Abstract
We sought to determine the effects of activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) on multilocularization of adipocytes in adult white adipose tissue (WAT). Male C57BL/6 normal, db/db, and ob/ob mice were treated with agonists of PPAR-γ, PPAR-α, or β3-adrenoceptor for 3 weeks. To distinguish multilocular adipocytes from unilocular adipocytes, whole-mounted adipose tissues were co-immunostained for perilipin and collagen IV. PPAR-γ activation with rosiglitazone or pioglitazone induced a profound change of unilocular adipocytes into smaller, multilocular adipocytes in adult WAT in a time-dependent, dose-dependent, and reversible manner. PPAR-α activation with fenofibrate did not affect the number of locules or remodeling. db/db and ob/ob obese mice exhibited less multilocularization in response to PPAR-γ activation compared to normal mice. Nevertheless, all adipocytes activated by PPAR-γ contained a single nucleus regardless of locule number. Multilocular adipocytes induced by PPAR-γ activation contained substantially increased mitochondrial content and enhanced expression of uncoupling protein-1, PPAR-γ coactivator-1-α , and perilipin. Taken together, PPAR-γ activation induces profound multilocularization and enhanced mitochondrial biogenesis in the adipocytes of adult WAT. These changes may affect the overall function of WAT.
Keywords: mitochondria; mitochondrial uncoupling protein; pioglitazone; receptors, adrenergic, β-3; rosiglitazone
Introduction
PPARγ agonists are commonly used as insulin sensitizers for treating patients with type II diabetes (Fonseca, 2003; Hammarstedt et al., 2005). Many in vitro studies (Rosen et al., 1999; 2000; Yamauchi et al., 2001; Albrektsen et al., 2002; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007) indicate that PPARγ activation with PPARγ agonists stimulates adipocyte differentiation through the activation of a battery of adipogenic genes. In fact, PPARγ is highly expressed in adipose tissue, where it plays a central role in adipose tissue function (Rosen et al., 1999; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007). PPARγ is essentially required for the formation, differentiation, and survival of both white adipose tissue (WAT) and brown adipose tissue (BAT) in vivo (Rosen et al., 1999; Imai et al., 2004; Zhang et al., 2004). Activation of PPARγ makes adipocytes in adult WAT more insulin-sensitive and better able to actively take up and efficiently retain lipid, possibly by enhancing the expression of genes that favor lipid uptake and retention, while reducing the expression of genes that lower adipocyte lipid content (Yamauchi et al., 2001; Albrektsen et al., 2002). However, activation of PPARγ causes structural remodeling of adipocytes in adult WAT. This remodeling is characterized by increased number of smaller adipocytes, reduced size of lipid droplet and enlarged rounded nuclei in adipocytes, and more matrix tissue surrounding the adipocytes, which have been visualized mainly by sectioned adipose tissues and conventional staining methods (Okuno et al., 1998; Toseland et al., 2001; Yamauchi et al., 2001). The origin of these smaller adipocytes is postulated to be the amplified differentiation of residential adipoblasts/preadipocytes or the active division of adipocytes themselves (Okuno et al., 1998; Yamauchi et al., 2001).
Adult WAT is mainly composed of mature unilocular adipocytes, which are characterized by a single, large perilipin-coated lipid droplet that fills the majority of the cytoplasm. BAT is mainly composed of mature multilocular adipocytes, which are characterized by multiple small but variably-shaped lipid droplets surrounded by perilipin (Rosen et al., 2000; Cinti, 2001, 2005; Prunet-Marcassus et al., 2006; Rosen and MacDougald, 2006). Obviously, the sizes, shapes, numbers, and even roles of these adipocytes vary depending on their localization and the obesity status of the animal (Cinti, 2001; Prunet-Marcassus et al., 2006). In obesity, it is common for adipocytes to increase in size, but most WAT are composed of large unilocular adipocytes. However, adipocytes in the adult WAT can become multilocular in special situations such as β3-adrenergic stimulation (Himms-Hagen et al., 2000; Granneman et al., 2005), exposure to cold temperature (Loncar et al., 1988), or deletion of translational inhibitor 4E-BP1 (Tsukiyama-Kohara et al., 2001). During our recent study (Koh et al., 2007), we intriguingly found that the most well-known PPARγ agonist, rosiglitazone, a thiazolidinedione derivative, induced a marked shift of unilocular adipocytes to multilocular adipocytes (multilocularization) in most WAT of adult mice. Previous studies (Wilson-Fritch et al., 2003; Bogacka et al., 2005) indicated that PPARγ agonist induced mitochondrial biogenesis and remodeling in vitro and in vivo. However, the underlying mechanism by which rosiglitazone causes the adipocytes to become multilocular and the significance of this remodeling in the regulation of lipid storage and mitochondrial biogenesis are poorly understood.
In this study, we sought to define the effect of PPARγ activation on the multilocularization of adipocytes in WAT of adult mice. To distinguish multilocular adipocytes from unilocular adipocytes in the multicellular adipose tissues, whole-mounted adipose tissues were co-immunostained for perilipin (a membrane protein that surrounds lipid droplets and is selectively expressed in adipocytes and steroidogenic cells) (Londos et al., 2005; Koh et al., 2007) and collagen IV (the matrix protein that surrounds each adipocyte individually as a basement membrane) (Londos et al., 2005). In addition, we examined whether PPARγ activation-induced multilocular adipocytes had multiple nuclei. We also examined the relationship between PPARγ activation-induced multilocularization and mitochondrial content and the expression of UCP-1, PGC-1α and perilipin. Finally, we compared the similarity and dissimilarity between the remodeling induced by PPARγ activation versus β3-adrenoceptor stimulation. Our findings revealed that activation of PPARγ with rosiglitazone and pioglitazone induced profound multilocularization of adipocytes in adult WAT in a dose-dependent, time-dependent, and reversible manner, with increased mitochondrial content and higher expression of UCP-1, PGC-1α and perilipin.
