Abstract
Recent phylogenetic studies based on multi-gene data have provided compelling evidence that the Mycosphaerellaceae and Teratosphaeriaceae represent numerous genera, many of which can be distinguished based on their anamorph morphology. The present study represents the second contribution in a series describing several novel species in different capnodealean genera defined in a previous study. Novelties on Eucalyptus from Australia include: Penidiella pseudotasmaniensis, P. tenuiramis, Phaeothecoidea intermedia, P. minutispora, Pseudocercospora tereticornis, Readeriella angustia, R. eucalyptigena, R. menaiensis, R. pseudocallista, R. tasmanica, Teratosphaeria alboconidia, T. complicata, T. majorizuluensis, T. miniata, T. profusa, Zasmidium aerohyalinosporum and Z. nabiacense, while Teratosphaeria xenocryptica is described on Eucalyptus from Chile. Novelties on other hosts include Phaeophleospora eugeniicola on Eugenia from Brazil, and Zasmidium nocoxi on twig litter from the USA.
Keywords: Mycosphaerella, Penidiella, Phaeophleospora, Phaeothecoidea, Pseudocercospora, Readeriella, Teratosphaeria, Zasmidium
INTRODUCTION
The genus Mycosphaerella s.l. includes around 3 000 names (Aptroot 2006), and together with its associated anamorph genera (especially Cercospora, Pseudocercospora, Septoria, Ramularia, etc.) represents more than 10 000 species names (Crous et al. 2000, 2001, 2004a, b, 2006a, b, c, 2007a, b, c, 2008a, b, Crous & Braun 2003, Arzanlou et al. 2007, 2008). Although Crous (1998) postulated that Mycosphaerella is polyphyletic, initial phylogenetic trees based on ITS DNA sequence data suggested the contrary (Crous et al. 1999, 2000, 2001, Stewart et al. 1999, Goodwin et al. 2001). However, adding additional loci later showed that Mycosphaerella is polyphyletic (Hunter et al. 2006, Crous et al. 2007a, b). Groups that have subsequently been separated from Mycosphaerella s.l. include Davidiella species with Cladosporium anamorphs (Davidiellaceae) (Braun et al. 2003, Crous et al. 2007b, Schubert et al. 2007, Zalar et al. 2007, Dugan et al. 2008), and Schizothyrium species with Zygophiala anamorphs (Schizothyriaceae) (Batzer et al. 2008).
In a recent study that considered generic boundaries in the Teratosphaeriaceae and Mycosphaerellaceae (Capnodiales, Dothideomycetes; Schoch et al. 2006), Crous et al. (2009b) suggested to use a single generic name per phylogenetically defined genus of fungi. In spite of the fact that not all clades could be defined, 12 genera were circumscribed in the Mycosphaerellaceae, representing Cercospora, Cercosporella, Dothistroma, Lecanosticta, Phaeophleospora, Polythrincium, Pseudocercospora, Ramularia, Ramulispora, Septoria, Sonderhenia and Zasmidium. The genera Ramichloridium and Dissoconium were excluded from the Mycosphaerellaceae, and shown to represent an undefined family (Crous et al. in prep.). A further nine genera were defined in the Teratosphaeriaceae, namely Baudoinia, Capnobotryella, Catenulostroma, Devriesia, Penidiella, Phaeothecoidea, Readeriella, Staninwardia and Teratosphaeria.
The aim of this study was to apply the generic concepts established in Crous et al. (2009b) for novel species collected from various substrates. Thus species descriptions are linked to genera represented by phylogenetically defined clades (Crous et al. 2009b), irrespective if the name applies to a sexual or asexual morph. The oldest generic name is given priority for each respective clade, with teleomorph species epithets having priority over anamorph epithets if both are known for the same holomorph.
MATERIALS AND METHODS
Isolates
Leaves with typical ‘Mycosphaerella’ leaf spots were chosen for study. Excised lesions were soaked in water for approximately 2 h, after which they were placed in the bottom of Petri dish lids, with the top half of the dish containing 2 % malt extract agar (MEA; Oxoid, Hampshire, England) (Crous et al. 1991). Ascospore germination patterns were examined after 24 h, and single ascospore and conidial cultures established as described by Crous (1998). Colonies were sub-cultured onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), MEA, and oatmeal agar (OA) (Crous et al. 2009c), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. All cultures obtained in this study are maintained in the culture collection of the CBS (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004a).
Table 1.
Details of the isolates for which novel sequences were generated and of other isolates of importance to this study.
| Species | Accession number1 | Host | Country | Collector | GenBank number (ITS)2 |
|---|---|---|---|---|---|
| Dissoconium commune | CPC 12397 | Eucalyptus globulus | Australia | I.W. Smith | GQ852738 |
| Dissoconium dekkeri | CPC 13097 | Corymbia variegata | Australia | A.J. Carnegie | GQ852739 |
| CPC 13098 | Eucalyptus "argophloia" | Australia | A.J. Carnegie | GQ852740 | |
| CPC 13264 | Eucalyptus molucana | Australia | B.A. Summerell | GQ852741 | |
| CPC 13279 | Eucalyptus sp. | Australia | P.W. Crous | GQ852742 | |
| CPC 13479 | Eucalyptus camaldulensis | Thailand | W. Himaman | GQ852743 | |
| ’Mycosphaerella’ heimii | CPC 11000 | Eucalyptus sp. | Colombia | M.J. Wingfield | GQ852744 |
| CPC 13099 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852745 | |
| ’Mycosphaerella’ konae | CPC 10992 | Eucalyptus sp. | Colombia | M.J. Wingfield | GQ852746 |
| ’Mycosphaerella’ marksii | CPC 13273 | Eucalyptus sp. | Australia | P.W. Crous | GQ852747 |
| CPC 13724 | Eucalyptus globulus | Australia | I.W. Smith | GQ852748 | |
| ’Penidiella’ pseudotasmaniensis | CBS 124991; CPC 12400 | Eucalyptus globulus | Australia | I.W. Smith | GQ852749 |
| Penidiella tenuiramis | CBS 124993; CPC 13692 | Eucalyptus tenuiramis | Tasmania | B.A. Summerell | GQ852750 |
| ’Phaeocryptopus’ qaeumannii | CPC 14373 | Pseudotsuga menziesii | – | – | GQ852751 |
| CPC 14374 | Pseudotsuga menziesii | – | – | GQ852752 | |
| CPC 14375 | Pseudotsuga menziesii | – | – | GQ852753 | |
| Phaeothecoidea intermedia | CBS 124994; CPC 13711 | Eucalyptus globulus | Australia | B.A. Summerell | GQ852754 |
| Phaeothecoidea minutispora | CBS 124995; CPC 13710 | Eucalyptus globulus | Australia | B.A. Summerell | GQ852755 |
| Pseudocercospora crousii | CBS 119487; Lynfield 1260 | Eucalyptus sp. | New Zealand | C.F. Hill | GQ852756 |
| Pseudocercospora pseudoeucalyptorum | CPC 12406 | Eucalyptus globulus | Australia | I.W. Smith | GQ852757 |
| CPC 12568 | Eucalyptus nitens | Australia | C. Mohammed | GQ852758 | |
| CPC 12802 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852759 | |
| CPC 12957 | Eucalyptus deonei | Australia | B.A. Summerell | GQ852760 | |
| CPC 13455 | Eucalyptus sp. | Portugal | P.W. Crous | GQ852761 | |
| CPC 13769 | Eucalyptus punctata | South Africa | P.W. Crous | GQ852762 | |
| CPC 13816 | Eucalyptus glanscens | United Kingdom | S. Denman | GQ852763 | |
| CPC 13926 | Eucalyptus sp. | USA:California | S. Denman | GQ852764 | |
| Pseudocercospora schizolobii | CBS 124990; CPC 13492 | Eucalyptus camaldulensis | Thailand | W. Himaman | GQ8527653 |
| Pseudocercospora sp. | CPC 12497 | Fraxinus rhynchophylla | South Korea | H.D. Shin | GQ852766 |
| CPC 14621 | Eucalyptus sp. | Madagascar | – | GQ852767 | |
| Pseudocercospora tereticornis | CBS 124996; CPC 12960 | Eucalyptus nitens | Australia | A.J. Carnegie | GQ852768 |
| CPC 13008 | Eucalyptus tereticornis | Australia | A.J. Carnegie | GQ852769 | |
| CPC 13299 | Eucalyptus tereticornis | Australia | P.W. Crous & B.A. Summerell | GQ852770 | |
| CPC 13315 | Eucalyptus tereticornis | Australia | P.W. Crous & B.A. Summerell | GQ852771 | |
| Readeriella angustia | CBS 124997; CPC 13608 | Eucalyptus delegatensis | Tasmania | B.A. Summerell | GQ852772 |
| CBS 124998; CPC 13618 | Eucalyptus delegatensis | Tasmania | B.A. Summerell | GQ852773 | |
| CPC 13621 | Eucalyptus regnans | Tasmania | B.A. Summerell, P. Summerell & A. Summerell | GQ852774 | |
| CPC 13630 | Eucalyptus delegatensis | Tasmania | B.A. Summerell | GQ852775 | |
| Readeriella callista | CBS 124986; CPC 13615 | Eucalyptus sclerophylla | Australia | B.A. Summerell | GQ852776 |
| CPC 12715 | Eucalyptus deanei | Australia | B.A. Summerell | GQ852777 | |
| CPC 12727 | Eucalyptus sp. | Australia | B.A. Summerell | GQ852778 | |
| CPC 12841 | Eucalyptus cannonii | Australia | B.A. Summerell | GQ852779 | |
| CPC 13605 | Eucalyptus multicaulis | Australia | B.A. Summerell | GQ852780 | |
| Readeriella eucalypti | CPC 13401 | Eucalyptus sp. | Portugal | P.W. Crous | GQ852781 |
| Readeriella eucalyptigena | CBS 124999; CPC 13026 | Eucalyptus dives | Australia | B.A. Summerell | GQ852782 |
| Readeriella menaiensis | CBS 125003; CPC 14447 | Eucalyptus oblonga | Australia | B.A. Summerell | GQ852783 |
| Readeriella mirabilis | CBS 125000; CPC 12370 | Eucalyptus sp. | Australia | R. Park | FJ493192 |
| CPC 12379 | Eucalyptus sp. | Australia | R. Park | GQ852784 | |
| CPC 13611 | Eucalyptus delegatensis | Tasmania | B.A. Summerell | GQ852785 | |
| Readeriella nontingens | CPC 14444 | Eucalyptus oblonga | Australia | B.A. Summerell | GQ852786 |
