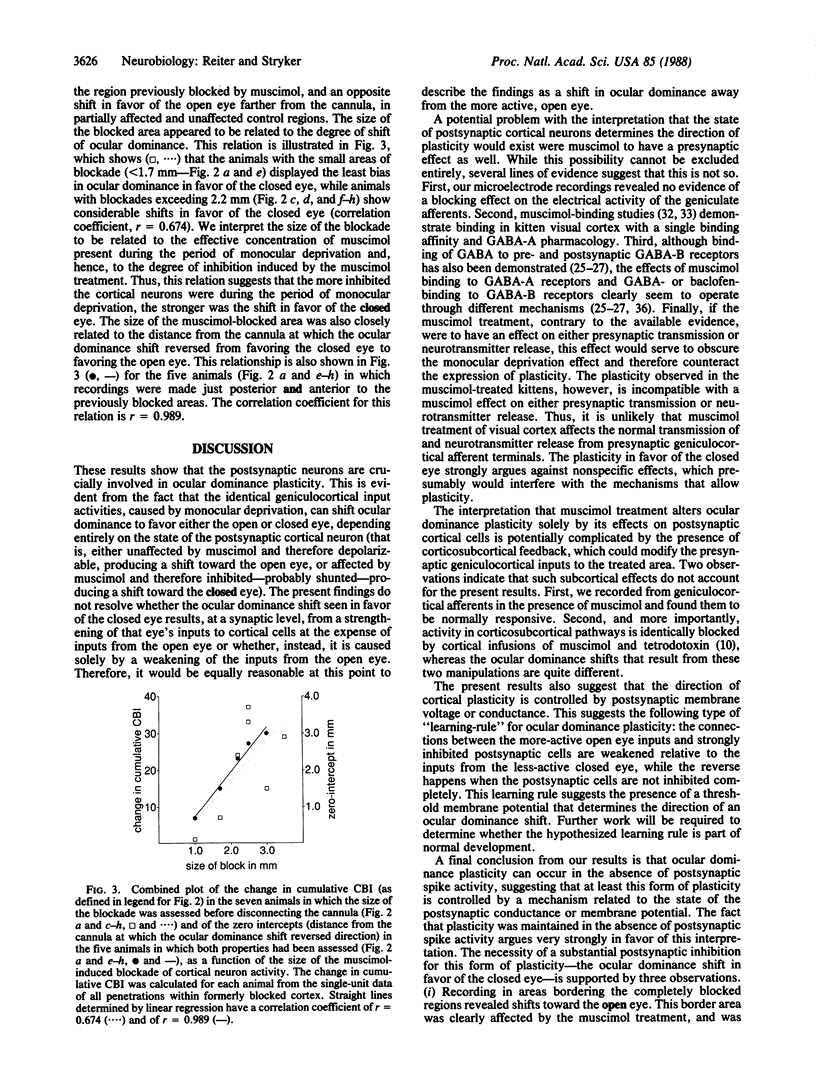
Models of synaptic plasticity in the nervous system have conventionally assumed a mechanism in which spike activity of a postsynaptic cell enhances the efficacy of recently active presynaptic inputs. Making use of the prompt and dramatic response of the visual cortex to occlusion of vision in one eye during the critical period, we tested the role of postsynaptic activity in ocular dominance plasticity. To do so, we selectively blocked cortical cell discharges with a continuous intracortical infusion of the inhibitory neurotransmitter agonist muscimol during a period of monocular deprivation. This drug inhibits cortical cell discharges with no apparent effect on the activity of their presynaptic geniculocortical inputs. Recording from single cortical cells after they had recovered from the muscimol-induced blockade, we found a consistent shift in the responsiveness of the visual cortex in favor of the less-active, closed eye, while the normal shift in favor of the more-active, open eye was evident in regions not affected by the treatment. Such an inhibition-coupled expression of plasticity in favor of the less-active, closed eye cannot be explained by a nonspecific disruption of cortical function. We interpret these results to indicate (i) that the postsynaptic cell is crucially involved in plasticity of the visual cortex, (ii) that the direction of cortical plasticity depends on postsynaptic membrane conductance or polarization, and (iii) that plasticity can occur in the absence of postsynaptic spike activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranyi A., Feher O. Long-term facilitation of excitatory synaptic transmission in single motor cortical neurones of the cat produced by repetitive pairing of synaptic potentials and action potentials following intracellular stimulation. Neurosci Lett. 1981 May 29;23(3):303–308. doi: 10.1016/0304-3940(81)90015-x. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986 Mar 13;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Abrams T. W., Kandel E. R. A test of Hebb's postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984 May;4(5):1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Pseudocholinesterase staining in the primary visual pathway of the macaque monkey. Nature. 1982 Sep 30;299(5882):439–442. doi: 10.1038/299439a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Kennedy M. B. Immunoreactivity for a calmodulin-dependent protein kinase is selectively increased in macaque striate cortex after monocular deprivation. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1536–1540. doi: 10.1073/pnas.83.5.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R., Murata K. Effects of glutamate and GABA on specific response properties of neurones in the visual cortex. Exp Brain Res. 1974;21(3):285–297. doi: 10.1007/BF00235748. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Mitchell J. F., Srinivasan V. The release of gamma-aminobutyric acid during inhibition in the cat visual cortex. J Physiol. 1971 Jan;212(2):519–534. doi: 10.1113/jphysiol.1971.sp009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T., Pettigrew J. D. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976 Oct 8;194(4261):206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Needler M. C., Shaw C., Cynader M. Characteristics and distribution of muscimol binding sites in cat visual cortex. Brain Res. 1984 Aug 13;308(2):347–353. doi: 10.1016/0006-8993(84)91076-x. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol. 1981 Jan;310:215–239. doi: 10.1113/jphysiol.1981.sp013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H. O., Waitzman D. M., Stryker M. P. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65(1):182–188. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C., Cynader M. Disruption of cortical activity prevents ocular dominance changes in monocularly deprived kittens. Nature. 1984 Apr 19;308(5961):731–734. doi: 10.1038/308731a0. [DOI] [PubMed] [Google Scholar]
- Shaw C., Needler M. C., Cynader M. Ontogenesis of muscimol binding sites in cat visual cortex. Brain Res Bull. 1984 Aug;13(2):331–334. doi: 10.1016/0361-9230(84)90134-5. [DOI] [PubMed] [Google Scholar]
- Skangiel-Kramska J., Kossut M. Increase of GABA receptor binding activity after short lasting monocular deprivation in kittens. Acta Neurobiol Exp (Wars) 1984;44(1):33–39. [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T., Sato H. GABAergic inhibition and orientation selectivity of neurons in the kitten visual cortex at the time of eye opening. Vision Res. 1985;25(3):383–388. doi: 10.1016/0042-6989(85)90063-x. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Waddington J. L., Cross A. J. Baclofen and muscimol: behavioural and neurochemical sequelae of unilateral intranigral administration and effects on 3H-GABA receptor binding. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):275–280. doi: 10.1007/BF00507114. [DOI] [PubMed] [Google Scholar]
- Willshaw D. J., von der Malsburg C. How patterned neural connections can be set up by self-organization. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):431–445. doi: 10.1098/rspb.1976.0087. [DOI] [PubMed] [Google Scholar]
- Wolf W., Hicks T. P., Albus K. The contribution of GABA-mediated inhibitory mechanisms to visual response properties of neurons in the kitten's striate cortex. J Neurosci. 1986 Oct;6(10):2779–2795. doi: 10.1523/JNEUROSCI.06-10-02779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




