Abstract
Ophiostomatoid fungi were isolated from Scolytus ratzeburgi infesting Betula pendula and B. pubescens in Norway. Fungi were identified based on morphology, DNA sequence comparison for two gene regions and phylogenetic analyses. The most abundant fungus was Ophiostoma karelicum, suggesting a specific relationship between the fungus, the vector insect and the host tree. Our results suggest that O. karelicum occurs across the geographic range of S. ratzeburgi and its close relatedness to the Dutch elm disease fungi suggests that it could be important if introduced into other parts of the world. Other fungi, only occasionally isolated from S. ratzeburgi, were identified as O. quercus and a novel taxon, described here as O. denticiliatum sp. nov.
Keywords: Betula, Ophiostoma, Scolytus, symbiosis
INTRODUCTION
The birch bark beetle, Scolytus ratzeburgi (Coleoptera: Curculionidae, Scolytinae), is the only Scolytus species known to infest birch. It is a very common bark beetle found on silver birch (Betula pendula) and downy birch (Betula pubescens) in Scandinavia and other parts of Europe (Postner 1974). Scolytus ratzeburgi typically infests weakened or dying trees, but can also attack apparently healthy trees (Saalas 1949). In a recent study, the occurrence of ophiostomatoid fungi in association with S. ratzeburgi was reported from the Karelia region of Finland and Russia (Linnakoski et al. 2008). A new Ophiostoma species, O. karelicum, together with O. quercus and a third taxon close to O. catonianum, were identified. Ophiostoma karelicum was the dominant fungus and consistently isolated from every beetle and gallery collected.
The pathogenicity of O. karelicum to birch and its role in the ecology of S. ratzeburgi remains unclear. The fact that the fungus is closely related to the Dutch elm disease fungi, Ophiostoma ulmi, O. novoulmi subsp. novoulmi and subsp. americana (Burges et al. 1979, Hubbes 1999, Brasier & Kirk 2001), is a matter of concern (Linnakoski et al. 2008). Another unanswered question is whether O. karelicum is also associated with the beetle in other geographical areas where this insect occurs. The aim of this study was thus to collect and identify ophiostomatoid fungi associated with S. ratzeburgi infesting birch in Norway.
MATERIALS AND METHODS
Collection of bark beetles
Sampling was conducted during the flight period (5–7 July) of Scolytus ratzeburgi during the summer of 2007 in Akerhus and Østfold counties, Norway. Adult bark beetles and their galleries were collected from piles of birch logs at three different sites. These included a sawmill yard in Spydeberg (Svenneby), and in forests at Vestby (near Haug, Garder) and Hobøl (near Kattås). At the time of sampling, adult beetles were found on birch trunk surfaces and under the bark where gallery construction was active. A total of 34 adults and 33 galleries were collected and stored individually at 5 °C until further study.
Fungi were isolated from the bark beetles as well as their galleries. Methods of isolation were the same as those described by Linnakoski et al. (2008). Representative isolates were deposited in the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands and the CMW-collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa. Isolates of the new taxon are also maintained in the VTT Culture Collection, VTT Technical Research Centre of Finland, Espoo with herbarium specimens deposited in Kuopio Museum of Natural History (KUO), Kuopio, Finland.
DNA extraction and PCR
Fungal isolates were grown on malt extract agar (MEA; 20 g Difco BactoTM malt extract from Becton, Dickinson & Company, Sparks, USA, 20 g Difco BactoTM agar and 1 L Milli-Q water). DNA was extracted using PrepMan Ultra Sample preparation reagent (Applied Biosystems, Foster City, CA, USA) as described by Linnakoski et al. (2008). For the ribosomal DNA operon, the internal transcribed spacers (ITS) 1 and 2, including the 5.8S gene, were amplified using primers ITS1-F (Gardes & Bruns 1993) and ITS4 (White et al. 1990). The β-tubulin gene was amplified using primers Bt2a and Bt2b (Glass & Donaldson 1995). Primer Bt2b was replaced in some cases with primer T10 (O’Donnel & Cigelnik 1997).
Gene fragments were amplified in 25 μL reaction mixture containing 0.25 μL of Super-Therm DNA Polymerase mixture (250 U) (Hoffmann-La Roche, Nutley, USA), 2.5 μL of reaction buffer (10×) and 2.5 μL of MgCl2 (25 mM) (supplied with the enzyme), 2.5 μL of dNTP’s and 0.25 μL of each primer (10 mM). PCR reactions were performed using an Eppendorf Mastercycler® Personal (Perkin-Elmer, Hamburg, Germany). The PCR conditions were: an initial denaturation step at 95 °C for 2 min, followed by 40 cycles of 30 s at 95 °C, 30 s at 54 °C and 1 min at 72 °C, and a final chain elongation at 72 °C for 8 min. Amplified products were sequenced with the BigDye v3.1 Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) on the ABI Prism 377 Autosequencer (Applied Biosystems, Foster City, CA, USA).