Results
PPARγ activation with rosiglitazone induces profound multilocularization in adipocytes of adult WAT
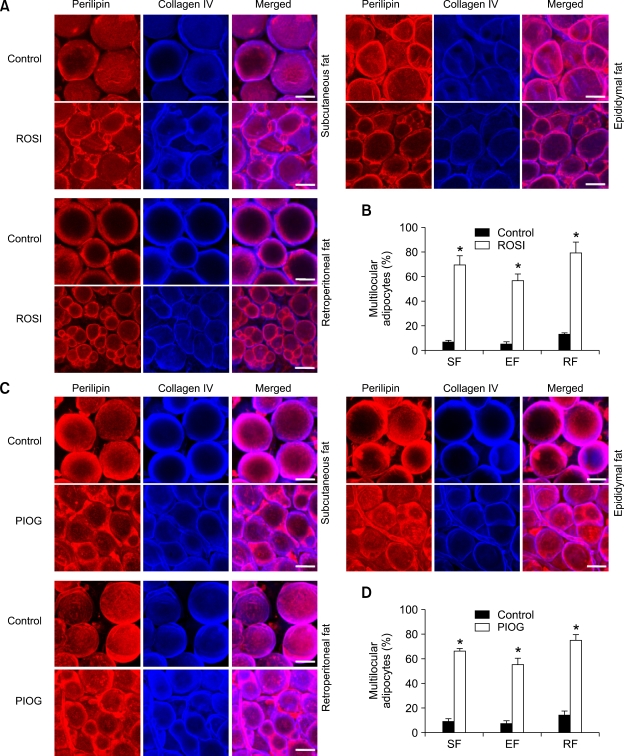
We defined any adipocyte having more than a single lipid droplet (≥ 2 locules) as a "multilocular adipocyte" and any adipocyte having only a single lipid droplet as a "unilocular adipocyte". To distinguish multilocular from unilocular adipocytes in adult WAT, whole-mounted WAT was co- immunostained for perilipin (Robenek et al., 2005; Koh et al., 2007) and collagen IV (Martinez-Hernandez and Amenta, 1983). This whole-mount co-immunostaining enabled us to clearly view the locules and to monitor the remodeling of individual adipocytes. In normal adult mice fed with normal diet (Control), most of the adipocytes in adult WAT were round-shaped unilocular adipocytes and a few are multilocular adipocytes in subcutaneous (6.7%, n = 3,000 cells), epididymal (4.9%, n = 3,000 cells), and retroperitoneal (12.7%, n = 3,000 cells) adipose tissues (Figure 1). Rosiglitazone treatment (~15 mg/kg/day) gradually increased the number of multilocular adipocytes in the subcutaneous adipose tissues (23.5%, n = 3,000 cells at 1 week, P < 0.01 and 44.8%, n = 3,000 cells at 2 weeks, P <0.01) versus at 0 week (6.1%, n =3, 000 cells). At 3 weeks after the start of the rosiglitazone treatment, percentages of multilocular adipocytes in total adipocytes were markedly increased in subcutaneous (69.5%, n = 4,000 cells, P < 0.01), epididymal (56.8%, n = 4,000 cells, P < 0.01), retroperitoneal (79.2%, n =4,000 cells, P < 0.01), and mesenteric (74.6%, n = 3,000 cells, P < 0.01) adipose tissues compared to the Control (Figures 1A and 1B, Supplementary data Figure S1). Moreover, the changes caused by rosiglitazone treatment in the percentages of multilocular adipocytes in subcutaneous, epididymal, retroperitoneal, and mesenteric adipose tissues were almost identical between male and female mice (Supplementary data Figure S1). Lower doses (~3.25 and ~7.5 mg/kg/day) of rosiglitazone induced proportionally smaller increases in the percentage of multilocular adipocytes in the subcutaneous adipose tissue (27.2%, n = 4,000 cells, P < 0.01 and 43.1%, n = 4, 000 cells, P < 0.01) versus the Control. Thus, rosiglitazone induced substantial multilocularization in adipocytes of the adult WAT in time- and dose-dependent manners regardless of sex.
Figure 1.
Rosiglitazone and pioglitazone induce profound multilocularization in the adipocytes of adult WAT of mice. The indicated adipose tissues were harvested from C57BL/6J mice that had treated with nothing (Control), rosiglitazone (~15 mg/kg/day) (A, B) or pioglitazone (~15 mg/kg/day) (C, D) for 3 weeks. (A, C) Tissues were whole-mounted, co-immunostained for perilipin (for lipid droplets) and collagen IV (the basement membrane that surrounds each adipocyte), and merged. Note that adipocytes with variable and smaller-sized perilipin+ lipid droplets are abundantly detected in rosiglitazone- or pioglitazone-treated adipose tissues. Scale bars, 20 µm. (B, D) Multilocular adipocytes were counted in 10 regions (~100 adipocytes/region) per adipose tissue in each mouse of Control (n = 3), rosiglitazone (B, n = 4) or pioglitazone (D, n = 4), and presented as a percentage of the total adipocytes counted. Bars represent the mean ± SD. *, P < 0.01 versus control. ROSI, rosiglitazone; PIOG, pioglitazone.
PPARγ activation with pioglitazone also induces profound multilocularization in adipocytes of adult WAT
To further support the mechanism of adipocyte multilocularization by PPARγ activation, we treated the mice with another thiazolidinedione-derived selective PPARγ agonist, pioglitazone (~15 mg/kg/day for 3 weeks). Similar to the rosiglitazone treatment, at 3 weeks after the start of pioglitazone treatment, percentages of multilocular adipocytes in total adipocytes were markedly increased in subcutaneous (65.6%, n = 4,000 cells, P < 0.01), epididymal (54.6%, n = 4,000 cells, P < 0.01), and retroperitoneal (74.7%, n = 4,000 cells, P <0.01) adipose tissues compared to the Control (Figures 1C and 1D). In comparison, activation of PPARα by treatment with fenofibrate (a selective PPARα agonist, ~150 mg/kg/ day for 3 weeks) did not significantly change percentages of multilocular adipocytes in total adipocytes in subcutaneous (Control versus fenofibrate; 7.4%, n = 3,000 cells versus 8.2%, n = 4,000 cells, P > 0.05), epididymal (Control versus fenofibrate; 5.8%, n=3,000 cells versus 6.1%, n = 4,000 cells, P > 0.05), and retroperitoneal (Control versus fenofibrate; 11.4%, n = 3,000 cells versus 10,9%, n = 4,000 cells, P > 0.05) adipose tissues (Supplementary data Figure S2). These data indicate that the rosiglitazone- and pioglitazone-induced adipocyte multilocularization result from the activation of PPARγ, but not from the activation of PPARα.
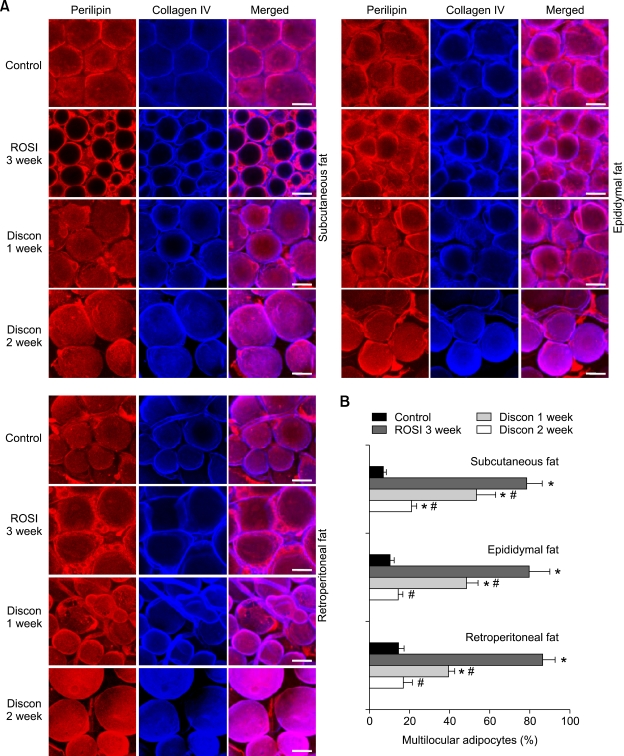
PPARγ activation with rosiglitazone induces reversible multilocularization and remodeling in adipocytes of adult WAT
To determine whether the observed multilocularization in adipocytes of adult WAT is reversible, we observed the number of multilocular adipocytes of WAT in mice that had discontinued treatment for 1 week or 2 weeks after rosiglitazone treatment (~15 mg/ kg/day for 3 weeks). At both time points after the discontinuation of rosiglitazone treatment (Discon-R), most multilocular adipocytes had changed back to unilocular adipocytes in subcutaneous (rosiglitazone, 3 week versus Discon-R, 1 week and 2 week; 78.3%, n = 4,000 cells versus 53.5%, n = 4,000 cells, P < 0.05 and 21.0%, n = 4,000, P <0.05), epididymal (rosiglitazone, 3 week versus Discon-R, 1 week and 2 week; 79.6%, n = 4,000 cells versus 48.4%, n = 4,000 cells, P < 0.05 and 14.6%, n = 4,000, P <0.05), and retroperitoneal (rosiglitazone, 3 week versus Discon-R, 1 week and 2 week; 86.3%, n = 4,000 cells versus 39.4%, n = 4,000 cells, P < 0.05 and 17.1%, n = 4,000, P < 0.05) adipose tissues (Figure 2). Thus, the rosiglitazone- induced multilocularization of adipocytes is reversible.
