Abstract
Founder populations, characterized by a single ancestor affected by LQTS and by a large number of individuals and families all related to the ancestor and thereby carrying the same disease-causing mutation, represent the ideal human model to study the role of “modifier genes” in the long QT syndrome (LQTS). This chapter reviews some of the fundamental concepts related to founder populations and provides the necessary historic background to understand why so many can be found in South Africa. The focus then moves onto a specific LQT1 founder population, carrier of the A341V mutation, that has been extensively studied during the last 10 years and has provided a significant number of previously unforeseen information. These novel findings range from an unusually high clinical severity not explained by the electrophysiological characteristics of the mutation, to the importance of the tonic and reflex control of heart rate for risk stratification, to the identification of the first modifier genes for the clinical severity of LQTS.
Keywords: Long QT syndrome, Genetics, Sudden cardiac death, Modifier Genes, IKs current
Often, the naiveté of non-senior investigators is best illustrated by the titles of their articles. Twenty-five years ago,1 one of our reviews on the long QT syndrome (LQTS) had as under-title “Progress and questions”; the accent being definitely on the “impressive progress” even though – sensibly – some room was left for “questions”. Little did we know! What happened after the discovery of the first 3 LQTS genes in 1995 and 19962–4 was truly a mind-boggling progress, reviewed elsewhere5–7; nonetheless, a significant number of questions still remain and one of the most important concerns the role of the so-called “modifier genes”, those relatively common genetic variants that may be associated with higher or lower severity of the clinical manifestations. The present essay will deal with some aspects of our own search for modifier genes and with the importance, as a unique human model, of founder populations. We will focus on a South-African LQTS founder population identified by one of us8 and which we have jointly been studying for more than 10 years.
This population now consists of 26 South African LQTS families all segregating the same KCNQ1 mutation (A341V) caused by a founder effect. The disease allele in all of these families descends from a common ancestor who migrated to South Africa from Western Europe more than 300 years ago (see below). Even though these individuals share the same disease-causing mutation, there are significant phenotypic differences, including a wide distribution of the QT interval and the presence or absence of cardiac events. This large kindred, therefore, represents a powerful resource to study other factors, such as modifier genes or environmental variables, that modulate the severity of the clinical manifestations of the disease.
Founder populations and their implications, with a bit of local history
The origin of this LQTS founder population, object of our interest and studies, is tightly associated with the history of South Africa. The Afrikaner population is predominantly of Dutch, German, and French descent with a sprinkling from other sources9,10. It harbours a variety of single gene diseases, often rare elsewhere, but relatively common in South Africa. Examples other than the KCNQ1-A341V LQTS-causing mutation include progressive familial heart block types I&II (PFHBI11 and PFHBII12), hypertrophic cardiomyopathy13, familial hypercholesterolemia14, arrhythmogenic right ventricular cardiomyopathy15, and numerous others non-cardiac diseases10. All of these are the result of founder effects.
Basically, the current Afrikaner population arose from a small gene pool. In 1730, the nascent Afrikaner population in the Cape was 2,540 and increased, almost exclusively by natural means, by a factor of almost 80 times over about 150 years to about 220,000 in 1878. The total white population was 340,000 at this time, the extra numbers being explained by significant migration from Great Britain during the 19th century after the British took over the Cape as a colony in 1806. Over the next 150 years, the Afrikaner population growth slowed down considerably until the 1990s, growing only 15 times to the current 3 million.
The foundation of modern South Africa has its origins in European trade with the Far East and the need for a replenishment station for ships sailing through such long distance and encountering major health problems such as scurvy, due to malnutrition. Indeed, the intention in 1752 when the Dutch East India Company established such a settlement at the foot of the “Tafelberg” (Table Mountain) was not colonization. However, very soon company officials were given “free citizen” status and land. Colonization of the current Southern Cape occurred by natural growth of the European population with successive generations moving onto adjacent land. Even though they were sharing the same geographic area with slaves, Khoi-San and blacks, the Afrikaners generally formed a breeding isolate due their perceptions of origin, culture and religion9.
Directly relevant to our studies, until late in the 19th century all of A341V ancestors were located in a small geographic area in the Southern Cape, within about 200 km from Cape Town (Geldenhuys, personal communication). Huge population movements, namely the “Groot Trek” (1834), where 15% of the Cape Colony moved north to escape British rule, the lure of quick wealth after the discovery of diamonds near Kimberley in 1867 and of gold near Johannesburg in 1886 ended the geographic relationship with the disease allele. Together with the urbanization of the 20th century these factors resulted in persons with the A341V mutation now mostly finding themselves in the big metropolitan areas of Cape Town, Johannesburg, Pretoria and some smaller cities.
Founder populations are not unique to South Africa. A similar story is present in Finland where for many diseases founding effects have been described16, including for long LQTS17. With a history dating back 1000 years to a small founder population, a small starting gene pool, the Finns spread into unpopulated virgin territory as their numbers increased and with the increase in numbers the number of individuals with disease genes that were present in the starting gene pool. In contrast, because of the shorter population history, the average preserved chromosomal segment size in the Afrikaner population will be larger, which generally means that fewer markers will be necessary to do genome wide scans. Other examples of founder effects are found in the French speaking Canadians in Quebec18, in the descendants of Anabaptist religious groups that migrated to the new world, namely, the Hutterites19, Mennonites20, Amish21, and the Newfoundland population22. All these groups formed breeding isolates, keeping separate from the indigenous people, similar to what the Afrikaners did, and thereby causing founder effects.