Sequence analyses
Raw sequences were aligned and consensus sequences determined manually using Geneious Pro v4.0.4 for MacIntosh (Biomatters, Auckland, New Zealand). BLAST searches were conducted for preliminary identifications, after which datasets were compiled that included published GenBank sequences. Since the sequence data for intron 5 of the β-tubulin gene varies greatly between different species groups in Ophiostoma, the β-tubulin dataset was treated in subsets in order to obtain improved alignments for species definition. Alignments were done online within MAFFT v6 (Katoh et al. 2002), using the E-INS-I method with a gap opening penalty of 1.53 and an offset value of 0.00. All sequences obtained in this study were deposited in GenBank (Table 1).
Table 1.
Fungal isolates obtained from Scolytus ratzeburgi infesting birch and used in this study.
| Species identity | Isolate no.1 |
Geographical origin | GenBank accession no. |
|||
|---|---|---|---|---|---|---|
| CBS | CMW | VTT | ITS | β-tubulin | ||
| Ophiostoma canum-like | 29490 | Hobøl, Norway | FJ804493 | |||
| 124499 | 29495 | Hobøl, Norway | FJ804505 | |||
| O. denticiliatum sp. nov. | 124497a,b | 29493a,b | D-091316 | Hobøl, Norway | FJ804490 | FJ804502 |
| 124498a,b | 29494a,b | D-091317 | Hobøl, Norway | FJ804491 | FJ804503 | |
| D-091318a,b | Hobøl, Norway | FJ804492 | FJ804504 | |||
| O. flexuosum-like | 124500 | 29496 | Hobøl, Norway | FJ804494 | FJ804506 | |
| O. karelicum | 124494 | 29484 | Spydeberg, Norway | FJ804482 | FJ804495 | |
| 124495 | 29485 | Vestby, Norway | FJ804483 | FJ804496 | ||
| 29486 | Hobøl, Norway | FJ804484 | FJ804497 | |||
| 29487 | Spydeberg, Norway | FJ804485 | FJ804498 | |||
| 29488 | Vestby, Norway | FJ804486 | FJ804499 | |||
| 29489 | Hobøl, Norway | FJ804487 | FJ804500 | |||
| O. multiannulatum-like | 29492 | Spydeberg, Norway | FJ804489 | |||
| O. quercus | 124496 | 29491 | Hobøl, Norway | FJ804488 | FJ804501 | |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; VTT: Culture Collection of the Technical Research Centre of Finland, Espoo, Finland. All isolates were collected by R. Linnakoski.
a Isolates used in growth studies.
b Isolates crossed with each other to obtain perithecia for morphological descriptions.
Neighbour-joining (NJ) analyses (with the Kimura-2 parameter active) and maximum parsimony (MP) were conducted using MEGA v4 (Kumar et al. 2008). Bootstrap values were calculated for 1 000 replicates (Felsenstein 1985).
Bayesian analyses were carried out with MrBayes v3.1.2 (Ronquist & Huelsenbeck 2003). Four independent Markov chains were run for 6 million generations. Substitution models were determined for each dataset using the Akaike Information Criterion (AIC) in MrModeltest v2.3 (http://www.abc.se/~nylander/). Trees were sampled every 100 generations resulting in 60 000 trees, discarding the burn-in of the chain, as calculated for the respective datasets. The remaining trees for both runs were used to construct a majority rule consensus tree for each dataset.
Morphological studies
DNA sequence analyses suggested that some of the isolates from S. ratzeburgi represent an undescribed species of Ophiostoma, but only anamorph structures had been found in the galleries and in culture. In order to ascertain whether mating of strains would result in the teleomorph of this species, single conidial isolates were crossed in all possible combinations. Each culture was also crossed against itself as a control. These sexual compatibility tests were conducted on three different media including water agar (WA; 15 g Difco BactoTM agar and 1 L Milli-Q water), MEA and oatmeal agar (OA; 15 g oatmeal, 15 g Difco BactoTM agar and 1 L Milli-Q water) with sterilised birch twigs placed on the surface of the medium. Cultures were incubated at 20 °C and inspected regularly for fruiting structures.
For the descriptions, anamorph and teleomorph (where present) fruiting structures were mounted in 85 % lactic acid on glass slides and observed using a Nikon Eclipse 50i phase contrast microscope (Nikon Corporation, Tokyo, Japan). A Nikon DS-Fi1 camera system (Nikon Corporation, Tokyo, Japan) was used to photograph images. Fifty measurements were made of each of the taxonomically informative anamorph and teleomorph structures. Averages, ranges and standard deviations of the measurements were computed and measurements are presented in the format (minimum–) mean minus standard deviation – mean plus standard deviation (–maximum).