Figure 2.
Rosiglitazone-induced multilocularization is reversible. The indicated adipose tissues were harvested from C57BL/6J mice that had been treated with nothing (Control), rosiglitazone (~15 mg/kg/day) for 3 weeks, or that had discontinued the rosiglitazone treatment for 1 week (Discon 1 week) or 2 weeks (Discon 2 week) after the rosiglitazone treatment for 3 weeks. (A) Tissues were whole-mounted, co-immunostained for perilipin and collagen IV, and merged. Note that rosiglitazone-induced multilocular adipocytes have reversibly recovered to unilocular adipocytes upon discontinuation of treatment. Scale bars, 20 µm. (B) Multilocular adipocytes were counted in 10 regions (~100 adipocytes/region) per adipose tissue in each mouse of Control (n = 3), rosiglitazone (n = 4), Discon 1 week (n = 4) or Discon 2 weeks (n = 4), and presented as a percentage of the total adipocytes counted. Bars represent the mean ± SD. *P < 0.01 versus control. #P < 0.05 versus rosiglitazone, 3 week.
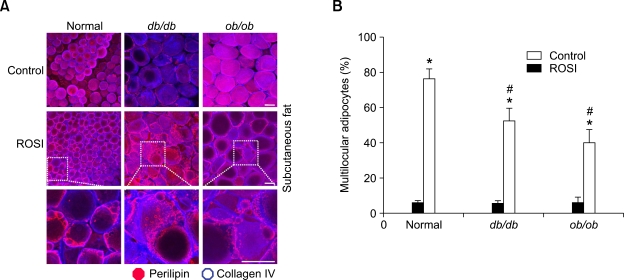
Obese mice exhibit less rosiglitazone-induced multilocularization and remodeling.
To compare responsiveness of rosiglitazone-induced changes between normal mice and age-matched male db/db and ob/ob obese mice, the mice were treated with rosiglitazone (~15 mg/kg/day) for 3 weeks. The mean diameters of adipocytes in db/db and ob/ob obese mice were ~3.0-4.0-fold larger than the mean diameter of adipocytes in normal mice (Figure 3A). Percentages of multilocular adipocytes to total adipocytes in the subcutaneous adipose tissues were increased by the rosiglitazone treatment in normal mice (from 5.7%, n = 3,000 cells to 76.1%, n = 4,000 cells, P < 0.01), db/db mice (from 5.1%, n = 3,000 cells to 52.8%, n = 4,000 cells, P < 0.01) and ob/ob mice (from 5.4%, n = 3,000 cells to 39.5%, n = 4,000 cells, P <0.01) (Figure 3). Thus, rosiglitazone-induced adipocyte remodeling was less in db/db- (10.4-fold, P < 0.05) and ob/ob (6.9-fold, P < 0.05) obese mice than in normal mice (13.4-fold) (Figure 3). Thus, db/db- and ob/ob obese mice exhibit less rosiglitazone-induced multilocularization compared to normal mice.
Figure 3.
Rosiglitazone induces less multilocularization in the adipocytes of adult subcutaneous adipose tissues of db/db and ob/ob obese mice than of normal mice. The indicated adipose tissues were harvested from C57BL/6J mice, db/db mice, and ob/ob mice that had treated with nothing (Control) or rosiglitazone (~15 mg/kg/day) for 3 weeks. (A) Tissues were whole-mounted, co-immunostained for perilipin (dark pink) and collagen IV (blue), and merged. Note that fewer small-sized adipocytes are detected in the subcutaneous adipose tissues of db/db mice and ob/ob mice compared those of normal mice. A higher magnification of the merged images (white dotted rectangles) reveals characteristic rosiglitazone-induced multilocular adipocytes. Scale bars, 50 µm. (B) Multilocular adipocytes were counted in 10 regions (~100 adipocytes/region) per subcutaneous adipose tissue in each mouse of Control (n = 3) or rosiglitazoneI (n = 4), and presented as a percentage of the total adipocytes counted. Bars represent the mean ± SD. *P < 0.01 versus control. #P < 0.05 versus Normal.
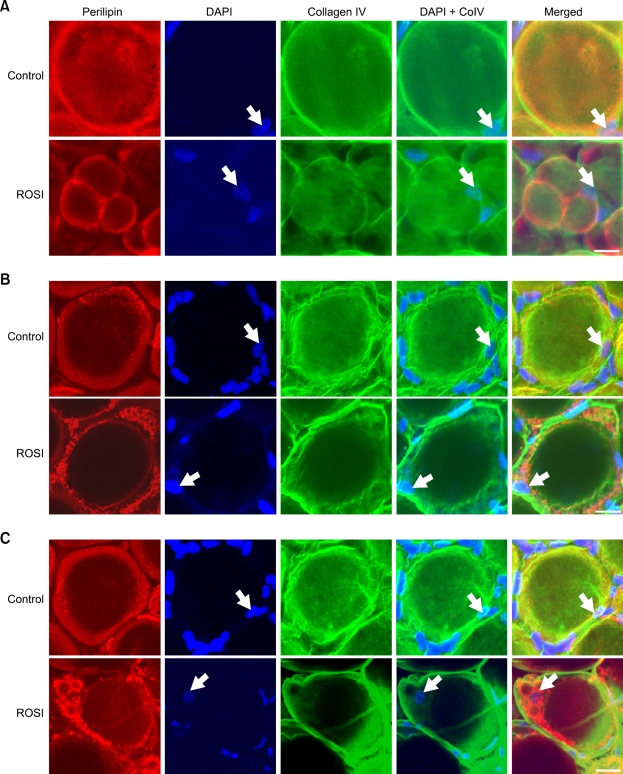
Rosiglitazone does not induce karyokinesis in the adipocytes of normal and obese adipose tissues.