The existence of founder mutations carries multiple implications. It is expected that with many descendants who survived and reproduced successfully, despite a deleterious disease-allele, there should be the opportunity for a better description of the natural history of the associated disease and for the possibility of studying the role of factors other than the primary cause, such as lifestyle, environment or of other genetic loci, which might modify the clinical phenotype.
Furthermore, the origin from a small gene pool and the residence in a single geographic area with a shared culture should reduce not only genetic variance but also variable influences due to different environment or customs. In other words, with less variability to be taken into consideration genetic studies could be simplified. As to the question of our own primary interest, the major advantage provided by founder populations seems to be the fact that they represent the ideal human model in which to study the existence and role of “modifier genes”. The simple reason is that a single mutation should provide the same, or extremely similar, substrate (arrhythmic substrate in the case of LQTS) and should then more easily allow assessment of the “modifier” role of other genetic variants. This is in sharp contrast to when these studies are attempted in populations of patients who carry different mutations because it will always be complex and fraught with probable errors in the attempt to interpret, for instance, different clinical severity on the basis of putative genetic modifiers when the different mutations may vary contribute per se to these differences.
Founder populations also have disadvantages. The selection of a study population is not independent but through kinship and family relatedness comes into play. Methods used for genome wide screening may have to be adapted for family-based studies23. If family relationships exist unknown to the investigator this may lead to false positive associations and this emphasizes the utility of genealogy24. Indeed, the knowledge of genealogy going back 10 to 12 generations, as it happens with the Afrikaner population, becomes very important and can prevent incorrect inferences or conclusions. This population is very useful for mapping purposes and also for the identification of modifying influences. Among the Afrikaners, there is reduced heterogeneity and greater linkage disequilibrium intervals than in European populations in general and also than in the Finnish population, one where LQTS founder effects have been described25. The Afrikaner ancestors can almost always be traced over three and a half centuries and, in principle, the degree of inbreeding can be calculated26.
In general, founding surnames carry little current prediction of risk. Surname attrition in an autosomal dominantly inherited disease would roughly be 75% per generation, depending on initial conditions and would be negligible after 10 to 12 generations. After all, if the founding couple by chance had only daughters the surname would have become separated in the first generation. Current associations of surname and disease are likely to be by chance and not by cosegregation. Similar to the small initial gene pool, the pool of surnames was small. Estimates made earlier this century suggest that nearly half of all the three million Afrikaners hold surnames that are derived from just 50 original colonists; this group of 50, besides the surname Swart also includes the surname Brink.
The origin of the A341V South African founder population
In South Africa, the Dutch East India Company had an excellent system, later continued under English rule, for recording births and deaths. Furthermore, church records and family information recorded in the family bibles as well as in cemeteries are good sources of genealogical information. This has been critical for our success in identifying the individuals most likely to have represented the original ancestors.
Thanks largely to the efforts of our partner, the genealogist Professor Gerhard Geldenhuys, we were able to trace the index case to two founding couples - both of mixed Dutch and French Huguenot origin, who married around 1720 (Fig. 1). For totally unscientific reasons (such as the role of destiny), but still with a 25% chance of being right, we favour the view that the most likely initial ancestor was Pieter Swart, the Dutch spelling of Peter Schwartz, who travelled with his parents from the Netherlands and settled in the Cape between 1785 and 1792. He then – on June 15, 1721 - married Sara du Bois, a French Huguenot girl born in the Cape, and their children were baptised in Stellenbosch, in the Dutch Reformed Church in the 1720s (Fig. 2). Pieter probably has been buried underneath the floor of a church as had been the custom in those days and we were unable to find his tomb. The lines of descent can be traced to the union of one of his children, Johannes, with a daughter in the Uys family (Fig.1), which represents the other possible founding line, as one of the four of them must have harboured the A341V allele.
Figure 1.
The ancestors of current living A341V carriers could be traced to children of the union between Susanna Swart, daughter of Pieter Swart and Sara du Buys, and Dirk Uys. They were residing in the Southern Cape in an area within 200 km from Cape Town where until about the end of the 19th century all obligate A341V carriers can be traced to.
Figure 2.
Record of the baptism of Johannes Swart, son of Pieter Swart and Sara (du) Buys, in 1722 (page 100 of the baptismal book) [highlighted in yellow] by the “scriba” (church secretary) of the Dutch Reformed Church in Stellenbosch, the second oldest European town in South Africa after Cape Town. Two of the Swart children were baptised here.
Characteristics of the LQTS South African founder population
Our initial goal was to describe this South African LQTS founder population (SA-KCNQ1-A341V) and examine some of its characteristics. These patients belong to the LQT1 genetic subgroup, with mutations affecting the IKs current. This is the most common variant of LQTS and the cardiac events of these patients are almost always triggered by sympathetic activation, i.e. physical or emotional stress27; swimming is especially dangerous for them.
Of 345 individuals in the original study population28 (currently 382), 166 were mutation carriers (MCs) (currently 184), 154 were noncarriers (nMCs) (currently 173), and 25 were not genetically tested. Among the 166 MCs, 131 (79%) have had symptoms, with a median age at first cardiac event of 6 years, and 23 (14%) suffered sudden cardiac death before age 20 years. We defined as asymptomatic 26 patients who were older than 15 years and with no events without therapy. Those below age 15 were too young to be designated as asymptomatic. Eighty-six MCs and 102 nMCs, with a basal ECG recorded after age 15, were analyzed for differences in QTc interval duration, heart rate (heart rate), and symptoms. Despite sharing the same genetic defect, mutation carriers as a group exhibited a wide range of QTc values (406 to 676 ms), with 12% of individuals having a normal QTc (<440 ms). This is a clear indication that something else, besides the A341V mutation, contributes to determine the duration of the QT interval among these MCs. A QTc >500 ms was associated with an increased risk of experiencing cardiac events (OR= 4.22; p=0.03), thus confirming our previous observations regarding this value as a meaningful cut-off for assessing high risk.
