Abstract
Despite decreases in the cancer death rates in high-resource countries, such as the USA, the number of cancer cases and deaths is projected to more than double worldwide over the next 20–40 years. Cancer is now the third leading cause of death, with >12 million new cases and 7.6 million cancer deaths estimated to have occurred globally in 2007 (1). By 2030, it is projected that there will be ∼26 million new cancer cases and 17 million cancer deaths per year. The projected increase will be driven largely by growth and aging of populations and will be largest in low- and medium-resource countries. Under current trends, increased longevity in developing countries will nearly triple the number of people who survive to age 65 by 2050. This demographic shift is compounded by the entrenchment of modifiable risk factors such as smoking and obesity in many low-and medium-resource countries and by the slower decline in cancers related to chronic infections (especially stomach, liver and uterine cervix) in economically developing than in industrialized countries. This paper identifies several preventive measures that offer the most feasible approach to mitigate the anticipated global increase in cancer in countries that can least afford it. Foremost among these are the need to strengthen efforts in international tobacco control and to increase the availability of vaccines against hepatitis B and human papilloma virus in countries where they are most needed.
Introduction
Cancer is now the third leading cause of death worldwide, with >12 million new cases and 7.6 million cancer deaths estimated to have occurred in 2007 (1). By 2030, it is projected that there will be ∼26 million new cancer cases and 17 million cancer deaths per year (2). Moreover, the global distribution of cancer and types of cancer that predominate continues to change, especially in economically developing countries. Low- and middle-income countries accounted for about half (51%) of all cancers worldwide in 1975; this proportion increased to 55% in 2007 and is projected to reach 61% by 2050 (3). Cancers of the lung, breast, colon/rectum and prostate are no longer largely confined to Western industrialized countries but are among the most common cancers worldwide.
This global increase in the cancer burden and its disproportionate impact on economically developing countries is being propelled by both demographic changes in the populations at risk and by temporal and geographic shifts in the distribution of major risk factors. The three most important factors that contribute to these trends are:
Growth and aging of populations;
The entrenchment of modifiable risk factors (particularly cigarette smoking, Western diet and physical inactivity) in developing countries and
The slower decline in cancers related to infectious etiologies in low-resource countries than in high-resource countries.
The first of these largely reflects progress in reducing death rates from acute infectious conditions among children and young adults. Unfortunately, this has not historically been matched by parallel efforts to control the major modifiable risk factors that cause cancer and other chronic diseases. Given the magnitude of the projected demographic trends and their disproportionate impact on countries that can least afford increased health care expenditures, preventive measures offer the most feasible approach. This paper identifies several preventive measures that could mitigate the expected increase in the number of people affected by cancer over the next 20–40 years. Foremost among these are the need to strengthen efforts in international tobacco control and to increase the availability of vaccines against hepatitis B virus (HBV) and human papilloma virus (HPV) in countries where they are most needed. Both tobacco control and universal childhood vaccination have been proven to be highly effective and cost effective in countries across a wide range of economic development. Both have the potential to provide the greatest benefit at the lowest cost.
Global population changes
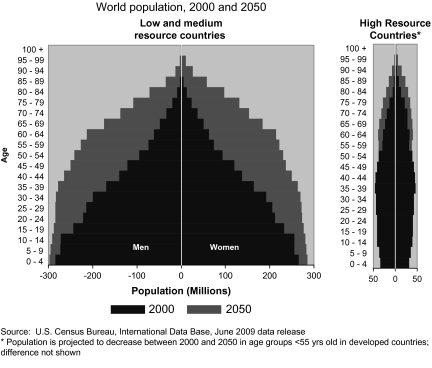
Population growth and aging are the largest contributors to the increasing total number of cancer cases and the shift in the burden of cancer and other chronic diseases toward economically developing countries. The world population in 2008 was 6.7 billion. Barring a catastrophe, this number is expected to increase to 8.3 billion in 2030 (2) and to 8.9 billion by 2050 (3). Figure 1 illustrates that the projected population growth will be much larger in low- and medium-resource countries than in high-resource countries, even in older age groups. For example, the number of people age 65 and above in low- and medium-resource countries is expected to increase from 247 million to 982 million between the year 2000 and 2050. The corresponding increase in people age 65 and above in high-resource countries will be from 171 million to 376 million. Aging of populations will disproportionately affect the number of people who develop or die from cancer since almost half (45%) of cancers diagnosed worldwide in 2002 occurred in people >65 years of age (3).
Fig. 1.
Growth and aging of world population, 2000 and 2050 by gender and level of economic development.
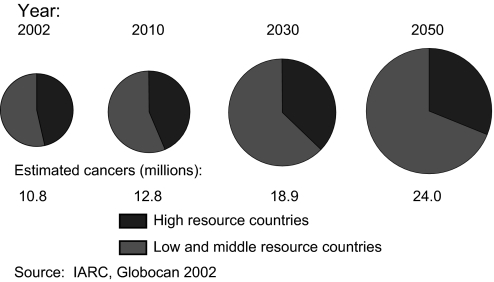
Estimates of the future impact of population growth and aging on the global cancer burden depend on assumptions about the anticipated changes in cancer incidence rates as well as changes in the underlying population at risk. However, it is more difficult to predict global trends in incidence rates than to project demographic changes because the information on cancer incidence rates is limited or non-existent for large areas of the world and there is great variation among countries. Hence, some projections of the future cancer burden assume that the global cancer incidence rates will remain constant over time and that the number of cancer cases and deaths will be affected only by growth and aging of the population. For example, Figure 2 illustrates the expected global increase in incident cancer cases from 2002 to 2050 under this assumption. Even without any increase in the cancer incidence rate, the number of cases anticipated in 2050 (24 million) would be more than twice the number in 2002 (10.8 million). Most of this increase would occur in low- and middle-resource countries (3). By comparison, the number of cancers that the International Agency for Research on Cancer projects by 2030 (26 million) is substantially higher than the estimate in Figure 2 (18.9 million) because the International Agency for Research on Cancer assumes a 1% annual increase in the incidence rate as well as the anticipated demographic changes in the population at risk (2).
Fig. 2.
Number and distribution of cancer cases by level of economic development and year assuming no change in the annual incidence rate.