Because PPARγ activation with thiazolidinedione derivatives is known to increase the population of small-sized adipocytes from adipoblasts/preadipocytes (Rosen et al., 2000; Zhang et al., 2004), and our HE staining revealed that rosiglitazone treatment induced reduced sizes but increased number of adipocytes, particularly in subcutaneous adipose tissue (Supplementary data Figure S3), we questioned whether rosiglitazone- induced multilocularization was indeed the source of the small multilocular adipocytes in the subcutaneous adipose tissue. To address this question, we extensively examined any nuclear doubling (karyokinesis) before cell division (cytokinesis) by visualization and quantification of the number of nuclei in multilocular and unilocular adipocytes in subcutaneous (5,000 multilocular and 1,000 unilocular adipocytes), epididymal (4,000 multilocular and 800 unilocular), and retroperitoneal (5,000 multilocular and 1,000 unilocular adipocytes) adipose tissues of normal mice treated with rosiglitazone (~15 mg/kg/day for 3 weeks) (n = 3) (Figure 4). If karyokinesis was induced by the PPARγ stimulation, two nuclei would be detected in single unilocular or multilocular adipocytes. However, all adipocytes contained a single nucleus regardless of whether they were multilocular or unilocular (Figure 4A). We also extensively examined the number of nuclei in each multilocular or unilocular adipocyte in subcutaneous (5,000 multilocular and 2,000 unilocular adipocytes), epididymal (4,000 multilocular and 2,000 unilocular adipocytes), and retroperitoneal (5,000 multilocular and 2,000 unilocular adipocytes) adipose tissues of db/db (n = 3) and ob/ob mice (n = 3) treated with rosiglitazone. However, all adipocytes contained a single nucleus regardless of their number of locules or their genetic background (Figures 4B and 4C). These results indicate that PPARγ stimulation may not induce karyokinesis and subsequent cytokinesis, even in adipocytes that have been profoundly changed by multilocularization.
Figure 4.
Rosiglitazone-induced multilocular adipocytes have a single nucleus in adult subcutaneous adipose tissues of normal, db/db, and ob/ob obese mice. The indicated adipose tissues were harvested from C57BL/6J mice (A), db/db mice (B), and ob/ob mice (C) that had been treated with nothing (Control) or rosiglitazone (~15 mg/kg/day) for 3 weeks. Tissues were whole-mounted, co-immunostained for perilipin and collagen IV, stained for DAPI, and merged. Note that all unilocular and multilocular adipocytes contain only a single nucleus (white arrows) in each adipocyte of all 3 strains of mice. Scale bars, 20 µm.
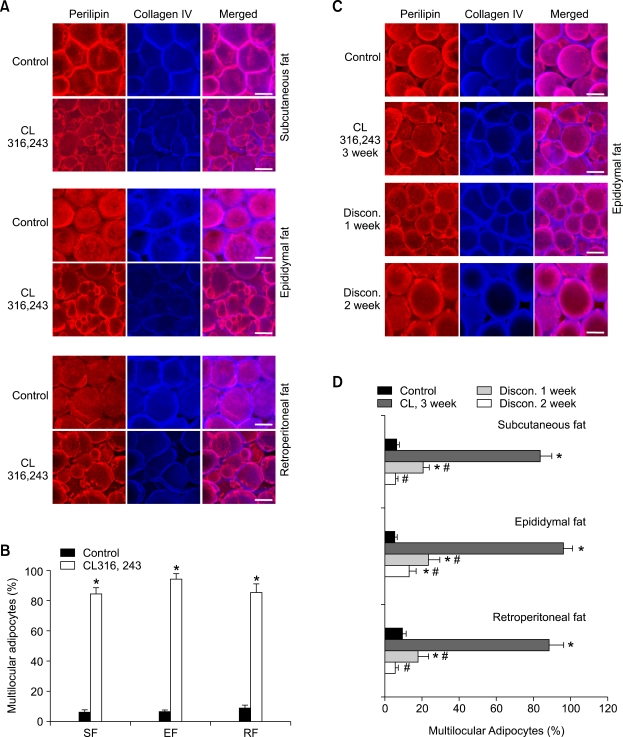
Activation of β3-adrenoceptor with CL316,243 also induces multilocularization and remodeling.
Activation of β3-adrenoceptor is known to induce profound multilocularization and remodeling, and to increase mitochondrial content in the adipocytes of adult adipose tissues (Himms-Hagen et al., 2000; Granneman et al., 2005). To find the degree of similarity or dissimilarity between β3-adrenoceptor activation versus PPARγ activation on these processes, we treated mice with CL316,243 (CL, 0.1 mg/kg/day for 3 weeks), and compared the changes with mice treated with the rosiglitazone. Consistent with previous reports (Himms-Hagen et al., 1994; Miyazaki et al., 2001; Carmona et al., 2005), body weights and weights of WAT decreased during the CL treatment, whereas these weights increased during the rosiglitazone treatment compared to the control treatment (data not shown). However, similar to the rosiglitazone treatment, at 3 weeks after start of the CL treatment, percentages of multilocular adipocytes to total adipocytes were markedly increased in subcutaneous (84.3%, n = 4,000 cells, P < 0.01), epididymal (94.1%, n = 4,000 cells, P < 0.01), and retroperitoneal (85.2%, n = 4,000 cells, P < 0.01) adipose tissues versus the control buffer treatment (Figures 5A and 5B). Moreover, similar to the rosiglitazone treatment, at 1 week and 2 week after the discontinuation of CL treatment (Discon-C), most multilocular adipocytes had recovered to unilocular adipocytes with round, smooth shape in subcutaneous (CL, 3 week versus Discon-C, 1 week and 2 week; 83.6%, n = 4,000 cells versus 20.6%, n = 4,000 cells, P < 0.05and 5.9%, n = 4,000, P < 0.05), epididymal (CL, 3 week versus Discon-C, 1 week and 2 week; 96.1%, n = 4,000 cells versus 23.5%, n = 4,000 cells, P < 0.05 and 13.1%, n = 4,000, P < 0.05), and retroperitoneal (CL, 3 week versus Discon-C, 1 week and 2 week; 88.5%, n = 4,000 cells versus 17.9%, n = 4,000 cells, P < 0.05 and 5.5%, n = 4,000, P < 0.05) adipose tissues (Figures 5C and 5D). In addition, at 3 weeks after start of the CL treatment, mitochondrial content in the multilocular adipocytes was markedly increased in subcutaneous, epididymal, and retroperitoneal adipose tissues (Supplementary data Figure S4). Moreover, at 1 week and 2 weeks after the discontinuation of CL treatment, the increased amount of mitochondria in the adipocytes had recovered to the normal level in parallel with the reversion of multilocular to unilocular adipocytes (data not shown). Thus, activation of β3-adrenoceptor with CL induced similar patterns of adipocyte multilocularization and mitochondrial biogenesis in time-dependent and reversible manners, similar to the changes induced by the rosiglitazone, although they produced opposite effects on body weight and WAT weight.
Figure 5.
Profound and reversible multilocularization occurs by β3-adrenoceptor CL316,243 in the adipocytes of adult adipose tissues. The indicated adipose tissues were harvested from C57BL/6J mice that had treated with PBS (Control) or CL316,243 (~0.1 mg/kg/day) for 3 weeks (A, B), or that had discontinued the CL316,243 treatment for 1 week (Discon 1 week) or 2 week (Discon 2 week) after the CL316,243 treatment for 3 week (C, D). (A, C) Tissues were whole-mounted, co-immunostained for perilipin and collagen IV, and merged. Note that CL316,243-induced multilocular adipocytes reversibly recovered to unilocular adipocytes upon discontinuation of treatment. Scale bars, 20 µm. (B, D) Multilocular adipocytes were counted in 10 regions (~100 adipocytes/region) per adipose tissue in each mouse treated with had been treated with Control (n = 3), CL316,243 for 3 week (n = 4), Discon 1 week (n = 4) or Discon 2 weeks (n = 4), and presented as a percentage of the total counted adipocytes. Bars represent the mean ± SD. *P < 0.01 versus control. #P < 0.05 versus CL316,243 3 week.