Three isolates (Table 1) of the unknown Ophiostoma species were chosen to determine the optimal temperature for growth in culture. Agar plugs (5 mm diam) bearing mycelium were transferred from the actively growing margins of one wk old cultures and placed at the centres of 9 cm Petri dishes containing 2 % MEA. The growth rates of isolates were determined at 5, 10, 15, 20, 25, 30 and 35 °C (± 0.5 °C) in the dark. Five replicate plates were used for each temperature interval and colony diameters (three colony diameter measurements per plate) were determined 8 d after inoculation. The results were calculated as mm/day (± standard deviation).
For scanning electron microscopy, small agar disks were cut from the sporulating colonies and fixed overnight in 2.5 % glutaraldehyde in a 0.15 M phosphate buffer (pH 7.4). After three buffer rinses, the specimens were post-fixed in 1 % osmium tetroxide for 60 min, dehydrated in an ethanol series, and critical point dried using CPD 010 (Balzers Union AG, Balzers, Liechtenstein). The specimens were coated with a 30 nm thick copper layer using an Emitech K675X sputter coater (Emitech, Ashford, UK). A LEO 1550 scanning electron microscope (Beamtech Nordiska AB, Gnesta, Sweden) operating at 5 kV was used to examine the specimens.
RESULTS
Fungal isolations
A total of 99 isolates were obtained from bark beetles and their galleries (Table 2). All isolates formed typical Pesotum and Sporothrix anamorphs in culture. No ascomata were observed. Six different morphological groups were recognized among the isolates. One of the taxa (87 isolates) was isolated from every gallery and every beetle sampled in the study. The other taxa were present in low numbers and were not consistently isolated from the samples.
Table 2.
Ophiostoma species isolated from Scolytus ratzeburgi beetles and galleries in Norway.
| Species | Site 1: Spydeberg |
Site 2: Vestby |
Site 3: Hobøl |
Total | |||
|---|---|---|---|---|---|---|---|
| Beetle | Gallery | Beetle | Gallery | Beetle | Gallery | ||
| Ophiostoma karelicum | 17 | 21 | 10 | 13 | 11 | 15 | 87 |
| O. quercus | 0 | 0 | 0 | 0 | 5 | 0 | 5 |
| O. denticiliatum sp. nov. | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| O. canum-like | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| O. flexuosum-like | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| O. multiannulatum-like | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
DNA sequence analyses
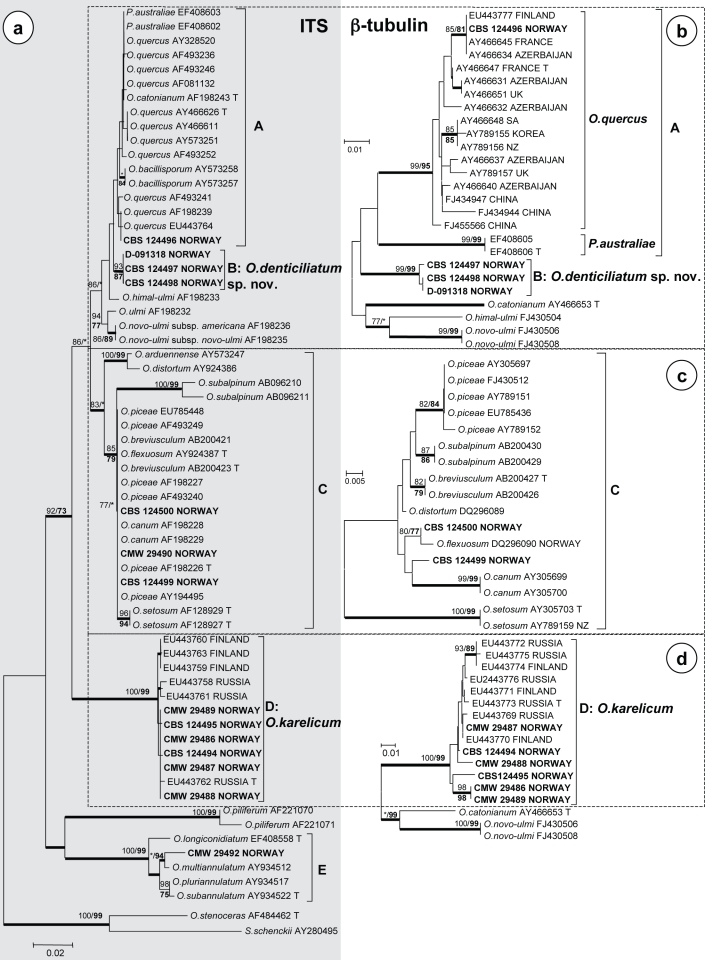
Aligned DNA sequence from the ITS regions yielded 715 characters including gaps (Fig. 1a), while alignments of the three β-tubulin data subsets consisted of 339, 332 and 329 characters, respectively, including gaps (Fig. 1b–d). All the trees presented were obtained from NJ analyses (Fig. 1). The Consistency Indices (CI) obtained from heuristic searches in MP analyses for the four datasets were 0.757, 0.819, 0.886 and 0.933, respectively, while the Retention Indices were 0.926, 0.889, 0.925 and 0.941, respectively. The Bayesian analyses for ITS and β-tubulin gene regions produced trees with topologies similar to those of the NJ and MP analyses. The best fitting substitution model selected for the Bayesian analyses was GTR+I+G for all the datasets.