Tobacco use and prevention of tobacco-related cancers
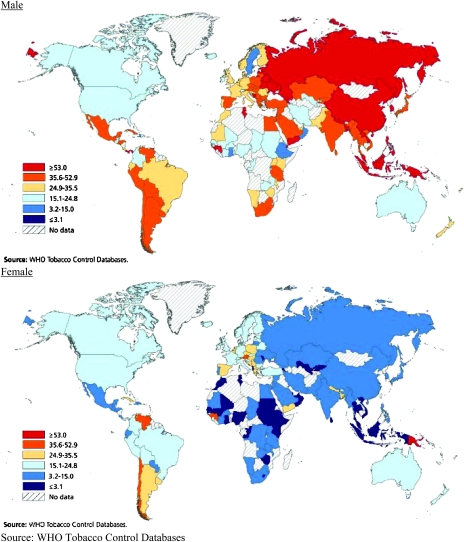
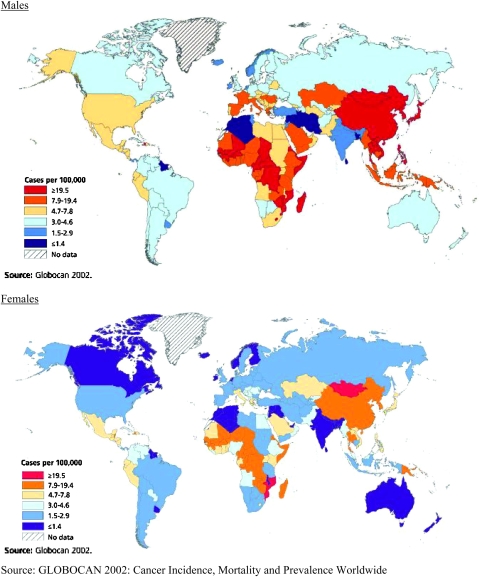
Tobacco use is the single largest preventable cause of cancer and premature death worldwide. An estimated 1.3 billion people in the world currently smoke tobacco; the vast majority of these smoke manufactured cigarettes (4). If current trends in smoking and population growth continue, the number of current smokers is expected to reach 2 billion worldwide by 2030 (5). As shown in Figure 3, smoking prevalence is highest among men in Eastern Europe, the former Soviet Union, China and Indonesia. Cigarette smoking among women has been decreasing in most high-resource countries but is stable or increasing in several countries in Southern, Central and Eastern Europe (4). With the decline of tobacco use in many industrialized countries, the geography of smoking has shifted from the developed to the developing world, especially for men. About 50% of men and 9% of women are current smokers in developing countries, compared with 35% of men and 22% of women in high-resource countries (4). Over 60% of all smokers in the world live in just 10 countries (in order): China, India, Indonesia, Russian Federation, USA, Japan, Brazil, Bangladesh, Germany and Turkey (6). The number of current smokers in China approximately equals the entire population of the USA. While most of these smokers are currently under age 40, the combination of continued smoking and aging will vastly increase the future adverse effects of tobacco use on cancer and other chronic diseases, unless effective campaigns can be mounted to promote cessation.
Fig. 3.
Prevalence of male and female smoking by geographic region.
The International Agency for Research on Cancer designates at least 15 different types or subtypes of cancer as causally related to smoking (7). These include cancers of the lung and bronchus (all histological subtypes), larynx, oral cavity, pharynx, lip, nasopharynx, nasal cavity and paranasal sinuses, esophagus (squamous and adenocarcinoma), bladder, kidney (parenchyma and renal pelvis), pancreas, uterine cervix, stomach, liver and acute myeloid leukemia (7). Cancers attributed to cigarette smoking accounted for more than one-fifth of the estimated 1.42 million cancer deaths that occurred globally in the year 2000 (8). Lung cancer is the most common cancer caused by smoking, even though it accounts for less than half of all smoking-attributable deaths (9). Cigarette smoking accounts for ∼80% of lung cancer cases in men and 50% in women worldwide (5). Lung cancer has been the most common cancer in the world since 1985 (10).
In many countries, temporal trends in smoking, and more recently in smoking cessation, have been so extreme that they have dominated national trends in cancer mortality (9). For example, the downturn in death rates from smoking-related cancers in men, ages 65–69 that began in the UK in the early 1960s, in the USA in the mid-1980s and in Poland in the early 1990s, has been the major driver in the downturn in death rates from all cancers combined in these countries (9). This has occurred despite the delay that occurs between reductions in smoking prevalence and reductions in lung cancer mortality, seen when comparing Figures 3 and 4 for men in North America and Western Europe. Progress in reducing male lung cancer rates was achieved principally by smoking cessation among adult men. Unfortunately, cessation rates are much lower in large developing countries like China than in the West. In China, many millions of young cigarette smokers who began smoking in adolescence are now aging. The future risks of cancer or other diseases caused by smoking may eventually be greater among these younger generations of Chinese men who began smoking in childhood than those now seen among Chinese smokers who initiated smoking later in life (9).
Fig. 4.
Lung cancer incidence in males and females.
Despite this bleak picture, much progress can and is being made to reduce tobacco smoking, especially in high-resource countries. In the USA, per capita cigarette consumption has decreased to levels not seen since the start of World War II. Projections based on data from the Food and Agriculture Organization of the United Nations indicate that tobacco consumption will continue to fall in economically developed countries but will rise in most low- and medium-resource countries (11). A number of interventions have proven to be effective in reducing the demand for tobacco products and in changing social norms about smoking. Tax policies that raise the price of tobacco products are the single most effective approach for reducing demand. Price increases are an especially powerful deterrent against the initiation of smoking in youth (12,13,14) and motivation for addicted smokers to quit. Although the impact of price on cigarette demand varies widely depending on the population studied and study design, a 10% increase in cigarette prices result in a 2.5–5% reduction in cigarette demand in high-resource countries. Estimates of price responsiveness in low- and middle-income countries are at least as large (15).
Smoke-free laws that prohibit smoking in public places substantially reduce non-smokers’ exposures to secondhand smoke, change social norms about smoking and motivate smokers to quit. Successful smoke-free interventions by Ireland and other countries have greatly broadened the protection of non-smokers. Public awareness about the harmful effects of tobacco on health can be increased by requiring prominent, graphical warnings on cigarette packaging (16) and by educational campaigns aimed at health professionals. These are especially important in low- and medium-resource countries, where baseline levels of awareness about the extent of tobacco's deleterious effects are low and doctors still smoke. In China, 61.3% of male physicians smoke, compared with 66.9% of the general male population.
Many of the adverse health effects of smoking can be averted by cessation. Smoking cessation is the only effective way to reduce risk for the 1.3 billion smokers in the world. As mentioned above, widespread smoking cessation among men in high-income countries since the 1950s is largely responsible for the significant decrease in death rates from lung and other smoking-related cancers in these countries. Although pharmacological treatment is less affordable in economically developing than in high-resource countries, brief counseling interventions by health care personnel are feasible. Counseling about smoking cessation can be integrated into other public health and health care delivery programs, such as tuberculosis, human immunodeficiency virus (HIV)/AIDS, family planning and maternal health programs.