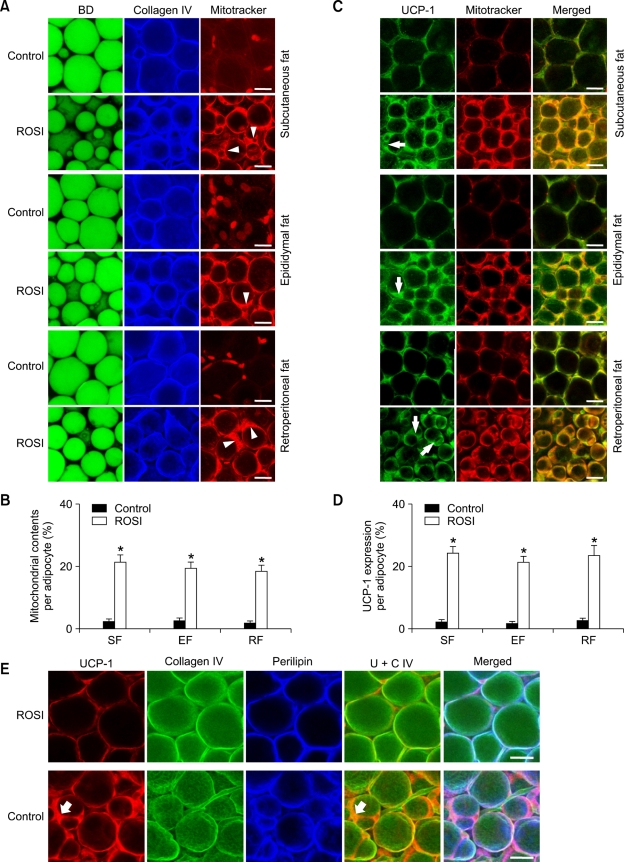
Rosiglitazone-induced multilocular adipocytes contain more mitochondria and higher expression of uncoupled protein-1 (UCP-1).
To determine any relationship between rosiglitazone-induced multilocularization and mitochondrial biogenesis, whole-mounted adipose tissues were co-stained with BD493/503 (fluorescent dye for selective binding to accumulated neutral lipid) and/or Mitotracker (fluorescent dye for selective binding to intracellular mitochondria), and immunostained for collagen IV or UCP-1. These double and triple co-stainings enabled us to visualize mitochondrial content (Figure 6, Supplementary data Figure S5) and UCP-1 expression (Figure 6) in the collagen IV-covered unilocular or multilocular adipocytes. rosiglitazone treatment (~15 mg/kg/ day) gradually increased mitochondrial content and UCP-1 expression in the mitochondria of multilocular adipocytes over time (data not shown). Compared to the Control, the mitochondrial content in the adipocytes was significantly higher in subcutaneous (9.7 fold, n = 4,500 cells, P < 0.01), epididymal (7.9 fold, n = 4,500 cells, P < 0.01), and retroperitoneal (10.6 fold, n =4,500 cells, P < 0.01) adipose tissues of the mice treated with rosiglitazone for 3 weeks (Figures 6A and 6B, Supplementary data Figure S5). UCP-1 protein was expressed only in the mitochondria of the adipocytes (Figure 6C). Accordingly, the amount of UCP-1 protein expression was markedly higher in subcutaneous (11.6 fold, n = 4,500 cells, P < 0.01), epididymal (13.4 fold, n = 4,500 cells, P < 0.01), and retroperitoneal (~9.1 fold, n = 4,500 cells, P < 0.01) adipose tissues of mice receiving rosiglitazone treatment compared to the Control (Figures 6C and 6D). Similarly, rosiglitazone treatment increased the amount of UCP-1 mRNA expression in subcutaneous adipose tissues (3.9 fold, n = 4, P < 0.05) compared to the Control (n =4) (Supplementary data Figure S6). Detailed images indicated that UCP-1 was more highly expressed in the multilocular adipocytes of the mice treated with rosiglitazone (Figure 6E). These data suggest that rosiglitazone may increase thermogenesis in the adipocytes of adult WAT by simultaneously increasing mitochondrial content, UCP-1 expression and multilocularization.
Figure 6.
Rosiglitazone concomitantly increases mitochondrial contents and UCP-1 expression in the multilocular adipocytes of the adult adipose tissues. The indicated adipose tissues were harvested from C57BL/6J mice that had been treated with nothing (Control) or rosiglitazone (~15 mg/kg/day) for 3 weeks. (A, C, E) Tissues were whole-mounted, co-immunostained for collagen IV and Mitotracker, and stained with BODIPY 493/503 (BD, for lipid droplets) (A); co-immunostained for UCP-1 and Mitotracker, and merged (B); or co-immunostained for UCP-1, collagen IV, and perilipin, and merged (E). White arrowheads in A and white arrows in C point to rosiglitazone-induced multilocular adipocytes that contain substantially increased mitochondrial content and UCP-1 expression. Scale bars, 20 µm. (B, D) Positive signals for Mitotracker or UCP-1 for a given cell number (~1,500; ~300/each region) at 5 regions per adipose tissue in each mouse of Control (n = 3) or rosiglitazone (n = 3) were quantified and presented as %, with the total cells as 100%. Bars represent the mean ± SD. *P < 0.01 versus control.
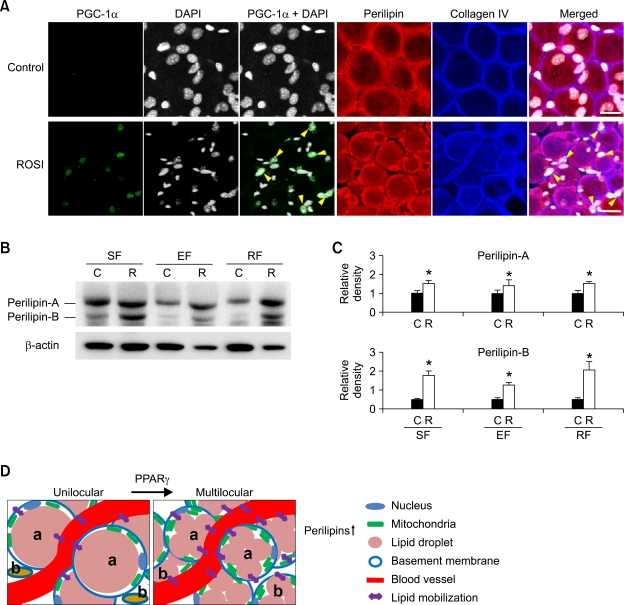
Rosiglitazone-induced multilocularization of adipocytes correlates with increased expressions of PPARγ coactivator-1α (PGC-1α), perilipin, and molecules that are involved in lipid metabolism.
