Abstract
Many fungal genera have been defined based on single characters considered to be informative at the generic level. In addition, many unrelated taxa have been aggregated in genera because they shared apparently similar morphological characters arising from adaptation to similar niches and convergent evolution. This problem is aptly illustrated in Mycosphaerella. In its broadest definition, this genus of mainly leaf infecting fungi incorporates more than 30 form genera that share similar phenotypic characters mostly associated with structures produced on plant tissue or in culture. DNA sequence data derived from the LSU gene in the present study distinguish several clades and families in what has hitherto been considered to represent the Mycosphaerellaceae. In some cases, these clades represent recognisable monophyletic lineages linked to well circumscribed anamorphs. This association is complicated, however, by the fact that morphologically similar form genera are scattered throughout the order (Capnodiales), and for some species more than one morph is expressed depending on cultural conditions and media employed for cultivation. The present study shows that Mycosphaerella s.s. should best be limited to taxa with Ramularia anamorphs, with other well defined clades in the Mycosphaerellaceae representing Cercospora, Cercosporella, Dothistroma, Lecanosticta, Phaeophleospora, Polythrincium, Pseudocercospora, Ramulispora, Septoria and Sonderhenia. The genus Teratosphaeria accommodates taxa with Kirramyces anamorphs, while other clades supported in the Teratosphaeriaceae include Baudoinea, Capnobotryella, Devriesia, Penidiella, Phaeothecoidea, Readeriella, Staninwardia and Stenella. The genus Schizothyrium with Zygophiala anamorphs is supported as belonging to the Schizothyriaceae, while Dissoconium and Ramichloridium appear to represent a distinct family. Several clades remain unresolved due to limited sampling. Mycosphaerella, which has hitherto been used as a term of convenience to describe ascomycetes with solitary ascomata, bitunicate asci and 1-septate ascospores, represents numerous genera and several families yet to be defined in future studies.
Keywords: Cibiessia, Colletogloeum, Dissoconium, Kirramyces, Mycosphaerella, Passalora, Penidiella, Phaeophleospora, Phaeothecoidea, Pseudocercospora, Ramularia, Readeriella, Stenella, Teratosphaeria, Zasmidium
INTRODUCTION
When Colin Booth delivered his Presidential address to the British Mycological Society in 1977, he chose the title ‘Do you believe in genera?’. This, interestingly, was the question Mr Mason asked him when he first arrived at the Commonwealth Mycological Institute. This question raised a very complex issue, and it was sufficient to silence anyone embarking on a career in mycology. However, Booth went on to research this topic, and delivered his interpretation in his published Presidential address (Booth 1978). In addressing this issue, he chose the Nectriaceae, a group that he knew very well. For the purpose of the present study, we focus on the genus Mycosphaerella that was a core focus of Booth’s colleague, J.A. von Arx, who worked at the ‘sister’ Institute, the Centraalbureau voor Schimmelcultures (CBS) in the Netherlands.
To believe in genera, Booth (1978) emphasised the need to clarify what a genus represents. Here he followed the definition of Singer (1975), namely that a genus represents an assemblage of species separated from others by a gap larger or more abrupt than that existing between species. Since 2009 is also the 150th celebration of Darwin’s ‘On the Origin of Species’, it is fitting to reflect on the quote Booth cited from this book, namely, ‘that our classifications will come to be genealogies: and that they will then truly give what may be called the plan of creation’. Booth (1978) made the point that particularly for microfungi, taxonomy was largely at the alpha or descriptive phase, and that mycology and generic concepts had suffered from what he referred to as ‘shoe-box taxonomy’.
The shoe-box taxonomy referred to by Booth led mycologists to place taxa with similar primary characters that were considered important at the time, in the same box. This resulted in many genera trivialia, their members often genetically widely separated reflecting distinct evolutionary histories. He further noted that conidiomatal and ascomatal morphology frequently reflected a response to a particular niche, rather than genealogical relationship. What this implied was that many generic names reflected ‘terms of convenience’, rather than genealogical relationships. This is especially true for Mycosphaerella and its anamorphs that we discuss in this study.
Subsequent to the time when Booth (1978) published his views on genera, mycology has undergone a major revolution in the way that fungal groupings at all levels are recognised. This has emerged from the now widely adopted application of DNA sequence comparisons to define fungal groups (Taylor et al. 2000). Phylogenetic relationships derived from various gene regions have allowed mycologists to revise the classification schemes to coincide with molecular phylogenetic relationships. This has resulted in major changes reflecting higher order relationships (James et al. 2006, Hibbett et al. 2007). Thus, many genera have been shown as poly- or paraphyletic (Halleen et al. 2004, Lee et al. 2004, Réblová et al. 2004, Verkley et al. 2004b, Crous et al. 2006b, c, 2007a, b, Arzanlou et al. 2007, Wang et al. 2007, Phillips et al. 2008), and cosmopolitan species have been shown to represent assemblages of often large numbers of cryptic taxa (Barnes et al. 2004, Crous et al. 2004b, c, 2006a, d, 2008a, b, Groenewald et al. 2005, Mostert et al. 2006, Andjic et al. 2007b, Cheewangkoon et al. 2008).
The genus Mycosphaerella s.l. together with its associated anamorph genera (especially Cercospora, Pseudocercospora, Septoria, Ramularia, etc.), represents more than 10 000 taxa (Crous et al. 2000, 2001, 2004b, c, 2006a, b, d, 2007a, b, c, 2008a, b, Crous & Braun 2003, Arzanlou et al. 2007, 2008). In a treatment of the Mycosphaerella species and associated anamorphs occurring on Eucalyptus, Crous (1998) showed that the genus is polyphyletic, and suggested that it would eventually be subdivided to reflect natural groups as defined by its anamorphs. However, results obtained in the first phylogenetic trees published for the genus based on ITS DNA sequence data, suggested that Mycosphaerella was monophyletic (Stewart et al. 1999, Crous et al. 1999, 2000, 2001, Goodwin et al. 2001).
As greater numbers of DNA sequences were included in phylogenetic analyses for Mycosphaerella species, the view of this genus as being monophyletic has gradually collapsed. Thus it has now been aptly demonstrated that Mycosphaerella is polyphyletic (Hunter et al. 2006, Crous et al. 2007a), and the complex has in recent years been separated into Davidiella species with Cladosporium anamorphs (Davidiellaceae) (Braun et al. 2003, Crous et al. 2007b, Schubert et al. 2007, Zalar et al. 2007, Dugan et al. 2008), Schizothyrium species with Zygophiala anamorphs (Schizothyriaceae) (Batzer et al. 2008), Teratosphaeria species with more than 12 anamorphs (Teratosphaeriaceae) (Crous et al. 2007a) and Mycosphaerella species with more than 20 anamorph genera (Mycosphaerellaceae) (Crous & Braun 2003). All of these groups reside in the Capnodiales in the Dothideomycetes (Schoch et al. 2006). Although Davidiella (Cladosporium) and Schizothyrium (Zygophiala) have a clear one to one relationship with anamorph genera, this is far from true for Mycosphaerella (Mycosphaerellaceae) and Teratosphaeria (Teratosphaeriaceae), where the teleo-morph morphology is relatively conserved throughout the two respective families. To complicate the situation further, similar anamorph morphologies have evolved in different clades, and in some cases even outside the family (Crous et al. 2007a).
Redefining generic concepts with the incorporation of molecular phylogenetic data has, in many cases, led to the recognition of several natural groups in larger assemblages formerly defined solely based on alpha taxonomy. A further complication arises from dual nomenclature, where generic names linked to anamorph genera have to be linked to teleomorph genera. Two options are thus available for mycologists. One is to use anamorph generic names as nouns, and to accept that they can be poly- and paraphyletic (Halleen et al. 2004, Lee et al. 2004, Réblová et al. 2004, Verkley et al. 2004b, Crous et al. 2006b, c, 2007a, b, Arzanlou et al. 2007, Wang et al. 2007, Phillips et al. 2008). The alternative is to provide new anamorph genus names for well-defined clades, and in the process identify the characters that can be used to distinguish them.
In order to halt the unnecessary proliferation of generic names, Crous et al. (2007a) proposed to use anamorph genera for the same phenotype, regardless of where it clustered within the Capnodiales. This approach has flaws as taxa in different clades inevitably end up with the same generic names suggesting that they are related, and such a situation has led to substantial disagreement among Mycosphaerella taxonomists (see Cortinas et al. 2006, Andjic et al. 2007a, Crous et al. 2007a, 2008a, 2009a). A solution to this dilemma lies in the introduction of generic names for discrete monophyletic lineages, but concurrently not to perpetuate the problems that arise from maintaining dual nomenclature. Here a single generic name, based on priority but regardless of whether it is an ‘anamorph’ or ‘teleomorph’ generic name, is used for all unambiguous monophyletic phylogenetic lineages, as also done recently in other groups of fungi (Rossman & Samuels 2005, Crous et al. 2006d, 2008a, b, Damm et al. 2008, Phillips et al. 2008).
In the present study this approach is applied to the Mycosphaerellaceae and Teratosphaeriaceae. The aim is to provide a more natural classification for the genera in these families.
MATERIALS AND METHODS
Isolates
Leaves with leaf spot symptoms typical of infection by ‘Mycosphaerella’ were collected from various parts of the world. Excised lesions were soaked in water for approximately 2 h, after which they were attached to the bottom of Petri dish lids, with the top half of the dish containing 2 % malt extract agar (MEA; Oxoid, Hampshire, England) (Crous et al. 1991). Ascospore germination patterns were examined after 24 h, and single ascospore cultures established as described by Crous (1998). For those symptoms where no teleomorph was observed, cultures were established from single conidia.
DNA phylogeny
Genomic DNA was extracted from mycelium taken from fungal colonies on MEA using the UltraCleanTM Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). A part of the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene (SSU), the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and the first 900 bp at the 5’ end of the 28S rRNA gene (LSU) was amplified and sequenced as described by Cheewangkoon et al. (2008).
The generated LSU sequences were compared with other fungal DNA sequences from NCBI’s GenBank sequence database using a megablast search of the nr database; sequences with high similarity were added to the alignment. The alignment was subjected to neighbour-joining phylogenetic analyses as described by Cheewangkoon et al. (2008) and to a RAxML v7.0.4 analysis (Stamatakis et al. 2005a, b) using a maximum likelihood (ML) search with 1 000 bootstrap replicates (Stamatakis et al. 2008) as implemented at the CIPRES portal v1.15 (http://www.phylo.org/portal/Home.do). Search parameters assigned by the search engine included a GAMMA model of rate heterogeneity, ML estimation of the alpha-parameter and a GTR substitution matrix. Novel sequences were lodged in GenBank (Table 1) and the alignments and phylogenetic trees in TreeBASE (http://www.treebase.org).
Table 1.