| Readeriella patrickii | CBS 124987; CPC 13602 | Eucalyptus amygdalina | Tasmania | P. Summerell & B.A. Summerell | GQ852787 |
| Readeriella pseudocallista | CBS 125001; CPC 13599 | Eucalyptus prominula | Australia | B.A. Summerell | GQ852788 |
| Readeriella tasmanica | CBS 125002; CPC 13631 | Eucalyptus delegatensis | Tasmania | B.A. Summerell | GQ852789 |
| Teratosphaeria alboconidia | CPC 14597 | Eucalyptus miniata | Australia | B.A. Summerell | FJ493197 |
| Teratosphaeria complicata | CPC 14535 | Leaf litter of Eucalyptus miniata | Australia | B.A. Summerell | GQ852790 |
| Teratosphaeria considenianae | CPC 13032 | Eucalyptus sp. | Australia | B.A. Summerell | GQ852791 |
| CPC 14057 | Eucalyptus stellatata | Australia | B.A. Summerell | GQ852792 | |
| Teratosphaeria corymbiae | CBS 124988; CPC 13125 | Eucalyptus henryii | Australia | A.J. Carnegie | FJ493185 |
| Teratosphaeria cryptica | CPC 12415 | Eucalyptus globulus | Australia | I.W. Smith | GQ852793 |
| CPC 12424 | Eucalyptus globulus | Australia | I.W. Smith | GQ852794 | |
| CPC 12559 | Eucalyptus nitens | Australia | C. Mohammed | GQ852795 | |
| CPC 12562 | Eucalyptus nitida | Australia | C. Mohammed | GQ852796 | |
| CPC 12565 | Eucalyptus amygdalina | – | C. Mohammed | GQ852797 | |
| CPC 13839 | Eucalyptus globulus | Australia | I.W. Smith | GQ852798 | |
| CPC 13842 | Eucalyptus globulus | Australia | I.W. Smith | GQ852799 | |
| Teratosphaeria destructans | CBS 111370; CPC 1368 | Eucalyptus sp. | Indonesia | P.W. Crous | GQ852800 |
| Teratosphaeria eucalypti | CPC 12552 | Eucalyptus nitens | Australia | C. Mohammed | GQ852801 |
| Teratosphaeria majorizuluensis | CBS 120040; CPC 12712 | Eucalyptus botryoides | Australia | B.A. Summerell | GQ852802 |
| Teratosphaeria miniata | CBS 125006; CPC 14514 | Leaf litter of Eucalyptus miniata | Australia | B.A. Summerell | GQ852803 |
| Teratosphaeria molleriana | CPC 12232 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852804 |
| CPC 12246 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852805 | |
| Teratosphaeria nubilosa | CPC 11926 | Acacia auriculiformis | Thailand | W. Himaman | GQ852806 |
| CPC 12235 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852807 | |
| CPC 12243 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852808 | |
| CPC 12830 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852809 | |
| CPC 13452 | Eucalyptus sp. | Portugal | P.W. Crous | GQ852810 | |
| CPC 13825 | Eucalyptus globulus | Australia | I.W. Smith | GQ852811 | |
| CPC 13828 | Eucalyptus globulus | Australia | I.W. Smith | GQ852812 | |
| CPC 13831 | Eucalyptus globulus | Australia | I.W. Smith | GQ852813 | |
| CPC 13833 | Eucalyptus globulus | Australia | I.W. Smith | GQ852814 | |
| CPC 13835 | Eucalyptus globulus | Australia | I.W. Smith | GQ852815 | |
| CPC 13837 | Eucalyptus globulus | Australia | I.W. Smith | GQ852816 | |
| CPC 13844 | Eucalyptus globulus | Australia | I.W. Smith | GQ852817 | |
| CPC 13847 | Eucalyptus coniocalyx | South Africa | P.W. Crous | GQ852818 | |
| CPC 13849 | Eucalyptus obliqua | South Africa | P.W. Crous | GQ852819 | |
| ’Teratosphaeria’ parva | CPC 12249 | Eucalyptus globulus | Portugal | A.J.L. Phillips | GQ852820 |
| CPC 12419 | Eucalyptus globulus | Australia | I.W. Smith | GQ852821 | |
| Teratosphaeria profusa | CBS 125007; CPC 12821 | Eucalyptus nitens | Australia | A.J. Carnegie | FJ493196 |
| Teratosphaeria sp. | CPC 13680 | Eucalyptus placita | Australia | B.A. Summerell | GQ852822 |
| Teratosphaeria stellenboschiana | CBS 124989; CPC 13767 | Eucalyptus punctata | South Africa | P.W. Crous | GQ852823 |
| CPC 12283 | Eucalyptus sp. | Corsica | J. Dijksterhuis | GQ852824 | |
| CPC 13764 | Eucalyptus punctata | South Africa | P.W. Crous | GQ852825 | |
| ’Teratosphaeria’ suberosa | CPC 13090 | Eucalyptus agglomerata | Australia | A.J. Carnegie | GQ852826 |
| CPC 13091 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852827 | |
| CPC 13093 | Eucalyptus mollucana | Australia | A.J. Carnegie | GQ852828 | |
| CPC 13094 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852829 | |
| CPC 13095 | Eucalyptus cloeziana | Australia | A.J. Carnegie | GQ852830 | |
| CPC 13096 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852831 | |
| CPC 13104 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852832 | |
| CPC 13106 | Eucalyptus argophloia | Australia | A.J. Carnegie | GQ852833 | |
| CPC 13111 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852834 | |
| CPC 13115 | Eucalyptus dunnii | Australia | A.J. Carnegie | GQ852835 | |
| CPC 13736 | Eucalyptus aquatica | Australia | B.A. Summerell | GQ852836 | |
| Teratosphaeria suttonii | CBS 119973; CMW 23440 | Eucalyptus pellita | Vietnam | T.I. Burgess | GQ852837 |
| CPC 12218 | Eucalyptus sp. | Indonesia | M.J. Wingfield | GQ852838 | |
| Teratosphaeria viscidus | CBS 124992; CPC 13306 | Eucalyptus sp. | Australia | P.W. Crous | FJ493186 |
| Teratosphaeria xenocryptica | CBS 122905; CPC 355 | Leaves of Eucalyptus sp. | Chile | M.J. Wingfield | AF309622 |
| ’Zasmidium’ aerohyalinosporum | CBS 125011; CPC 14636 | Eucalyptus tectifica | Australia | B.A. Summerell | GQ852839 |
| Zasmidium citri | CPC 13467 | Eucalyptus sp. | Thailand | W. Himaman | GQ852840 |
| Zasmidium lonicericola | CBS 125008; CPC 11671 | Lonicera japonica | South Korea | H.D. Shin | DQ303097 |
| ’Zasmidium’ nabiacense | CBS 125010; CPC 12748 | Eucalyptus sp. (red gum) | Australia | A.J. Carnegie | GQ852841 |
| Zasmidium nocoxi | CBS 125009; CPC 14044 | Litter | USA | P.W. Crous | GQ852842 |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI) of the University of Pretoria, Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; Lynfield: Private culture collection of Frank Hill.
2 ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA.
3 This isolate was not included in the analyses; see Crous et al. 2009b for position in 28S nrDNA phylogeny.
DNA phylogeny
Genomic DNA was extracted from mycelia taken from fungal colonies on MEA using the UltraCleanTM Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). A part of the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene (SSU), the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and the first 900 bp at the 5’ end of the 28S rRNA gene (LSU) was amplified and sequenced as described by Cheewangkoon et al. (2008). The generated ITS sequences were compared with other fungal DNA sequences from NCBI’s GenBank sequence database using a megablast search of the nr database; sequences with high similarity were added to the alignments. The ITS sequences were subsequently divided into a Mycosphaerellaceae and a Teratosphaeriaceae alignment to improve the alignment quality. Both alignments were subjected to phylogenetic analyses as described by Cheewangkoon et al. (2008) and only the first 1 000 equally parsimonious trees were saved. Novel sequences were lodged in GenBank (Table 1) and the alignments and phylogenetic trees in TreeBASE (http://www.treebase.org).
Taxonomy
Wherever possible, 30 measurements (× 1 000 magnification) were made of structures mounted in lactic acid, with the extremes of spore measurements given in parentheses. Colony colours (surface and reverse) were assessed after 1 mo on MEA, OA and PDA at 25 °C in the dark, using the colour charts of Rayner (1970).
RESULTS
Phylogenetic analyses
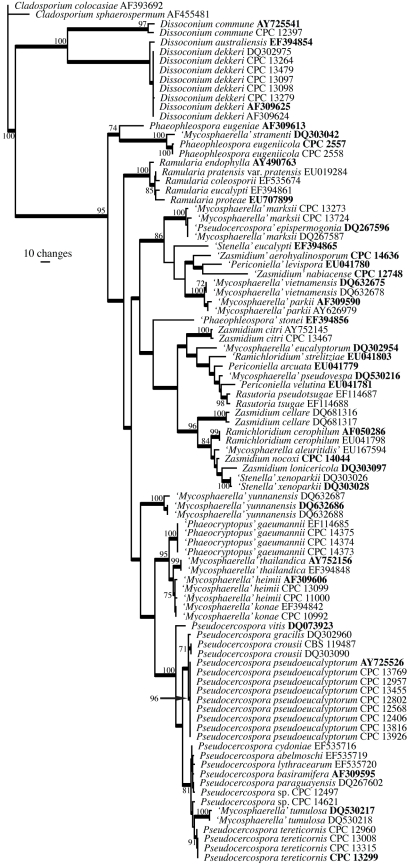
The manually adjusted ITS alignment for the Mycosphaerellaceae contained 93 taxa (including the two outgroup sequences) and, of the 547 characters used in the phylogenetic analysis, 268 were parsimony-informative, 30 were variable and parsimony-uninformative, and 249 were constant. Neighbour-joining analysis using the three substitution models on the sequence data yielded trees with identical topology and bootstrap values. For the parsimony analysis, only the first 1 000 equally most parsimonious trees were retained, the first of which is shown in Fig. 1 (TL = 1234, CI = 0.484, RI = 0.876, RC = 0.424).
Fig. 1.
First of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the Mycosphaerellaceae ITS sequence alignment using PAUP v4.0b10 (Swofford 2003). The scale bar shows 10 changes, and bootstrap support values 1 000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and ex-type sequences are printed in bold face. The tree was rooted to Cladosporium colocasiae (GenBank accession AF393692) and Cladosporium sphaerospermum (GenBank accession AF455481).
The manually adjusted ITS alignment for the Teratosphaeriaceae contained 93 taxa (including the two outgroup sequences) and, of the 521 characters used in the phylogenetic analysis, 265 were parsimony-informative, 35 were variable and parsimony-uninformative, and 221 were constant. Neighbour-joining analysis using the three substitution models on the sequence data yielded trees with identical topology and bootstrap values. For the parsimony analysis only the first 1 000 equally most parsimonious trees were retained, the first of which is shown in Fig. 2 (TL = 1473, CI = 0.411, RI = 0.892, RC = 0.367).
Fig. 2.