Activation of PPARγ induces expression of various genes and activation of various kinases; these gene products are related to adipocyte differentiation, lipid metabolism, and insulin sensitivity (Rosen et al., 1999, 2000; Yamauchi et al., 2001; Albrektsen et al., 2002; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007). Among them, we chose PGC-1α and perilipin to test their relationship in PPARγ activation-induced multilocularization, mitochondrial biogenesis and UCP-1 expression. Firstly, rosiglitazone treatment increased PCG-1α expression in the nuclei of multilocular adipocytes to a detectable range, whereas the control adipocytes did not show any PCG-1α expression (Figure 7A). Similarly, the amount of PGC-1α mRNA expression was markedly increased in subcutaneous adipose tissues by the rosiglitazone treatment (2.3 fold, n = 4, P < 0.05) compared to the Control (n = 4) (Supplementary data Figure S6). Secondly, an increased amount of perilipin is required to surround each lipid droplet in multilocular adipocytes upon PPARγ activation. In fact, rosiglitazone treatment increased the protein levels of perilipin-A and -B in subcutaneous (1.5-fold, n = 3, P < 0.05 and 3.6-fold, n = 3, P < 0.05), epididymal (1.4-fold, n = 3, P < 0.05 and 2.5-fold, n = 3, P < 0.05), and retroperitoneal (1.5-fold, n = 3, P < 0.05 and 4.1-fold, n = 3, P < 0.05) adipose tissues of the mice versus the Control (Figures 7B and 7C). These data indicate that rosiglitazone-induced multilocularization of adipocytes is related to the expressions of perilipin and PGC-1α in the adult WAT (Figure 7D).
Figure 7.
Rosiglitazone-induced multilocularization of adipocytes is related to the expression of PCG-1α and perilipin. The indicated adipose tissues (SF, subcutaneous fat; EF, epididymal fat; RF, retroperitoneal fat) were harvested from C57BL/6J mice that had treated with nothing (Control or C) or rosiglitazone (~15 mg/kg/day; R) for 3 weeks. (A) Subcutaneous adipose tissues were whole-mounted, co-immunostained for PGC-1α (green), DAPI (white), perilipin (red), and collagen IV (blue), and merged. Note that rosiglitazone markedly induces PGC-1α expression in the nuclei of multilocular adipocytes (yellow arrowheads). Results were similar from 3 independent experiments. (B) Tissue lysates from the isolated adipocytes were separated by SDS-PAGE and immunoblotted with anti-perilipin antibody (upper panel). Each protein lysate was also immunoblotted with anti-β-actin antibody to quantify the amount of total protein loaded (lower panel). (C) The relative ratio measured for each control is arbitrarily presented as 1. Bars represent the mean ± SD from 3 experiments. *P < 0.05 versus each C. Scale bars, 20 µm. (D) Schematic diagram of a proposed model for the changes in adipocytes of adult WAT induced by PPARγ activation. The processes depicted are: multilocularization with increased expression of perilipins, an increased number of smaller adipocytes, which are the result of the reduced size of differentiated adipocytes (a) and adipogenesis of resident preadipocytes (b), and increased mitochondrial content with increased expression of perilipin. These changes provide favorable conditions for the mobilization of lipids from adipocytes into circulation and vice versa with enhanced lipolytic and lipogenic activities.
Discussion
Thiazolidinedione derivatives such as rosiglitazone and pioglitazone have become well-established in the treatment of type 2 diabetes (Fonseca, 2003; Hammarstedt et al., 2005; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007). The mechanism of action of rosiglitazone and pioglitazone is centered on their ability to activate PPARγ, which is abundantly expressed in adipose tissues (Rosen et al., 1999; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007). Our findings clearly show that PPARγ activation with rosiglitazone or pioglitazone treatment induces profound multilocularization of adipocytes in adult WAT. Co-immunostaining for perilipin and collagen IV in the whole-mounted adipose tissues is critical and of paramount importance for assessing the abundance of multilocular and unilocular adipocytes. Because perilipin is found exclusively on the outer surface of lipid storage droplets in every adipocyte (Koh et al., 2007), the visualization of perilipin by specific immunostaining clearly distinguishes adipocytes from non-adipocytes in multicellular adipose tissues. Moreover, because collagen IV surrounds each individual adipocyte as a basement membrane (Martinez-Hernandez and Amenta, 1983), the visualization of collagen IV and perilipin by co-immunostaining clearly distinguishes multilocular adipocytes from unilocular adipocytes. Using these reliable and convincing visualization methods, we found that PPARγ activation induces profound multilocularization in adipocytes of adult adipose tissues in time- and dose-dependent and reversible manners, whereas PPARα activation did not have these effects.
PPARγ is a ligand-activated nuclear receptor and is highly expressed in WAT where it regulates the expression of a number of genes involved in adipogenesis as well as catabolic lipid metabolism (Rosen et al., 1999, 2000; Yamauchi et al., 2001; Albrektsen et al., 2002; Berger and Moller, 2002; Lehrke and Lazar, 2005; Heikkinen et al., 2007; Powell et al., 2007). However, interestingly, the appearance of a large number of smaller adipocytes with reduced size of lipid droplet and enlarged rounded nuclei in the WAT is one of the key features by PPARγ activation with thiazolidinedione derivatives (Okuno et al., 1998; Yamauchi et al., 2001). However, the origin of these smaller adipocytes has not been previously known. Our findings provide convincing evidence that PPARγ activation with thiazolidinedione derivatives induces synchronous multilocularization and size reduction of adipocytes without cell division, indicating that the majority of the smaller adipocytes have originated from extensive multilocularization of already differentiated unilocular adipocytes. However, not only is the number of adipocytes in the WAT notably increased, but also the weight of WAT is increased by PPARγ activation with thiazolidinedione derivatives, suggesting that certain populations of smaller adipocytes could be derived from amplified differentiation of residential adipoblasts/preadipocytes by PPARγ activation, and these cells may contribute to the increased weight of WAT. However, because specific makers for the smaller adipoctytse derived residential adipoblasts/preadipocytes are not fully identified, assessment of differentiating smaller adipocytes is not currently available. Nevertheless, PPARγ may have simultaneous dual roles in adult WAT; first to increase lipid utilization from differentiated adipocytes and second to increase adipogenesis for undifferentiated adipoblasts/preadipocytes (Figure 7D). Furthermore, the db/db- and ob/ob obese mice exhibited less rosiglitazone-induced multilocularization and remodeling compared to normal mice. It could be possible that their adipose tissues display blunted PPARγ activation by thiazolidinedione derivatives or attenuated PPARγ-induced transcriptional activation.
The present findings also show that activation of β3-adrenoceptor with CL316,243 induced similar patterns of adipocyte multilocularization in time-dependent and reversible manners compared to those changes induced by rosiglitazone, although they produced opposite effects on body weight and WAT weight. In addition, consistent with previous reports (Himms-Hagen et al., 2000; Granneman et al., 2005), we also found that activation of β3-adrenoceptor increased mitochondrial biogenesis and expression of UCP-1. Therefore, we predict that the β3-adrenoceptor-induced decrease in the weight of WAT is caused by substantial remodeling and enhanced FA oxidation and oxygen consumption in adipocytes without amplified differentiation of residential adipoblasts/preadipocytes.