Details of the isolates for which novel sequences were generated.
| Species | Accession number1 | GenBank number (28S nrDNA) |
|---|---|---|
| Baudoinia compniacensis | CBS 123031; DAOM 238773; UAMH 10808 | GQ852580 |
| CBS 123032; DAOM 237864; UAMH 10764 | GQ852581 | |
| Capnobotryella renispora | CBS 215.90; IAM 13015 | GQ852582 |
| Cercospora apii | CBS 118712 | GQ852583 |
| Cercospora zebrinae | CBS 118790; IMI 262766; WAC 7973 | GQ852584 |
| Cercosporella virgaureae | CBS 113304 | GQ852585 |
| Dissoconium aciculare | CBS 201.89 | GQ852586 |
| CBS 204.89 | GQ852587 | |
| Dissoconium australiensis | CBS 120729; CPC 13282 | GQ852588 |
| Dissoconium commune | CBS 110747; CPC 831 | GQ852589 |
| CBS 114239; CPC 10492 | GQ852590 | |
| CPC 12397 | GQ852591 | |
| Dissoconium dekkeri | CPC 13098 | GQ852592 |
| CPC 13264 | GQ852593 | |
| CPC 13279 | GQ852594 | |
| CPC 13479 | GQ852595 | |
| Dothistroma pini | CBS 116487; CMW 10951 | GQ852596 |
| Dothistroma septosporum | CBS 112498; CPC 3779 | GQ852597 |
| Lecanosticta acicola | CBS 871.95; MPFN 314 | GQ852598 |
| ‘Mycosphaerella’ acaciigena | CBS 112515; CPC 3837 | GQ852599 |
| CBS 112516; CPC 3838 | GQ852600 | |
| ‘Mycosphaerella’ africana | CBS 116154; CMW 4945; CPC 794 | GQ852601 |
| ‘Mycosphaerella’ ellipsoidea | CBS 110843; CPC 850 | GQ852602 |
| ‘Mycosphaerella’ endophytica | CBS 114662; CPC 1193 | GQ852603 |
| ‘Mycosphaerella’ heimii | CBS 110682; CMW 4942; CPC 760 | GQ852604 |
| CPC 11000 | GQ852605 | |
| CPC 13099 | GQ852606 | |
| ‘Mycosphaerella’ heimioides | CBS 111190; CMW 3046; CPC 1312 | GQ852607 |
| ‘Mycosphaerella’ holualoana | CBS 110699; CPC 2155 | GQ852608 |
| ‘Mycosphaerella’ irregulariramosa | CBS 111211; CPC 1362 | GQ852609 |
| ‘Mycosphaerella’ keniensis | CBS 111001; CMW 5147; CPC 1084 | GQ852610 |
| ‘Mycosphaerella’ konae | CPC 10992 | GQ852611 |
| ‘Mycosphaerella’ marksii | CBS 110942; CPC 982 | GQ852612 |
| CPC 11222 | GQ852613 | |
| CPC 13273 | GQ852614 | |
| CPC 13724 | GQ852615 | |
| ‘Mycosphaerella’ parkii | CBS 387.92; CMW 14775; CPC 353 | GQ852616 |
| Mycosphaerella pyri | CBS 222.31; CPC 3677 | GQ852617 |
| Passalora bellynckii | CBS 150.49; CPC 3635 | GQ852618 |
| Passalora brachycarpa | CBS 115124 | GQ852619 |
| ‘Passalora’ eucalypti | CBS 111318; CPC 1457 | GQ852620 |
| ‘Passalora’ graminis | CBS 113303 | GQ852621 |
| ‘Passalora’ sp. | CPC 11876 | GQ852622 |
| CPC 12319 | GQ852623 | |
| ‘Passalora’ vaginae | CBS 140.34; DSM 1148; IMI 303641 | GQ852624 |
| ‘Penidiella’ sp. | CBS 124991; CPC 12400 | GQ852625 |
| CBS 124993; CPC 13692 | GQ852626 | |
| ‘Phacellium’ paspali | CBS 113093; RoKI 1144 | GQ852627 |
| Phaeothecoidea sp. | CBS 124994; CPC 13711 | GQ852628 |
| CBS 124995; CPC 13710 | GQ852629 | |
| Pseudocercospora bixae | CBS 111804; CPC 2554 | GQ852630 |
| Pseudocercospora crousii | CBS 119487; Lynfield 1260 | GQ852631 |
| Pseudocercospora fijiensis | X300 | GQ852632 |
| Pseudocercospora griseola forma griseola | CPC 10779 | GQ852633 |
| Pseudocercospora paraguayensis | CBS 111317; CPC 1458 | GQ852634 |
| Pseudocercospora platani | CBS 110755; IMI 136770; CPC 4299 | GQ852635 |
| Pseudocercospora pseudoeucalyptorum | CBS 114242; CMW 14908; CPC 10390 | GQ852636 |
| CPC 12406 | GQ852637 | |
| CPC 12568 | GQ852638 | |
| CPC 12802 | GQ852639 | |
| CPC 12957 | GQ852640 | |
| CPC 13455 | GQ852641 | |
| CPC 13769 | GQ852642 | |
| CPC 13816 | GQ852643 | |
| CPC 13926 | GQ852644 | |
| Pseudocercospora punctata | CPC 10532 | GQ852645 |
| Pseudocercospora schizolobii | CBS 124990; CPC 13492 | GQ852646 |
| Pseudocercospora sp. | CBS 124996; CPC 12960 | GQ852647 |
| CPC 13008 | GQ852648 | |
| CPC 13299 | GQ852649 | |
| CPC 13315 | GQ852650 | |
| CPC 14621 | GQ852651 | |
| Pseudocercospora sphaerulinae | CBS 112621; CPC 4314 | GQ852652 |
| Ramulispora sorghi | CBS 110578; CPC 905 | GQ852653 |
| CBS 110579; CPC 906 | GQ852654 | |
| Readeriella callista | CBS 124986; CPC 13615 | GQ852655 |
| CPC 12715 | GQ852656 | |
| CPC 12727 | GQ852657 | |
| CPC 12841 | GQ852658 | |
| CPC 13605 | GQ852659 | |
| Readeriella eucalypti | CPC 13401 | GQ852660 |
| Readeriella mirabilis | CPC 12379 | GQ852661 |
| CPC 13611 | GQ852662 | |
| Readeriella nontingens | CPC 14444 | GQ852663 |
| Readeriella patrickii | CBS 124987; CPC 13602 | GQ852664 |
| Readeriella sp. | CBS 124997; CPC 13608 | GQ852665 |
| CBS 124998; CPC 13618 | GQ852666 | |
| CBS 124999; CPC 13026 | GQ852667 | |
| CBS 125001; CPC 13599 | GQ852668 | |
| CBS 125002; CPC 13631 | GQ852669 | |
| CBS 125003; CPC 14447 | GQ852670 | |
| CPC 13621 | GQ852671 | |
| CPC 13630 | GQ852672 | |
| Septoria aceris | CBS 652.85 | GQ852673 |
| Septoria apiicola | CBS 400.54; IMI 092628 | GQ852674 |
| Septoria convolvuli | CBS 102325 | GQ852675 |
| Septoria cucubali | CBS 102368 | GQ852676 |
| Septoria leucanthemi | CBS 109090 | GQ852677 |
| Septoria senecionis | CBS 102366 | GQ852678 |
| Sonderhenia eucalypticola | CPC 11252 | GQ852679 |
| Teratosphaeria considenianae | CPC 13032 | GQ852680 |
| CPC 14057 | GQ852681 | |
| Teratosphaeria cryptica | CBS 110975; CMW 3279; CPC 936 | GQ852682 |
| CPC 12415 | GQ852683 | |
| CPC 12424 | GQ852684 | |
| CPC 12559 | GQ852685 | |
| CPC 12562 | GQ852686 | |
| CPC 12565 | GQ852687 | |
| CPC 13839 | GQ852688 | |
| CPC 13842 | GQ852689 | |
| Teratosphaeria destructans | CBS 111370; CPC 1368 | GQ852690 |
| Teratosphaeria eucalypti | CPC 12552 | GQ852691 |
| Teratosphaeria molleriana | CPC 12232 | GQ852692 |
| CPC 12246 | GQ852693 | |
| Teratosphaeria nubilosa | CPC 11926 | GQ852694 |
| CPC 12235 | GQ852695 | |
| CPC 12243 | GQ852696 | |
| CPC 12830 | GQ852697 | |
| CPC 13452 | GQ852698 | |
| CPC 13825 | GQ852699 | |
| CPC 13828 | GQ852700 | |
| CPC 13831 | GQ852701 | |
| CPC 13833 | GQ852702 | |
| CPC 13835 | GQ852703 | |
| CPC 13837 | GQ852704 | |
| CPC 13844 | GQ852705 | |
| CPC 13847 | GQ852706 | |
| CPC 13849 | GQ852707 | |
| ‘Teratosphaeria’ parva | CPC 12249 | GQ852708 |
| CPC 12419 | GQ852709 | |
| ‘Teratosphaeria’ sp. | CBS 120040; CPC 12712 | GQ852710 |
| CBS 125006; CPC 14514 | GQ852711 | |
| CPC 12200 | GQ852712 | |
| CPC 13680 | GQ852713 | |
| CPC 14535 | GQ852714 | |
| Teratosphaeria stellenboschiana | CBS 124989; CPC 13767 | GQ852715 |
| CPC 12283 | GQ852716 | |
| CPC 13764 | GQ852717 | |
| ‘Teratosphaeria’ suberosa | CPC 11032 | GQ852718 |
| CPC 13091 | GQ852719 | |
| CPC 13093 | GQ852720 | |
| CPC 13094 | GQ852721 | |
| CPC 13095 | GQ852722 | |
| CPC 13096 | GQ852723 | |
| CPC 13104 | GQ852724 | |
| CPC 13106 | GQ852725 | |
| CPC 13111 | GQ852726 | |
| CPC 13115 | GQ852727 | |
| CPC 13736 | GQ852728 | |
| Teratosphaeria suttonii | CBS 119973, CMW 23440 | GQ852729 |
| Verrucisporota daviesiae | CBS 116002; VPRI 31767 | GQ852730 |
| Verrucisporota proteacearum | CBS 116003; VPRI 31812 | GQ852731 |
| Zasmidium anthuriicola | CBS 118742 | GQ852732 |
| Zasmidium citri | CPC 13467 | GQ852733 |
| ‘Zasmidium’ sp. | CPC 12748 | GQ852734 |
| CPC 14044 | GQ852735 | |
| CPC 14636 | GQ852736 |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI) of the University of Pretoria, Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; DSM: Deutsche Sammlung von Mikrorrganismen und Zellkulturen GmbH, Braunschweig, Germany; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; MPFN: Culture collection at the Laboratoire de Pathologie Forestiére, INRA, Centre de Recherches de Nancy, 54280 Champenoux, France; UAMH: University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada; VPRI: Victorian Department of Primary Industries, Knoxfield, Australia; WAC: Department of Agriculture Western Australia Plant Pathogen Collection, Perth, Australia; X: Private culture collection of Mahdi Arzanlou; RoKI: Private culture collection Roland Kirschner; Lynfield: Private culture collection Frank Hill.
Taxonomy
To confirm the morphology of the included strains, fungal structures were mounted in lactic acid for microscopic examination. Colonies were sub-cultured onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), MEA, and oatmeal agar (OA) (Crous et al. 2009b), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. Colony colours were rated according to the colour charts of Rayner (1970). All cultures obtained in this study are maintained in the culture collection of the CBS (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004a).