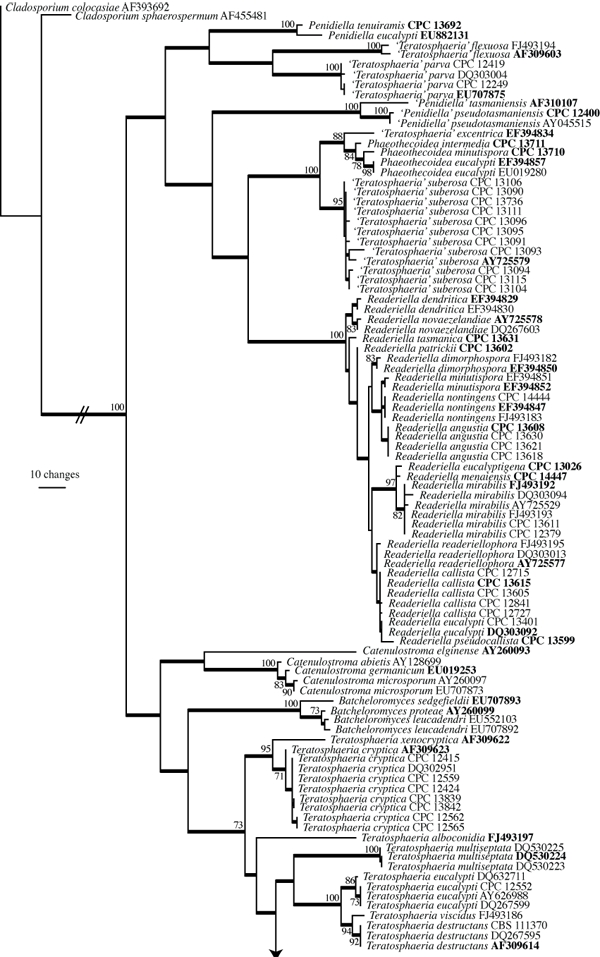
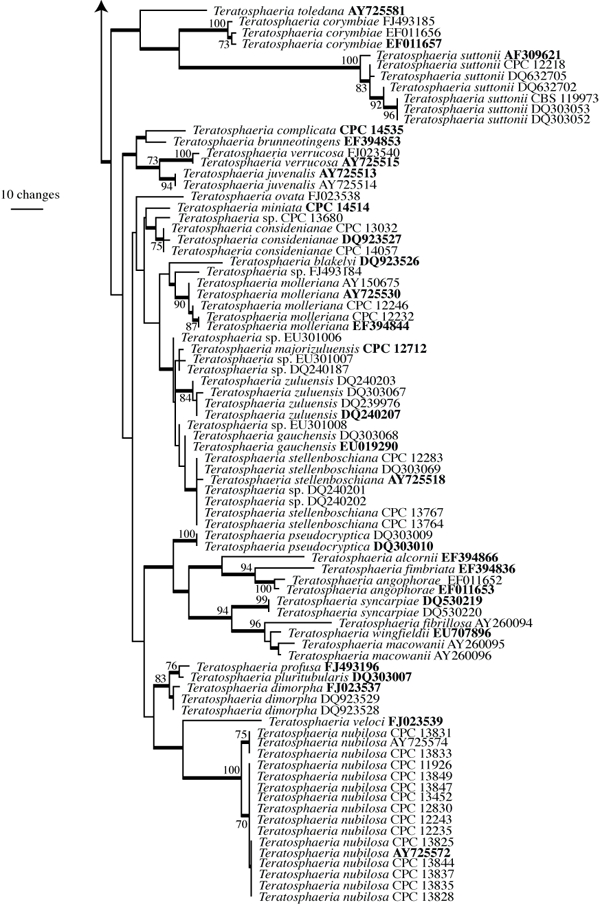
(two parts) First of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the Teratosphaeriaceae ITS sequence alignment using PAUP v4.0b10 (Swofford 2003). The scale bar shows 10 changes, and bootstrap support values 1 000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and ex-type sequences are printed in bold face. The tree was rooted to Cladosporium colocasiae (GenBank accession AF393692) and Cladosporium sphaerospermum (GenBank accession AF455481).
Taxonomy
This study has lead to the discovery of several novel taxa, which are treated in alphabetical order below.
Penidiella complex (Teratosphaeriaceae; Clades 21, 23 in Crous et al. 2009b)
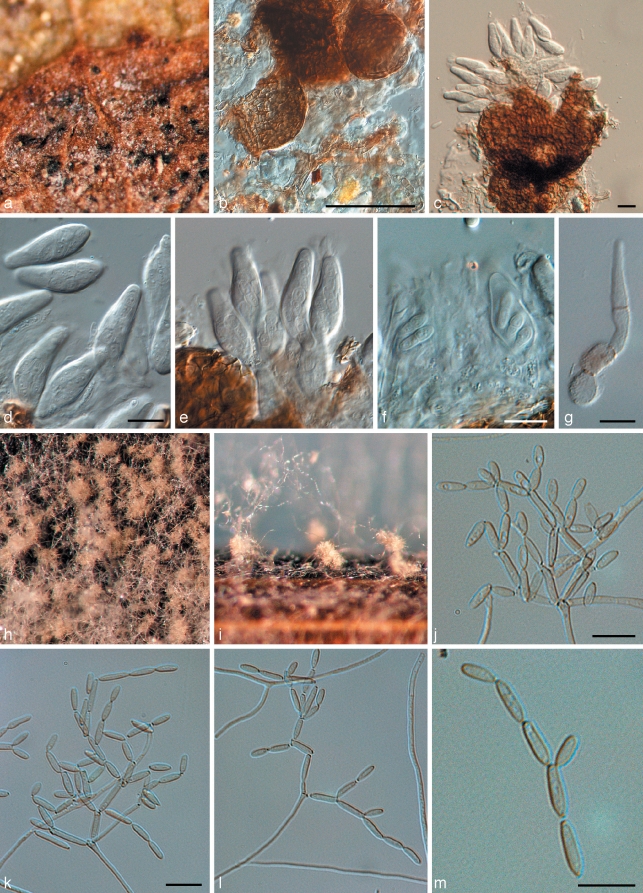
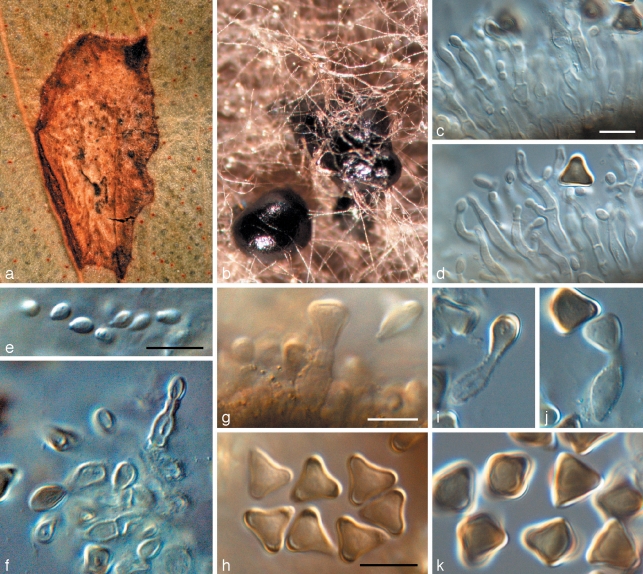
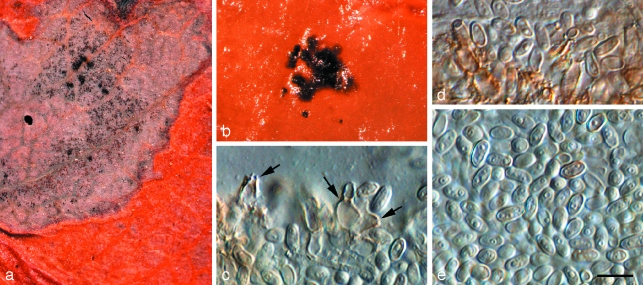
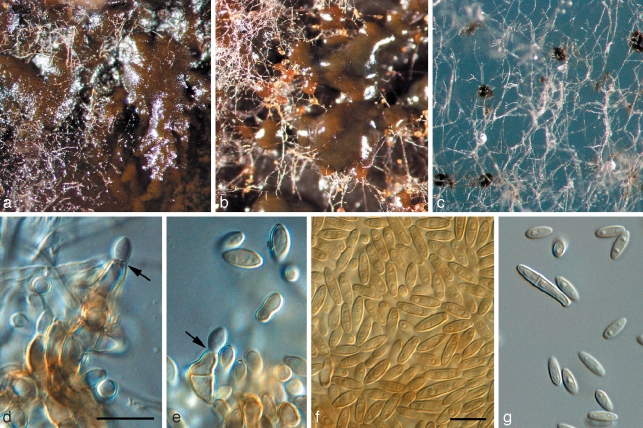
Penidiella pseudotasmaniensis Crous, sp. nov. — MycoBank MB509742; Fig. 3
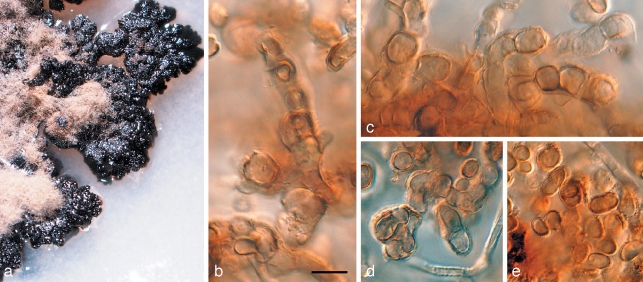
Fig. 3.
Penidiella pseudotasmaniensis (CPC 12400). a. Leaf spot; b. somewhat superficial ascomata; c. broken ascoma with asci; d–f. asci; g. germinating ascospore; h. colony on PDA; i. sporulation on pine needle; j–m. conidiophores giving rise to catenulate conidia. — Scale bars = 10 μm.
Statui anamorpho Teratosphaeriae tasmaniensis similis, sed germinatione ascosporarum typi G, ascosporis minoribus, (8–)9(–10) × 3(–3.5) μm.
Etymology. Name refers to the similarity of the Penidiella anamorph to that of ‘Teratosphaeria’ tasmaniensis.
Leaf spots amphigenous, irregular to subcircular or circular, 2–5 mm diam, medium brown, with a raised border and thin, red-purple margin. Ascomata pseudothecial, amphigenous, black, erumpent to almost superficial and loose, attached to the surface by means of a hyphal stroma; hyphae brown, septate, branched, 2–6 μm diam, arising from the base and sides of ascomatal body, but predominantly basal; globose, up to 100 μm wide; apical ostiole 5–10 μm wide; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoidal, straight to slightly curved, 8-spored, 25–35 × 8–10 μm. Ascospores bi- to tri-seriate, overlapping, hyaline, guttulate, thin-walled, straight, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, medianly 1-septate, constricted at the septum, tapering towards both ends, but more prominently towards the lower end, prominently guttulate, covered in mucilaginous sheath, which largely disappears at maturity, (8–)9(–10) × 3(–3.5) μm; ascospores germinate from one or both polar ends, with germ tubes parallel to the long axis of the spore; spore swelling, distorting and becoming prominently constricted at the septum, verruculose and brown, up to 6 μm wide (also observed on host surface) (germination Type G sensu Crous 1998). Conidiophores brown, erect, variable in length when on ends of creeping hyphae, 1–2 μm wide. Conidiogenous cells predominantly terminal, but at times also lateral, apex swollen with 1–4 subdenticulate loci that are thickened and somewhat darkened. Secondary ramoconidia (Schubert et al. 2007) subcylindrical, aseptate, pale brown, smooth, subcylindrical to narrowly fusoid, 5–9 × 1.5–2 μm. Intercalary and terminal conidia aseptate, pale brown, smooth, subcylindrical to narrowly fusoid, 5–9 × 1.5–2 μm; scars thickened and somewhat darkened.
Culture characteristics — Colonies on MEA erumpent, somewhat spreading, powdery due to profuse sporulation, sparse to moderate aerial mycelium, margin even but catenulate; surface honey to buff; reverse isabelline; colonies reaching 20 mm after 1 mo; on OA smoke-grey with moderate aerial mycelium and smooth, catenulate margins; reaching 30 mm diam after 1 mo; fertile.
Specimen examined. Australia, Victoria, on leaves of Eucalyptus globulus, Sept. 2005, coll. I.W. Smith, CBS H-20252 holotype, isol. P.W. Crous, cultures ex-type CPC 12400 = CBS 124991, CPC 12401, 12402.
Notes — Penidiella pseudotasmaniensis is phylogenetically related to T. tasmaniensis. Morphologically, however, the two species are quite distinct, and they also have different ascospore germination patterns (Crous 1998). Although the present collection was made in Victoria, a sequence lodged in GenBank by A. Milgate (AY045515), suggests that this species is also present in Tasmania.