Unusual clinical severity
Because our ascertainment revealed, to our surprise, relatively few asymptomatic patients in the SA-A341V population and a 14% incidence of sudden death before the age of 40 years, we considered the possibility that the KCNQ1-A341V mutation segregating in these families might be associated with a greater incidence of cardiac events compared with that reported for LQT1 subjects in general. To test this hypothesis in a preliminary way, we compared clinical severity between the SA-A341V and our own LQT1 population in Pavia. These analyses supported the unexpected observation that the clinical severity of LQTS observed in the SA-A341V population is significantly greater than that in LQT1 in general.
Even though we immediately assumed that the unexpected clinical phenotype was caused directly by this particular missense mutation, we could not exclude the possibility that the clinical severity was mediated not by the KCNQ1-A341V mutation per se but by some other probably genetic or epigenetic factors present in these families all living in South Africa for approximately 300 years. To answer this question and to determine whether the high arrhythmic risk observed in the South African (SA) families was indeed due solely to the KCNQ1-A341V mutation, one of relatively few “hot spot” missense mutations, we performed another study29 on non-SA patients with LQT1 secondary to A341V. The study population was obtained through an international collaborative project involving 8 countries worldwide (Finland, France, Germany, Italy, Japan, the Netherlands, Russia, and the United States). Data were recorded for a total of 78 patients from 24 unrelated, non-SA families harboring the KCNQ1-A341V mutation. For comparison, we used 205 LQT1 non-A341V patients followed at our center in Pavia.
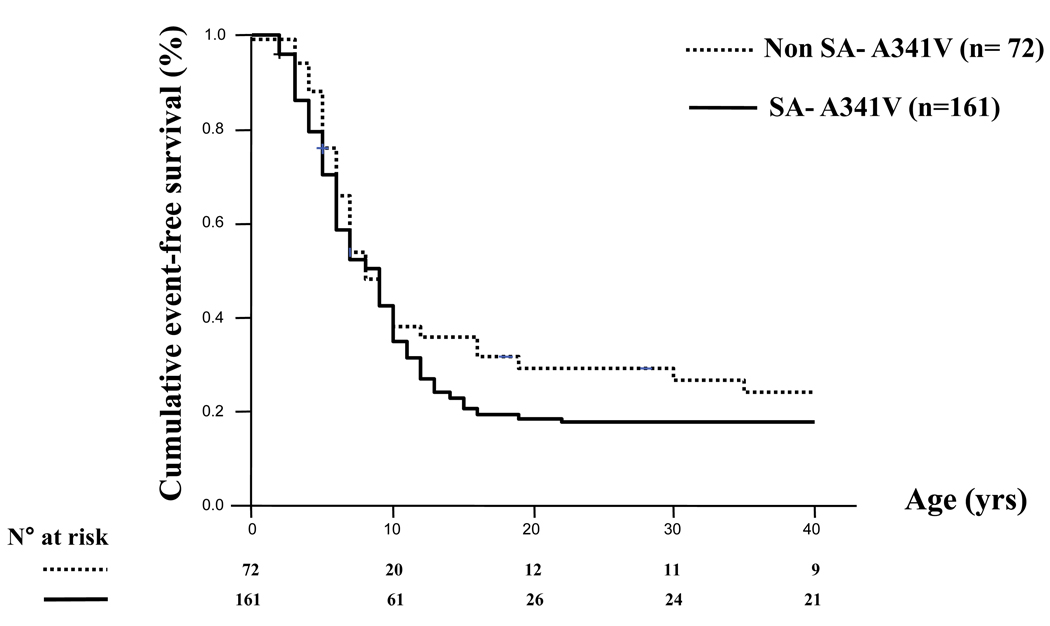
First, we observed that there were almost no differences between the 166 SA patients and the 78 non-SA patients all carriers of A341V mutation as their cumulative event-free survival was extremely similar (Fig. 3), with 79% and 68% respectively suffering a first cardiac event before age 40 and in the absence of β-blocker therapy. Impressively, by age 10 in both groups, approximately 60% of the mutation carriers had already become symptomatic. This indicated that the high incidence of cardiac events was related to the A341V mutation itself and not to some unknown factor associated with their permanence in South Africa.
Figure 3.