No single country can successfully combat the resources of the international tobacco companies. Thus, nations have united behind the Framework Convention on Tobacco Control (FCTC), the first health treaty negotiated under the auspices of the World Health Organization (WHO). The FCTC provides a framework for national legislation and enforcement of tobacco control measures. The FCTC was promulgated in May 2003 in response to the global tobacco pandemic with the objective of substantially reducing the worldwide prevalence of tobacco use and exposure to tobacco smoke. As of August 2009, 166 countries have ratified the treaty, representing ∼80% of the world's population. FCTC provisions establish international standards for tobacco taxation, advertising and sponsorship, regulation, trade, etc. Effective measures against smuggling are especially important. Estimates suggest that 6–8% of cigarettes consumed globally are contraband products, shipped to avoid taxes. Smuggling can be deterred by prominent tax stamps that cannot be counterfeited easily, local language warnings on cigarette packs and aggressive enforcement of anti-smuggling measures and penalties.
Diet, obesity and physical inactivity
Like tobacco, obesity, physical inactivity and poor nutrition are established causes of several types of cancer. Rapid increases in the prevalence of obesity (defined by WHO as body mass index >30 in adults) have occurred in the USA since the 1970s and more globally since the 1980s (17). The trend toward overweight and obesity is even greater in children than in adults and has occurred not only in high-resource countries but also in urban and even rural areas of many low- and middle-income countries (17). WHO estimates that the number of overweight adults (age >15) in 2005 was ∼1.6 billion, of whom 300 million were obese. The number of overweight was projected to increase to 2.3 billion by 2015 (18).
The global increase in overweight and obesity is attributed to increased availability of calorie-dense foods and decreased physical activity. Food supplies have become more plentiful and foods, especially processed foods, have become more energy dense in fats and sugars (17). Coinciding with this, humans have become more sedentary as mechanization has eliminated much of the need for involuntary physical activity. Excess adiposity, especially visceral adiposity, is known to increase risk of a variety of cancers, including breast (postmenopausal), endometrium, colon/rectum, esophagus (adenocarcinoma) and kidney. Despite the negative health, social and economic consequences of obesity, efforts are just beginning to develop solutions to the problem. People who have gained weight generally have great difficulty losing it and maintaining weight loss. There is great interest in identifying policy measures to help people maintain a healthy weight throughout life. However, research in this area is currently at an early developmental stage and evaluation of these methods is ongoing.
Cancers related to chronic infections
Persistent infection with various microbial organisms accounts for ∼18% of cancers worldwide (19). The most common malignancies caused by chronic infections are cancer of the uterine cervix, stomach and liver, caused by HPV, Helicobacter pylori (H.pylori) and HBV and Hepatitis C (HCV) virus, respectively (Table I). Several other cancer sites related to infectious agents that occur mostly regionally are also listed in Table I. These include Kaposi’s sarcoma (KS) caused by human herpes virus 8 (HHV-8), nasopharyngeal cancer and certain lymphomas from Epstein Barr virus (EBV), bladder cancer from Schistosoma haematobium, leukemia from human t-cell lymphotropic virus and biliary tract cancer from onchocerciasis.
Table I.
The burden of cancer caused by Infectious agents worldwide
| Infectious agent | IARC classificationa | Cancer site/cancer | Number of cancer cases | Percentage of cancer cases worldwide |
| Helicobacter pylori | 1 | Stomach | 490 000 | 5.4 |
| HPV | 1, 2A | Cervix and other sites | 550 000 | 6.1 |
| HBV, HCV | 1 | Liver | 390 000 | 4.3 |
| EBV | 1 | Lymphomas and nasopharyngeal carcinoma | 99 000 | 1.1 |
| HHV-8 | 2A | KS | 54 000 | 0.6 |
| Schistosoma haematobium | 1 | Bladder | 9000 | 0.1 |
| HTLV-1 | 1 | Leukemia | 2700 | 0.1 |
| Liver flukes | Cholangiocarcinoma (biliary system) | 800 | ||
| Opisthorchis viverrini | 1 | |||
| Clonochis sinensis | 2A | |||
| Total infection-related cancer | 1 600 000 | 17.7 | ||
| Total cancer in 1995 | 9 000 000 | 100 |
HTLV-1, human t-cell lymphotropic virus.
Source: World Cancer Report 2008 (2).
Group 1 = carcinogenic to humans; Group 2A = probably carcinogenic to humans.
In terms of global patterns, the incidence rates from cancers of the stomach and liver have been falling in most countries worldwide, although the decrease has been slower in low- and medium-resource countries than in the West. The percentage of cancers attributed to infections remains about three times higher in developing (26%) than in developed (8%) countries (20). For most cancers related to infectious organisms, chronic inflammation is an important component of pathogenesis. Some viruses incorporate their genetic material directly into host cell DNA (21). The remaining sections of this paper will focus on the most common infection-related cancers for which vaccines and other preventive approaches are available.
HPV-related cancers
Cervical cancer is the second most common cancer among women worldwide, with an estimated 555 000 new cases and 310 000 deaths in 2007. About 80% of cervical cancer cases occur in developing countries where, in many regions, it is the most common cancer among women (2). Although the age-standardized incidence rate is considerably lower in Asia than in Central America, Asia accounts for more than half of the world's cervical cancer cases and deaths because of its immense population.
Incidence and mortality rates for cervical cancer in developed countries have decreased dramatically in the past 25 years, due largely to cervical cancer screening using Pap tests, which allows for detection and treatment of precancerous lesions. The incidence and death rates remain high in countries that cannot afford cytologic screening, where oncogenic strains of HPV are still common. Worldwide, the highest incidence rates are in South America and the Caribbean, Sub-Saharan Africa and South and Southeastern Asia. The disproportionate burden of cervical cancer on developing countries is due mainly to lack of resources for cervical cancer screening.