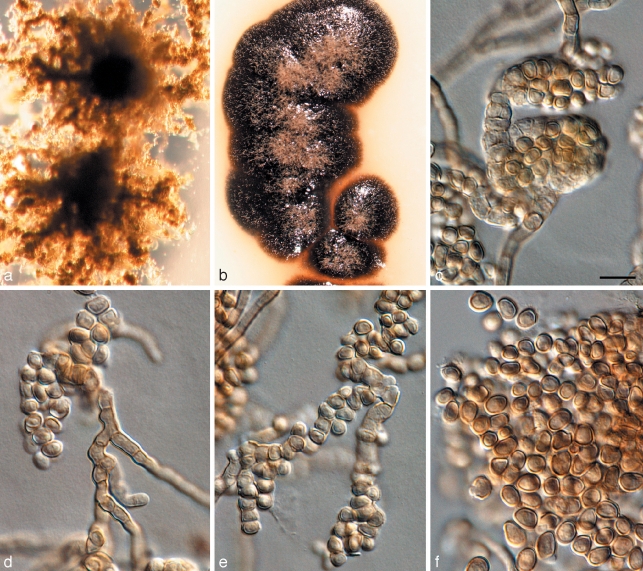
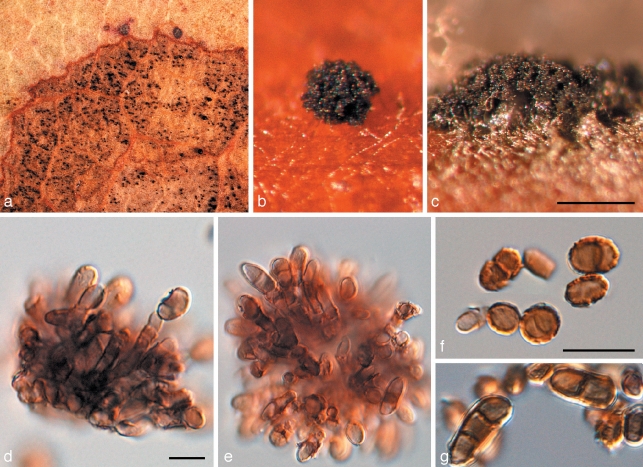
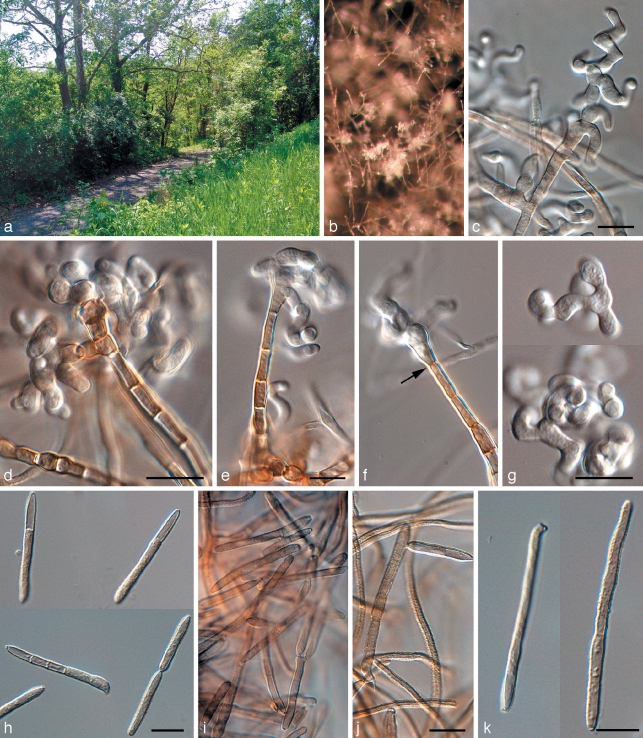
Penidiella tenuiramis Crous & Summerell, sp. nov. — MycoBank MB509743; Fig. 4
Fig. 4.
Penidiella tenuiramis (CPC 13692). a. Colony on PDA; b. colony sporulating on pine needle; c–f. macroconidiophores and catenulate conidia; g, h. micro-conidiophores; i, j. conidia. — Scale bars = 10 μm.
Penidiellae columbianae similis, sed conidiophoris apice non ramoso, sine apparato conidiogeno, et conidiis (6–)8–10(–12) × 3–4 μm.
Etymology. Name refers to the host species, Eucalyptus tenuiramis.
Isolated from leaves incubated in damp chambers. Mycelium spreading, superficial, but also internal in host tissue, pale brown, smooth, septate, branched, 1.5–2.5 μm, becoming dark brown, warty, up to 5 μm wide. Conidiophores dimorphic. Microconidiophores reduced to conidiogenous cells, lateral on hyphae, with or without a basal septum, up to 15 μm long, 2–3 μm wide. Macroconidiophores erect, arising as lateral branches from superficial hyphae, or as terminal ends of creeping hyphae, variable in length, pale to dark brown, smooth, 2–4 μm wide. Conidiogenous cells pale brown, smooth, subcylindrical, at times with clavate apex, 5–15 × 2.5–3.5 μm; scars somewhat thickened and darkened. Ramoconidia subcylindrical, pale to medium brown, smooth, 10–25 × 3–3.5 μm. Secondary ramoconidia narrowly to broadly ellipsoidal or subcylindrical, with 1–3 apical or lateral loci. Intercalary and terminal conidia ellipsoid to narrowly fusoid with obtuse ends, brown, smooth, (6–)8–10(–12) × 3–4 μm.
Culture characteristics — Colonies on MEA cottony, spreading with moderate aerial mycelium, and uneven margins, grey-olivaceous, reverse olivaceous-black; colonies covering plate after 1 mo; on OA cottony, smoke-grey to grey-olivaceous, covering plate after 1 mo.
Specimen examined. Australia, Tasmania, Tasman Peninsula, Brown Mountain walk, 43°11’13.9"S, 147°51’00.8"E, on leaves of Eucalyptus tenuiramis, 14 Oct. 2006, coll. B.A. Summerell & P. Summerell, CBS H-20253 holotype, isol. P.W. Crous, cultures ex-type CPC 13692 = CBS 124993, CPC 12693, 13694.
Notes — Penidiella tenuiramis clusters with the type species, P. columbiana (culture ex-type CBS 486.80, fig. 1 in Crous et al. 2009b). Morphologically it is distinct from the latter species, having microconidiophores, and pale brown conidia.
Phaeophleospora (Mycosphaerellaceae; Clade 5 in Crous et al. 2009b)
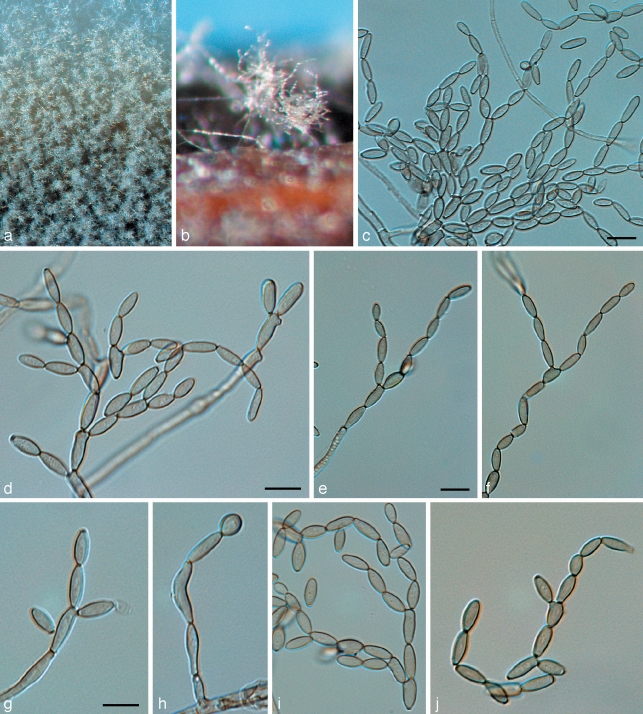
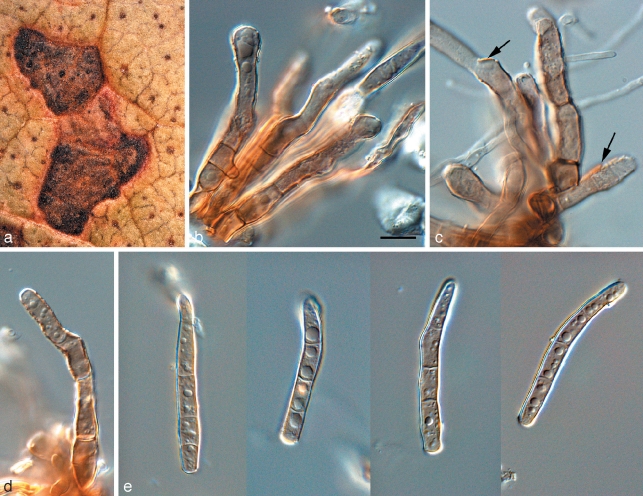
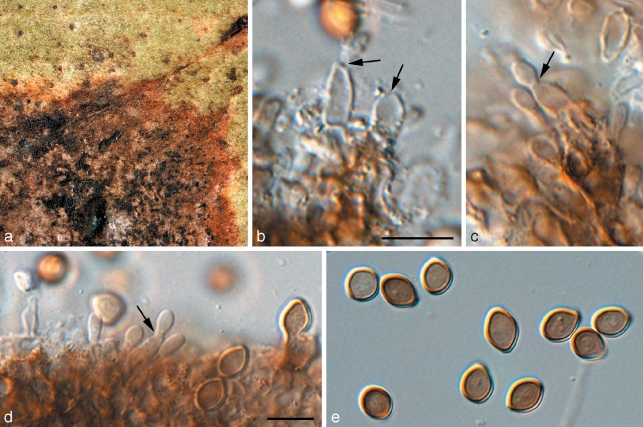
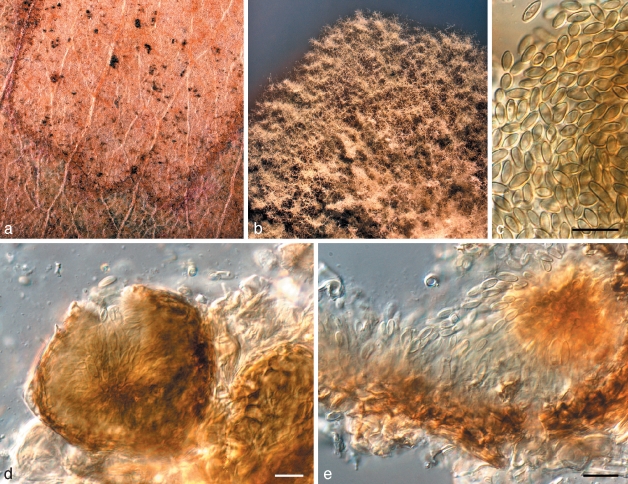
Phaeophleospora eugeniicola Crous & Alfenas, sp. nov. — MycoBank MB509744; Fig. 5
Fig. 5.
Phaeophleospora spp. a–g. Phaeophleospora eugenia. a. Colony on OA; b–d. conidiogenous cells giving rise to conidia; e, f. macroconidia; g. microconidia. — h–o. P. eugeniicola (CPC 2555). h–j. Vertical sections through conidiomata; k. hyphal elements in conidioma; l. conidiogenous cells; m–o. conidia. — p–s. P. stonei. p. Leaf spot with conidia cirrus (arrow); q. colony on OA; r. conidiogenous cells giving rise to conidia; s. conidia. — Scale bars = 10 μm.
Conidia solitaria, exsudata in cirris, subcylindrica ad obclavata, apice obtuso, basis obconice truncata, latissima in medio vel in triente basale conidii, crassitunicata, (9–)11–13(–15)-euseptata, recta vel irregulatiter curvata, hyalina vel subhyalina, levia, granulata, (40–)60–80(–90) × (4–)5–6 μm; hila cum segmento marginali minuto.
Etymology. Named after Eugenia, the host on which it occurs.