Our findings further indicate that PPARγ activation with rosiglitazone induces 'morphologic and biochemical conversion of WAT to BAT' (Hansen and Kristiansen, 2006), as evidenced by: (1) multilocularization and reduced size of adipocytes, (2) substantial increases of mitochondrial content and UCP-1 expression, and (3) marked increase of PGC-1α expression (Figure 7A). PGC-1α is a thermogenic nuclear hormone receptor coactivator, and its principal in vivo roles are to promote cold-induced thermogenesis, mitochondrial biogenesis, hepatic gluconeogenesis, and fatty acid β-oxidation (Rosen, et al., 2000). During mitochondrial biogenesis, the role of PGC-1α is cooperative with PPARγ and other coactivators to induce mitochondrial genes including UCP-1 (Puigserver et al., 1998; Wu et al., 1999; Lin et al., 2005; Hansen and Kristiansen, 2006). Consistent with previous results (Wilson-Fritch et al., 2004; Bogacka et al., 2005; Guan et al., 2005), our findings also revealed that PPARγ activation with rosiglitazone increases mitochondrial biogenesis and PGC-1α level. Thus, the rosiglitazone-induced increased expression of PGC-1α may contribute to enhanced UCP-1 expression and mitochondrial biogenesis. In fact, overexpression of PGC-1α in WAT induced mitochondrial biogenesis and expression of UCP-1, and subsequently those changes stimulate heat dissipation from the WAT (Puigserver et al., 1998; Wu et al., 1999). Moreover, PPARγ stimulation with rosiglitazone strikingly induces mitochondrial biogenesis and results in mitochondrial remodeling to a cristae-rich morphology (Toseland et al., 2001; Wilson-Fritch et al., 2004; Choo et al., 2006). UCP-1 is a facultative proton transporter localized to the mitochondrial inner membrane, where it can uncouple the oxidation of fuel substrates from the production of ATP, thus generating heat (Hansen and Kristiansen, 2006). Therefore, the PPARγ- stimulated multilocular adipocytes may have enhanced fatty acid oxidation and oxygen consumption through markedly increased mitochondrial content and mitochondrial UCP-1 protein. These data imply that rosiglitazone-induced multilocularization enhances lipid metabolism by simultaneous promotion of triglyceride lipolysis/FA oxidation and FA transport/lipogenesis through an increase in the surface area of lipid droplets per adipocyte (Figure 7D). Thus, multiple lipid droplets per adipocyte may lead to an increase in their accessibility not only to triglyceride lipolysis/FA oxidation but also to FA transport/lipogenesis in the adult WAT. Eventually, this effect of rosiglitazone can be envisioned to contribute directly and indirectly to changes in whole-body energy metabolism and insulin sensitivity.
Finally, our findings reveal that rosiglitazone-induced multilocularization of adipocytes is also related to the expression of perilipin. Perilipin is mainly located at the surface of lipid droplets and regulates lipid content in adipocytes (Londos et al., 2005; Robenek et al., 2005; Granneman and Moore, 2008). Perilipin regulates lipolysis by phosphorylation-dependent and -independent pathways by interactions with different domains of the protein (Zhang et al., 2003; Granneman and Moore, 2008) and it inhibits lipolysis by acting as a barrier against hydrolysis of triacylglycerol by lipases. However, when perilipin is phosphorylated by cAMP-dependent protein kinase, it undergoes a conformational change that allows access of hormone-sensitive lipase to the surface of the lipid droplet and enhances lipase activity (Clifford et al., 2000). Our findings indicate that the amount of perilipin per adipocyte must be proportionally increased to match the increased number of lipid droplets induced by PPARγ activation with rosiglitazone or PLOG. In fact, the western blot analyses revealed that rosiglitazone increased the expression of perilipin-A and, intriguingly, to a greater extent, perilipin-B in the adipose tissues. In fact, there are two forms of perilipin, perilipin A and perilipin B in adipocytes, and the two forms are expressed from differentially spliced mRNAs. Perilipin A is present at a much higher concentration than perilipin B (Londos et al., 2005; Robenek et al., 2005). Perilipin A affects triacylglycerol lipases present in adipocytes, whereas the action of perilipin B is mainly exerted to hormone-sensitive lipase (Zhang et al., 2003). Therefore, we postulate that PPARγ-induced multilocular adipocytes may have enhanced lipid modulating activity through increased amounts of perilipins covering the multiple lipid droplets.
Taken together, PPARγ activation induces profound multilocularization and reduces adipocyte size without karyokinesis in adipocytes of adult WAT. PPARγ activation also causes a substantial increase in mitochondrial biogenesis, and marked enhancement in the expression of UCP-1, PGC-1α, and perilipin. These PPARγ activation-induced changes may contribute to the modulation of the overall physiology of adipose tissue.
Methods
Mouse
Specific pathogen-free C57BL/6J, db/db (C57BL/6J genetic background) and ob/ob (C57BL/6J genetic background) mice were purchased from Jackson Laboratory (Jackson Labs, Bar Harbor, ME). Mice were bred in our pathogen-free animal facility, and 8-9-week-old male mice were used for this study unless otherwise indicated. Animal care and experimental procedures were performed under approval from the Animal Care Committees of KAIST. All animals were fed a standard laboratory diet (PMI LabDiet, St. Louis, MO) ad libitum with free access to water unless otherwise indicated.
Treatments
All treatments were performed in mice that were 8-9 weeks old. For treatment with PPARγ or PPARα agonists, the mice were fed normal diet food containing rosiglitazone (~15 mg/kg/day, SmithKline Beecham Pharmaceuticals, Middlesex, UK), pioglitazone (~15 mg/kg/day, Eli Lilly and Company Korea, Seoul, Korea) or fenofibrate (~150 mg/kg/day, Eli Lilly and Company Korea) ad libitum for the indicated times. For treatment of β3-adrenoceptor agonist or control buffer, the mice were treated with CL316,243 (intraperitoneal injection, 0.1 mg/kg/day, Sigma-Aldrich, MO) or PBS for 3 weeks. Body weight and food intake were recorded on an every alternate day throughout the treatments.