Virtually, all cases of cervical cancer result from recurrent cervical infections with HPV (22). About 40 HPV viral types have been found to infect the anogenital tract (23). A recent pooled analysis of case–control studies from nine countries identified 15 HPV types that are associated with an increased risk of cervical cancer. Among these types, HPV 16 and HPV 18 have the highest prevalence among cervical cancer patients (50.5 and 13.1%, respectively) and are associated with a >200-fold increased risk of cervical cancer (24). Although infection with HPV (types 16 and 18 in particular) greatly increase the risk of developing cervical cancer, such infection is common and most infected women do not develop cervical cancer. The prevalence of HPV infection among women as measured among women without cervical cancer measured in a recent international study was 15.5%, with 5% being positive for HPV types 16 or 18. The cumulative lifetime probability of acquiring a cervical infection with at least one type of HPV is extremely high for sexually active individuals. However, most HPV infections disappear spontaneously within 2–4 years, and only a small percentage progress to low- and high-grade squamous intraepithelial lesions. High-grade squamous intraepithelial lesion, which includes carcinoma in situ, may progress to invasive cancer if not detected and treated. Factors that may influence progression include immunosupression, smoking, increasing parity (number of children) and coinfection with herpes simplex virus or chlamydia (25).
In addition to cervical cancer, HPV infection is associated with vulvar carcinoma, some oral carcinomas, penile carcinoma and anal carcinoma. The anus is similar to the cervix in that both have a transformation zone that is susceptible to HPV infection. The risk of anal carcinoma is increased among men who have sex with men (26).
Primary prevention
Recently developed cervical cancer vaccines have the potential to prevent HPV infection; however, effective vaccines against HPV infection are too costly to be available in high-risk areas. Consequently, negotiations for tiered pricing must reduce the cost from about $360 per woman and identify alternative sources of funding if the vaccine is to be made available in the areas of greatest need in the next 10–20 years.
Although some HPV subtypes cause genital warts, most infections with HPV cause no symptoms. Since HPV can be present in skin throughout the anogenital area as well as in genital secretions, use of condoms may be somewhat but not completely protective (26). Circumcision apparently reduces the risk of transmission and acquisition of HPV as well as the risk of cervical cancer among female partners (26).
Detection of high-grade squamous intraepithelial lesion and carcinoma in situ by Pap testing, with effective treatment of precancerous lesions, is currently the most effective way to prevent cervical cancer. Pap testing is not available to most women in developing countries. Barriers to Pap testing in developing countries are substantial and include lack of public health and medical infrastructure to organize population screening and follow-up, as well as the high level of expertize and quality control needed to ensure accuracy of cytologic diagnosis. In some countries, cultural taboos may also prevent women from seeking or receiving appropriate screening from male health care providers. Development and evaluation of alternative screening strategies for use in developing countries has been a high priority for international health agencies. Visual inspection of the cervix after applying acetic acid is one cost-effective technique (27). Training and employing midwives and other female health workers offers some potential for overcoming cultural barriers.
Secondary prevention
In addition to preventing invasive cervical cancer, Pap testing can detect cervical cancer at an early stage, when it is most readily treated.
Liver cancer, HBV and hepatitis C virus
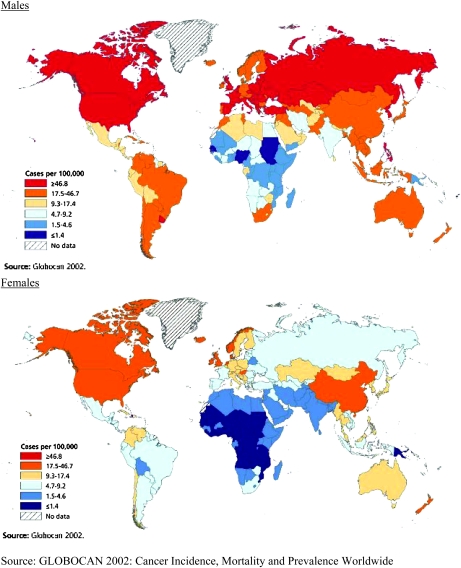
Liver cancer is the fifth most common cancer in men and the eighth most common in women (1). An estimated 711 000 cases of primary liver cancer occured worldwide in 2007; about three-quarters of these are hepatocellular carcinoma (HCC). Liver cancer is the third leading cause of cancer death worldwide with an estimated 680 000 deaths occurring in 2007. More than 80% of cases occur in developing countries; >55% occur in China alone. Figure 5 illustrates that liver cancer incidence rates are highest in West and Central Africa and in Asia, especially among men.
Fig. 5.
Liver cancer incidence in males and females.
Globally, ∼75% of all liver cancer cases and 50% of all deaths are caused by chronic infection with either HBV or HCV (1). The fraction of cases attributable to HBV is greater in developing countries (59%) than in developed countries (23%) The corresponding percentages for HCV are 33 and 20%, respectively (1). Both HBV and HCV are directly transmitted by person-to-person contact with blood or other body fluids. Hepatitis A, another common agent of viral hepatitis, is usually transmitted through contaminated food and does not cause liver cancer.
A safe and effective vaccine against HBV has been available since 1982. No vaccine has yet been developed for HCV, which accounts for ∼10% of liver cancer deaths worldwide. Vaccination for HBV is the most cost-effective strategy to reduce liver cancer. Effective vaccination programs could avert at least 300 000 liver cancer deaths per year. The cost of the vaccine has been reduced from $100 to $1 (for three doses of treatment) through the efforts of the Global Alliance for Vaccines and Immunization. Over three-quarters of WHO member states have introduced hepatitis B vaccine into routine infant immunization schedules, although in many places it is difficult to deliver the vaccine within 24 h of birth. Vaccine delivery is particularly challenging in high risk, low-resource areas of Africa.
HBV
Approximately 54% of liver cancer worldwide is attributed to chronic infection with HBV (20). Chronic (long term) infection with HBV is indicated by persistence of hepatitis B surface antigen (a marker of active Hepatitis B infection) in the blood after the acute infection has passed. The probability of developing chronic infection is much higher for infants and children than for adults. People with chronic infection are also referred to as ‘chronic carriers’ because they are able to infect others. Among people who have had HBV infection, only those with chronic infection are at increased risk of developing chronic liver disease, cirrhosis and liver cancer (28).
About 350 million people in the world today (29), and 1.25 million people in the USA (30), are chronic carriers of HBV. HBV may be transmitted by mucous membrane or skin contact only when there are breaks (even small and insignificant ones) in these barriers. It may be transmitted by injection or sexual intercourse or pass from a mother to her baby during birth. Because HBV is able to remain infectious for about a week on any surface, minute exposures from sharing of toothbrushes, razors and other items in household settings can result in transmission (31). An important cause of HBV infection in developing countries is reuse of syringes or needles between patients without sterilization. An estimated 20% of new HBV infections worldwide, or 8–16 million infections per year, arise from unsafe injections in medical care settings (32).