RESULTS
Phylogenetic analysis
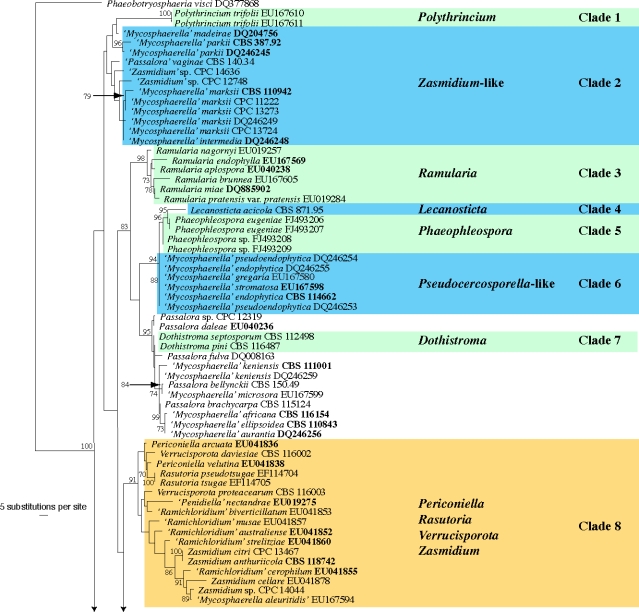
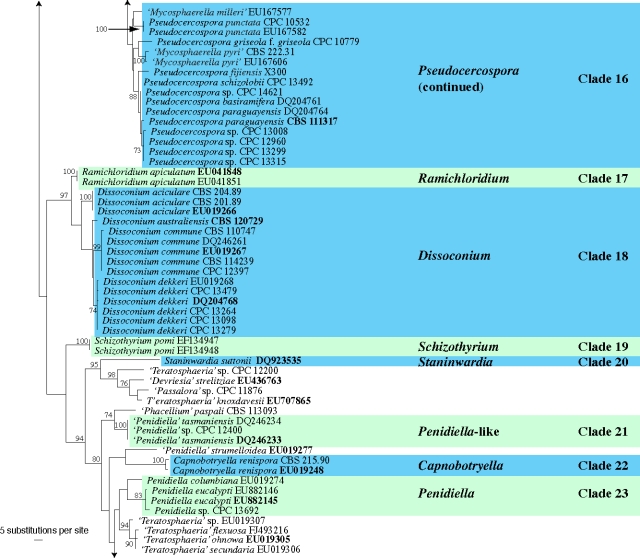
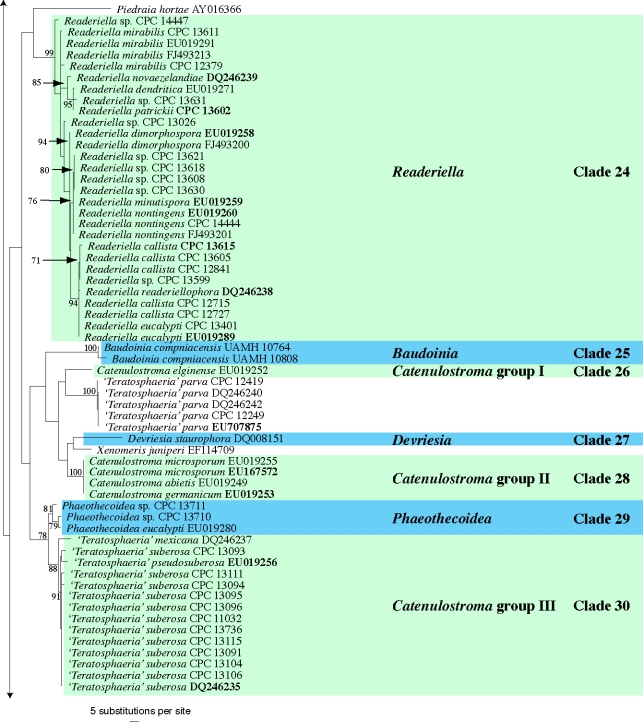
The manually adjusted LSU alignment contained 316 taxa (including the outgroup sequence) and 773 characters were included in the phylogenetic analysis. As the focus of this paper was the higher-order phylogeny of these fungi, the ITS sequences obtained were not used in the phylogenetic analyses. They were, however, used in a follow-up study on species (Crous et al. 2009c) and lodged in GenBank as part of that study, if not present there already. The three distance analyses yielded trees with identical overall topologies and supported the same lineages as the RAxML phylogeny but with some rearrangements of lineages at the deeper nodes (data not shown). Examples of these rearrangements include the swapping of Clade 18 (Dissoconium) and Clade 19 (Schizothyrium) from the Teratosphaeriaceae to the Mycosphaerellaceae compared to the RAxML phylogeny, highlighting the insecure phylogenetic position of these two genera. No significant increase or decrease in bootstrap support values was observed between the distance and RAxML analyses and the low bootstrap support values observed for some clades (see below) could be due to the choice of gene and/or the sampling for the analyses. The obtained RAxML phylogeny with a tree length of 2 357 547 is shown in Fig. 1. The final ML optimisation likelihood value obtained was −9197.49319 and the alpha value was estimated as 0.236078.
Fig. 1.
(five parts) Maximum likelihood tree from RAxML showing the phylogenetic relationships based on the LSU sequence alignment. The scale bar shows 5 substitutions per site, and bootstrap support values (> 69 %) from 1 000 replicates are shown at the nodes. Ex-type sequences are printed in bold face. The tree was rooted to Phaeobotryosphaeria visci (GenBank accession DQ377868).
Taxonomy
Numerous anamorph genera have been associated with ‘Mycosphaerella’, although the genus has largely been used as a convenient mycological concept, rather than a phylogenetic entity. As increasing numbers of asexual ‘genera’ are collected and subjected to DNA sequence analysis, many of these reside in the Capnodiales (Schoch et al. 2006, Crous et al. 2007a). The present study addresses the question of logical groupings for some of these genera. Many remain unresolved, chiefly due to low numbers of taxa presently available in culture that can thus be used for DNA sequence analyses. As greater numbers of taxa are collected, the generic boundaries of more clades will be resolved. For the present, however, we treat only those genera that could be resolved based on available cultures. In each case the generic name to use for a specific clade is indicated if that clade is resolved. Many phylogenetically distinct taxa still remain in ‘Mycosphaerella’, ‘Teratosphaeria’ or in one of the associated asexual genera, and these can only be disposed to their correct genera as their taxonomy and DNA phylogeny are clarified. The clade numbers below refer to the numbers indicated on Fig. 1. Several unresolved clades are left untreated and are thus not discussed.
Clade 1: Polythrincium (Cymadothea teleomorph; Mycosphaerellaceae)
Polythrincium trifolii (teleomorph Cymadothea trifolii), an important foliar pathogen of clover, was recently treated by Simon et al. (2009). The anamorph genus Polythrincium (1817) predates the Mycosphaerella-like teleomorph genus, Cymadothea (1935), and morphologically the most informative morph. The older generic name Polythrincium is thus preferred for this clade.
Clade 2: Zasmidium-like (Mycosphaerellaceae)
Although the following taxa resemble others in the Zasmidium clade, they cluster as sister to Zasmidium s.s. (Clade 8), which is poorly resolved. Taxa in this clade all form hyaline propagules of a synanamorph in their aerial mycelium, although this feature is not restricted to taxa in this clade. Presently it is still unclear which features separate this clade from Zasmidium s.s., and thus the latter name is applied to both clades.
Clade 3. Ramularia (Mycosphaerella s.s. teleomorphs; Mycosphaerellaceae)
The genus Mycosphaerella is typified by M. punctiformis, which has a Ramularia anamorph, R. endophylla (Verkley et al. 2004a). Ramularia represents a well-known genus of anamorphs that has been monographed (Braun 1998), representing hyaline hyphomycetes with solitary to fasciculate conidiophores, and aseptate to transversely septate hyaline conidia with thickened, darkened, refractive scars. Given the fact that Mycosphaerella has been applied in the broad sense to many diverse genera in the family, and has become a ‘name of convenience’ rather than one indicative of genealogical relationship, we consider that it would be best to use the older name for this clade, namely Ramularia (1833), rather than Mycosphaerella (1884). The recently reported unique scar structure separating Cercosporella from Ramularia should also be noted here. Based on these observations on Cercosporella centaureicola (CBS 120253) by Kirschner (2009), as well as its phylogenetic placement, C. centaureicola is accepted as a likely synonym of R. nagornyi, as discussed by Kirschner (2009).
Clade 4: Lecanosticta (Mycosphaerellaceae)
This lineage includes only Lecanosticta acicola (teleomorph: M. dearnessii) and additional taxa will need to be added before it can be adequately resolved. Lecanosticta acicola (= L. pini) is the type species of the genus Lecanosticta and represents the generic name that should be used for this clade.
Clade 5: Phaeophleospora (Mycosphaerellaceae)
Phaeophleospora is characterised by pycnidia that give rise to conidia via brown, percurrently proliferating conidiogenous cells (Crous et al. 1997), and by brown, scolecosporous conidia with transverse septa. This morphology has evolved several times in the Capnodiales. Andjic et al. (2007a) separated Phaeophleospora from the phylogenetically distant Kirramyces based on the pigment gradient observed in conidia of P. eugeniae, the type species of Phaeophleospora. Crous et al. (2007a) showed that Phaeophleospora belonged to the Mycosphaerellaceae, whilst Kirramyces belonged to the Teratosphaeriaceae. Very few species of Phaeophleospora are presently known from culture, and most need to be recollected, and their morphological features and classification re-evaluated.
Clade 6: Pseudocercosporella-like (Mycosphaerellaceae)
Taxa residing in this clade have anamorphs and teleomorphs that resemble Pseudocercosporella and Mycosphaerella, respectively. However, the type species of Pseudocercosporella, P. ipomoeae, needs to be recollected before the generic name applicable to this clade can be resolved.
Clade 7: Dothistroma (Mycosphaerellaceae)
Dothistroma (1941) is based on D. pini, and is linked to a Mycosphaerella-like (or Scirrhia, Eruptio) teleomorph. The two species of Dothistroma that have been subjected to DNA sequence analysis cluster together in this clade, which is closely related to Passalora-like fungi, for which the status remains unclear. The appropriate name for this clade is still unclear, as we suspect that adding more taxa would lead to a better resolution of morphological types within the larger clade in which Dothistroma resides.
Clade 8: Zasmidium-complex (Mycosphaerella-like and Rasutoria teleomorphs; Mycosphaerellaceae)
Zasmidium is characterised by coarsely verrucose, olivaceous-green hyphae, that give rise to conidiophores with integrated conidiogenous cells that proliferate sympodially near the apex, with conspicuously pigmented, darkened, somewhat refractive planate scars. Conidia are formed singly or in short chains, and are cylindrical to fusiform, verrucose, obovate to obconical, subhyaline to pigmented, 0–pluri-septate, with conspicuous, slightly pigmented, thickened, refractive hila. Morphologically, Zasmidium resembles Stenella, but the type species of the latter genus clusters in the Teratosphaeriaceae (Arzanlou et al. 2008), whereas Zasmidium clusters in the Mycosphaerellaceae. Conidia of Stenella (S. araguata) have pileate scars (David 1993), while those of Zasmidium (Z. cellare) and former Stenella species belonging in the Mycosphaerellaceae are planate, i.e. Cercospora-like.
The Zasmidium clade remains poorly resolved (Fig. 1, part 1), and it also includes the type species of Periconiella (P. velutina) and Verrucisporota (V. proteacearum). Furthermore, the Zasmidium-like morphology has also evolved elsewhere in the Mycosphaerellaceae (Fig. 1, clade 2). Additional collections need to be added to clarify the relationships among taxa with the morphology type (verrucose superficial hyphae with pigmented structures, and thickened, darkened, refractive, convex scars). The identity of Mycosphaerella aleuritidis (CBS 282.62) could not be confirmed in culture, and hence its position in this clade, and purported Pseudocercospora aleuritidis anamorph, remains uncertain. Zasmidium is presently paraphyletic in the Mycosphaerellaceae. The genus Zasmidium s.s. should be applied to this clade, though more taxa need to be added to resolve the status of other morphotypes (genera) clustering in this clade.
Zasmidium anthuriicola (U. Braun & C.F. Hill) Crous & U. Braun, comb. nov. — MycoBank MB509715
Basionym. Stenella anthuriicola U. Braun & C.F. Hill, Fung. Diversity 22: 33. 2006.
Zasmidium citri (Whiteside) Crous, comb. nov. — MycoBank MB509716
Basionym. Mycosphaerella citri Whiteside, Phytopathology 62: 263. 1972.
Anamorph. Cercospora citrigrisea F.E. Fisher, Phytopathology 51: 300. 1961.
≡ Stenella citrigrisea (F.E. Fisher) Sivan., in Sivanesan, Bitunicate ascomycetes and their anamorphs: 226. 1984.
Clade 9: Cercosporella (Mycosphaerellaceae)
Cercosporella (1880), which is based on C. virgaureae (= C. cana), has hyaline conidiophores and conidia with planate, slightly thickened and somewhat refractive, inconspicuous, smooth conidial scars (Braun 1995, Kirschner 2009). Although hardly any species are known from culture, the genus appears to be phylogenetically distinct.