Leaf spots amphigenous, circular, 1–5 mm diam, brown, surrounded by a wide red-purple margin. Conidiomata epiphyllous, pycnidial, aggregated in the center of lesions, becoming erumpent, lifting the epidermis, which turns grey, until the spore mass breaks through in a black cirrus; pycnidia black, subglobose or somewhat flattened, unilocular, up to 150 μm high and 250 μm wide; wall of brown textura epidermoidea in surface view, and of textura angularis to textura intricata in vertical section, base of 2–3 layers, but lateral walls wider, consisting of up to 7 layers, with hyphal elements that frequently extend into the cavity (possibly as precursors to conidiophores); ostiole irregular, central. Conidiophores mostly reduced to conidiogenous cells, or multi-septate, subcylindrical, branched or not, brown, verruculose, 15–25 × 4–5 μm. Conidiogenous cells terminal, discrete, brown, verruculose, subcylindrical or doliiform, with 1–5 inconspicuous percurrent proliferations, or at times with sympodial proliferation, 8–15 × (3–)4–6(–8) μm. Conidia solitary, exuded in cirri, subcylindrical to obclavate, apex obtuse, base obconically truncate, widest in middle or basal third of conidium, thick-walled, (9–)11–13(–15)-euseptate, straight to irregularly curved, hyaline to subhyaline, smooth, granular, (40–)60–80(–90) × (4–)5–6 μm; hila with a minute marginal frill. Spermatogenous cells developing in vitro on CLA in conidiomata before the development of conidia, hyaline, ampulliform, 5–7 × 4–6 μm. Spermatia hyaline, smooth, rod-shaped, 3–4 × 1 μm.
Culture characteristics — Colonies pale white (surface), and pale mouse-grey (reverse), with smooth, slightly irregular margins, obtaining 13 mm diam after 14 d at 25 °C in the dark on MEA.
Specimen examined. Brazil, Pará, Belém, living leaves of Eugenia klotzschiana, 28 Apr. 1999, coll. A.C. Alfenas, PREM 56551 holotype, isol. P.W. Crous, cultures ex-type CPC 2555–2558 (cultures no longer viable, and therefore not deposited in CBS).
Notes — Phaeophleospora is based on P. eugeniae, which commonly occurs on leaf spots of Eugenia uniflora in Brazil (Crous et al. 1997), and is an anamorph of the Mycosphaerellaceae. Phaeophleospora eugeniicola is unusual in the sense that it has brown, verruculose hyphal elements that have also been observed to extend from the pycnidial wall into the cavity, giving rise to conidiogenous cells in some instances, but mostly appearing as sterile paraphyses between fertile, aseptate conidiophores. Phaeophleospora eugeniicola is also distinguished by its pycnidia with thick, almost stromatic lateral walls. This is in contrast to the type species, which tends to have more uniform walls, 3–4 cell layers thick. Phaeophleospora eugeniae and Ph. eugeniicola cluster apart (Fig. 1) from the recently described Ph. stonei (Crous et al. 2007c), which does not appear to be genetically related to the type species of Phaeophleospora.
Phaeothecoidea (Teratosphaeriaceae; Clade 29 in Crous et al. 2009b)
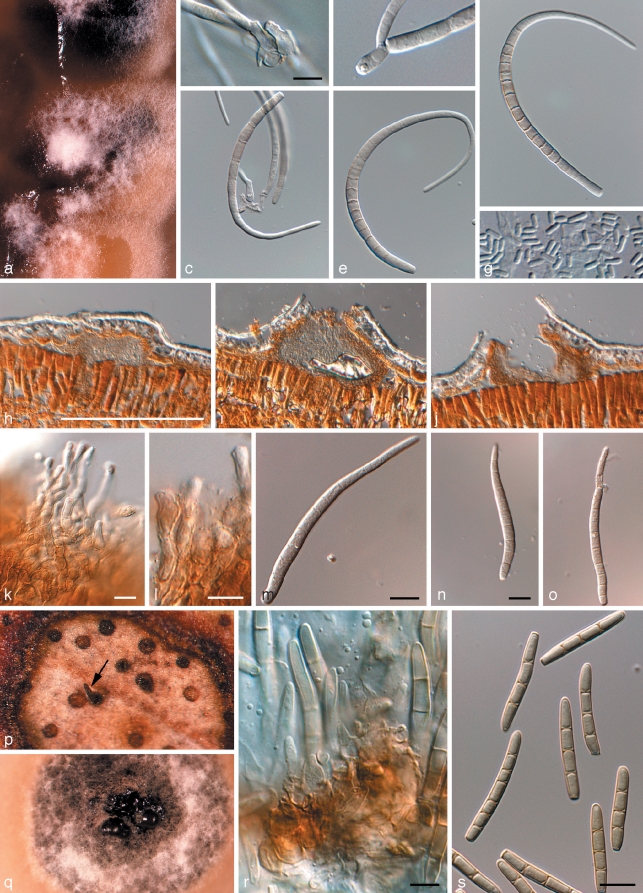
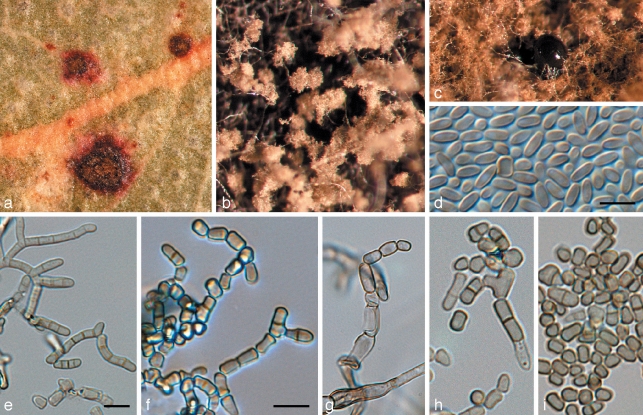
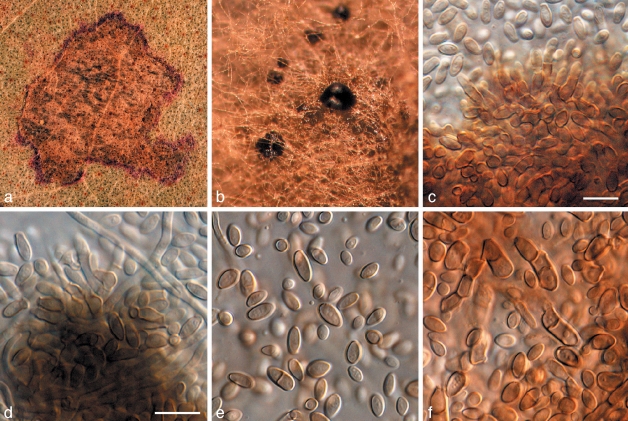
Phaeothecoidea intermedia Crous & Summerell, sp. nov. — MycoBank MB509745; Fig. 6
Fig. 6.
Phaeothecoidea intermedia (CPC 13711). a. Colony on OA; b–d. hyphae giving rise to endoconidia; e. conidia. — Scale bar = 10 μm.
Phaeothecoideae eucalypi similis, sed conidiis minoribus, 10–13 × 5–8 μm.
Etymology. Name refers to the conidia that are intermediate in size between those of P. eucalypti and P. minutispora.
Hyphae in vitro creeping, subhyaline, verruculose, branched, septate, 3–6 μm wide, becoming swollen, up to 15 μm wide, verruculose, medium brown; end cells dividing into several endoconidia, which are released upon rupture of the cell wall. Endoconidia pale to medium brown, smooth to verruculose, thick-walled, ellipsoid to obovoid or obclavate, 4–7 μm diam; after liberation swelling, becoming 1-septate, medium to dark brown, verruculose to verrucose, 10–13 × 5–8 μm.
Culture characteristics — Colonies on OA irregular, erumpent, with sparse aerial mycelium and feathery margins; center hazel, outer zone olivaceous-black, reaching 12 mm diam after 1 mo; fertile.
Specimen examined. Australia, Bruny Island, Adventure Bay Beach, 43°20’55.3"S, 147°19’21.8"E, on leaves of Eucalyptus globulus, 11 Oct. 2006, coll. B.A Summerell, P. Summerell & A. Summerell, CBS H-20254 holotype, isol. P.W. Crous, cultures ex-type CPC 13711 = CBS 124994.
Notes — Conidia of P. intermedia are slightly smaller than those of P. eucalypti, 10–15 × 5–7 μm (Crous et al. 2007c), but larger than those of P. minutispora, 5–8 × 4–6 μm. The two Phaeothecoidea species described here were isolated from the same leaves, and were originally separated based on their distinct cultural morphologies. Colonies of P. intermedia are very irregular and erumpent in growth, while those of P. minutispora are much less so, and more uniform.
Phaeothecoidea minutispora Crous & Summerell, sp. nov. — MycoBank MB509746; Fig. 7
Fig. 7.
Phaeothecoidea minutispora (CPC 13710). a. Colony on SNA; b. colony on OA; c–e. hyphae giving rise to endoconidia; f. conidia. — Scale bar = 10 μm.
Phaeothecoideae eucalypi similis, sed conidiis 5–8 × 4–6 μm.
Etymology. Name refers to the small conidia observed in this fungus.
Hyphae in vitro creeping, subhyaline, verruculose, branched, septate, 3–5 μm wide, becoming swollen, up to 10 μm wide, verruculose, medium brown; end cells dividing into several endoconidia, which are released upon rupture of the cell wall. Endoconidia pale to medium brown, smooth to verruculose, thick-walled, ellipsoid to obovoid or obclavate, 3–5 μm diam; after liberation swelling, becoming 1-septate, medium to dark brown, verruculose to verrucose, 5–8 × 4–6 μm.
Culture characteristics — Colonies on MEA slimy, erumpent, without aerial mycelium, and irregular, crenate margin, and folded surface, iron-grey, reverse greenish black; colonies reaching 15 mm diam after 1 mo; on OA fuscous-black, erumpent, with sparse to no aerial mycelium, and even margin; reaching 8 mm diam after 1 mo; fertile.
Specimen examined. Australia, Bruny Island, Adventure Bay Beach, 43°20’55.3"S, 147°19’21.8"E, on leaves of Eucalyptus globulus, 11 Oct. 2006, coll. B.A Summerell, P. Summerell & A. Summerell, CBS H-20255 holotype, isol. P.W. Crous, cultures ex-type CPC 13710 = CBS 124995.
Notes — Phaeothecoidea minutispora can easily be distinguished from P. eucalypti (conidia 10–15 × 5–7 μm; Crous et al. 2007c), in that it has smaller conidia, 5–8 × 4–6 μm. Phylogenetically these species also cluster apart (Fig. 2, part 1).
Pseudocercospora (Mycosphaerellaceae; Clade 16 in Crous et al. 2009b)
Pseudocercospora tereticornis Crous & Carnegie, sp. nov. — MycoBank MB509747; Fig. 8
Fig. 8.
Pseudocercospora tereticornis (CPC 13299). a. Leaf spots; b–d. conidiophores with conidiogenous cells (arrow indicates percurrent proliferation); e. conidia with guttules. — Scale bar = 10 μm.
Statui anamorpho Mycosphaerellae tumulosae similis, sed conidiis subcylindricis, (30–)40–65(–85) × (4–)5(–6) μm, hilis truncatis.
Etymology. Name refers to Eucalyptus tereticornis, on which it causes a prominent leaf spot disease.
Leaf spots amphigenous, irregular to subcircular, 1–5 mm diam, medium brown with a raised border and thin, red-purple margin. Mycelium predominantly internal, medium brown, consisting of septate, branched, roughened hyphae, 2–4 μm wide. Caespituli fasciculate, amphigenous, medium brown on leaves, up to 80 μm wide and 170 μm high. Conidiophores aggregated in loose fascicles arising from the upper cells of a brown stroma, up to 40 μm wide and 30 μm high; conidiophores medium brown, roughened, 2–10-septate, subcylindrical, straight to variously curved or geniculate-sinuous, unbranched, 40–140 × 4–6 μm. Conidiogenous cells terminal, unbranched, medium brown, roughened, tapering to a flat-tipped apex, proliferating percurrently at apex by means of several irregular proliferations, 10–30 × 4–5 μm. Conidia solitary, medium brown, smooth, guttulate, subcylindrical, apex obtuse, at times conidia somewhat elongated, then apex subobtuse, widest at truncate base, straight to curved, 1–6-septate, (30–)40–65(–85) × (4–)5(–6) μm; hila inconspicuous, with minute marginal frill.