Unadjusted Kaplan Meier estimate of the cumulative event-free survival in the non-SA and SAA341V groups. Any cardiac event (syncope, cardiac arrest or LQTS-related sudden cardiac death), whichever occurred first, was considered from birth through age 40 years and before β-blocker therapy. Numbers at risk are indicated. (From ref. 29)
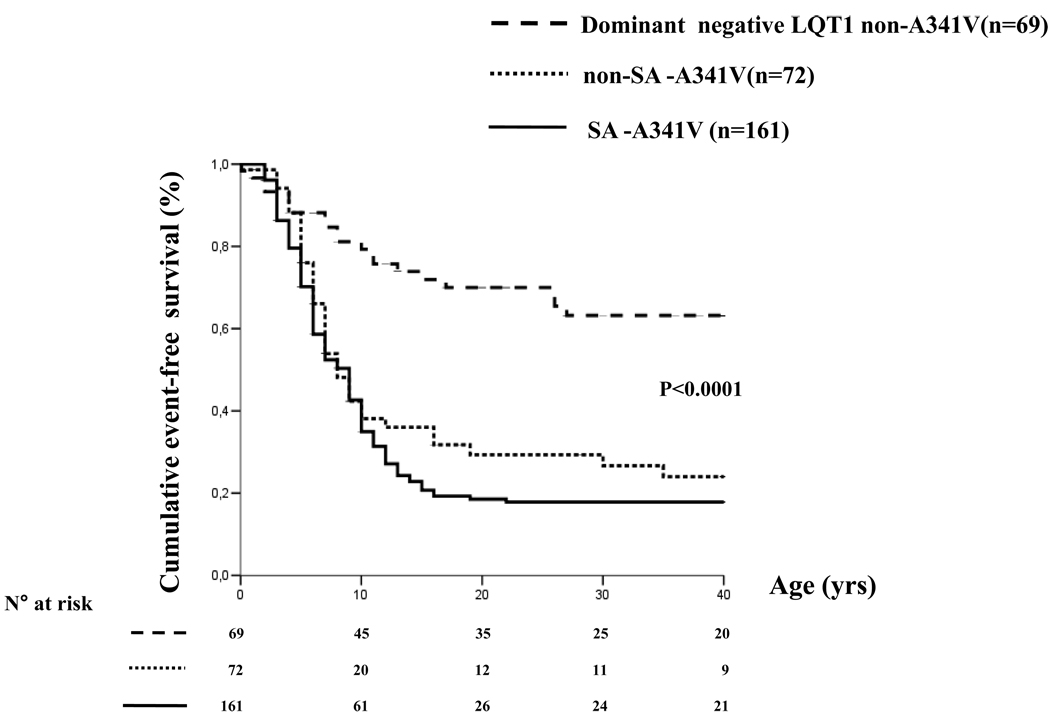
At this point, all A341V carriers could be analyzed together as a group and compared to the non-A341V patients. This analysis showed striking differences, as exemplified in Table 1. Among the most impressive are the incidence of cardiac events (75% vs 24%, p<0.001) and of cardiac arrest or sudden death (30% vs 7%, p<0.001) before age 40 and off β-blocker therapy. The curves on the cumulative event-free survival speak for themselves (Fig. 4).
TABLE 1.
Clinical characteristics of the entire A341V population and comparison with a LQT1 non-A341V group.
| All A341V | LQT1 non-A341V | p-value | |
|---|---|---|---|
| N. of genotype positive patients | 244 | 205 | |
| Symptomatic (any first event before age 40) |
184 (75%) | 49 (24%) | <0.001 |
| Median age at onset | 6 | 11 | 0.001 |
| CA/SCD | 74 (30%) | 14 (7%) | <0.001 |
| QTc, ms | 485±43 | 465±38 | <0.001 |
| ≥ 500 ms | 45 (29%) | 26 (14%) | 0.001 |
| Median follow-up, yrs | 30 | 32 | 0.35 |
Figure 4.
Unadjusted Kaplan Meier estimate of the cumulative event-free survival (any first event) in the whole (non-SA + SA) A341V population plotted versus the LQT1 non-A341V group. Any cardiac event, whichever occurred first, was considered from birth through age 40 and before β-blocker therapy. Numbers at risk are indicated. (From ref. 29)
As recent findings indicated that both transmembrane mutations and dominant-negative functional mutations in KCNQ1 were associated with increased disease severity30, we also considered the possible effect of the mutation site and the possibility that the clinical severity of A341V might be a consequence of its dominant-negative nature. Therefore, we compared all A341V genotype-positive patients with the LQT1 population stratified for mutation site and the LQT1 patients with dominant-negative mutations and observed that the clinical severity of the A341V group was always much greater. We then compared our two A341V populations with the non-A341V group comprising only mutations with a dominant-negative effect functionally demonstrated (Fig. 5). Even in this case, patients with the dominant-negative A341V mutation had a significantly higher probability of becoming symptomatic than patients with other dominant negative LQT1-causing mutations (p<0.0001).
Figure 5.
Unadjusted Kaplan Meier estimate of the cumulative event free survival (any first event) only in patients with LQT1 secondary to dominant negative KCNQ1 mutations: the two A341V groups are plotted versus the LQT1 non-A341V group. Any cardiac event, whichever occurred first, was considered from birth through age 40 and before β-blocker therapy. Numbers at risk are indicated. (From ref. 29)
Finally, because our own non-A341V population appeared to be somewhat less symptomatic than other LQT1 populations previously reported, for the sake of safety, we also made a comparison with the largest non-A341V population available to us, namely the 573 patients who were part of the recent study by Moss et al.30. Figure 6 shows Kaplan-Meier curves for these 573 patients, for the 19 A341V patients from the same study, and for our own 233 A341V patients. Two important points become apparent. The first is that the probability of arrhythmic symptoms is twice as large (80% versus 40%; p<0.0001) among the A341V compared with the non-A341V patients. The second is the very impressive and practically identical Kaplan-Meier curves of the 19 A341V patients studied by Moss et al.30 and of the 233 A341V patients from our study.
Figure 6.