Fig. 1.
Phylograms obtained from Neighbour-joining analyses of DNA sequences of (a) the nuclear ITS region and (b–d) the β-tubulin (BT) gene. Bootstrap support values (1 000 replicates) above 75 % are indicated at the nodes (normal type for Neighbour-joining, bold type for maximum parsimony). Posterior probabilities (above 90 %) obtained from Bayesian analyses are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 75 %. Isolate numbers of sequences obtained in this study are printed in bold type. — Scale bar = total nucleotide difference between taxa.
Comparisons of ITS sequences of the isolates from this study with sequences from GenBank, showed that S. ratzeburgi fungi resided in five groups which we designated A to E (Fig. 1a). Of these, groups A and C were not resolved in the ITS tree (Fig 1a), thus β-tubulin was used to distinguish between the species representing them (Fig. 1b, c). The groups representing O. karelicum (B and D) and an apparently undescribed species were well-resolved in the ITS tree (Fig. 1a) and in these cases β-tubulin sequence data were used to confirm their identities (Fig. 1b, d). For the isolate in group E, the ITS sequence data showed that it was closely related to, but distinct from, O. multiannulatum (Fig. 1a). No published β-tubulin sequences are available for species in this group and it could not be resolved further.
Analyses of the sequence data for the β-tubulin gene region revealed more variation among the so-called O. piceae-complex species (group C) than was present in the ITS tree. The isolates clustering in this group (Fig. 1c) were closely related to, but distinct from, O. flexuosum and O. canum.
Sexual compatibility tests
Pairing of single conidial cultures of the undescribed Ophiostoma species resulted in several successful crosses where ascomata with viable ascospores were produced. None of the single spore cultures formed ascomata when mated with themselves, indicating that the species is heterothallic.
Growth in culture
Isolates of the undescribed Ophiostoma species reached an average diam of 28 (± 4) mm within 8 d on MEA at 25 °C, which was also the optimal temperature for growth. The mean radial growth rate of isolates was 3.5 (± 0.5) mm/d at 25 °C. Very little growth occurred at 5 and 30 °C.
Taxonomy
Ophiostoma denticiliatum Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB509666; Fig. 2, 3
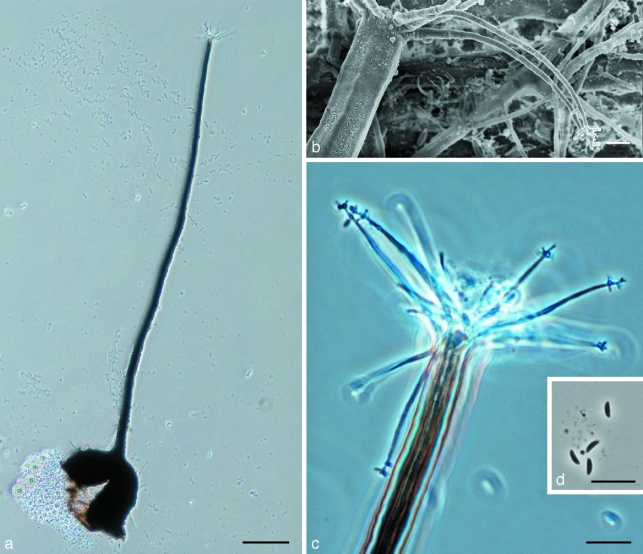
Fig. 2.
Morphological characters of Ophiostoma denticiliatum (holotype) teleomorph structures. a. Ascoma; b. scanning electron micrograph (SEM) of ostiolar hyphae with denticulate apex, starting to form Sporothrix-like anamorph; c. ostiolar hyphae; d. ascospores. — Scale bars: a = 100 μm; b = 3 μm; c, d = 10 μm.