Clade 10: Ramulispora (Mycosphaerellaceae)
Ramulispora is typified by R. sorghi, a pathogen that causes prominent leaf spots on sorghum called sooty stripe, due to the abundant production of microsclerotia on the leaf surface (Braun 1995, Crous et al. 2003a). It is further characterised by forming sporodochia with hyaline, transversely euseptate, scolecosporous conidia.
Clade 11: Cercospora (Mycosphaerellaceae)
The genus Cercospora, which is based on Cercospora penicillata (= C. depazeoides), contains more than 600 species that are saprobic or plant pathogenic (Crous & Braun 2003, Groenewald et al. 2005, 2006, Crous et al 2006a). Conidiophores are solitary to fasciculate, arising from internal hyphae or stromata, erect, continuous to pluriseptate, subhyaline to pigmented, smooth to finely roughened, with integrated, terminal or intercalary conidiogenous cells, proliferating sympodially, and conspicuously thickened, darkened, planate scars. Conidia are predominantly solitary, scolecosporous, obclavate to cylindrical-filiform, acicular, hyaline or subhyaline, mostly pluriseptate, smooth, with thickened, darkened, planate hila. Species of Cercospora form a well-defined clade in the Mycosphaerellaceae. Species with brown, pigmented conidia are accommodated in Passalora, though the latter concept has evolved in several clades in the Capnodiales, and remains to be resolved. The name to use for this clade is Cercospora, which represents a monophyletic genus (J.Z. Groenewald et al. in prep.).
Clade 12: Septoria (Mycosphaerellaceae)
Septoria (1884) includes more than 2 000 plant pathogenic coelomycetes that are associated with leaf spot diseases. The genus is characterised by pycnidial conidiomata, and hyaline conidiogenous cells with sympodial and/or percurrent proliferation, giving rise to filiform, hyaline, smooth-walled, multiseptate conidia (Verkley & Priest 2000). The majority of known species cluster within the Mycosphaerellaceae, although this morphology type has also evolved outside the family, and the genus is poly- and paraphyletic. The type species, S. cytisi, needs to be recollected to determine which Septoria-like clade is applicable to Septoria s.s. This clade, including Clade 11, was supported with a bootstrap support value of 68 % (not shown).
Clade 13. Sonderhenia (Mycosphaerellaceae)
Swart & Walker (1988) introduced the genus Sonderhenia to accommodate pycnidial anamorphs of Mycosphaerella that formed brown, transversely distoseptate conidia on brown, percurrently proliferating conidiogenous cells. Two species are known from the genus, namely S. eucalypticola and S. eucalyptorum, which appear to form a monophyletic clade (68 % bootstrap support value, not shown).
Clade 14. Pseudocercospora-like (Mycosphaerellaceae)
The fact that Pseudocercospora species cluster in two well defined clades is not surprising. What was unexpected, is that Pseudocercospora s.s. (Clade 16), clusters apart from a complex commonly referred to as the Ps. heimii clade, including species such as Ps. crystallina, Ps. heimii, Ps. heimioides, Ps. konae, Ps. irregulariramosa, Ps. thailandica, etc. These species all have smooth, pale brown, subcylindrical to narrowly obclavate conidia, conidiogenous cells that proliferate sympodially and occur singly on hyphae in culture, and colonies that form red crystals in agar when cultivated (Crous & Wingfield 1996, Crous 1998). The taxonomic status of this clade remains unresolved, and will be dealt with in a revision of the Pseudocercospora complex (Hunter et al. in prep.).
Clade 15. Passalora-like (Mycosphaerellaceae)
Hyphomycetes with pigmented conidia, and darkened, thickened, refractive scars, formed on fasciculate conidiophores, have traditionally been placed in Passalora (Braun 1995). Crous & Braun (2003) extended this concept to include taxa with superficial mycelium (Mycovellosiella, based on M. cajani) and conidia in chains (Phaeoramularia, based on Ph. gomphrenicola). This definition, however, appears to be inordinately wide, as several clades have taxa exhibiting the Passalora-like morphology. The type species of Passalora, P. bacilligera, must be recollected before the taxonomy of this complex can be fully resolved.
Clade 16. Pseudocercospora (Mycosphaerellaceae)
The genus Pseudocercospora represents species with pigmented conidiophores arranged singly on superficial hyphae, synnemata (in the type species, P. vitis, and in Phaeoisariopsis) to fascicles arising from a submerged to erumpent stroma, almost becoming sporodochial to acervular in some cases (Crous et al. 2006b). Conidiophores give rise to terminal and intercalary conidiogenous cells that form conidia via sympodial and/or percurrent proliferations. Proliferations can be rough and irregular (Cercostigmina), or smooth and inconspicuous. Conidia are mostly scolecosporous, smooth or finely roughened, pigmented, thin- to thick-walled (Scolecostigmina), transverse or with oblique eu- to distosepta (Stigmina). Conidiogenous loci are inconspicuous, or slightly thickened around the rim (Paracercospora and Passalora-like). Pseudocercospora has recently been conserved over Stigmina (Braun & Crous 2006), and is the recommended generic name for this clade. Cultures of some taxa in this clade could not be confirmed morphologically, and probably represent misidentifications, namely Mycosphaerella milleri (CBS 541.63), which is supposed to have Passalora magnoliae as an anamorph, and Mycosphaerella pyri (CBS 222.31), which is supposedly linked to Septoria pyricola. This clade was supported with a bootstrap support value of 62 % (not shown).
Pseudocercospora fori (G.C. Hunter, Crous & M.J. Wingf.) G.C. Hunter, Crous & M.J. Wingf., comb. nov. — MycoBank MB509717
Basionym. Mycosphaerella fori G.C. Hunter, Crous & M.J. Wingf., Mycol. Res. 108: 677. 2004.
Notes — This species commonly forms its Pseudocercospora state in culture and on host material. Although originally named in ‘Mycosphaerella’ (Hunter et al. 2004), this fungus is better accommodated in Pseudocercospora. Both sexual and asexual states are fully described in the original publication by Hunter et al. (2004).
Pseudocercospora schizolobii (M.J. Wingf. & Crous) M.J. Wingf. & Crous, comb. nov. — MycoBank MB509718
Basionym. Passalora schizolobii M.J. Wingf. & Crous, Fungal Planet 2. 2006.
Culture characteristics — Colonies on MEA erumpent, irregular, sectored, with sparse aerial mycelium, margin catenulate, smooth, surface crenate, olivaceous-grey; reverse iron-grey; reaching 20 mm diam after 1 mo; on OA erumpent, spreading with moderate aerial mycelium, and smooth, catenulate margins, pale olivaceous-grey to olivaceous-grey; reaching 25 mm after 1 mo.
Specimens examined. Ecuador, Buenos Aires, Pacheco, on leaves of Schizolobium parahybum, 17 Jan. 2006, M.J. Wingfield, culture ex-type CPC 12962 = CBS 120029. – Thailand, on leaves of Eucalyptus camaldulensis, Oct. 2006, W. Himaman, CPC 13492 = CBS 124990.
Notes — Passalora schizolobii was described as a leaf spot pathogen of Schizolobium parahybum from Ecuador (Wingfield et al. 2006). The present collection represents what appears to be the same species (on ITS sequence data, and morphology), but occurring on Eucalyptus. Conidia are 1–7-septate, and (30–)40–55(–80) × (2.5–)3(–3.5) μm, with inconspicuous hila, 1–1.5 μm wide. Passalora schizolobii was placed in Passalora due to the slightly darkened, thickened hila. Morphologically it represents an intermediate between Passalora and Pseudocercospora, which explains why it clusters among other species of Pseudocercospora, suggesting that taxa with scars and hila that are slightly darkened and thickened, but not refractive, should rather be placed in Pseudocercospora than in Passalora (Crous & Braun 2003). A multi-gene approach and inoculation studies are required to clarify if the Eucalyptus isolates are really the same as those causing a serious disease on Schizolobium.
Clade 17: Ramichloridium
In the past Ramichloridium included a heterogeneous group of fungi with diverse life styles, viz. saprobes, human and plant pathogens. Ramichloridium is characterised by taxa with erect, dark, more or less differentiated, branched or unbranched conidiophores and predominantly aseptate conidia produced on a sympodially proliferating rachis (de Hoog 1977). No teleomorph has been linked to the genus. Ramichloridium was accepted as paraphyletic by Arzanlou et al. (2008), with taxa clustering in the Mycosphaerellaceae and Teratosphaeriaceae. The type species of the genus, R. apiculatum, clusters with Dissoconium, but its higher order phylogenetic relationship has not been resolved.
Clade 18. Dissoconium (Mycosphaerella-like teleomorphs)
Dissoconium is unique in the Capnodiales in that it is characterised by producing pairs of forcibly discharged primary and secondary conidia on sympodially proliferating conidiogeneous cells, which gives rise to a conidium-bearing rachis (Crous et al. 2007c, 2008a, Arzanlou et al. 2008). The genus is peculiar in that the two conidial types frequently anastomose after being forcibly discharged, and some species form microsclerotial bodies in culture (Crous et al. 2008a). Where known, teleomorphs have been accommodated in ‘Mycosphaerella’ (Crous et al. 2004b). Morphologically there is little to choose between these teleomorphs and Mycosphaerella s.s., except that they tend to have a non-persistent mucilaginous sheath surrounding their ascospores, and periphyses lining the ostiolar cavity. These features, however, also occur in several species of Mycosphaerella s.s. It has thus not been possible to identify morphological features in the teleomorph that could be used to separate these clades. The recommended genus name for this clade is Dissoconium, although additional taxa need to be added to clarify its higher order phylogeny. This clade was supported with a bootstrap support value of 60 % (not shown).
Clade 19: Schizothyrium (Zygophiala anamorphs)
In former classification schemes, the genus Schizothyrium was placed in the Schizothyriaceae, Dothideales (von Arx & Müller 1975), Microthyriales (Kirk et al. 2001), and Dothideomycetes (Eriksson 2006). Schizothyrium is characterised by strongly flattened, crustose, rounded or elongated ascomata, opening by irregular splits, with bitunicate asci, and some interascal tissue composed of remnants of stromatal cells, and transversely 1-septate, hyaline to pale brown ascospores. Recent phylogenetic studies (Schoch et al. 2006, Batzer et al. 2008) support Schizothyrium as residing in the Dothideomycetes, subclass Dothideomycetidae, order Capnodiales. Results obtained here support the family Schizothyriaceae as sister to the Mycosphaerellaceae. It should be noted, however, that the type species, S. acerinum, is presently not known from culture, and needs to be recollected.
Clade 20: Staninwardia (Teratosphaeriaceae)
Staninwardia is known from two species occurring on leaf spots of Eucalyptus (Summerell et al. 2006). It is characterised by acervuli with brown, catenulate conidia covered in a mucilaginous sheath. No teleomorph state has been reported for this genus.
Clades 21, 23: Penidiella complex (Teratosphaeriaceae)
The genus Penidiella, which is based on P. columbiana, is polyphyletic. Species of Penidiella have synnematous to solitary conidiophores, consisting of a single terminal conidiogenous cell giving rise to several ramoconidia that form secondary ramoconidia, or a branched apparatus composed of several terminal and sometimes lateral conidiogenous cells giving rise to sequences of ramoconidia. The branched apparatus may be loose to dense, metula-like. The conidiogenous cells have only few, usually 1–3(–4), terminal or subterminal subdenticulate loci, and ramoconidia are prominent and numerous, giving rise to branched chains of secondary conidia with flat-tipped hila. Species that have thus far been found to cluster apart from the type, P. columbiana, appear to have a different conidiogenous apparatus. Inordinately few taxa are known from this complex, and it is premature to subdivide Penidiella. We thus retain it as paraphyletic taxon.
Clade 22: Capnobotryella (Teratosphaeriaceae)
Capnobotryella (based on C. renispora) is characterised by forming brown, septate, thick-walled hyphae, with ellipsoidal, 0–1-septate conidia forming directly on the hyphae, via minute phialides, and the production of endoconidia (Sugiyama & Amano 1987). No teleomorph state has been reported for this genus.