Culture characteristics — Colonies on MEA erumpent, with moderate aerial mycelium and smooth, lobate margins; olivaceous-grey with patches of white; reverse iron-grey; reaching 35 mm diam after 1 mo; on OA flat, spreading with moderate aerial mycelium, and even catenulate margins, olivaceous-grey; reaching 35 mm after 1 mo.
Specimens examined. Australia, Queensland, Cairns, 48 km from Mareeba, Eureka Creek, 17°17’13.2"S, 145°02’27.4"E, 468 m, on leaves of E. tereticornis, 27 Sept. 2006, leg. P.W. Crous & B. Summerell, holotype CBS H-20256, isol. P.W. Crous, cultures ex-type CPC 13299–13301, 13315–13317; Bago State Forest, on leaves of Eucalyptus nitens, 22 Dec. 2005, A.J. Carnegie, CBS H-20257, culture CPC 12960 = CBS 124996; New South Wales, Bonalbo, Morpeth Park, on leaves of E. tereticornis, 8 Feb. 2006, coll. A.J. Carnegie, CBS H-20258, isol. P.W. Crous, cultures CPC 13008, 13009.
Notes — Phylogenetically Ps. tereticornis is closely related to Mycosphaerella tumulosa (Fig. 1), which also occurs on E. tereticornis (Carnegie et al. 2007). Morphologically, however, conidia of M. tumulosa have more obconically subtruncate basal cells than the truncate bases observed in Ps. tereticornis. Pseudocercospora tereticornis is most similar to Ps. pseudobasitruncata (Braun & Dick 2002), but distinct in that it has longer conidiophores, several irregular, percurrent proliferations on its conidiogenous cells, and shorter, more subcylindrical conidia with less septa.
Readeriella (incl. Nothostrasseria, with Teratosphaeria-like teleomorphs, and Cibiessia synanamorphs) (Teratosphaeriaceae; Clade 24 in Crous et al. 2009b)
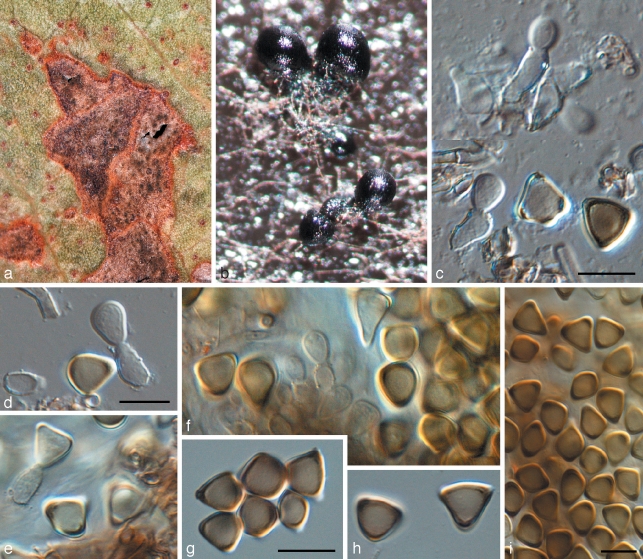
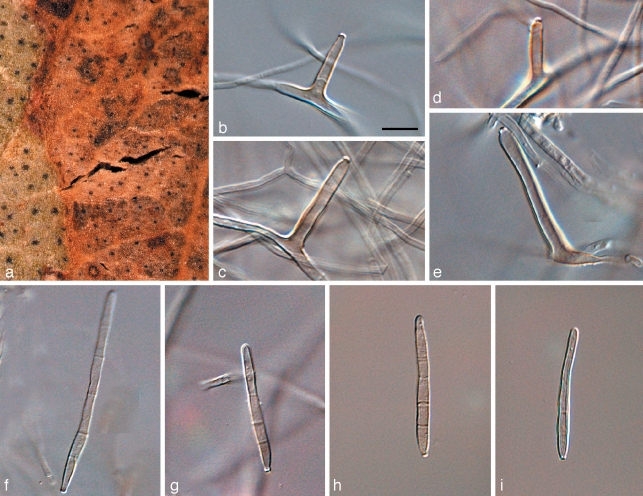
Readeriella angustia Crous & Summerell, sp. nov. — MycoBank MB509748; Fig. 9
Fig. 9.
Readeriella angustia (CPC 13608). a. Leaf spots; b. colony on PDA; c. Readeriella state among Cibiessia conidiophores; d. Readeriella conidia; e–i. conidia of Cibiessia state. — Scale bars = 10 μm.
Readeriellae minutisporae similis, sed conidiis angustioribus, 3–5 × 1.5–2 μm.
Etymology. Name refers to the narrow conidia in this fungus.
Leaf spots amphigenous, raised, dark brown with a raised border and thin red-brown margin, subcircular to angular, 1–3 mm diam. Hyphae pale brown, smooth, 2–3 μm wide, disarticulating at septa to form short, pale brown, cylindrical conidia with obtusely rounded to subtruncate ends; aseptate conidia 2–4 × 2–3 μm, 1(–3)-septate conidia 5–10 × 2–4.5 μm; conidia developing further, becoming medium brown, predominantly aseptate, verruculose, ellipsoidal to subglobose or globose, with dehiscence scars clearly visible on conidial body; inner layer of the dehiscence scar extends past the outer layer. Readeriella synanamorph: only observed in culture, with Cibiessia state dominant. Conidiomata oozing a dark brown conidial masses; conidiomata pycnidial, subglobose, unilocular; wall consisting of 3–4 layers of brown textura angularis. Conidiophores 0–1-septate, subcylindrical to ampulliform, hyaline to pale brown, smooth, 5–10 × 3–4 μm, mono- or polyphialide with visible periclinal thickening, or phialides proliferating percurrently near apex. Conidia narrowly ellipsoid to subcylindrical with rounded ends, pale brown, smooth to finely verruculose, 3–5 × 1.5–2 μm.
Culture characteristics — Colonies on MEA spreading, flat, with sparse to moderate aerial mycelium, and smooth, catenulate margins; surface olivaceous-grey with outer region iron-grey; reverse iron-grey; colonies reaching 60 mm diam after 1 mo; on OA spreading, flat, with sparse to moderate aerial mycelium, olivaceous-grey; colonies covering the plate after 1 mo.
Specimens examined. Australia, Tasmania, Mount Wellington, 42°55’0"S, 147°15’0"E, on leaves of Eucalyptus delegatensis, 14 Oct. 2006, coll. B.A. Summerell, CBS H-20259 holotype, isol. P.W. Crous, cultures ex-type CPC 13608 = CBS 124997, CPC 13609, 13610, CPC 13618 = CBS 124998, CPC 13619, 13620; Mt Wellington, 42°55’0"S, 147°15’0"E, on leaves of Eucalyptus delegatensis, 14 Oct. 2006, B.A. Summerell, CPC 13630; Mt Wellington, 42°41’0"S, 146°44’0"E, on leaves of Eucalyptus regnans, 14 Oct. 2006, coll. B.A. Summerell, P. Summerell & A. Summerell, CBS H-20260, isol. P.W. Crous, cultures CPC 13621–13623.
Notes — The anamorph genus Cibiessia was erected for Teratosphaeria-like species that form anamorphs that have disarticulating chains of brown conidia born in aerial mycelium, and Readeriella synanamorphs (Crous et al. 2007a). Presently three species of Cibiessia are known, from which R. angustia can be distinguished based on its shorter, narrower conidia. In an attempt to deal with pleomorphism in this clade, we proposed that the older genus name, Readeriella is applied (Crous et al. 2009b).
Readeriella eucalyptigena Crous & Summerell, sp. nov. — MycoBank MB509749; Fig. 10
Fig. 10.
Readeriella eucalyptigena (CPC 13026). a. Leaf spot; b. colony on PDA; c, d. microconidiophores; e. microconidia; f, g, i, j. conidiogenous cells giving rise to conidia; h, k. conidia. — Scale bars = 10 μm.
Readeriellae mirabilis similis, sed conidiis minoribus, (7–)8–9(–10) μm longis, ad apicem (7–)8(–9) μm latis.
Etymology. Name refers to an association with Eucalyptus.
Leaf spots amphigenous, irregular to angular, pale to medium brown, with a raised, dark brown border, 2–5 mm diam. Description on OA. Conidiomata pycnidial, brown, up to 500 μm diam; wall consisting of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, pale brown, finely verruculose, ampulliform to dolliform, proliferating several times percurrently near apex, mono- or polyphialidic, 6–15 × 3–6 μm. Conidia solitary, medium brown, aseptate, smooth, granular, base truncate, with three apical, lateral, obtuse projections, deltoid, thick-walled, with darker pigmentation in the lateral projections, but with more prominent constriction between the projections and the base, (7–)8–9(–10) μm long, (7–)8(–9) μm wide at apex. Microconidiophores occurring in same conidioma, subcylindrical, irregularly branched, 1–3-septate, up to 35 μm long, 3–5 μm wide. Microconidia ellipsoidal with subtruncate bases and obtuse apices, hyaline, smooth, 3–5 × 2–3.5 μm.
Culture characteristics — Colonies on MEA spreading with moderate aerial mycelium and uneven, feathery margins, pale olivaceous-grey, reverse iron-grey; covering the dish after 1 mo; on OA olivaceous-grey, with patches of pale olivaceous-grey to white, and moderate aerial mycelium, covering the dish after 1 mo; colonies fertile.
Specimen examined. Australia, New South Wales, Southern Tablelands, on leaves of Eucalyptus dives, Apr. 2006, coll. B.A. Summerell, CBS H-20261 holotype, isol. P.W. Crous, cultures ex-type CPC 13026 = CBS 124999, CPC 13027, 13028.
Notes — Although conidia of R. eucalyptigena (7–10 × 7–9 μm) are somewhat smaller than those of R. mirabilis and R. menaiensis, the most obvious difference lies in the more prominent constrictions between the lateral projections and base of R. eucalyptigena conidia.
Readeriella mirabilis Syd. & P. Syd., Ann. Mycol. 6: 484. 1908.
Illustration — Crous et al. (2007a).
Leaf spots amphigenous, irregular to subcircular, medium brown, border raised, dark brown, 2–8 mm diam. Description on OA. Conidiomata pycnidial, brown, up to 300 μm diam; wall consisting of 2–3 layers of brown textura angularis. Conidiophores ampulliform to dolliform, 0–1-septate, pale brown, finely verruculose, predominantly unbranched, 10–20 × 3–5 μm. Conidiogenous cells terminal, unbranched, hyaline, becoming pale brown, smooth to finely verruculose, proliferating several times percurrently near apex, mono- or polyphialidic, ampulliform to dolliform, 6–15 × 3–5 μm. Conidia solitary, medium brown, aseptate, smooth, granular, base truncate, with three apical, lateral, obtuse projections, deltoid, thick-walled, with darker pigmentation in the lateral projections, (7–)9–10(–11) μm long, (7–)8–9(–10) μm wide at apex.
Culture characteristics — Colonies on MEA spreading, erumpent, with moderate aerial mycelium and droplets of slime; surface uneven, pale olivaceous-grey to olivaceous-grey, with feathery margins; reverse olivaceous-grey; reaching 20 mm after 2 wk; on OA spreading, woolly with moderate aerial mycelium, and even, catenulate margins; surface pale olivaceous-grey to olivaceous-grey; reaching 30 mm diam after 2 wk; colonies fertile.