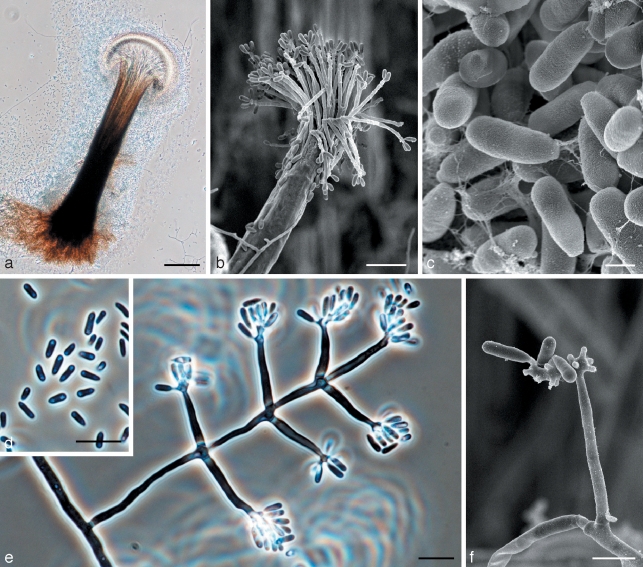
Fig. 3.
Morphological characters of Ophiostoma denticiliatum (holotype) anamorph structures. a. Pesotum anamorph; b. scanning electron micrograph (SEM) of Pesotum anamorph; c. SEM of conidia of Pesotum; d. conidia of Pesotum; e. Sporothrix-like anamorph with conidia; f. SEM of Sporothrix anamorph with conidia. — Scale bars: a = 100 μm; b, d, e = 10 μm; c = 1 μm; f = 3 μm.
Anamorphs. Pesotum sp.
Synanamorph. Sporothrix sp.
Bases atrae, globosae vel subglobosae (88–)132–181(–196) μm diametro, inornatae vel sparse pilis brunneis hypharum ornatae. Hyphae ostiolares (7–)9–13(–16) hyalinae divergentes (17–)25–42(–56) μm longae, basi 1–1.5 μm latae, apice denticulata conidia formanti; denticulis acutis 0.5–1.5 μm longis, primo crescentibus simul ac generatione hypharum. Ascosporae hyalinae, unicellulares, lateraliter visae allantoides 3.5–4.5(–5.5) × 1–1.5 μm, e fronte visae ellipsoideae (3–)3.5–4.5(–5) × 1–1.5 μm, ab extremo visae globosae. Anamorpha Pesotum macronema dominans. Anamorpha Sporothriciformis adest.
Etymology. The epithet denticiliatum refers to the denticulate apices of the ostiolar hyphae.
Ascomata developing after 3 wk when cultures of opposite mating type are paired on agar with birch twigs; superficial or partly embedded in agar (Fig. 2). Bases dark, globose to subglobose, (88–)132–181(–196) μm diam; unornamented or sparsely ornamented with brown hyphal hairs. Ascomatal necks smooth, dark at base, becoming paler towards apex, straight or slightly curved, (394–)667–986(–1286) μm long excluding ostiolar hyphae, (22–)26–34(–43) μm wide at base, (7–)8.5–10.5(–12) μm wide at the apex (Fig. 2a). Ostiolar hyphae (7–)9–13(–16) in number, hyaline, divergent, (17–)25–42(–56) μm long, 1–1.5 μm wide at the base, with denticulate apices producing conidia; denticles pointed, 0.5–1.5 μm long, appearing soon after the appearance of ostiolar hyphae (Fig. 2b, c). Asci not observed. Ascospores hyaline, 1-celled, allantoid in side view, 3.5–4.5(–5.5) × 1–1.5 μm, ellipsoidal in frontal view, (3–)3.5–4.5(–5) × 1–1.5 μm, globose in end view (Fig. 2d). Pesotum macronematal anamorph predominant (Fig. 3). Synnemata simple, dark brown at the base, becoming paler toward the apex, (346–)421–542(–647) μm long including conidiogenous apparatus, (42–)75–126(–159) μm wide at base; conidiogenous cells (14–)16–21(–26) × 1–1.5 μm; conidia hyaline, 1-celled, smooth, oblong, clavate or obovoid (2.5–)3–4.5(–5.5) × 1–1.5 μm. Synnematal anamorph usually common on different agar media with birch twigs. Attached to substrate by brown rhizoid-like hyphae (Fig. 3a–d). Sporothrix synanamorph present. Conidiogenous cells micronematous, mononematous, hyaline, (10–)13–22(–30) × 1–1.5(–2) μm, apical part consisting of swollen clusters bearing pointed denticles, 0.5–1(–1.5) μm; conidia hyaline, 1-celled, smooth, oblong, clavate or obovoid (3–)4–5(–6) × 1–1.5 μm (Fig. 3e, f).