Clade 24: Readeriella (incl. Nothostrasseria, with Teratosphaeria-like teleomorphs, and Cibiessia synanamorphs) (Teratosphaeriaceae)
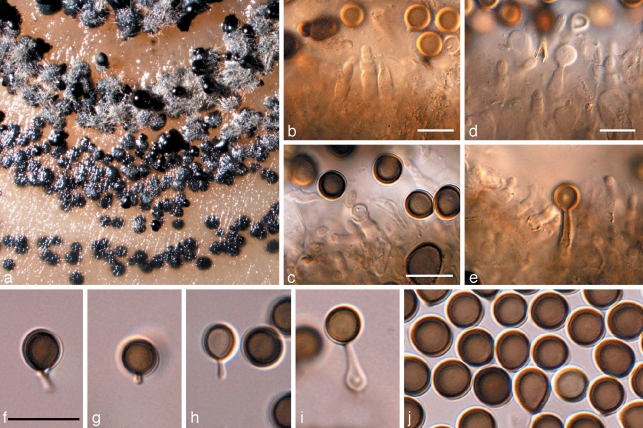
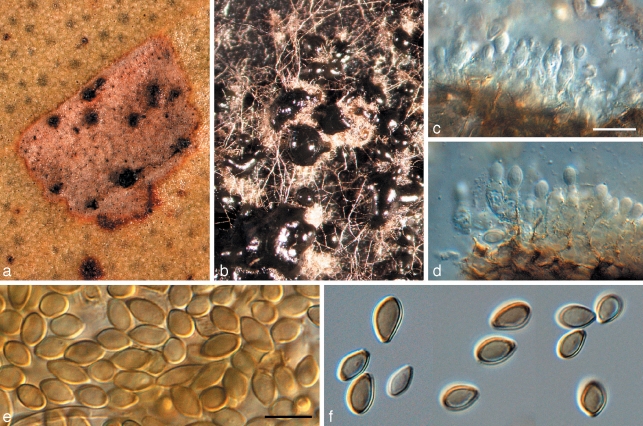
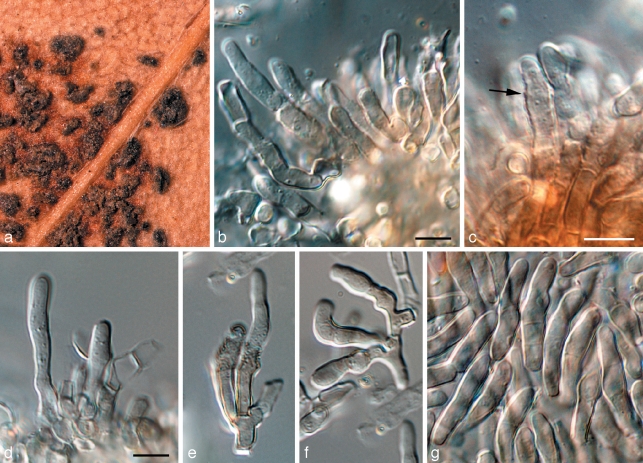
Crous et al. (2007a) used a wider concept for Readeriella, and recognised it as being polyphyletic within the Teratosphaeriaceae. Furthermore, based on its conidiogenesis that is very similar to that of Kirramyces, with conidiogenous cells ranging from mono- to polyphialides with periclinal thickening, to phialides with percurrent proliferation, the two genera were seen as synonymous. However, the present analysis shows that these two clades cluster apart within the Teratosphaeriaceae (Fig. 1, part 4). Although they are morphologically similar, Readeriella species have conidia that tend to have tapering subtruncate bases, and frequently form Cibiessia synanamorphs. In contrast, Kirramyces and Colletogloeopsis anamorphs have truncate conidial bases, and are never found associated with Cibiessia synanamorphs. Nothostrasseria (1983) has conidiogenesis similar to that in Readeriella, and forms conidia with basal appendages, which can also occur in Readeriella eucalypti (Fig. 2, Crous et al. 2007a). The generic name for this clade is the older name, Readeriella (1908).
Fig. 2.
Readeriella eucalypti (CPC 14950). a. Colony on OA; b–e. conidiogenous cells giving rise to conidia; f–i. conidia with basal appendages; j. conidia. — Scale bars = 10 μm.
Readeriella Syd. & P. Syd., Ann. Mycol. 6: 484. 1908.
Teleomorph. Teratosphaeria-like
= Nothostrasseria Nag Raj, Canad. J. Bot. 61: 23. 1983.
= Cibiessia Crous, Fung. Diversity 26: 151. 2007.
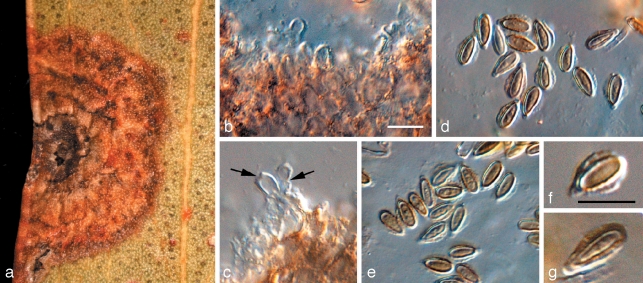
Readeriella callista (Syd.) Crous & Summerell, comb. nov. — MycoBank MB509719; Fig. 3
Fig. 3.
Readeriella callista (CPC 13615). a. Leaf spot with black pycnidia; b. colony on OA; c, d. conidiogenous cells giving rise to conidia; e, f. conidia. — Scale bars = 10 μm.
Basionym. Coniothyrium callistum Syd., Ann. Mycol. 35: 358. 1937.
≡ Microsphaeropsis callista (Syd.) B. Sutton, Mycol. Pap. 123: 35. 1971.
Leaf spots amphigenous, irregular, 2–5 mm diam, pale brown, with a thin, raised, dark brown border; associated with wasp damage in some collections. Conidiomata amphigenous, pycnidial, brown, up to 350 μm diam; wall consisting of 3–4 layers of brown textura angularis. Description on OA. Conidiophores subcylindrical, pale brown, finely verruculose, 0–2-septate, unbranched or branched above, frequently covered in a mucilaginous layer, 10–15 × 3–4 μm. Conidiogenous cells pale brown, finely verruculose, aseptate, dolliform to subcylindrical, proliferating percurrently near apex, 5–7 × 3–4 μm. Conidia solitary, brown, aseptate, smooth to finely verruculose, ellipsoid to fusoid, thick-walled, apex subobtuse, base subtruncate, apex and base with darker pigmentation, (7–)8–10(–11) × (3–)4–5(–5.5) μm; base 1–2 μm wide. Microconidiophores intermixed among macroconidiophores, cylindrical, straight to flexuous, 2 μm wide, variable in length. Microconidia ellipsoid, hyaline, smooth, 3–5 × 2 μm, apex obtuse, base subtruncate.
Culture characteristics — Colonies on MEA spreading, woolly, with moderate aerial mycelium, erumpent with uneven, catenulate margins; olivaceous-grey on surface, iron-grey in reverse; colonies reaching 50 mm diam after 1 mo; on OA woolly with moderate aerial mycelium, iron-grey with patches of olivaceous-grey, covering the plate after 1 mo; fertile.
Specimens examined. Australia, New South Wales, Bulli, on leaves of Eucalyptus haemastoma, Aug. 1935, F. Fraser, IMI 21230 holotype; New South Wales, Woodford 33°43’30"S, 150°29’25"E, on leaves of Eucalyptus sclerophylla, Oct. 2006, coll. B.A. Summerell, CBS H-20246 epitype designated here, isol. P.W. Crous, cultures ex-type CPC 13615 = CBS 124986, CPC13616, 13617; New South Wales, Rylestone, 32°39’31"S, 150°12’30"E, on leaves of Eucalyptus sp., Jan. 2006, coll. B.A. Summerell, isol. P.W. Crous, CPC 12727–12729; New South Wales, Rylestone, 32°39’31"S, 150°12’30"E, on leaves of Eucalyptus deanei, Jan. 2006, coll. B.A. Summerell, isol. P.W. Crous, CPC 12715–12717; New South Wales, Capertee 33°08’13"S, 150°04’46"E, on leaves of Eucalyptus cannonii, Jan. 2006, coll. B.A. Summerell, isol. P.W. Crous, CPC 12841–12843; New South Wales, Faulconbridge 33°40’18"S, 150°32’54"E, on leaves of Eucalyptus multicaulis, Oct. 2006, coll. B.A. Summerell, CBS H-20247, isol. P.W. Crous, CPC 13605–13607.
Notes — On OA, conidia of R. callista are somewhat longer and narrower, (7–)8–10(–11) × (3–)4–5(–5.5) μm, than those observed on host material, 7–8.5 × 4–5.5 μm. The overall conidial shape, thicker appearance of the conidial wall, and pigmentation, are very characteristic for this species.
Readeriella dendritica (Crous & Summerell) Crous & Summerell, comb. nov. — MycoBank MB509720
Basionym. Mycosphaerella dendritica Crous & Summerell, Fung. Diversity 26: 161. 2007.
Teleomorph. ‘Teratosphaeria’ dendritica (Crous & Summerell) Crous & U. Braun, Stud. Mycol. 58: 10. 2007.
Anamorph. Spilomyces dendriticus Hansf., Proc. Linn. Soc. New South Wales 81: 32. 1956.
≡ Nothostrasseria dendritica (Hansf.) Nag Raj, Canad. J. Bot. 61: 25. 1983.
Specimens examined. Australia, New South Wales, Rylestone, 32°39’31"S, 150°12’30"E, on leaves of Eucalyptus deanei, Feb. 2006, coll. B.A. Summerell, holotype CBS H-19772, isol. P.W. Crous, cultures ex-type CPC 12709 = CBS 120032, CPC 12710–12711; New South Wales, Laurel Hill, Bago State Forest, research trial, on leaves of E. nitens, 22 Dec. 2005, coll. A.J. Carnegie, isol. P.W. Crous, CPC 12820 = CBS 120733; Tasmania, on leaves of E. globulus, 31 Aug. 2006, coll. C. Mohammed, isol. P.W. Crous, CPC 13296 = CBS 120734, CPC 13297–13298.
Notes — The anamorph genus Nothostrasseria was introduced for a single species, N. dendritica, having conidia with characteristic basal appendages (Nag Raj 1993). The teleomorph was recently collected by Crous et al. (2007c), and subsequently shown to reside in the Teratosphaeriaceae (Crous et al. 2007a). Although the conidiogenesis is similar to that of Readeriella, the genus was tentatively retained as separate, as species of Readeriella generally lack persistent basal appendages. However, a recent collection of Readeriella eucalypti (CPC 14950, Fig. 2), was found to have basal appendages in vivo. Once cultured, conidia were found to frequently retain their basal appendages, similar to those observed in Harknessia, where species have appendages of different lengths (see Lee et al. 2004, Summerell et al. 2006). Because some species of Readeriella can have basal appendages, and as they cluster in a single well-supported clade (Fig. 1, part 4), there is no reason to retain Nothostrasseria as separate from Readeriella.
Readeriella dimorphospora (Crous & C. Mohammed) Crous, comb. nov. — MycoBank MB509721
Basionym. Cibiessia dimorphospora Crous & C. Mohammed, Fung. Diversity 26: 151. 2007.
≡ Readeriella minutispora (Crous & Carnegie) Crous & Carnegie, comb. nov. — MycoBank MB509722
Basionym. Cibiessia minutispora Crous & Carnegie, Fung. Diversity 26: 153. 2007.
Readeriella nontingens (Crous & Summerell) Crous & Summerell, comb. nov. — MycoBank MB509723
Basionym. Cibiessia nontingens Crous & Summerell, Fung. Diversity 26: 154. 2007.
Readeriella patrickii Crous & Summerell, sp. nov. — MycoBank MB509724; Fig. 4
Fig. 4.