Specimens examined. Australia, Victoria, on leaves of Eucalyptus capitellata, June 1907, F.M. Reader, S, holotype; Victoria, on leaves of Eucalyptus globulus, Sept. 2005, coll. I.W. Smith, CBS H-20262 epitype designated here, isol. P.W. Crous, cultures ex-type CPC 12370 = CBS 125000, CPC 12371, 12372.
Notes — Readeriella mirabilis is compared here with two similar species that have the same conidial shape and dimensions. Although R. menaiensis has conidia that are 9–10(–11) μm long, (7–)8–9 μm wide and thus similar to those in R. mirabilis, it has somewhat longer conidiophores (up to 30 μm long), and has faster growing, darker colonies. Readeriella eucalyptigena has somewhat smaller conidia to these two species, (7–)8–9(–10) μm long, (7–)8(–9) μm wide, with a more prominent lateral constriction between the projections and the conidial base.
Readeriella pseudocallista Crous & Summerell, sp. nov. — MycoBank MB509750; Fig. 11
Fig. 11.
Readeriella pseudocallista (CPC 13599). a. Leaf spot; b–d. conidiogenous cells giving rise to conidia (arrows denote loci); e. conidia. — Scale bars = 10 μm.
Readeriellae callistae similis, sed conidiis uniformiter modice brunneis et latitudine maximo in medio.
Etymology. Name refers to the fact that this fungus is very similar to Readeriella callista.
Leaf spots amphigenous, circular to irregular, medium brown, with a raised, brown border, and thin red-purple margin, up to 6 mm diam. Description on OA. Conidiomata pycnidial, brown, up to 300 μm diam; wall consisting of 3–4 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, hyaline to pale brown, smooth, aseptate, ampulliform to dolliform, with visible periclinal thickening near apex, 4–7 × 2–4 μm; polyphialides also observed in culture. Conidia solitary, medium brown, aseptate, finely verruculose, ellipsoidal, tapering towards a subobtuse apex and subtruncate or truncate base (1 μm wide), (5–)7–8 × (4.5–)5–5.5 μm.
Culture characteristics — Colonies on MEA spreading, woolly, pale olivaceous-grey to olivaceous-grey; reverse iron-grey; covering the plate in 1 mo; on OA pale olivaceous-grey, with patches of olivaceous-grey; covering the plate in 1 mo; fertile.
Specimen examined. Australia, New South Wales, Central Coast, 33°05’01"S, 151°07’39"E, on leaves of Eucalyptus prominula, Oct. 2006, coll. B.A. Summerell, CBS H-20263 holotype, isol. P.W. Crous, cultures ex-type CPC 13599 = CBS 125001, CPC 13600, 13601.
Notes — This fungus is very similar to R. callista in conidial dimensions, 7–8.5 × 4–5 μm, and conidiogenesis. It has a very distinct conidial shape, and pigmentation, with conidia being more uniformly medium brown, being widest in the middle, and lacking the prominent taper towards an obtuse apex observed in R. callista.
Readeriella tasmanica Crous & Summerell, sp. nov. — MycoBank MB509751; Fig. 12
Fig. 12.
Readeriella tasmanica (CPC 13631). a. Leaf spot; b. colony on OA; c–e. conidiogenous cells giving rise to conidia; f, g. conidia. — Scale bars = 10 μm.
Readeriellae dendriticae similis, sed conidiis minoribus, (7–)8–10(–11) × (2.5–)3–3.5(–4) μm.
Etymology. Name refers to Tasmania, where the type was collected.
Leaf spots predominantly hypophyllous, irregular to subcircular, medium brown, 1–4 mm diam, raised above the leaf lamina, with pycnidia oozing black spore masses onto the leaf surface. Description on OA. Conidiomata black, globose, pycnidial, almost superficial on agar surface, oozing black conidial masses, at times appearing multilocular, up to 400 μm diam. Conidiophores ampulliform to lageniform, hyaline, smooth, 0–3-septate, mono- to polyphialidic, rarely proliferating percurrently, rarely branched, with loci terminal and lateral, 10–20 × 3–5 μm. Conidia consisting of an ellipsoid body with obtuse apex, tapering to a tubular basal appendage; body medium brown, verruculose, (7–)8–10(–11) × (2.5–)3–3.5(–4) μm; tubular appendage separated from the conidium body by a septum, unbranched, hyaline, smooth, (5–)6–7(–8) × 1–1.5 μm.
Culture characteristics — Colonies on MEA erumpent, spreading with moderate to abundant aerial mycelium, and feathery margins; surface pale olivaceous-grey, margins olivaceous-grey; reverse olivaceous-grey; colonies reaching 35 mm diam after 1 mo; on OA erumpent, cottony, with moderate aerial mycelium, uneven but with smooth margins, pale olivaceous-grey; colonies reaching 40 mm after 1 mo.
Specimen examined. Australia, Tasmania, Mount Wellington Park, 42°55’0"S, 147°15’0"E, on leaves of Eucalyptus delegatensis, 14 Oct. 2006, coll. B.A. Summerell, CBS H-20264 holotype, isol. P.W. Crous, cultures ex-type CPC 13631 = CBS 125002, CPC 13632.
Notes — Conidia of Readeriella tasmanica (7–11 × 2.5–4 μm, appendages 5–8 × 1–1.5 μm) are smaller than those of R. dendritica (6–12 × 3–4 μm, appendages 4–15 × 1–1.5 μm; Summerell et al. 2006).
Readeriella menaiensis Crous & Summerell, sp. nov. — MycoBank MB509752; Fig. 13
Fig. 13.
Readeriella menaiensis (CPC 14447). a. Leaf spot; b. colony on OA; c–f. conidiogenous cells giving rise to conidia; g–i. conidia. — Scale bars = 10 μm.
Readeriellae mirabilis similis, sed conidiophoris longioribus et coloniis in vitro (MEA) cito crescentibus.
Etymology. Name refers to Menai, Australia where the fungus was first collected.
Leaf spots amphigenous, irregular to subcircular, medium brown, frequently grey-brown in center, border raised, dark brown, 2–5 mm diam. Description on OA. Conidiomata pycnidial, brown, up to 300 μm diam; wall consisting of 2–3 layers of brown textura angularis. Conidiophores ampulliform to dolliform, 0–2-septate, pale brown, finely verruculose, predominantly unbranched, 8–30 × 3–6 μm. Conidiogenous cells terminal, unbranched, medium brown, finely verruculose, proliferating several times percurrently near apex, mono- or polyphialidic, ampulliform to dolliform, 8–15 × 3–6 μm. Conidia solitary, medium brown, aseptate, smooth, granular, base truncate, with three apical, lateral, obtuse projections, deltoid, thick-walled, with darker pigmentation in the lateral projections, 9–10(–11) μm long, (7–)8–9 μm wide at apex.
Culture characteristics — Colonies on MEA spreading with moderate aerial mycelium and uneven, feathery margins, fuscous-black, reverse black; reaching 70 mm after 1 mo; on OA black, covering the dish after 1 mo, with sparse aerial mycelium; colonies fertile.
Specimen examined. Australia, New South Wales, Menai, Central Coast, 34°00’38"S, 151°00’57"E, on leaves of Eucalyptus oblonga, 23 Sept. 2007, coll. B.A. Summerell, CBS H-20265 holotype, isol. P.W. Crous, cultures ex-type CPC 14447 = CBS 125003, CPC 14448, 14449.
Notes — Readeriella menaiensis is distinguished from R. mirabilis based on its longer conidiophores and darker, faster growing colonies.
Teratosphaeria (with Kirramyces and Colletogloeopsis anamorphs, and Batcheloromyces-like synanamorphs) (Teratosphaeriaceae; Clade 31 in Crous et al. 2009b)
Teratosphaeria alboconidia Crous & Summerell, sp. nov. — MycoBank MB509753; Fig. 14
Fig. 14.
Teratosphaeria alboconidia (CPC 14597). a. Leaf spot; b. colony on OA; c, d. conidiogenous cells giving rise to conidia (arrows denote loci); e. conidia. — Scale bar = 10 μm.
Teratosphaeriae zuluensis similis, sed conidiis (4–)5–6(–7) × (2–)2.5–3 μm, pallide brunneis.
Etymology. Name refers to the pale brown conidia in this fungus.
Leaf spots amphigenous, irregular, 3–6 mm diam, grey, with a thin, raised, brown border. Conidiomata amphigenous, pycnidial, substomatal, brown, globose, up to 120 μm diam, exuding black conidial masses onto the leaf surface; wall consisting of 3–4 layers of brown textura angularis; becoming acervular in culture. Description on OA. Conidiophores reduced to conidiogenous cells, brown, verruculose, aseptate, dolliform, proliferating percurrently near apex, 5–8 × 2.5–3.5 μm. Conidia solitary, pale brown to brown, aseptate, guttulate, verruculose, ellipsoidal to subcylindrical, apex obtuse, base truncate or bluntly rounded, (4–)5–6(–7) × (2–)2.5–3 μm.
Culture characteristics — Colonies on MEA white, erumpent, spreading with moderate aerial mycelium and smooth, even margins; reverse scarlet; colonies reaching 25 mm diam after 1 mo; on OA flat, spreading, with even, catenulate margins, lacking aerial mycelium; surface slimy, flesh coloured with patches of scarlet, reaching 30 mm diam after 1 mo; fertile.
Specimen examined. Australia, Northern Territory, ENE Pine Creek, 13°40’49"S, 131°59’04.9"E, on leaf litter of Eucalyptus miniata, 23 Sept. 2007, coll. B.A. Summerell, CBS H-20266 holotype, isol. P.W. Crous, cultures ex-type CPC 14597, 14598 = CBS 125004, CPC 14599.
Notes — Morphologically T. alboconidia is quite distinct from other taxa in the genus, based on its rather small, (4–)5–6(–7) × (2–)2.5–3 μm, ellipsoid, pale brown conidia.
Teratosphaeria complicata Crous & Summerell, sp. nov. — MycoBank MB509754; Fig. 15
Fig. 15.
Teratosphaeria complicata (CPC 14535). a. Leaf spot; b. colony on OA; c. colony on inoculated pine needle; d, e. fascicles with conidiogenous cells giving rise to conidia; f, g. conidia. — Scale bars = 10 μm.
Conidiomata sporodochiales, amphigena. Cellulae conidiogenae terminals, subcylindricae, brunneae, verruculosae, ad apicem cum proliferationibus irregularibus, percurrentibus, 5–12 × 3–5 μm. Conidia subcylindrica vel ellipsoidea, recta vel curvata, brunnea, verruculosa, 0–3-septata; in vitro conidia 3-septata usque ad 30 μm longa, conidia 2-septata usque ad 20 μm, conidia 1-septata usque ad 13 μm, conidia non septata (6–)7–8 × (4.5–)5–6 μm.
Etymology. Name reflects the complicated situation surrounding the generic affinity of this fungus.
Leaf spots amphigenous, circular, 10–20 mm diam, brown, with a raised, thin, red-purple border. Conidiomata sporodochial, amphigenous, black, up to 60 μm diam on leaves, but up to 1 mm diam in culture on sterile pine needles on WA, and even larger on MEA. Conidiophores reduced to conidiogenous cells, or 1–3-septate, brown, verruculose, subcylindrical, unbranched or branched at apex, 10–20 × 3–5 μm. Conidiogenous cells terminal, subcylindrical, brown, verruculose, with several irregular, rough, flaring percurrent proliferations near apex, 5–12 × 3–5 μm; in culture conidiogenous cells frequently without percurrent proliferation visible. Conidia subcylindrical to ellipsoid, straight to curved, brown, verruculose, almost warty, with longitudinal striations, apex obtuse, base bluntly rounded or truncate, with a flaring marginal frill; wall thick, 0–3-septate, eventually disarticulating with age into aseptate conidia; 3-septate conidia up to 30 μm long, 2-septate up to 20 μm long, 1-septate up to 13 μm long, aseptate conidia, (6–)7–8 × (4.5–)5–6 μm in vivo.