Culture characteristics — Colonies at first hyaline, later becoming moderately to dark brown at the centre. Mycelium superficial on the agar, white to pale greyish aerial mycelium present. Pesotum anamorph dominant in cultures. Optimal temperature for growth 25 °C, growth reduced at 5 and 30 °C. The mean radial growth rate 3.5 (± 0.5) mm/d.
Sexuality — Heterothallic.
Host range — Associated with Scolytus ratzeburgi and their galleries in Betula pendula and B. pubescens.
Distribution — Presently known only from Southern Norway.
Specimens examined. Norway, Hobøl, on Scolytus ratzeburgi infesting Betula sp., July 2007, R. Linnakoski, holotype KUO 020520 (dried culture obtained from cross between CBS 124497 × CBS 124498, Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CBS 124497, CBS 124498; paratype KUO 020521 (dried culture CBS 124497); paratype KUO 020522 (dried culture CBS 124498); paratype KUO 020523 (dried culture VTT D-091318).
Notes — Ophiostoma denticiliatum is morphologically similar to other hardwood species in the so-called Ophiostoma piceae-complex, especially to O. quercus and the Dutch elm disease fungi. Species in the complex are characterised by ascospores that are orange-section shaped, with Pesotum and Sporothrix synanamorphs that are often produced simultaneously in culture (Harrington et al. 2001). Ophiostoma denticiliatum can be distinguished from other species in the complex by its relatively long ascomatal necks and especially the ostiolar hyphae that rapidly transform into conidiogenous cells with distinct denticles that produce conidia. In species such as O. quercus, ostiolar hyphae in old perithecia occasionally transform into Sporothrix-like conidiogenous cells (Z.W. de Beer, pers. comm.) but in O. denticiliatum these structures are a consistent character with all of the 50 examined ascomata sharing this feature. The denticles at the apices of the ostiolar hyphae are formed as soon as the ostiolar hyphae appear, and within days they begin to produce conidia and elongate. A character shared by O. denti-ciliatum and the Dutch elm disease fungi is that they are associated with Scolytus species infesting hardwoods (Burges et al. 1979, Webber 1990, Hubbes 1999), but O. denticiliatum was not consistently isolated from S. ratzeburgi, suggesting that the beetle might be only a casual vector of the fungus.
DISCUSSION
Ophiostomatoid fungi were frequently isolated from the adult beetles of Scolytus ratzeburgi and their galleries on birch in Norway. Of the 99 fungal isolates collected, these represented six Ophiostoma species. The only species consistently isolated from every gallery and beetle sampled was O. karelicum. Other species isolated infrequently included O. quercus and four apparently undescribed taxa. Of these, O. denticiliatum was described as new and the remaining three taxa were not described formally because an inordinately small number of isolates was available for them.
The results of this study are consistent with those of Linnakoski et al. (2008), who showed that O. karelicum is the predominant fungus associated with S. ratzeburgi in B. pendula. This fungus appears to have a specific association with the bark beetle species and its host tree. Our results further suggest that O. karelicum occurs across the geographic range of S. ratzeburgi, which includes various parts of Europe, Central and Eastern Russia, Mongolia and Japan (Postner 1974, Yanovskij 1996).
Ophiostoma quercus was found only occasionally associated with S. ratzeburgi in Norway, and this was also found previously for Finland and Russia (Linnakoski et al. 2008). The fungus was first described by Georgévitch (1926) from Quercus pedunculata (= Q. robur) in the present day Serbia. It is a common sap-staining agent on mostly hardwood hosts (de Beer et al. 2003, Geldenhuis et al. 2004, Kamgan Nkuekam et al. 2008, Grobbelaar et al. in press, Paciura et al. in press), but also causes economically relevant sap stain on commercially produced pine timber (Zhou et al. 2004, Thwaites et al. 2005, Kim et al. 2007). Ophiostoma quercus is morphologically and genetically highly diverse (Przybyl & Morelet 1993, Grobbelaar et al. 2009) with a worldwide distribution (Brasier & Kirk 1993, de Beer et al. 2003, Geldenhuis et al. 2004, Thwaites et al. 2005, Kamgan Nkuekam et al. 2008, Paciura et al. in press). It is vectored by many different insect species, especially bark beetles, but the association with these beetles appears to be casual (Brasier & Kirk 1993, Kirisits 2004, Zhou et al. 2004, 2006, Romón et al. 2007, Paciura et al. in press). The occasional occurrence of O. quercus with S. ratzeburgi is therefore not surprising.