Unadjusted Kaplan Meier estimate of the cumulative probability of a cardiac event (syncope, cardiac arrest or LQTS-related sudden death, whichever occurred first) in the LQT1 population from the U.S.-Netherlands-Japan collaborative study (8). Carriers of A341V mutation are compared with all the other LQT1 non-A341V patients. Superimposed is the curve representing the cumulative probability of a first cardiac event in the entire (SA+ non-SA) A341V population from the present study. Numbers at risk are indicated. (From ref. 29)
These data conclusively demonstrate the striking clinical severity associated with the A341V mutation and prove that cellular electrophysiological studies cannot always predict the clinical phenotype. Indeed, in the A341V patients, neither the location (transmembrane) nor the functional consequence of the mutation (dominant-negative effect) fully explains the unusually high clinical severity.
On this basis, we surmise that the current biophysical assessments of the electrophysiological effects of LQTS causing mutations do not provide the whole gamut of information necessary to make a complete genotype-phenotype correlation.
This study has provided the largest data set on patients affected by LQTS who carry the exact same mutation. The data unequivocally show that KCNQ1-A341V is a mutation associated with unusual clinical severity. We do not believe that this mutation is unique in its clinical phenotype, and we believe that other mutations may confer a risk for life-threatening arrhythmias higher than that associated with other mutations. Thus, one can envision not only genotype-specific treatment algorithms but even mutation-specific approaches.
Resting heart rate and risk for cardiac events
Because the magnitude of IKs, the current encoded by KCNQ1, is rate-dependent, we examined the possible role of heart rate as a predictor of events.
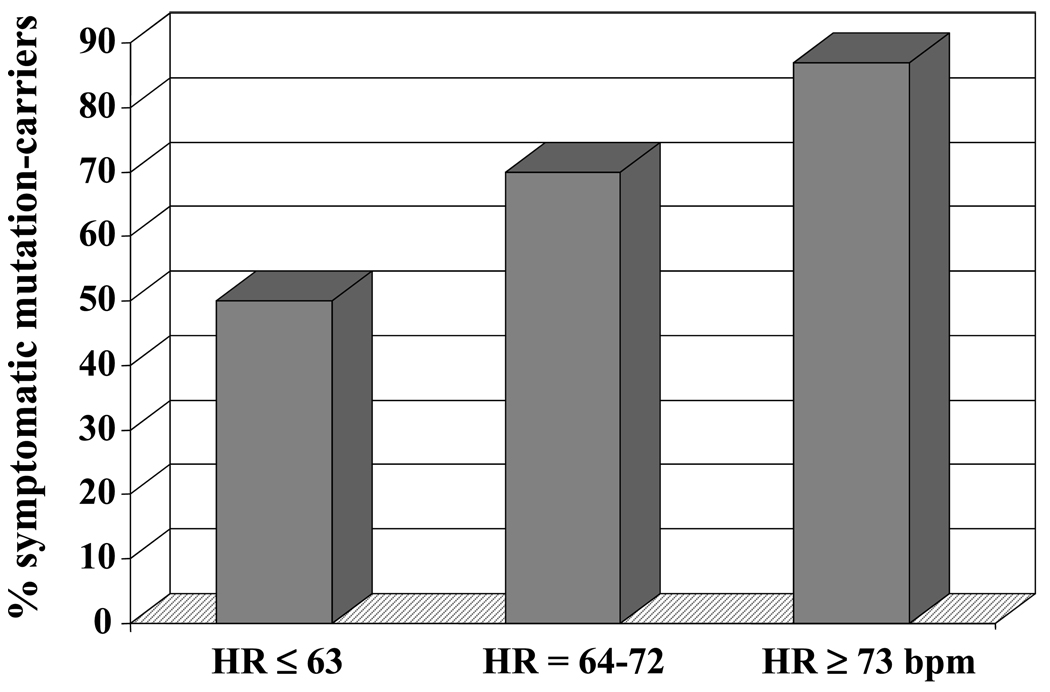
In a first study,28 we calculated heart rate from a standard ECG and, interestingly, we found that asymptomatic carriers had a significantly lower heart rate compared with symptomatic individuals (65±13 versus 71±11 bpm, p=0.026). MCs in the lowest two tertiles, defined by heart rate <73 bpm, were at lower risk for cardiac events compared with those with in the highest tertile, heart rate ≥73 bpm (OR=0.23; p=0.035). Results from both univariate and multivariate analyses identified heart rate and QTc as important factors in determining disease expression in this population. MCs with a heart rate < 73 bpm and a QTc <500 ms had a risk of cardiac events significantly lower compared to all other subjects combined (OR=0.19; p=0.005). Of note, the impact of heart rate in risk stratification was stronger in the subgroup of patients with a QTc <500 ms compared with that with a QTc ≥500 ms. Indeed, there was a linearly increasing proportion of symptomatic mutation carriers from the lower to the upper tertile of heart rate, representing an incremental risk (OR=2.5; p<0.03) (Fig. 7).
Figure 7.
Percentage of symptomatic patients with a basal QTc < 500 ms in each tertile of heart rate. (From ref. 28)
In a second study,31 we were determined to rule out any possible confounding variable. Accordingly, heart rate was measured in truly resting conditions with the recordings lasting 10 minutes while the patients were lying comfortably on a bed. In addition, the study was repeated with β-blockers on and off. In essence, the results fully confirmed the initial observations; the only difference being represented, as logically expected, by lower values in the second study. As in the first study, there was a significant difference between asymptomatic and symptomatic MCs because the asymptomatic MCs had a significantly lower heart rate (61±8 bpm vs. 66±9 bpm, p<0.04). The difference remained also during β-blocker treatment.