In high-prevalence areas of the world, HBV infection in ∼70% of cases is acquired at birth or in early childhood. In industrialized countries, infection more often occurs in adolescence and young adulthood and is associated with high-risk behaviors such as injection drug use and multiple sex partners. Health care workers and patients with exposure to pooled blood products, such as hemophiliacs, are also at increased risk.
The exact mechanism by which HBV infection causes HCC is unknown. Tumor cells of patients with liver cancer often have DNA from HBV inserted into cellular DNA. Inflammation and scarring of the liver (cirrhosis) may contribute to malignant transformation. Although 60–90% of patients with HCC due to chronic HBV have cirrhosis, it is possible to develop HCC without cirrhosis (33). Exposure to aflatoxins (toxic products of mold in grains and other foods products) may be a significant cofactor in localized areas (34).
Primary prevention.
Three doses of vaccine against HBV are needed to achieve adequate long-term immunity. It is recommended that all infants, unvaccinated adolescents and adults in high-risk groups receive the vaccine. The first dose for infants should be administered within 24 h. This is difficult in many parts of the worlds since about half of all babies born worldwide are born at home. In countries such as Indonesia, however, trained birth attendants have been taught to administer the vaccine to babies at home. It is also recommended that all pregnant women be tested for HBV carrier status; if positive, the newborn infant receives Hepatitis B immune globulin as well as Hepatitis B vaccine within 12 h after birth (35). This treatment is highly effective in preventing infection in newborns.
In high-resource countries, vaccination of infants and children and screening of blood donors for HBV surface antigen have greatly decreased the incidence of acute HBV, especially among people under the age of 20 (30,31). The burden of HBV infection and resulting liver disease and HCC is much greater in the developing world than in developed countries due to greater barriers to prevention. Although the WHO recommends HBV vaccination for all infants, many poor countries have been unable to implement this recommendation due to the cost of obtaining and administering the vaccine. In 1999, a commitment by the Bill and Melinda Gates Foundation of $750 million over 5 years led to the development of the Global Alliance for Vaccines and Immunization , an international partnership created to improve access to sustainable immunization services.
Screening of blood products and sterilization of injection equipment is essential to reduce HBV transmission in medical settings. Another potential way to reduce the incidence of HCC in some developing countries is to reduce consumption of foods contaminated with aflatoxins, which may contribute to the development of HCC among HBV carriers. Preventing aflatoxin contamination of the food supply can be accomplished by implementing changes in pre-and post-harvest agricultural practices and dietary patterns (34).
Secondary prevention.
No treatment has been proven to be effective in preventing progression to chronic liver disease and HCC among HBV carriers. Treatment with α-interferon or lamivudine reduces viral replication in some patients but the improvement is often transient (31,36). A recent study of treatment with Vitamin K showed some promise in reducing liver cancer rates among women with cirrhosis (37).
There have been no randomized controlled trials to evaluate the cost or efficacy of screening for liver cancer in chronic HBV carriers. In the USA, many physicians screen healthy carriers yearly or twice yearly for high levels of serum α-fetoprotein. Carriers with additional risk factors, including active chronic hepatitis or cirrhosis, are screened twice yearly with serum α-fetoprotein measurement and ultrasound (38).
HCV
About 31% of liver cancer worldwide is attributed to HCV compared with 8% in North America (20). First identified in 1988, HCV was confirmed as the major cause of ‘non-A, non-B’ hepatitis in 1990 (39). Although HCV infection can cause symptoms similar to HBV, the majority of newly infected people have no symptoms. A person infected with HCV has over an 80% probability of becoming a chronic carrier. Worldwide, 170 million persons are infected with HCV, and the global prevalence of HCV infection is 3.1%, ranging from 1.0% in Europe to 5.3% in Africa (40).
Direct transmission by blood contamination, usually though a needle, is the most important mode of HCV transmission. In the USA, 60% of chronic HCV infections are attributed to intravenous drug use, 15% to receipt of blood transfusions before 1989 (when a test to screen for HCV in donated blood became available) and 15% to sexual transmission (41). The rate of acquisition of HCV infection among intravenous drug users is very high, with a number of studies showing 20–40% being infected within the first year of using needles (42). HCV infection occurs in 3–8% of health care workers who experience needlestick exposures to HCV-infected patients and in 2–8% of infants born to mothers with active HCV infection (31). Approximately 50% of infants are able to eradicate the disease with no intervention. Post-exposure prophylaxis with immunoglobulin has not been proven effective in preventing HCV infection among infants or adults (43).
A decline in the rate of HCV infection in developed countries has been attributed to changes in the blood donor population and transfusion practice, mandatory screening of donated blood and a decline in injection drug use (44). Although liver cancer incidence increased in some developed countries such as the USA during the same time, this increase is thought to be due to the aging of the population previously infected (45).
About 25% of people with chronic HCV infection will develop hepatic fibrosis that evolves into cirrhosis. Among those who develop cirrhosis, liver cancer develops at a rate of 1–4% per year. Cofactors that may influence this progression are alcohol intake, age at infection and coinfection with HBV or HIV (43).
In developing countries, use of unscreened blood and blood products and reuse of injection equipment in medical settings are major routes of HCV transmission (42).
Primary prevention.
There is no vaccine available for HCV. In most developed countries and in some developing countries, the main route of transmission is intravenous drug use. Needle and syringe exchange programs have been shown, in a limited number of studies, to reduce rates of HCV infection (42). Universal precautions (a set of precautions designed to prevent transmission of blood-borne pathogens when providing first aid or health care) and safer needle design have reduced risks of infection among health care workers.
The Centers for Disease Control and Prevention recommend that routine HCV testing be offered to individuals in high-risk groups, although this practice may not be feasible in developing countries. Individuals who test positive are provided medical referral and counseling to reduce the risk of HCV transmission to others (44).
Other important elements of primary prevention are screening of donated blood and blood products for antibody to HCV and instituting infection control practices during all medical, surgical and dental procedures. While these measures are particularly important in parts of the world with high prevalence of HBV and HCV, they have not been implemented in many developing countries due to resource constraints (42).
Secondary prevention.
Patients with HCV should be evaluated to determine the extent of their liver disease, and if appropriate, receive counseling to limit their alcohol intake, receive anti-viral treatment, immunization with hepatitis A, hepatitis B, pneumococcal and influenza vaccines and treatment for drug or alcohol abuse. Treatment with α-interferon and ribavirin produces sustained response rates (meaning that HCV RNA is undetectable at 24 weeks after therapy) of 54–56%, with a low probability of subsequent relapse (43), and reduces the incidence of liver cancer among sustained responders (46). However, there are numerous adverse effects and contraindications to this treatment and the high cost makes it unaffordable for most HCV carriers in developing countries (43).