DNA sequences for two gene regions of the new species described here as O. denticiliatum, confirmed that the species resides in a discrete, well-supported phylogenetic group in Ophiostoma. The fungus is related and morphologically similar to other Ophiostoma species from hardwoods, but closest to Pesotum australiae and O. quercus. Pesotum australiae is at present known only from wounds on the native hardwood, Acacia mearnsii, in Australia, and no teleomorph has been observed for it (Kamgan Nkuekam et al. 2008). Although it is likely that P. australiae is carried to wounds on trees by insects, no insect-fungus associations are known for it.
The identity of three morphologically distinct groups of isolates could not be resolved in this study. Two of these groups were represented by single isolates (CBS 124500 = CMW 29496 and CMW 29492), and were closely related to O. flexuosum and O. multiannulatum, respectively. The third group included two isolates (CMW 29490, CBS 124499 = CMW 29495), which are related to, but clearly distinct from, O. canum. Due to the very low numbers of isolates of these three taxa, we have chosen not to provide names for them at the present time.
Several fungi vectored by bark beetles cause blue stain in timber, and some are serious forest pathogens. The best-known examples of such pathogens are the Dutch elm disease fungi, Ophiostoma ulmi and O. novoulmi subsp. novoulmi and subsp. americana (Burges et al. 1979, Hubbes 1999, Brasier & Kirk 2001). Both O. karelicum and O. denticiliatum are closely related to the Dutch elm disease fungi. Their relatedness to these highly pathogenic fungi suggests that O. karelicum and O. denticiliatum have the potential to cause a vascular disease. It will, therefore, be important to consider their virulence on Betula pendula and other Betula spp.
Several native Betula species occur, for example, in North America, but thus far Scolytus ratzeburgi has not been reported from that part of the world. Russian timber is exported to the USA (UNECE/FAO 2008) and the possibility of the insect and its associated fungi being introduced is relatively high. It would thus be useful to test the susceptibility of North American white birches to S. ratzeburgi and O. karelicum. Introduction of these fungi into North America would be analogous to the introduction of the Dutch elm disease pathogens, presumably from the Himalayas (Brasier 1983) into Europe and North America, to encounter tree hosts that had not evolved together with them. The resulting disease has been devastating and the same could occur for fungi such as O. karelicum if they are introduced into new environments.
Acknowledgments
We are deeply grateful to Dr Hugh Glen for the Latin diagnosis. We also thank Prof. Pekka Niemelä (Faculty of Mathematics and Natural Sciences, Department of Biology, University of Turku) and Prof. Emeritus Teuvo Ahti (Finnish Museum of Natural History, University of Helsinki) for their advice. We acknowledge the Graduate School in Forest Sciences (GSForest) and the Finnish Forest Research Institute (Metla), Finland; the members of the Tree Protection Co-operative Programme (TPCP) and the THRIP initiative of the Department of Trade and Industry, South Africa, and Norwegian Forest and Landscape Institute, Norway for financial support to undertake this study. We also thank the Finnish IT Centre for Science (CSC) for providing computational resources.
REFERENCES
- Beer ZW de, Wingfield BD, Wingfield MJ. 2003. The Ophiostoma piceae-complex in the southern hemisphere: a phylogenetic study.. Mycological Research 107: 469 – 476 [DOI] [PubMed] [Google Scholar]
- Brasier CM. 1983. Dutch elm disease. The origin of Dutch elm disease. Report on Forest Research: 32 HMSO, London: [Google Scholar]
- Brasier CM, Kirk SA. 1993. Sibling species within Ophiostoma piceae.. Mycological Research 97: 811 – 816 [Google Scholar]
- Brasier CM, Kirk SA. 2001. Designation of the EAN and NAN races of Ophiostoma novoulmi as subspecies.. Mycological Research 105: 547 – 554 [Google Scholar]
- Burges HD, Grove JF, Pople M. 1979. The internal microbial flora of the elm bark beetle, Scolytus scolytus, at all stages of its development.. Journal of Invertebrate Pathology 34: 21 – 25 [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap.. Evolution 39: 783 – 791 [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for Basidiomycetes – application to the identification of mycorrhiza and rusts.. Molecular Ecology 2: 113 – 118 [DOI] [PubMed] [Google Scholar]
- Geldenhuis MM, Roux J, Montenegro F, Beer ZW de, Wingfield MJ, Wingfield BD. 2004. Identification and pathogenicity of Graphium and Pesotum species from machete wounds on Schizolobium parahybum in Ecuador.. Fungal Diversity 15: 135 – 149 [Google Scholar]