We then assessed whether the level of resting heart rate was associated with a history of arrhythmic events in the subgroup of patients with a QTc <500 ms (above 500 ms almost all had symptoms) and found that MCs in the first tertile (heart rate<60 bpm) were less frequently symptomatic compared with MCs in the other 2 tertiles (OR 0.19, p=0.02) (Fig. 8). Figure 8 also provides evidence that a QTc >500 ms represents a more severe arrhythmogenic substrate in our population, on the basis of the fact that 15 of 16 (94%) MCs with a QTc >500 ms were symptomatic. These results demonstrate that asymptomatic MCs had a significantly lower heart rate than symptomatic MCs. This was true with and without β-blockers.
Figure 8.
Differential risk for arrhythmic events among 56 MCs according to resting heart rate off- β-blockers and QTc. The dashed horizontal line represents the predefined cut-off for QTc (≤ or >500 ms) whereas the dashed vertical line corresponds to the first tertile (≤60 bpm) of the heart rate values distribution. With a QTc > 500 ms all but one patient have symptoms. When the QTc is < 50 ms the level of heart rate plays a major role as while above 60 b/min the probability of events is 80% vs 38% when heart rate is bellow 60 b/min. (Modified from ref. 31)
Reflex control of heart rate and risk for cardiac events
We had previously provided experimental32 and clinical33 evidence that alterations in the autonomic control of the heart, highlighted by depressed baroreflex sensitivity (BRS) –largely, but not only, a marker of reduced cardiac vagal activity - are associated with increased risk for arrhythmias and sudden death after a myocardial infarction. The wide distribution of BRS values in normal animals32 suggested that these and other autonomic responses are under at least partial genetic control. To define whether individual differences in autonomic responsiveness are associated with a higher or lower propensity for life-threatening arrhythmias, we assessed the reflex control of heart rate in the South African LQT1 founder population by determining BRS31 by the phenylephrine method34. Given the important influence of age on BRS, we focused our analysis on the second and third age quartiles that included 38 subjects (age 26 to 47 years) and represented the central 50% of the tested population. In this group, the correlation with age was not significant. This approach allowed a reliable assessment of BRS while controlling for the age effect.
The mean value of BRS in the entire group of 38 subjects was 16.7 ± 8.8 ms/mm Hg (Fig. 9A). As expected by a distribution of autonomic parameters independent of the LQTS mutation, the BRS values between MCs (n=22) and non-MCs (n=16) were no different (17.1 ± 9.7 vs. 16.3 ± 7.6, p=NS) (Fig. 9B). By contrast, a significant difference emerged when the analysis compared the asymptomatics (n=8) with the symptomatics (n = 14) MCs (11.8 ± 3.5 vs. 20.1 ± 10.9; p < 0.05) (Fig. 4C). A BRS ≤ 12 ms/mm Hg, which corresponds to the first tertile of the distribution among all the 38 subjects, was associated with a significantly lower probability of having suffered cardiac events compared with a BRS in the upper 2 tertiles (OR 0.13, p < 0.05) (Fig. 9C). Indeed, among MCs with a BRS > 12 ms/mm Hg, 10 of 12 (83%) subjects were symptomatic.
Figure 9.
BRS values off-βB in the entire group under study aged 26–47 years (9A), in MCs and in non-MCs (9B), and in symptomatic and asymptomatic MCs (9C). Mean and standard deviation are shown for each subgroup. The dashed horizontal line represents the lower tertile of BRS values. (From ref. 31)
When we assessed whether the level of BRS was associated with a history of cardiac events in the 15 patients with a QTc ≤ 500 ms, we found that none of the MCs in the first tertile (BRS ≤ 12 ms/mm Hg) had cardiac events, whereas 80% of those with a BRS > 12 ms/mm Hg were symptomatic (p < 0.01).
There is a plausible biological explanation for this finding. LQT1 subjects are at greatest risk for arrhythmias whenever heart rate changes too rapidly, at variance with patients affected by ischemic heart disease who are at risk when there is insufficient vagal activity to antagonize the arrhythmogenic sympathetic activity. When heart rate increases quickly, reduced IKs prevents the necessary QT adaptation (QT shortening) and a new ventricular depolarization might encroach the vulnerable phase of the T-wave, thus initiating ventricular tachycardia or fibrillation. When heart rate decreases quickly, the sudden RR lengthening might increase the amplitude of early after-depolarizations and initiate torsades-de-pointes through triggered activity. This is why for LQT1subjects a “blunted” autonomic response, as revealed by relatively low BRS, acts as a protective factor.
Altogether, these data provide evidence that the individual autonomic make-up effectively contributes to modulate the probability of being symptomatic or asymptomatic in patients with the KCNQ1-A341V mutation and probably also in most LQT1 patients.
Modifier Genes
The study just described on the interaction between BRS values and risk for cardiac events in our founder population did carry the search for arrhythmia modifiers in a novel direction.
In LQT1 patients, for whom the main arrhythmogenic trigger is sympathetic activation27, it was reasonable to postulate a relation between autonomic responses and arrhythmic risk35. Baroreflex sensitivity, a validated quantitative marker of autonomic responses, is partly under genetic control36. We assumed that the wide range of BRS among individuals without structural heart disease could reflect this genetic control and that unavoidably also the carriers of LQT1 mutations would find themselves genetically bound to a propensity for having a low, intermediate, or high BRS. Our study suggested that the outcome of this association by chance between LQT1 mutations and varying levels of BRS would be a differential probability of being symptomatic. We also thought that the evidence that a specific autonomic phenotype (i.e., relatively low BRS) is associated with a reduced clinical severity of an LQT1 mutation could indicate that genes controlling BRS or, more broadly, autonomic responses involved in the neural control of the heart might include key modifier genes that might increase or reduce the risk for sudden cardiac death in LQTS and perhaps in other more common disorders.