Readeriella patrickii (CPC 13602). a. Leaf spot; b, c. conidiogenous cells giving rise to conidia (arrows denote loci); d–g. conidia with mucoid sheaths. — Scale bars = 10 μm.
Readeriellae mirabilis similis, sed conidiis sine projecturis lateralis, sed conidiis persistente mucilagine vaginatis, (6–)7–8(–9) × (2.5–)3(–3.5) μm.
Etymology. Named after Patrick Summerell, who collected this fungus when he accompanied the Council of Heads of Australasian Herbaria on their annual day out in the field after their meeting in Tasmania.
Leaf spots amphigenous, subcircular to irregular, pale to medium brown, with a raised, dark brown border, up to 5 mm diam. Description on OA. Conidiomata pycnidial, brown, globose, up to 250 μm diam; wall consisting of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, hyaline to pale brown, smooth to finely verruculose, dolliform, proliferating several times percurrently near apex, 5–7 × 3–4 μm. Conidia solitary, medium brown, aseptate, granular, finely verruculose, thick-walled, ellipsoid to obclavate, widest below the obtuse apex, base subtruncate to truncate, 1 μm wide, with inconspicuous marginal frill, (6–)7–8(–9) × (2.5–)3(–3.5) μm; covered in a persistent mucilaginous sheath.
Culture characteristics — Colonies on MEA spreading with moderate aerial mycelium and smooth, even margins, surface hazel, reverse umber to chestnut; on OA woolly, spreading with abundant aerial mycelium, and smooth, regular margins, pale olivaceous-grey to olivaceous-grey, covering the dish after 1 mo; colonies fertile on OA.
Specimen examined. Australia, Tasmania, Tasman Peninsula, 43°11’29.7"S, 147°51’00.7"E, on leaves of Eucalyptus amygdalina, 14 Oct. 2006, coll. P. Summerell & B.A. Summerell, CBS H-20248 holotype, isol. P.W. Crous, cultures ex-type CPC 13602 = CBS 124987, CPC 13603, 13604.
Notes — The collection on which this species is based sporulates poorly, and only after 1–2 mo were conidiomata observed on OA. Readeriella patrickii is distinct in that it has pycnidia, and pigmented, percurrently proliferating conidiogenous cells that give rise to aseptate, brown conidia with a persistent mucilaginous sheath. The latter feature has not been reported previously for any species of Readeriella.
Clade 25: Baudoinia (Teratosphaeriaceae)
Baudoinia, which is based on B. compniacensis, was erected by Scott et al. (2007) for a genus of hyphomycetes occurring on exposed surfaces exposed to substantial temperature and relative humidity shifts, characterised by brown, verrucose chains of conidia. No teleomorph has been reported for this fungus.
Clade 27: Devriesia (Teratosphaeriaceae)
Devriesia, which is based on D. staurophora, was erected by Seifert et al. (2004) for a heat-tolerant genus of hyphomycetes occurring in soil, with pigmented, catenulate conidia, somewhat darkened scars, and chlamydospores. No teleomorph state has been found.
Clades 26, 28, 30: Catenulostroma (Teratosphaeriaceae)
Catenulostroma is morphologically similar to Trimmatostroma, though the latter belongs to the Helotiales (Crous et al. 2007a). Trimmatostroma s.s. is well-distinguished from most Catenulostroma species by being saprobic, living on twigs and branches of woody plants, or occasionally isolated from leaf litter, i.e., they are not associated with leaf spots. However, it is clear that the concept of Catenulostroma as proposed by Crous et al. (2007a) is polyphyletic, but before this can be resolved, the type species, C. protearum, needs to be recollected.
Clade 29: Phaeothecoidea (Teratosphaeriaceae)
Crous et al. (2007c) established the genus Phaeothecoidea, based on P. eucalypti, and distinguished it from Hyphospora (teleomorph: Cumminutispora) and Phaeotheca, which both have endoconidia, on the basis of it having more thin-walled conidia, that become pigmented with age (Zalar et al. 1999). Colonies of Phaeothecoidea are wet and slimy. No teleomorphs have been reported to date (Crous et al. 2008a).
Clade 31: Teratosphaeria (with Kirramyces and Colletogloeopsis anamorphs, and Batcheloromyces-like synanamorphs) (Teratosphaeriaceae)
The genus Kirramyces (Walker et al. 1992) was established for a group of coelomycetes occurring on Eucalyptus with smooth to rough, brown-walled, percurrently proliferating conidiogenous cells, and brown, cylindrical to obclavate conidia with truncate bases and a marginal frill. It was distinguished from Sonderhenia on the basis of having distoseptate conidia, and from Stagonospora, by having pigmented conidia. Although two series of species were recognised, namely those with pale, finely verruculose conidia, and those with brown, rough conidia, it was decided to place them in a single genus, Kirramyces (B. Sutton, pers. comm.). The recollection of the type species of Phaeophleospora, namely P. eugeniae, showed that it had the same conidiogenesis as Kirramyces, leading Crous et al. (1997) to transfer all these taxa to Phaeophleospora (1916), which at the time appeared to be the older name for the complex. Crous & Wingfield (1996) introduced Colletogloeopsis, characterised by acervuli, and percurrently as well as sympodially proliferating conidiogenous cells, and 0–1-septate conidia. Based on cultural studies, Cortinas et al. (2006) found that species of Colletogloeopsis could have pycnidia as well as acervuli, concluding that conidiomatal structure was minimally useful in this group of anamorphic genera.
Employing DNA phylogenetic data, Andjic et al. (2007a) concluded that conidial septation was not informative at the generic level because taxa with aseptate conidia clustered with those having septate conidia. Furthermore, Andjic et al. (2007a) showed that Phaeophleospora eugeniae, the type species of Phaeophleospora clustered apart from Kirramyces epicoccoides, the type species of Kirramyces. Although conidia of P. eugeniae have an uneven pigmentation (more pale brown at the ends), this feature does not appear to hold up for other species of Phaeophleospora s.s. (P.W. Crous, in prep.). Crous et al. (2007a) showed Phaeophleospora to reside in the Mycosphaerellaceae and Kirramyces in the Teratosphaeriaceae, respectively. Phaeophloeospora was retained for P. eugeniae by Andjic et al. (2007a), while other taxa were recombined in Kirramyces, with Colletogloeopsis treated as synonym. Kirramyces was subsequently emended to incorporate conidia that vary from aseptate to euseptate, fusoid to cylindrical to long obclavate to ellipsoidal.
Crous et al. (2007a) placed the Teratosphaeria coelomycete anamorphs in Readeriella (1908), which they regarded as an older name for the coelomycetes accommodated in Kirramyces (1992). However, in the present study (Fig. 1, part 4, 5), we have shown that Readeriella is phylogenetically distinct from Kirramyces anamorphs. Morphologically, the distinction between Readeriella and Kirramyces is subtle, and lies in the conidial bases, with conidia of Readeriella having tapering subtrucate bases, in contrast to those of Kirramyces (= Colletogloeopsis) that tend to be more truncate.
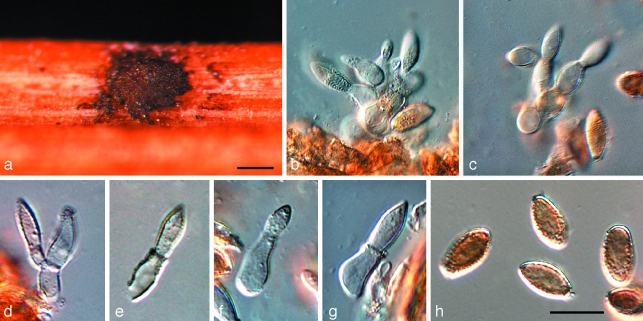
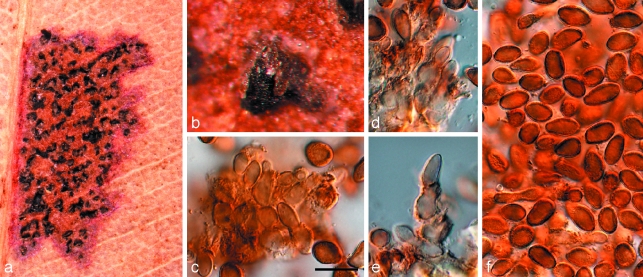
Whether Kirramyces is the oldest generic name to use for anamorphs in this clade is open to debate. Phaeoseptoria (1908) appears to be an anamorph of the Phaeosphaeriopsis complex (Arzanlou & Crous 2006), rendering it unavailable for this group of anamorphs. However, the status of the type species of Leptomelanconium (1923) remains unknown (Fig. 5), the species occurring on Corymbia, namely L. australiensis (Fig. 6), is clearly an anamorph of Teratosphaeria. The same is true for several species of ‘Coniothyrium’ treated by Sutton (1980) and Crous (1998). Colletogloeum (1953) has hitherto been a somewhat confused genus, including many species that appear to belong to Teratosphaeria. However, the ITS sequence from DNA extracted from a specimen representative of the type species, C. sissoo (IMI 119162) (Fig. 7), clearly revealed Colletogloeum to be allied to the Pseudocercospora (1910) complex, clustering in the Mycosphaerellaceae (data not shown). Jubispora (1986) is another interesting candidate genus that predates Kirramyces, having conidia partially covered by a mucoid sheath, as observed in Readeriella patrickii. The phylogenetic position of Jubispora is, however, unknown. Due to the uncertainty surrounding available anamorph names in this clade, we apply a single generic name to this genus. The oldest name, Teratosphaeria (1912), was thus selected to apply to all taxa in this clade (Crous et al. 2009a).
Fig. 5.
Leptomelanconium allescheri (WINF 4022). a. Conidioma on pine needle; b–g. conidiogenous cells giving rise to conidia; h. conidia. — Scale bars = 10 μm.
Fig. 6.
Teratosphaeria australiensis (IMI 159079a). a. Leaf spot; b. erumpent pycnidium; c–e. conidiogenous cells giving rise to conidia. f. conidia. — Scale bar = 10 μm.
Fig. 7.
Colletogloeum sissoo (IMI 119162). a. Conidiomata on leaf; b–f. conidiogenous cells giving rise to conidia; g. conidia. — Scale bars = 10 μm.
Teratosphaeria Syd. & P. Syd., Ann. Mycol. 10: 39. 1912.
Anamorph. Kirramyces J. Walker, B. Sutton & Pascoe, Mycol. Res. 96: 919. 1992.
Synanamorph. Batcheloromyces-like
= Colletogloeopsis Crous & M.J. Wingf., Canad. J. Bot. 75: 668. 1997.
Teratosphaeria alcornii Crous, nom. nov. — MycoBank MB509725
Basionym. Stigmina eucalypti Alcorn, Trans. Brit. Mycol. Soc. 60: 151. 1973.
≡ Batcheloromyces eucalypti (Alcorn) Crous & U. Braun, Stud. Mycol. 58: 12. 2007.
Notes — The epithet ‘eucalypti’ is already occupied by Teratosphaeria eucalypti, based on Cercospora eucalypti Cooke & Massee (1889) as shown below.
Teratosphaeria angophorae (Andjic, Carnegie & P.A. Barber) Andjic, Carnegie & P.A. Barber, comb. nov. — MycoBank MB509726
Basionym. Kirramyces angophorae Andjic, Carnegie & P.A. Barber, Mycol. Res. 111: 1193. 2007.
Notes— Teratosphaeria angophorae represents the transition of Colletogloeopsis (conidia 0–1-septate) to Kirramyces (conidia 3 or more septate). Although the majority of conidia are aseptate, this species provides support for the fact that there is a morphological range in conidial septation from Colletogloeopsis to Kirramyces.
Teratosphaeria australiensis (B. Sutton) Crous, comb. nov. — MycoBank MB509727; Fig. 6
Basionym. Leptomelanconium australiense B. Sutton, Nova Hedwigia 25: 163. 1974.
Specimen examined. Australia, York, on leaves of Corymbia ficifolia, H.L. Harvey, IMI 159079a holotype.