Other infection-related cancers
A number of other chronic infections are known to cause various types of cancer. Although these were not included among the top priorities for prevention, they do contribute substantially to the cancer burden in certain geographic areas and are discussed briefly below.
H.pylori and stomach cancer
Incidence and death rates from stomach cancer have steadily declined over the last 50 years, even though stomach cancer remains the fourth most common malignancy, and is second only to lung cancer as the leading cause of cancer deaths (10). Stomach cancer accounted for >1 million estimated cases and 800 230 deaths in 2007, with an estimated 60% of new cases occur in developing countries (1, 2). Despite falling incidence rates, the number of new cases and deaths continues to increase globally because of population growth and aging in high-risk countries. Risks are generally highest in Asia (Korea, Japan and China) and parts of Central and South America (Costa Rica, El Salvador and Columbia) and lowest in North America and most of Africa.
Chronic or recurrent infection with H.pylori is the main cause of chronic gastritis and peptic ulcers and increases risk for developing gastric lymphoma and cancer of the distal stomach (47). H.pylori is a spiral, gram-negative bacterium that colonizes the stomach. It is not known with absolute certainty how H.pylori is transmitted, but the most probable route of spread from person to person is through fecal-oral or oral-oral routes. Possible environmental reservoirs include contaminated water sources. Based on prevalence surveys conducted in the USA in 1988–1991, about one-third of adults had evidence of H.pylori infection (48). Prevalence increased steadily with increasing age, peaking at >50% among persons age ≥50. In developing countries, the age at onset of infection is generally lower and peaks at >90% among young adults (47).
The exact causes of the worldwide decline in gastric cancer incidence in the past decades are not known but are thought to include improvements in diet, food storage and a decline in H.pylori infection due to a general improvement in sanitary conditions and increasing use of antibiotics (49, 50). The eradication of H.pylori in asymptomatic carriers has been proposed as a potential method to prevent stomach cancer. Trials of the efficacy of this approach in preventing the development of atrophic gastritis should be conducted in high-risk areas. However, this approach does not preclude the existing practice of screening for gastric cancer in high-risk populations.
Primary prevention.
Since the source of H.pylori is not yet known, recommendations for avoiding infection have not been made. In general, it is always wise for persons to wash hands thoroughly, to eat food that has been properly prepared and to drink water from a safe, clean source. Trials of H.pylori eradication in high-risk populations have been attempted. Treatment with bismuth salts, amoxicillin and clarithromycin is currently the regimen of choice; however, suboptimal results (clearance of infection in <50% of persons treated) have been observed in some studies. Eradication will also be ineffective in preventing stomach cancer if people are rapidly reinfected (47). There is concern that extensive use of antibiotics may lead to antibiotic resistant strains of H.pylori or other pathogens (51). Chemoprevention trials using vitamin supplements have shown some promise and additional trials are underway (47).
Efforts are also underway to develop an H.pylori vaccine, but trials have thus far been disappointing (51). Furthermore, it has been hypothesized that the reduction in gastric acid secretion due to H.pylori infection may protect against the adverse effects of gastroesophageal reflux disease such as Barrett esophagus and adenocarcinoma of the gastric cardia and esophagus. Efforts to eradicate H.pylori with antibiotic treatment or vaccination should consider the potential risks as well as benefits (50).
Secondary prevention.
Screening for stomach cancer has been practiced in Japan (where the incidence of stomach cancer is about six times higher than the incidence in the USA) since 1963. The main screening technique used in Japan is indirect X-ray examination by the double contrast method. Record linkage between participants in the screening and a population-based cancer registry indicate that the sensitivity (ability to identify correctly those who have the disease) and specificity (ability to identify correctly those who do not have the disease) of this method were 88.5 and 92.0%, respectively (52). As a result of population screening and perhaps greater awareness of early symptoms and a low threshold for diagnostic evaluation, 50% of stomach cancers in Japan are diagnosed at a localized stage, and the overall 5 year survival has increased from 20% in 1962 to 40% in 1992. Over the same time period, the 5 year survival rate in the USA remained stable at 20% (53). Although general population screening is not recommended in low-incidence countries such as the USA, a low threshold for testing symptomatic individuals may be warranted.
KS, HHV-8 and HIV
It is estimated that 77 600 new cases of KS occurred worldwide in 2005. KS has been common in central Africa since the early 20th century, and was also found in some Mediterranean countries and the Middle East, but was rare elsewhere. It was also observed in developed countries among immigrants from endemic areas, recipients of organ transplants and patients with immune suppression from chemotherapeutic drugs. In 1981, an aggressive form of KS began to appear in the USA among homosexual men, one of the first signals of the AIDS epidemic (54).
Only one of the four subtypes of KS is associated with AIDS. The AIDS-related KS has identical histopathology to the other subtypes; it is more probable to involve multiple lesions and worse prognosis. In areas of Africa where KS was relatively common before the AIDS epidemic, the incidence of KS has increased ∼20-fold. In several African countries (Malawi, Swaziland, Uganda and Zimbawe), it is now the most common cancer in men and the second most common cancer in women (54).
It is now thought that infection with HHV-8 (also known as KS-associated herpes virus), rather than the AIDS virus itself, is the principal cause of KS. Sexual contact is thought to be the major mode of transmission of HHV-8. HHV-8 prevalence increases steadily with age among children in Africa, indicating that alternate modes of transmission exist (54).
In the USA, the risk of KS among men having sex with men is much greater than among other persons infected with HIV (55). The incidence of KS peaked among men age 20–54 in 1989 and then declined markedly. Since highly active antiretroviral therapy (HAART) did not become widely available in the USA until 1996, some of this decline is probably due to changes in sexual practices that reduced transmission of both HIV and HHV-8 (55,56). KS rates peaked somewhat later (1991–1996) among African-American men, and when first reported in 1992, rates among Hispanics were higher than among white and African-American men. Incidence rates among all five racial and ethnic groups declined from 1992 to 2001 but show some evidence of stabilizing in the past 3–4 years.
Primary prevention
The most effective methods for preventing KS are those that protect against exposure to HIV and HHV-8. Preventive behaviors include sexual abstinence, sex only with an uninfected partner, consistent and correct condom use, abstinence from injection drug use and consistent use of sterile equipment by those unable to cease injection drug use (57). Similar precautions are recommended for those who are already HIV positive, both to prevent infecting others and to avoid infection with other sexually transmitted and blood-borne diseases. There is some evidence that progression to KS might be accelerated among persons who become infected with HHV-8 after being infected with HIV (58).