These considerations prompted our screening of selected adrenergic receptor gene polymorphisms, the ADRA2B-Del301-303 and ADRA”C-Del322-325. Because ADRA2C-Del322-325 is associated with an increased release of norepinephrine from cardiac sympathetic nerves and because ADRB1-R389 is associated with a greater ability to couple to adenylyl cyclase than does ADRB1-G389, we hypothesized that the 2 receptor polymorphisms could act synergistically to modify BRS. To test this hypothesis, we combined subjects heterozygous for ADRA2C-Del322-325 or homozygous for ADRB1-R389 (Group 1, 11 families) and compared them with subjects without ADRA2C-Del322-325 and not homozygous for the ADRB1-R389 allele (Group 2, 7 families). Adrenergic responses were predicted to be enhanced in Group 1 and blunted in Group 2. Indeed, Group 1 subjects had a higher frequency of BRS values above the upper tertile compared with Group 2 subjects (46% vs. 8%; p < 0.05) (Fig. 10). Thus, subjects with a genetic profile predicted to have augmented adrenergic responses had brisker baroreceptor reflexes.
Figure 10.
BRS values off-βB in subjects aged 26–47 years (MCs and non-MCs), according to the specific genotype. Group 1: subjects with the ADRA2C-Del322-325 and subjects homozygous for ADRB1-R389. Group 2: subjects without ADRA2C-Del322-325 and not homozygous for the ADRB1-R389 allele. The solid horizontal line represents the upper tertile of BRS values. (From ref. 31)
A novel concept arises from our study. Some individuals might inherit a disease-causing mutation (e.g., a LQTS mutation such as KCNQ1-A341V). The same individuals might inherit certain specific and relatively common gene variants (e.g., the adrenoreceptor polymorphisms investigated in our study). The genetic transmissions of the LQTS mutations and of the adrenoreceptor polymorphisms are obviously independent of each other. The coexistence of a LQTS mutation with a polymorphism creating a genetic propensity toward brisk autonomic reflexes, a combination that our data indicate increases arrhythmic risk, is simply the play of chance.
Very recently, we have considered that other polymorphisms might contribute to the modulation of the clinical severity of LQTS. Genetic variations in NOS1AP, which encodes a nitric oxide synthase adaptor protein, contribute to QT interval duration in the general population37. Accordingly, we tested in our South African founder population the hypothesis that NOS1AP is a genetic modifier of LQTS. There were two main findings as two NOS1AP variants: 1) increased the probability of having a more prolonged QT interval even for mutation-carriers, thus despite their having an already markedly prolonged QTc; and, 2) increased by almost two times the risk for sudden cardiac death. This is the first evidence, demonstrated in subjects sharing the same mutation, that NOS1AP variants are associated with a greater risk for cardiac arrest and sudden death in LQTS38.
Conclusions
As we suspected from the outset, the South African LQT1 founder population has proved to be a very rich source for novel findings. Our studies have clearly illustrated the phenotypic heterogeneity of LQTS within a large population of related LQTS individuals carrying the same primary mutation. Given the expectation that all individuals carrying KCNQ1-A341V should have similar reductions in IKs, and thereby of similar clinical consequences, our findings point to the existence within this South African population of additional genetic influences affecting duration of the QT interval and the probability of cardiac events. This unique human model has already provided important information and we are confident that, especially in the area of modifier genes, by carefully conducted studies we will be able to successfully pursue our quest for a more complete understanding of this intriguing disorder. We would not be surprised if these findings would also prove relevant to the identification of risk factors for life-threatening arrhythmias in more common conditions such as acute myocardial infarction and heart failure.
ACKNOWLEDGMENTS
This work was supported by NIH grant HL068880, by Telethon – Italy Grant no. GGP07016, and by the Italian Ministry for Foreign Affairs grant Identificazione di “geni modificatori” modulanti il rischio di morte improvvisa in malattie cardiache aritmogene ereditarie.