Notes— Teratosphaeria australiensis is a typical species of Teratosphaeria, not congeneric with Leptomelanconium allescheri, the type species of Leptomelanconium. We examined herbarium material of the latter (WINF 4022, Fig. 5), and found that it was morphologically quite distinct from typical Colletogloeopsis/Kirramyces anamorphs, in having conidiophores that can be branched, and pigmented only in the apical conidiogenous region. This species is presently not known from culture.
Teratosphaeria blakelyi (Crous & Summerell) Crous & Summerell, comb. nov. — MycoBank MB509728
Basionym. Colletogloeopsis blakelyi Crous & Summerell, Fung. Diversity 23: 342. 2006.
≡ Readeriella blakelyi (Crous & Summerell) Crous & U. Braun, Stud. Mycol. 58: 26. 2007.
Teratosphaeria brunneotingens (Crous & Summerell) Crous & Summerell, comb. nov. — MycoBank MB509729
Basionym. Readeriella brunneotingens Crous & Summerell, Stud. Mycol. 58: 26. 2007.
Teratosphaeria considenianae (Crous & Summerell) Crous & Summerell, comb. nov. — MycoBank MB509730
Basionym. Colletogloeopsis considenianae Crous & Summerell, Fung. Diversity 23: 343. 2006.
≡ Readeriella considenianae (Crous & Summerell) Crous & U. Braun, Stud. Mycol. 58: 26. 2007.
Teratosphaeria corymbiae (Carnegie, Andjic & P.A. Barber) Carnegie, Andjic & P.A. Barber, comb. nov. — MycoBank MB509731
Basionym. Kirramyces corymbiae Carnegie, Andjic & P.A. Barber, Mycol. Res. 111: 1193. 2007.
Specimen examined. Australia, New South Wales, South Grafton, Grafton City Council, Landfill Plantation, 152°54’38"E, 29°46’21"S, on leaves of Corymbia henryii, 16 Feb. 2006, coll. A.J. Carnegie, isol. P.W. Crous, cultures CPC 13125 = CBS 124988, CPC 13126, 13127.
Teratosphaeria destructans (M.J. Wingf. & Crous) M.J. Wingf. & Crous, comb. nov. — MycoBank MB509732
Basionym. Kirramyces destructans M.J. Wingf. & Crous, S. African J. Bot. 62: 325. 1996.
≡ Phaeophleospora destructans (M.J. Wingf. & Crous) Crous, F.A. Ferreira & B. Sutton, S. African J. Bot. 63: 113. 1997.
≡ Readeriella destructans (M.J. Wingf. & Crous) Crous & U. Braun, Stud. Mycol. 58: 26. 2007.
Teratosphaeria eucalypti (Cooke & Massee) Crous, comb. nov. — MycoBank MB509733
Basionym. Cercospora eucalypti Cooke & Massee, Grevillea 18: 7. 1889.
≡ Kirramyces eucalypti (Cooke & Massee) J. Walker, B. Sutton & Pascoe, Mycol. Res. 96: 920. 1992.
≡ Phaeophleospora eucalypti (Cooke & Massee) Crous, F.A. Ferreira & B. Sutton, S. African J. Bot. 63: 113. 1997.
= Septoria pulcherrima Gadgil & M.A. Dick, New Zealand J. Bot. 21: 49. 1983.
≡ Stagonospora pulcherrima (Gadgil & M.A. Dick) H.J. Swart, Trans. Brit. Mycol. Soc. 90: 285. 1988.
≡ Readeriella pulcherrima (Gadgil & M.A. Dick) Crous & U. Braun, Stud. Mycol. 58: 26. 2007.
Teratosphaeria lilianiae (J. Walker, B. Sutton & Pascoe) Crous & Andjic, comb. nov. — MycoBank MB509734
Basionym. Kirramyces lilianiae J. Walker, B. Sutton & Pascoe, Mycol. Res. 96: 921. 1992.
≡ Phaeophleospora lilianiae (J. Walker, B. Sutton & Pascoe) Crous, F.A. Ferreira & B. Sutton, S. African J. Bot. 63: 115. 1997.
Teratosphaeria macowanii (Sacc.) Crous, comb. nov. — MycoBank MB509735
Basionym. Coniothecium macowanii Sacc., Syll. Fung. 4: 512. 1886, nom. nov., based on Coniothecium punctiforme G. Winter, Hedwigia 24: 33. 1885, non C. punctiforme Corda, Icon. Fungorum (Corda) 1: 2. 1837.
≡ Trimmatostroma macowanii (Sacc.) M.B. Ellis, More Dematiacous Hyphomycetes: 29. 1976.
≡ Catenulostroma macowanii (Sacc.) Crous & U. Braun, Stud. Mycol. 58: 17. 2007.
Teratosphaeria multiseptata (Carnegie) Carnegie, comb. nov. — MycoBank MB509736
Basionym. Mycosphaerella multiseptata Carnegie, Mycologia 99: 471. 2007.
Teratosphaeria obscuris (P.A. Barber & T.I. Burgess) P.A. Barber & T.I. Burgess, comb. nov. — MycoBank MB509737
Basionym. Mycosphaerella obscuris P.A. Barber & T.I. Burgess, Fung. Diversity 24: 146. 2007.
Teratosphaeria stellenboschiana (Crous) Crous, comb. nov. — MycoBank MB509738
Basionym. Colletogloeopsis stellenboschiana Crous, Stud. Mycol. 55: 110. 2006.
≡ Readeriella stellenboschiana (Crous) Crous & U. Braun, Stud. Mycol. 58. 26. 2007.
Specimens examined. Corsica, on leaves of Eucalyptus sp., Aug. 2005, coll. J. Dijksterhuis, CBS H-20249, isol. P.W. Crous, cultures CPC 12283–12285. – South Africa, Western Cape Province, Stellenbosch Mountain, on leaves of Eucalyptus sp., 4 Dec. 2004, P.W. Crous, CBS H-19688 holotype, culture ex-type CBS 116428 = CPC 10886; Gauteng, Pretoria, on leaves of Eucalyptus punctata, 28 Feb. 2007, P.W. Crous, CBS H-20250, CPC 13767 = CBS 124989, CPC 13764–13769.
Notes — Although there are two nucleotide differences between the ex-type strain and these new collections, conidial dimensions of the latter are (6–)7–8(–9) × (3–)4(–4.5) μm, thus being very similar to those of the ex-type strain, (6.5–)7–9(–10) × (3–)3.5(–4) μm (Crous et al. 2006e), suggesting this to be intraspecific variation.
Teratosphaeria syncarpiae (Carnegie & M.J. Wingf.) Carnegie & M.J. Wingf., comb. nov. — MycoBank MB509739
Basionym. Mycosphaerella syncarpiae Carnegie & M.J. Wingf., Mycologia 99: 469. 2007.
Teratosphaeria viscidus (Andjic, P.A. Barber & T.I. Burgess) Andjic, P.A. Barber & T.I. Burgess, comb. nov. — MycoBank MB509740
Basionym. Kirramyces viscidus Andjic, P.A. Barber & T.I. Burgess, Australas. Plant Pathol. 36. 485. 2007.
Specimen examined. Australia, Queensland, 24 km outside Mareeba Dimbulah, 17°8’21.2"S, 145°14’58.6"E, 503 m, on leaves of Eucalyptus sp., 26 Aug. 2006, coll. B.A. Summerell & P.W. Crous, CBS H-20251, isol. P.W. Crous, cultures CPC 13306 = CBS 124992, CPC 13307, 13308.
Teratosphaeria wingfieldii (Crous) Crous, comb. nov. — MycoBank MB509741
Basionym. Catenulostroma wingfieldii Crous, Persoonia 20: 67. 2008.
DISCUSSION
In an attempt to delineate natural genera within the Mycosphaerella complex, the present study integrates anamorph and teleomorph morphologies with a molecular phylogeny derived from the LSU gene sequences. Because most of these ‘morphological genera’ have been shown to be poly- and paraphyletic, a generic name (s.s.) can be applied only to the clade in which the type species resides. Furthermore, an attempt has been made to integrate anamorph and teleomorph names, and not introduce further genera. Thus, the oldest available generic name was chosen for each clade (irrespective of anamorph or teleomorph), and the oldest available epithet was chosen for each species, with priority given to teleomorph species epithets for the holomorph. Where a taxon is asexual, but is genetically identical to the teleomorph (thus contains an element of the teleomorph, in this case the DNA), it has been described (or combined) in sexual genera. Likewise, we have described (or combined) sexual taxa in asexual genera only where an asexual genus is available for the clade, and where it contains the DNA element typical of that anamorph genus. We thus accept the similarity in DNA sequence to be equal in value to the presence of certain morphological features, such as asci and ascospores. Sexual and asexual taxa are treated as equal. Where they are genetically similar, preference has been given to the oldest name (date of publication).
Mycosphaerella (1884) sensu Aptroot (2006) is heterogeneous. In the strict sense, Mycosphaerella is linked only to Ramularia (1833) anamorphs, with preference given to the latter name, due to the confusion surrounding Mycosphaerella, as well as date of publication. Teratosphaeria (1912) species have Kirramyces (1992) (incl. Colletogloeopsis 1997) anamorphs, with Teratosphaeria used as the generic name for this well-defined clade of foliar pathogens (Crous et al. 2009a). In addition, numerous other genera have been recognised as distinct in the present study, many of which have Mycosphaerella-like teleomorphs.
Results of this study have shown that the Mycosphaerella complex as it is presently defined in the literature encompasses numerous genera, many of which remain unnamed. Before DNA-based phylogenetic inference was available for this group, these genera were obscured by the fact that the teleomorph morphology, namely a submerged to erumpent ascoma, in most cases without residual hamathecial tissue, with bitunicate asci and 1-celled ascospores, had evolved throughout the order. Due to the unavailability of cultures, these hypotheses could never be tested. The result was that Mycosphaerella became known as a genus with up to 30 different anamorph genera (Crous & Braun 2003, Crous et al. 2000, 2001, 2004b, c, 2006a, b, c, d, 2007a, b, c). A further problem arose from the fact that many of these anamorph forms evolved in more than one clade, and they thus occurred in different families, and represent different genera. This phenomenon added to the confusion, and it provided support for a wider generic concept for Mycosphaerella. As more taxa and DNA sequence data have become available for study, it has increasingly appeared that the minute features observed among the various anamorphs, were in many cases indicative of different phylogenetic lineages.
Many clades remain unresolved in this study. This is due to the fact that they are poorly populated by taxa, and in some cases the absence of cultures has made it impossible to place them appropriately. These will hopefully be resolved in the future as additional collections are made and cultures and DNA sequence data become available. Nevertheless, the proposed system of a single generic name per clade is infinitely more stable than the one used in the past and in which both the anamorphs and teleomorphs needed to undergo nomenclatural changes. An added advantage of this new taxonomic scheme is that it does not suffer from synanamorphs developing in various places throughout the tree, resulting in a further proliferation of names.
In the title of this paper, we pose the question: ‘Do you believe in genera?’ In 1943, Bisby & Ainsworth stated that ‘Nature may make species, but man has made the genera’. Before the incorporation of DNA sequence-based phylogenies, the Saccardoian system based on spore septation defined numerous artificial boundaries in the Mycosphaerellaceae (Crous et al. 2003b). Mycosphaerella has until now been used as a convenient receptacle concept to incorporate numerous morphologically diverse anamorphs. A startling fact is that so many solitary lineages and anamorph morphology types remain unresolved in the present phylogeny. This shows that a concerted effort is needed to make collections that will ultimately provide a more robust representation of various morphology types, common ancestors and sister taxa. We now have the ability to use DNA phylogenies to reflect evolutionary history. By integrating the phylogenetic species concept with morphology, we can now select meaningful break points in lineages which can be attributed to genera.