Secondary prevention
HAART treatment reduces the risk of KS among patients infected with HIV and may also be effective in treating the tumors (55). In 2003, the WHO estimated that 6 million people in developing countries needed immediate HAART and <8% would get it. On World AIDS Day in 2003, the WHO launched ‘The 3 by 5 Initiative’, whose goal is to provide antiretroviral treatment to 3 million people in developing countries by 2005 (11).
HIV, EBV and non-Hodgkin’s lymphoma
It is estimated that there were 300 000 new cases of non-Hodgkin’s lymphoma (NHL) worldwide in 2007. NHL is an extremely varied group of neoplasms. Some forms of NHL, including Burkitt’s lymphoma, are associated with EBV. Most people are infected with EBV at sometime in their lives. When infection occurs during adolescence or young adulthood, it causes infectious mononucleosis 35–50% of the time. EBV infection is also associated with some AIDS-related NHL and Hodgkin’s lymphoma.
AIDS-related NHL is generally aggressive, high-grade lymphomas of B-cell origin. They include primary central nervous system lymphomas, high-grade immunoblastic and Burkitt’s lymphoma, and to a lesser extent, intermediate-grade large cell diffuse lymphomas. Unlike KS, which occurs primarily in men who have sex with men, all HIV risk groups, including intravenous drug users and children of HIV-positive individuals have equivalent risks of AIDS-related NHL. The diagnosis of AIDS precedes the onset of NHL in 57% of patients, but in 30% of cases, the diagnosis of AIDS is made at the same time as NHL and HIV positivity. In general, the clinical course of patients with AIDS-related lymphoma is more aggressive, and the disease is more extensive and less responsive to therapy than that of non-HIV patients with non-Hodgkin’s lymphoma (59).
There has also been some evidence of an increased risk of Hodgkin’s lymphoma among HIV-infected individuals, but the evidence is less strong (59).
Primary prevention
Primary prevention of AIDS-related lymphomas rests on primary prevention of HIV infection, as described under KS. Since EBV is transmitted through contact with saliva, and may be present in saliva of healthy people, there are no methods for primary prevention of EBV infection.
Secondary prevention
Some AIDS-related lymphomas are associated with high levels of immunosuppression, as reflected by very low CD4 counts. The incidence of primary central nervous system lymphoma, which is associated with very low CD4 counts, fell rapidly after introduction of HAART therapy, whereas the incidence of intermediate type lymphoma has remained stable since 1995. Overall, epidemiologic data show that the risk of AIDS-related lymphoma has decreased ∼50% with HAART therapy, but risk is unchanged within groups of patients stratified by CD4 count. As the proportion of patients receiving HAART therapy has increased, there has been a shift to higher CD4 strata, with a change in the distribution of lymphoma types. Those lymphomas that do occur are more responsive to treatment, which may account for recent trends of improved survival for AIDS-related NHL (60).
Conclusions
In summary, preventive measures provide the only feasible approach to slow and ultimately reverse the global increase in cancer and its disproportionate impact on countries that can least afford it. We have emphasized why national and international efforts to strengthen tobacco control and to make childhood vaccination against HBV and HPV universally available and affordable in countries where these infections are prevalent should be among the highest priorities. Successful implementation of these programs will require both national leadership and international collaboration to overcome the economic and political barriers that often impede public health. It will also require action-oriented translational research to adapt programs that have proven to be effective in high-income countries to every setting in which they are needed.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- EBV
Epstein Barr virus
- FCTC
Framework Convention on Tobacco Control
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HAART
highly active antiretroviral therapy
- HHV-8
human herpes virus 8
- HIV
human immunodeficiency virus
- HPV
human papilloma virus
- KS
Kaposi’s sarcoma
- NHL
non-Hodgkin’s lymphoma
- WHO
World Health Organization
References
- 1.American Cancer Society. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.IARC. Lyon: IARC Press; 2008. World Cancer Report, 2008. Boyle,P. and Levin,B.E. (eds), [Google Scholar]
- 3.Bray F, et al. Predicting the future burden of cancer. Nat. Rev. Cancer. 2006;6:63–74. doi: 10.1038/nrc1781. [DOI] [PubMed] [Google Scholar]
- 4.Shafey O, et al. The Tobacco Atlas. 3rd edn. Atlanta, GA: American Cancer Society; 2009. p. 128. [Google Scholar]
- 5.Mackay J, et al. The Tobacco Atlas. 2nd edn. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 6.Jha P. Avoiding global cancer deaths and total deaths from smoking. Nat. Rev. Cancer. 2009;9:655–664. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- 7.IARC. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 83. Lyon: International Agency for Research on Cancer; 2004. Tobacco smoke and involuntary smoking; p. 1452. [Google Scholar]
- 8.Ezzati M, et al. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int. J. Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 9.IARC. IARC Handbooks of Cancer Prevention, Tobacco Control. Reversal of Risk After Quitting Smoking. Vol. 11. Lyon: IARC; 2007. [Google Scholar]
- 10.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Food and Agriculture Organization of the United Nations. Projections of tobacco production, consumption and trade to the year 2010. Rome: FAO; 2003. [Google Scholar]
- 12.Ranson MK, et al. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 2002;4:311–319. doi: 10.1080/14622200210141000. [DOI] [PubMed] [Google Scholar]
- 13.Chaloupka FJ, et al. Tax, price and cigarette smoking: evidence from the tobacco documents and implications for tobacco company marketing strategies. Tob. Control. 2002;11(suppl. 1):s62–s72. doi: 10.1136/tc.11.suppl_1.i62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guindon GE, et al. Trends and affordability of cigarette prices: ample room for tax increases and related health gains. Tob. Control. 2002;11:35–43. doi: 10.1136/tc.11.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross H, et al. Economic policies to tobacco control in developing countries. Salud Publica Mex. 2006;48(suppl. 1):s113–s120. doi: 10.1590/s0036-36342006000700014. [DOI] [PubMed] [Google Scholar]
- 16.Hammond D, et al. Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Tob. Control. 2006;15(suppl. 3):iii19–iii25. doi: 10.1136/tc.2005.012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 18.World Health Organization. Preparing for the Introduction of HPV Vaccines: policy and Programme Guidance for Countries. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 19.Mackay J, et al. The Cancer Atlas. Atlanta, GA: American Cancer Society; 2006. p. 128. [Google Scholar]