- Georgévitch P. 1926. Ceratostomella querci n. sp. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 183: 759 – 761 [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes.. Applied and Environmental Microbiology 61: 1323 – 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobbelaar J, Beer ZW de, Aghayeva DN, Bloomer P, Wingfield MJ, Wingfield BD. 2009. Delimitation of Ophiostoma quercus and its synonyms using multiple gene phylogenies.. Mycological Progress 8: 221 – 236 [Google Scholar]
- Harrington TC, McNew D, Steimel J, Hofstra D, Farrell R. 2001. Phylogeny and taxonomy of the Ophiostoma piceae-complex and the Dutch elm disease.. Mycologia 93: 111 – 136 [Google Scholar]
- Hubbes T. 1999. The American elm and Dutch elm disease.. Forestry Chronicle 75: 265 – 273 [Google Scholar]
- Kamgan Nkuekam G, Jacobs K, Beer ZW de, Wingfield MJ, Roux J. 2008. Pesotum australi sp. nov. and Ophiostoma quercus associated with Acacia mearnsii trees in Australia and Uganda, respectively. Australasian Plant Pathology 37: 406 – 416 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.. Nucleic Acids Research 30: 3059 – 3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G-H, Kim J-J, Breuil C. 2007. Sap-staining fungi from logs and boards of two commercially important pines in Korea.. Holzforschung 61: 333 – 336 [Google Scholar]
- Kirisits T. 2004. Fungal associates of European bark beetles with special emphasis on the Ophiostomatoid fungi . In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans H. (eds), Bark and wood boring insects in living trees in Europe, a synthesis: 81–235 Kluwer Academic Publishers, Dordrecht: [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: A biologist-centric software for evolutionary analyses of DNA and protein sequences.. Briefings in Bioinformatics 9: 299 – 306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnakoski R, Beer ZW de, Rousi M, Niemelä P, Pappinen A, Wingfield MJ. 2008. Fungi, including Ophiostoma karelicum sp. nov., associated with Scolytus ratzeburgi infesting birch in Finland and Russia. Mycological Research 112: 1475 – 1488 [DOI] [PubMed] [Google Scholar]
- O’Donnel K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous.. Molecular Phylogenetics and Evolution 7: 103 – 116 [DOI] [PubMed] [Google Scholar]
- Paciura D, Zhou XD, Beer ZW de, Jacobs K, Wingfield MJ. Characterisation of synnematous bark beetle-associated fungi from China, including Graphium carbonis sp. nov. Fungal Diversity In press [Google Scholar]
- Postner M. 1974. Scolytidae (Ipidae), Borkenkäfer. In: Schwencke W. (ed), Die Forstschädlinge Europas. Band. 2 (Käfer): 334–482 Paul Parey Verlag, Hamburg, Berlin, Germany: [Google Scholar]
- Przybyl K, Morelet M. 1993. Morphological differences between Ophiostoma piceae and O. querci, and among O. querci isolates. Cryptogamie Mycologie 14: 219 – 228 [Google Scholar]
- Romón P, Zhou X, Iturrondobeitia JC, Wingfield MJ, Goldarazena A. 2007. Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain.. Canadian Journal of Microbiology 53: 756 – 767 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models.. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Saalas U. Suomen metsähyönteiset. [Finnish forest insects] WSOY; Porvoo, Helsinki: 1949. [Google Scholar]
- Thwaites JM, Farrell RL, Duncan SM, Reay SD, Blanchette RA, Hadar E, Hadar Y, Harrington TC, McNew D. 2005. Survey of potential sapstain fungi on Pinus radiata in New Zealand.. New Zealand Journal of Botany 43: 653 – 663 [Google Scholar]
- UNECE/FAO 2008. Forest products annual market review 2007–2008. Geneva Timber and Forest Study Paper 23, ECE/TIM/SP/23, United Nations, New York / Geneva: [Google Scholar]
- Webber JF. 1990. Relative effectiveness of Scolytus scolytus, S. multistriatus and S. kirschi as vectors of Dutch elm disease. European Journal of Forest Pathology 20: 184 – 192 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–324 Academic Press, San Diego, California: [Google Scholar]
- Yanovskij VM. 1996. Annotated list of Scolytids (Coleoptera: Scolytidae) of North Asia.. Entomological Review 79: 493 – 522 [Google Scholar]
- Zhou XD, Beer ZW de, Ahumada R, Wingfield BD, Wingfield MJ. 2004. Ophiostoma and Ceratocystiopsis spp. associated with two pine-infesting bark beetles in Chile. Fungal Diversity 15: 261 – 274 [Google Scholar]
- Zhou XD, Beer ZW de, Wingfield MJ. 2006. DNA sequence comparisons of Ophiostoma spp., including Ophiostoma aurorae sp. nov., associated with pine bark beetles in South Africa. Studies in Mycology 55: 269 – 277 [DOI] [PMC free article] [PubMed] [Google Scholar]