Acknowledgments
Prof. dr U. Braun (Martin-Luther-Universität, Halle, Germany) is thanked for providing the Latin diagnoses. The authors thank technical staff A. van Iperen (cultures), M. Vermaas (photo plates), and M. Starink (DNA isolation, amplification and sequencing) for their invaluable assistance. Various colleagues collected material used in this study, for which we are grateful, namely Prof. dr H-D Shin (Korea University Seoul, Korea), Prof. dr A.C. Alfenas (University of Viçosa, MG, Brazil), Dr C. Mohammed (CSIRO, Tasmania, Australia), Mr I.W. Smith (University of Melbourne, Australia), and Dr J. Dijksterhuis (CBS, Netherlands). We are also grateful to Alex Buchanan, Tasmanian Herbarium and Ian Cowie, Northern Territory Herbarium for assistance with the collection and identification of Eucalyptus spp. and to Richard Johnston and Andrew Orme, Royal Botanic Gardens and Domain Trust for the collection of Eucalyptus in New South Wales.
Some years ago, Prof. dr DL Hawksworth asked PWC why he regarded Colin Booth as one of his favourite mycologists. From the present paper it is clear that the answer partly lies in his Presidential address (Booth 1978), which continues to inspire to this day.
REFERENCES
- Andjic V, Barber PA, Carnegie AJ, Hardy GEStJ, Wingfield MJ, Burgess TI. 2007a. Phylogenetic reassessment supports accommodation of Phaeophleospora and Colletogloeopsis from eucalypts in Kirramyces. Mycological Research 111: 1184 – 1198 [DOI] [PubMed] [Google Scholar]
- Andjic V, Barber PA, Carnegie AJ, Pegg G, Hardy GEStJ, Wingfield MJ, Burgess TI. 2007b. Kirramyces viscidus sp. nov., a new eucalypt pathogen from tropical Australia closely related to the serious leaf pathogen, Kirramyces destructans. Australasian Plant Pathology 36: 478 – 487 [Google Scholar]
- Aptroot A. 2006. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1 – 231 [Google Scholar]
- Arx JA von, Müller E. 1975. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology 9: 1 – 159 [Google Scholar]
- Arzanlou M, Crous PW. 2006. Phaeosphaeriopsis musae. Fungal Planet 9 [Google Scholar]
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater M-F, Buddenhagen IW, Viljoen A, Crous PW. 2008. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19 – 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes I, Crous PW, Wingfield BD, Wingfield MJ. 2004. Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology 50: 551 – 565 [Google Scholar]
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 232 – 244 [DOI] [PubMed] [Google Scholar]
- Bisby GR, Ainsworth GC. 1943. The numbers of fungi. Transactions of the British Mycological Society 26: 16 – 19 [Google Scholar]
- Booth C. 1978. Presidential address: Do you believe in genera? Transactions of the British Mycological Society 71: 1 – 9 [Google Scholar]
- Braun U. 1995. A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 1 IHW-Verlag, Eching, Germany: [Google Scholar]
- Braun U. 1998. A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 2 IHW-Verlag, Eching, Germany: [Google Scholar]
- Braun U, Crous PW. 2006. (1732) Proposal to conserve the name Pseudocercospora against Stigmina and Phaeoisariopsis (Hyphomycetes). Taxon 55: 803 [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JG, Hoog SG de. 2003. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3 – 18 [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, Toanan C. 2008. Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77 – 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinas MN, Crous PW, Wingfield BD, Wingfield MJ. 2006. Multi-gene phylogenies and phenotypic characters distinguish two species within the Colletogloeopsis zuluensis complex associated with Eucalyptus stem cankers. Studies in Mycology 55: 133 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. 1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 [Google Scholar]
- Crous PW, Aptroot A, Kang J-C, Braun U, Wingfield MJ. 2000. The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107 – 121 [Google Scholar]
- Crous PW, Braun U. 2003. Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1 – 571 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33 – 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Ferreira FA, Sutton BC. 1997. A comparison of the fungal genera Phaeophleospora and Kirramyces (coelomycetes). South African Journal of Botany: 63: 111 – 115 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004a. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Crous PW, Groenewald JZ, Gams W. 2003a. Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology 109: 841 – 850 [Google Scholar]
- Crous PW, Groenewald JZ, Groenewald M, Caldwell P, Braun U, Harrington TC. 2006a. Species of Cercospora associated with grey leaf spot of maize. Studies in Mycology 55: 189 – 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. 2004b. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195 – 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. 2004c. Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457 – 469 [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. 2009a. Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38 – 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Wingfield MJ, Aptroot A. 2003b. The value of ascospore septation in separating Mycosphaerella from Sphaerulina in the Dothideales: a Saccardoan myth? Sydowia 55: 136 – 152 [Google Scholar]
- Crous PW, Hong L, Wingfield MJ, Wingfield BD, Kang J. 1999. Uwebraunia and Dissoconium, two morphologically similar anamorph genera with distinct teleomorph affinity. Sydowia 52: 155 – 166 [Google Scholar]
- Crous PW, Kang J-C, Braun U. 2001. A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081 – 1101 [Google Scholar]
- Crous PW, Liebenberg MM, Braun U, Groenewald JZ. 2006b. Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163 – 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schroers H-J, Groenewald JZ, Braun U, Schubert K. 2006c. Metulocladosporiella gen. nov. for the causal organism of Cladosporium speckle disease of banana. Mycological Research 110: 264 – 275 [DOI] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006d. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Mohammed C, Himaman W, Groenewald JZ. 2007c. Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26: 143 – 185 [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Groenewald JZ. 2009c. Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia 23: 119 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ. 2008a. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59 – 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds). 2009b. Fungal Biodiversity. CBS Laboratory Manual Series 1 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ. 1996. Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia 88: 441 – 458 [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. 2006e. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99 – 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. 1991. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628 – 632 [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ. 2008b. Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Crous PW, Fourie PH. 2008. A fissitunicate ascus mechanism in the Calosphaeriaceae, and novel species of Jattaea and Calosphaeria on Prunus wood. Persoonia 20: 39 – 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JC. 1993. A revision of taxa referred to Heterosporium Klotzsch ex Cooke (Mitosporic Fungi). PhD Thesis University of Reading, UK: [Google Scholar]
- Dugan FM, Braun U, Groenewald JZ, Crous PW. 2008. Morphological plasticity in Cladosporium sphaerospermum. Persoonia 21: 9 – 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson OE. 2006. Outline of Ascomycota – 2006. Myconet 12: 1 – 82 [Google Scholar]
- Goodwin SB, Dunkle DL, Zismann VL. 2001. Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91: 648 – 658 [DOI] [PubMed] [Google Scholar]
- Groenewald M, Groenewald JZ, Braun U, Crous PW. 2006. Host range of Cercospora apii and C. beticola, and description of C. apiicola, a novel species from celery. Mycologia 98: 275 – 285 [DOI] [PubMed] [Google Scholar]
- Groenewald M, Groenewald JZ, Crous PW. 2005. Distinct species exist within the Cercospora apii morphotype. Phytopathology 95: 951 – 959 [DOI] [PubMed] [Google Scholar]
- Halleen F, Schroers H-J, Groenewald JZ, Crous PW. 2004. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.). Studies in Mycology 50: 431 – 455 [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509 – 547 [DOI] [PubMed] [Google Scholar]
- Hoog GS de. 1977. Rhinocladiella and allied genera. Studies in Mycology 15: 1 – 140 [Google Scholar]
- Hunter GC, Roux J, Wingfield BD, Crous PW, Wingfield MJ. 2004. Mycosphaerella species causing leaf disease in South African Eucalyptus plantations. Mycological Research 108: 672 – 681 [DOI] [PubMed] [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. 2006. A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147 – 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, et al. 2006. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443: 818 – 822 [DOI] [PubMed] [Google Scholar]
- Kirschner R. 2009. Cercosporella and Ramularia. Mycologia 101: 110 – 119 [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. 2001. Dictionary of fungi 9th edition CAB International, Wallingford: [Google Scholar]
- Lee S, Groenewald JZ, Crous PW. 2004. Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology 50: 235 – 252 [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW. 2006. Taxonomy and pathology of Togninia (Diaportales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1 – 115 [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia Mycologue Publications, Waterloo, Ontario, Canada: [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW. 2008. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29 – 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK: [Google Scholar]
- Réblová M, Mostert L, Gams W, Crous PW. 2004. New genera in the Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology 50: 533 – 550 [Google Scholar]
- Rossman AY, Samuels GJ. 2005. Towards a single scientific name for species of fungi. Inoculum 56, 3: 3 – 6 [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1043 – 1054 [DOI] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, Hoog GS de, Crous PW. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105 – 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Untereiner WA, Ewaze JO, Wong B, Doyle D. 2007. Baudoinia, a new genus to accommodate Torula compniacensis. Mycologia 99: 592 – 601 [DOI] [PubMed] [Google Scholar]
- Seifert KA, Nickerson NL, Corlett M, Jackson ED, Louis-Seize G, Davies RJ. 2004. Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany 82: 914 – 926 [Google Scholar]
- Simon UK, Groenewald JZ, Crous PW. 2009. Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia 22: 49 – 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. 1975. The Agaricales in modern taxonomy, preface II Cramer, Lehre, Germany: [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A fast bootstrapping algorithm for the RAxML web-servers. Systematic Biology 57: 758 – 771 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. 2005a. RAxML-III: a fast program for maximum likelihood-based inferences of large phylogenetic trees. Bioinformatics 21: 456 – 463 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Ott M, Ludwig T. 2005b. RAxML-OMP: An efficient program for phylogenetic inference on SMPs In: Malyshkin VE. (ed), Proceedings of 8th International Conference on Parallel Computing Technologies (PaCT2005), Krasnoyarsk, Russia, September 2005, volume 3606 of Lecture Notes in Computer Science: 288–302 Springer Verlag, Berlin, Germany: [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabo L. 1999. Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491 – 1499 [Google Scholar]
- Sugiyama J, Amano N. 1987. Two metacapnodiaceous sooty moulds from Japan: their identity and behavior in pure culture. In: Sugiyama J. (ed), Pleomorphic fungi: the diversity and its taxonomic implications: 141–156 Kodansha, Tokyo/Elsevier, Amsterdam: [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, Summerbell RC, Crous PW. 2006. Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323 – 350 [Google Scholar]
- Sutton BC. 1980. The coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Swart HJ, Walker J. 1988. Australian leaf-inhabiting fungi XXVIII. Hendersonia on Eucalyptus. Transactions of the British Mycological Society 90: 633 – 641 [Google Scholar]
- Taylor JW, Jacobsen DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics & Biology 31: 21 – 32 [DOI] [PubMed] [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. 2004a. Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271 – 1282 [DOI] [PubMed] [Google Scholar]
- Verkley GJM, Priest MJ. 2000. Septoria and similar coelomycetous anamorphs of Mycosphaerella. Studies in Mycology 45: 123 – 128 [Google Scholar]
- Verkley GJM, Silva M da, Wicklow DT, Crous PW. 2004b. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323 – 335 [Google Scholar]
- Walker J, Sutton BC, Pascoe IG. 1992. Phaeoseptoria eucalypti and similar fungi on Eucalyptus with description of Kirramyces gen. nov. (Coelomycetes). Mycological Research 96: 911 – 924 [Google Scholar]
- Wang H-K, Aptroot A, Crous PW, Hyde KD, Jeewon R. 2007. The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RPB2 gene phylogenies. Mycological Research 111: 1268 – 1276 [DOI] [PubMed] [Google Scholar]
- Wingfield MJ, Crous PW, Groenewald JZ. 2006. Passalora schizolobii. Fungal Planet 2 [Google Scholar]
- Zalar P, Hoog GS de, Gunde-Cimerman N. 1999. Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Studies in Mycology 43: 49 – 56 [Google Scholar]
- Zalar P, Hoog GS de, Schroers H-J, Crous PW, Groenewald JZ, Gunde-Cimerman N. 2007. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157 – 183 [DOI] [PMC free article] [PubMed] [Google Scholar]