Histological and morphometric analysis
Mice were anesthetized by intramuscular injection of the combination of anesthetics (80 mg/kg ketamine and 12 mg/kg xylazine). In some cases, inguinal subcutaneous fat, epididymal fat, and retroperitoneal fat were sampled with careful dissection, photographed with a digital camera (Coolpix 8400, Nikon), and weighed. In most cases, the indicated adipose tissues were fixed by vascular perfusion of 1% paraformaldehyde in PBS. The harvested tissues were either stained for hematoxylin and eosin (HE), embedded in paraffin, and sectioned at 5 µm or were whole-mounted for immunostaining (Cho et al., 2007; Koh et al., 2007). Whole-mount tissues were prepared and incubated for 1 h at room temperature with blocking solution containing 5% donkey serum (Jackson Immuno-Research Laboratories Inc.) in PBST (0.3% Triton X-100 in PBS). After blocking, the whole-mounted tissues were incubated overnight at 4℃ with one or more of the following primary antibodies: (a) for lipid droplets of adipocytes, guinea pig anti-perilipin antibody (diluted 1:1000; Research Diagnostics); (b) for basement membrane of individual adipocytes, rabbit anti-collagen IV antibody (diluted 1:1000; Cosmo Bio Co., Ltd., Tokyo, Japan) or goat anti-collagen IV antibody (diluted 1:1000; Chemicon International, Temecula, CA); (c) for uncoupling protein-1 (UCP-1), rabbit anti-UCP-1 antibody (diluted 1:1000; Abcam, Cambridge, UK); or (d) for PGC-1α, rabbit anti-PGC-1α antibody (diluted 1:500; Calbiochem). After several washes in PBST, whole-mounted tissues were incubated for 1 h at room temperature with one or more secondary antibodies: (a) Cy3- or Cy5-conjugated anti-guinea pig antibody (diluted 1:500; Jackson Immuno-Research Laboratories); (b) Cy3- or Cy5-conjugated anti-rabbit antibody (diluted 1:500; Jackson ImmunoResearch Laboratories); or (c) Cy3-conjugated anti-goat antibody (diluted 1:500; Jackson ImmunoResearch Laboratories). For special staining applied after the antibody incubations, whole mounted tissues were stained for 30 min at room temperature with one or more of the following: (a) for neutral lipid in adipocytes, 4,4-difluoro-1,3,5,7,8- pentamethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY 493/ 503, 1.0 µg/ml in PBS; Invitrogen, Carlsbad, CA); (b) for active mitochondria, MitoTracker Red CMXRos (MitoTracker, 100 nM in PBS; Invitrogen); (c) for nuclei, 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1 µg/ml in PBS; Invitrogen). For control experiments, the primary antibody was omitted or substituted with preimmune serum. Signals were visualized and digital images were obtained using a Zeiss ApoTome microscope and a Zeiss LSM 510 confocal microscope equipped with argon and helium-neon lasers (Carl Zeiss). For determining the unilocular and multilocular adipocytes, double-immunostained color images for perilipin and collagen IV were captured with a Zeiss LSM 510 confocal microscope. For determination of percentage of multilocular adipocytes in total adipocytes in the indicated adipose tissue, adipocytes were counted in 10 random regions (~100 adipocytes/each region) per adipose tissue treated with indicated agents, and presented as a percentage of the total counted adipocytes. For determination of nuclei per adipocyte in the indicated adipose tissue, adipocytes were counted by the 2 investigators in 10 regions (~200 adipocytes/each region) per adipose tissue treated with indicated agents. For calculating the mitochondrial contents and UCP-1 expression, immunostained color images for MitoTracker or UCP-1 were captured with a Zeiss LSM 510 confocal microscope. Using ImageJ software (http://rsb.info.nih.gov/ij), we selected the MitoTracker or UCP-1 area as a region-of-interest from the images, and they were converted to 8-bit gray scale. Area densities of the MitoTracker or UCP-1-stained images were measured from the pixels in the region-of-interest; only pixels over a certain level (> 50 intensity value) were taken to exclude background fluorescence. The mean number from the 2 investigators was used to estimate the percentage of the multilocular adipocytes, the mitochondrial contents and UCP-1 expression. Interinvestigator variation was < 5%.
Semi-quantitative RT-PCR, quantitative real-time RT-PCR and Western blot analysis
After mice were anesthetized by intramuscular injection of the combination of anesthetics, adipocytes from the indicated adipose tissues were harvested by incubation with 0.2% type 2 collagenase (Worthington, Lakewood, NJ) for 1 h at 37℃, filtered through a 100 µm nylon filter (BD Bioscience), and centrifuged at 400 × g for 5 min. For RT-PCR, total RNA was extracted from the isolated adipocytes by using Total RNA Isolation System (Promega) according to manufacturer's instructions. Each cDNA was made with Reverse Transcription System (Promega), and sqPCR reactions were performed using Taq DNA polymerase (Bioneer Inc., Daejon, Korea) with 30 cycles used for the PCR of UCP-1 and PGC-1α and 25 cycles used for the PCR of GAPDH with appropriate primers (Supplementary data Table S1). The expression level of each indicated gene was normalized by mouse GAPDH. qrRT-PCR was performed with the SYBR Premix Ex Taq™ (Takara, Japan) using the iCycler iQ5 Real-time PCR system (Bio-Rad, Hercules, CA). qrPCR reactions were performed with the appropriate primers (Supplementary data Table S1) for 40 cycles. For Western blotting, isolated adipocytes were dissolved in lysis sample buffer, and equal amounts of the protein lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with one of following primary antibodies: (a) rabbit anti-perilipin antibody (diluted 1:1000; Sigma-Aldrich) or (b) rabbit anti-β-actin (diluted 1:1000; Sigma-Aldrich). All signals were detected and analyzed by densitometric scanning (LAS-1000, Fuji Film, Tokyo, Japan).
Statistics
Values presented are means ± standard deviation (SD). Significant differences between means were determined by analysis of variance followed by the Student-Newman-Keuls test. Statistical significance was set at P < 0.05.
Supplemental data
Supplemental Data include six figures and one table and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-41-12-01.pdf.
Acknowledgments
This work was supported by KOSEF grant funded by the MEST, Korea [R2009-0079390 (GYK)].
Abbreviations
- BAT
brown adipose tissue
- FA
fatty acid
- HE
hematoxylin and eosin
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- qrRT-PCR
quantitative real-time RT-PCR
- sqRT-PCR
semi-quantitative RT-PCR
- UCP-1
uncoupling protein-1
- PGC-1-α
PPAR-γ coactivator-1-α
- WAT
white adipose tissue
Supplementary Material
Supplemental data
References
- 1.Albrektsen T, Frederiksen KS, Holmes WE, Boel E, Taylor K, Fleckner J. Novel genes regulated by the insulin sensitizer rosiglitazone during adipocyte differentiation. Diabetes. 2002;51:1042–1051. doi: 10.2337/diabetes.51.4.1042. [DOI] [PubMed] [Google Scholar]
- 2.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 3.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 4.Carmona MC, Louche K, Nibbelink M, Prunet B, Bross A, Desbazeille M, Dacquet C, Renard P, Casteilla L, Penicaud L. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int J Obes (Lond) 2005;29:864–871. doi: 10.1038/sj.ijo.0802943. [DOI] [PubMed] [Google Scholar]
- 5.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 6.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 7.Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- 8.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem. 2000;275:5011–5015. doi: 10.1074/jbc.275.7.5011. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115(Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 12.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U. The effect of PPARgamma ligands on the adipose tissue in insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2005;73:65–75. doi: 10.1016/j.plefa.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 18.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W, Chambon P, Metzger D. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101:199–209. doi: 10.1016/0889-1605(88)90010-9. [DOI] [PubMed] [Google Scholar]
- 24.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Hernandez A, Amenta PS. The basement membrane in pathology. Lab Invest. 1983;48:656–677. [PubMed] [Google Scholar]
- 26.Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001;24:710–719. doi: 10.2337/diacare.24.4.710. [DOI] [PubMed] [Google Scholar]
- 27.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell E, Kuhn P, Xu W. Nuclear Receptor Cofactors in PPARgamma-Mediated Adipogenesis and Adipocyte Energy Metabolism. PPAR Res. 2007;2007:53843. doi: 10.1155/2007/53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prunet-Marcassus B, Cousin B, Caton D, Andre M, Penicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 31.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–1338. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 33.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 34.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 35.Toseland CD, Campbell S, Francis I, Bugelski PJ, Mehdi N. Comparison of adipose tissue changes following administration of rosiglitazone in the dog and rat. Diabetes Obes Metab. 2001;3:163–170. doi: 10.1046/j.1463-1326.2001.00117.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 37.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to theinsulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang HH, Souza SC, Muliro KV, Kraemer FB, Obin MS, Greenberg AS. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem. 2003;278:51535–51542. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA. 2004;101:10703–10708. doi: 10.1073/pnas.0403652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data