- 20.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 21.Mueller NB, et al. The best practices use of the guidelines by ten state tobacco control programs. Am. J. Prev. Med. 2006;31:300–306. doi: 10.1016/j.amepre.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: IARC Press; 1995. Human papillomaviruses; pp. 35–378. [Google Scholar]
- 23.World Health Organization. The World Health Report 2003. Geneva: WHO; 2003. [Google Scholar]
- 24.Munoz N, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman MH, et al. The epidemiology of cervical carcinogenesis. Cancer. 1995;76:1888–1901. doi: 10.1002/1097-0142(19951115)76:10+<1888::aid-cncr2820761305>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.Schiffman M, et al. Human papillomavirus: epidemiology and public health. Arch. Pathol. Lab. Med. 2003;127:930–934. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 27.IARC. Screening for cervical cancer. In: Stewart B, Kliehues P, editors. World Cancer Report. Lyon: IARC Press; 2003. pp. 167–171. [Google Scholar]
- 28.WHO. Hepatitis B. 2002. http://www.who.int/csr/resources/publications/hepatitis/WHO_CDS_CSR_LYO_2002_2/en (8 July 2009, date last accessed) [Google Scholar]
- 29.IARC. Chronic infections. In: Kliehues BSaP, editor. World Cancer Report. Lyon: International Agency for Research on Cancer; 2003. pp. 56–61. [Google Scholar]
- 30.CDC. Incidence of acute hepatitis B—United States, 1990–2002. MMWR Morb. Mortal. Wkly. Rep. 2004;52:1252–1254. [PubMed] [Google Scholar]
- 31.Nelson K, et al. Viral hepatitis. In: Nelson K, Williams C, Grahm N, editors. Infectious Disease Epidemiology: theory and Practise. Gaithersburg, MD: Aspen Publishers, Inc.; 2001. [Google Scholar]
- 32.Kane A, et al. Transmission of hepatitis B, hepaptitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull. World Health Organ. 1999;77:801–807. [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey MA, et al. Hepatocellular carcinoma: predisposing conditions and precursor lesions. Gastroenterol. Clin. North Am. 2002;31:641–662. doi: 10.1016/s0889-8553(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 34.Wild CP, et al. Primary prevention of hepatocellular carcinoma in developing countries. Mutat. Res. 2000;462:381–393. doi: 10.1016/s1383-5742(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Achievements in public health: hepatitis B vaccination—United States, 1982–2002. MMWR Morb. Mortal. Wkly. Rep. 2002;51:549–552. [PubMed] [Google Scholar]
- 36.Camma C, et al. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J. Hepatol. 2001;34:593–602. doi: 10.1016/s0168-8278(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 37.Habu D, et al. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358–360. doi: 10.1001/jama.292.3.358. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen MH, et al. Screening for hepatocellular carcinoma. J. Clin. Gastroenterol. 2002;35(5 suppl. 2):S86–S91. doi: 10.1097/00004836-200211002-00004. [DOI] [PubMed] [Google Scholar]
- 39.Alter MJ, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA. 1990;264:2231–2235. [PubMed] [Google Scholar]
- 40.WHO. Hepatitis C. 2000. http://www.who.int/mediacentre/factsheets/fs164/en/ (8 July 2009, date last accessed) [Google Scholar]
- 41.CDC. Viral hepatitis C. 2004. http://www.cdc.gov/ncidod/diseases/he[patitis/c/plan/HCV_infection.htm (24 August 2004, date last accessed) [Google Scholar]
- 42.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J. Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 43.Flamm SL. Chronic hepatitis C virus infection. (Reprinted) JAMA. 2003;289:2413–2417. doi: 10.1001/jama.289.18.2413. [DOI] [PubMed] [Google Scholar]
- 44.CDC. National Hepatitis C Prevention Strategy. 2004. http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/HCV_infection.htm (24 August 2004, date last accessed) [Google Scholar]
- 45.El-Serag H, et al. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 46.Sung M, et al. Hepatitis Viruses. In: Holland J, Frei EI, editors. Cancer Medicine. Hamilton, London: BC Decker Inc.; 2003. pp. 349–358. [Google Scholar]
- 47.Plummer M, et al. Epidemiology of gastric cancer. IARC Sci. Publ. 2004;157:311–326. [PubMed] [Google Scholar]
- 48.Everhart JE, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J. Infect. Dis. 2000;181:1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 49.Pisani P, et al. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol. Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 50.Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J. Infect. Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 51.Newton R, et al. Subgroup report: stomach cancer. IARC Sci. Publ. 2004;157:29–32. [PubMed] [Google Scholar]
- 52.Murakami R, et al. Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer. 1990;65:1255–1260. doi: 10.1002/1097-0142(19900301)65:5<1255::aid-cncr2820650536>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 53.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database:Incidence–SEER 9 Regs Public Use, Nov 2002 Sub (1973-2000) < 18 Age Groups >, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2003, based on the November 2002 submission http://www.seer.cancer.gov. (8 July 2009, date last accessed) [Google Scholar]
- 54.Parkin DM, Ferlay J, Hamdi-Cherif M, Sitas F, Thomas J, Wabinga H, Whelan SL, editors. Cancer in Africa: Epidemiology and Prevention. IARC Scientific Publications No. 153. Lyon: International Agency for Research on Cancer; 2003. Kaposi Sarcoma; pp. 286–294. [PubMed] [Google Scholar]
- 55.Scadden D. Neoplasms in Acquired Immunodeficiency Syndrome. In: Holland JF, Frei E, editors. Cancer Medicine. Hamilton, London: BC Decker Inc; 2003. pp. 2259–2275. [Google Scholar]
- 56.Eltom MA, et al. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J. Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 57.CDC. Management of possible sexual, injecting-drug-use, or other non-occupational exposure to HIV, including considerations related to antiretroviral therapy. Public Health Service statement. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 1998;47:1–14. [PubMed] [Google Scholar]
- 58.Kaplan JE, et al. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm. Rep. 2002;51:1–52. [PubMed] [Google Scholar]
- 59.Babus V, et al. Helicobacter pylori and gastric cancer in Croatia. Cancer Lett. 1998;125:9–15. doi: 10.1016/s0304-3835(97)00446-1. [DOI] [PubMed] [Google Scholar]
- 60.Little RF. AIDS-related non-Hodgkin's lymphoma: etiology, epidemiology, and impact of highly active antiretroviral therapy. Leuk. Lymphoma. 2003;44(suppl. 3):S63–S8. doi: 10.1080/10428190310001623748. [DOI] [PubMed] [Google Scholar]