The Authors are grateful to Drs. Valerie Corfield, Lia Crotti, Gerhard Gendenhuys, Alfred L. George, Jr., Marshall Heradien, Emilio Vanoli, and to Althea Goosen for significant contributions to the studies reviewed in this chapter. Also, they are thankful to Pinuccia De Tomasi for expert editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schwartz PJ. Idiopathic long QT syndrome: Progress and Questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 3.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Priori SG, Napolitano C. The long QT syndrome. In: Zipes DP, Jalife J, editors. CARDIAC ELECTROPHYSIOLOGY. FROM CELL TO BEDSIDE. III EDITION. Philadelphia: WB Saunders Co; 2000. pp. 597–615. [Google Scholar]
- 6.Moss AJ, Schwartz PJ. 25th Anniversary of the international Long QT Syndrome Registry: an ongoing quest to uncover the secrets of LQTS. Circulation. 2005;111:1199–1201. doi: 10.1161/01.CIR.0000157069.91834.DA. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Crotti L. Long QT and short QT syndromes. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5th Edition. Philadelphia: Elsevier/Saunders; 2009. pp. 731–744. [Google Scholar]
- 8.de Jager T, Corbett CH, Badenhorst JC, Brink PA, Corfield VA. Evidence of a long QT founder gene with varying phenotypic expression in South African families. J Med Genet. 1996;33:567–573. doi: 10.1136/jmg.33.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giliomee H. The Afrikaners. Tafelberg Publishers Ltd.; University of Virginia Press; 2003. [Google Scholar]
- 10.Botha MC, Beighton P. Inherited disorders in the Afrikaner population of southern Africa. Part I. Historical and demographic background, cardiovascular, neurological, metabolic and intestinal conditions. S Afr Med J. 1983;64:609–612. [PubMed] [Google Scholar]
- 11.Kruse M, Schulze-Bahr E, Corfield VA, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Schulze-Bahr E, Brink PA, Pongs O. Impaired endocytosis of TRPM4 channel associated with progressive familial heart block type I. J Clin Invest. 2009 doi: 10.1172/JCI38292. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez P, Moolman-Smook J, Brink PA, Corfield VA. A gene locus for progressive familial heart block type II (PFHBII) maps to chromosome 1q32.2-q32.3. Hum Genet. 2005;118:133–137. doi: 10.1007/s00439-005-0029-5. [DOI] [PubMed] [Google Scholar]
- 13.Moolman-Smook JC, De Lange WJ, Bruwer EC, Brink PA, Corfield VA. The origins of hypertrophic cardiomyopathy-causing mutations in two South African subpopulations: a unique profile of both independent and founder events. Am J Hum Genet. 1999;65:1308–1320. doi: 10.1086/302623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotze MJ, Langenhoven E, Warnich L, du Plessis L, Retief AE. The molecular basis and diagnosis of familial hypercholesterolaemia in South African Afrikaners. Ann Hum Genet. 1991;55:115–121. doi: 10.1111/j.1469-1809.1991.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 15.ARVC Registry of the Cardiac Arrhythmia Society of Southern Africa (CASSA) Watkins DA, Hendricks N, Shaboodien G, et al. Clinical features, survival experience and profile of Plakophylin-2 gene mutations in participants of the arrhythmogenic right ventricular cardiomyopathy registry of South Africa. Heart Rhythm. doi: 10.1016/j.hrthm.2009.08.018. this supplement. [DOI] [PubMed] [Google Scholar]
- 16.Norio R. The Finnish Disease Heritage III: the individual diseases. Hum Genet. 2003;112:470–526. doi: 10.1007/s00439-002-0877-1. [DOI] [PubMed] [Google Scholar]
- 17.Fodstad H, Swan H, Laitinen P, et al. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med. 2004;36 Suppl 1:53–63. doi: 10.1080/17431380410032689. [DOI] [PubMed] [Google Scholar]
- 18.Laberge AM, Michaud J, Richter A, et al. Population history and its impact on medical genetics in Quebec. Clin Genet. 2005;68:287–301. doi: 10.1111/j.1399-0004.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 19.Boycott KM, Parboosingh JS, Chodirker BN, et al. Clinical genetics and the Hutterite population: a review of Mendelian disorders. Am J Med Genet A. 2008;146A:1088–1098. doi: 10.1002/ajmg.a.32245. [DOI] [PubMed] [Google Scholar]
- 20.Orton NC, Innes AM, Chudley AE, Bech-Hansen NT. Unique disease heritage of the Dutch-German Mennonite population. Am J Med Genet A. 2008;46A:1072–1087. doi: 10.1002/ajmg.a.32061. [DOI] [PubMed] [Google Scholar]
- 21.Patton MA. Genetic studies in the Amish community. Ann Hum Biol. 2005;32:163–167. doi: 10.1080/03014460500075274. [DOI] [PubMed] [Google Scholar]
- 22.Rahman P, Jones A, Curtis J, et al. The Newfoundland population: a unique resource for genetic investigation of complex diseases. Hum Mol Genet. 2003;12(Spec No 2):R167–R172. doi: 10.1093/hmg/ddg257. [DOI] [PubMed] [Google Scholar]
- 23.Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet. 2000;67:146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman DL, Abney M, McPeek MS, Ober C, Cox NJ. The importance of genealogy in determining genetic associations with complex traits. Am J Hum Genet. 2001;69:1146–1148. doi: 10.1086/323659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Service S, DeYoung J, Karayiorgou M, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38:556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- 26.Greeff JM. Deconstructing Jaco: genetic heritage of an Afrikaner. Ann Hum Genet. 2007;71:674–688. doi: 10.1111/j.1469-1809.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long QT syndrome. Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital Long QT Syndrome in a founder population. Circulation. 2005;112:2602–2610. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 29.Crotti L, Spazzolini C, Schwartz PJ, et al. The common Long QT Syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification. Circulation. 2007;116:2366–2375. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]
- 30.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz PJ, Vanoli E, Crotti L, et al. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–929. doi: 10.1016/j.jacc.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 33.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators: Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. for the. [DOI] [PubMed] [Google Scholar]
- 34.La Rovere MT, Schwartz PJ. Baroreflex sensitivity. In: Zipes DP, Jalife J, editors. CARDIAC ELECTROPHYSIOLOGY. FROM CELL TO BEDSIDE. III EDITION. Philadelphia: WB Saunders Co; 2000. pp. 771–781. [Google Scholar]
- 35.Schwartz PJ. Another role for the sympathetic nervous system in the long QT syndrome? J Cardiovasc Electrophysiol. 2001;12:500–502. doi: 10.1046/j.1540-8167.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- 36.Tank J, Jordan J, Diedrich A, et al. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- 37.Arking DE, Pfeufer A, Post W, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nature Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 38.Crotti L, Monti MC, Insolia R, et al. NOS1AP is a genetic modifier of congenital long-QT syndrome. Circulation. doi: 10.1161/CIRCULATIONAHA.109.879643. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]