Culture characteristics — Colonies on MEA spreading, with sparse aerial mycelium; surface catenulate, uneven; margin feathery; olivaceous-grey with patches of iron-grey due to sporulation; reverse greenish black; reaching 25 mm after 1 mo; on OA spreading, woolly, with moderate aerial mycelium, olivaceous-grey; margin irregular, forming a diffuse red-brown pigment in agar; colonies reaching 18 mm diam after 1 mo; fertile.
Specimen examined. Australia, Northern Territory, ENE Pine Creek, 13°40’49"S, 131°59’04.9"E, on leaf litter of Eucalyptus miniata, 23 Sept. 2007, coll. B.A. Summerell, CBS H-20267 holotype, isol. P.W. Crous, cultures ex-type CPC 14535–14537.
Notes — Following Ellis (1971), the present collection would be defined as a species of Stigmina. Morphologically it is similar to T. eucalyptorum, described from E. maidenii leaves in Tanzania (Crous 1998). Conidia of the latter species are smaller, however, being 0–2-septate, 8–15 × 3.5–6 μm. However, based on the findings of Crous et al. (2006b), Stigmina was shown to be a synonym of Pseudocercospora, and hence Crous et al. (2007a) decided to place these taxa occurring on Eucalyptus in Batcheloromyces (see Crous et al. 2008a). This morphology type has evolved in several clades, and the Eucalyptus species, which lack the superficial mycelial plaques typical of Batcheloromyces (Taylor et al. 1999), belong elsewhere. Because they cluster in Teratosphaeria s.s., we choose to name the fungus in this genus, rather than erecting a new anamorph genus to accommodate it. Furthermore, Crous (1998) illustrated T. suttonii, which has a Kirramyces anamorph, to form such a cercosporoid synanamorph in culture.
Teratosphaeria majorizuluensis Crous & Summerell, sp. nov. — MycoBank MB509755; Fig. 16
Fig. 16.
Teratosphaeria majorizuluensis (CPC 12712). a. Leaf spot; b. colony on PDA; c. conidia; d. globose pycnidium; e. conidiogenous cells giving rise to conidia. — Scale bars = 10 μm.
Teratosphaeriae zuluensis similis, sed conidiis majoribus, (4–)5–6(–7) × (1.5–)2(–2.5) μm.
Etymology. Name refers to the fact that the fungus is morphologically similar to Teratosphaeria zuluensis, but with larger conidia.
Leaf spots amphigenous, irregular blotches up to 3 cm diam, medium brown with a thin, raised, dark brown border, and red-purple margin. Conidiomata hypophyllous, substomatal, exuding conidia in black masses; conidiomata pycnidial on host, globose, brown to black, up to 50 μm diam, but in agar becoming acervular, convulated and huge, up to 1 mm diam; wall consisting of 3–4 cell layers of brown cells of textura angularis. Conidiogenous cells brown, verruculose, aseptate, doliiform to ampulliform, proliferating percurrently near the apex, 5–7 × 2–3 μm; sympodial proliferation also observed in culture. Conidia brown, verruculose, ellipsoidal to subcylindrical, apex obtuse to subobtuse, tapering to a subtruncate or truncate base (1–2 μm wide) with inconspicuous, minute marginal frill, (4–)5–6(–7) × (1.5–)2(–2.5) μm in vivo; 6–9 × 2.5–4 μm in vitro.
Culture characteristics — Colonies on PDA reaching 35 mm diam after 5 wk at 25 °C; colonies spreading but erumpent, with moderate aerial mycelium and irregular margins; surface grey-olivaceous to dull green or green-olivaceous; reverse iron-grey.
Specimen examined. Australia, New South Wales, Middle Head, Sydney Harbour National Park, 33°49’51"S, 151°15’31"E, on leaves of Eucalyptus botryoides, Feb. 2006, coll. B.A. Summerell, CBS H-19773 holotype, isol. P.W. Crous, cultures ex-type CPC 12712 = CBS 120040, CPC 12713, 12714.
Notes — Teratosphaeria majorizuluensis resembles several species known from Eucalyptus in conidial shape, and is phylogenetically closely related to T. zuluensis (Crous et al. 2009a). Conidia of T. majorizuluensis are longer and narrower than those of T. zuluensis (4–)4.5–5(–6) × 2–2.5(–3.5) μm, and narrower than those of T. gauchensis (4–)5–6(–7.5) × (2–)2.5(–3) μm (Cortinas et al. 2006). Teratosphaeria majorizuluensis is associated with prominent leaf spots, whereas T. zuluensis and T. gauchensis primarily cause stem cankers. Based on their nucleotide phylogeny, it is clear that the latter two taxa as defined by Cortinas et al. (2006) incorporate several phylogenetic species. Furthermore, it is possible that certain clades or groups of isolates have the ability to cause both leaf spots and stem cankers, and that T. majorizuluensis represents such an isolate within this complex (see Fig. 2, part 2). Further pathogenicity tests will have to be conducted, however, to test this hypothesis.
Teratosphaeria miniata Crous & Summerell, sp. nov. — MycoBank MB509756; Fig. 17
Fig. 17.
Teratosphaeria miniata (CPC 14514). a. Leaf spot; b. colony on PDA; c, d. conidiogenous cells giving rise to conidia; e, f. conidia. — Scale bars = 10 μm.
Teratosphaeriae profusae similis, sed conidiis saepe aseptatis, (5–)6–7(–8) × (2.5–)3(–3.5) μm.
Etymology. Name refers to the Eucalyptus miniata host on which the fungus is found.
Leaf spots amphigenous, irregular, 3–12 mm diam, medium brown, with a raised, brown border, and thin, red-purple margin. Conidiomata hypophyllous (Pseudocercospora sp. on some spots, epiphyllous), pycnidial, substomatal, brown, globose, up to 90 μm diam, exuding black conidial masses onto the leaf surface; wall consisting of 3–4 layers of brown textura angularis; becoming acervular in culture, up to 1 mm diam. Description on OA. Conidiophores brown, verruculose, reduced to conidiogenous cells, or up to 2-septate with terminal and lateral conidiogenous cells, subcylindrical, branched or not, up to 15 μm long, 3–4 μm wide. Conidiogenous cells, brown, verruculose, aseptate, dolliform to ampulliform, proliferating percurrently near apex, 4–8 × 2.5–3.5 μm; sympodial proliferation also observed in culture. Conidia solitary, brown, aseptate, verruculose, ellipsoidal to subcylindrical, apex obtuse to subobtuse, tapering to a subtruncate or truncate base (1–2 μm wide), with inconspicuous marginal frill, (5–)6–7(–8) × (2.5–)3(–3.5) μm; older cultures have conidia that become swollen, broadly ellipsoid, 1-septate, up to 10 μm long and 5 μm wide (more pronounced on MEA than on OA).
Culture characteristics — Colonies on MEA spreading, erumpent, surface sectored, with deep creases, fuscous-black, with umber aerial mycelium in middle; margins catenulate, smooth, reverse fuscous-black; reaching 40 mm after 1 mo; on OA spreading with sparse to moderate aerial mycelium, pale olivaceous-grey with olivaceous-grey margins; reaching 50 mm diam after 1 mo.
Specimen examined. Australia, Northern Territory, ENE Pine Creek, 13°40’49"S, 131°59’04.9"E, on leaf litter of Eucalyptus miniata, 23 Sept. 2007, coll. B.A. Summerell, CBS H-20269 holotype, isol. P.W. Crous, cultures ex-type CPC 14514 = CBS 125006, CPC 14515, 14516.
Notes — Teratosphaeria miniata is somewhat reminiscent of T. angophorae, in that conidia appear to develop further in culture, obtaining additional septa, and also undergoing microcyclic conidiation. Morphologically, however, these two species differ markedly in their conidial dimensions (Andjic et al. 2007).
Teratosphaeria profusa Crous & Carnegie, sp. nov. — MycoBank MB509757; Fig. 18
Fig. 18.
Teratosphaeria profusa (CPC 12821). a. Colony on PDA; b. colony on OA; c. colony on SNA; d, e. conidiogenous cells giving rise to conidia; f, g. conidia. — Scale bars = 10 μm.
Teratosphaeriae miniatae similis, sed conidiis majoribus, (7–)8–10(–13) × (2.5–)3(–3.5) μm.
Etymology. Name refers to the profuse sporulation on agar.
Isolated from leaf spots together with several other fungi. Conidiomata amphigenous, pycnidial, substomatal, brown, globose, up to 90 μm diam; wall consisting of 3–4 layers of brown textura angularis; becoming very large in agar, and acervular. Description on OA. Conidiophores brown, verruculose, subcylindrical, frequently branched, up to 4-septate, variable in length, but also reduced to conidiogenous cells when arranged in a dense stroma (less so on OA, more prominent on MEA). Conidiogenous cells brown, verruculose, aseptate, dolliform to ampulliform, proliferating percurrently near apex, 5–12 × 3.5–6 μm; sympodial proliferation also observed in culture. Conidia solitary, brown, verruculose, with two prominent guttules, ellipsoidal to subcylindrical, 0(–1)-septate, apex subobtuse, tapering to a subtruncate or truncate base (1–2 μm wide), with inconspicuous marginal frill, (7–)8–10(–13) × (2.5–)3(–3.5) μm.
Culture characteristics — Colonies on MEA erumpent, with moderate aerial mycelium, sectored, with uneven, catenulate margin, smoke-grey with patches of olivaceous-black due to sporulation; reverse olivaceous-black; reaching 25 mm after 1 mo; on OA spreading with sparse to moderate smoke-grey aerial mycelium; surface iron-grey, with smooth, catenulate margin, reaching 50 mm diam after 1 mo.
Specimen examined. Australia, Bago State Forest, on leaves of Eucalyptus nitens, 22 Dec. 2005, coll. A.J. Carnegie, CBS H-20270 holotype, isol. P.W. Crous, culture ex-type CPC 12821 = CBS 125007.
Notes — Teratosphaeria profusa is phylogenetically (Fig. 2, part 2) closely related to T. pluritubularis, a sexual species originally described from E. globulus leaves collected in Spain (Crous et al. 2006f). Morphologically it resembles several species recently described in Colletogloeopsis or Kirramyces, but is distinct based on its conidial dimensions, and conidia that also develop an additional septum with age (Crous et al. 2006f, 2007a, c, Andjic et al. 2007).
Teratosphaeria xenocryptica Crous & M.J. Wingf., sp. nov. — MycoBank MB509758
Anamorph. Kirramyces sp.
Teratosphaeriae crypticae similis, sed ascosporis minoribus, (10–)11–12(–13) × 3–3.5(–4) μm.
Etymology. Name refers to Teratosphaeria cryptica, to which it is morphologically similar.
Leaf spots occurring on juvenile and older leaves, circular to irregular, forming larger blotches with age, red-brown, with a prominent purple, raised margin. Ascomata amphigenous, black, globose, subepidermal to erumpent, ostiolate, up to 110 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci obovoid to broadly ellipsoid, aparaphysate, subsessile, bitunicate, 8-spored, 30–40 × 7–10 μm. Ascospores tri- to multi-seriate, hyaline, ellipsoidal with rounded ends, medianly 1-septate, constricted at the septum, guttulate, thick-walled, straight, (10–)11–12(–13) × 3–3.5(–4) μm; mucoid sheath visible on some ascospores. Acervuli amphigenous, dark brown to black, subcuticular, erumpent, up to 150 μm diam, intermingled among ascomata. Conidiogenous cells arising from upper cells of the stroma, doliiform to subcylindrical, 5–15 × 3–5 μm, thin-walled, pale to medium brown, proliferating 1–4 times percurrently. Conidia single, pale to medium brown, aseptate, smooth to verruculose, subcylindrical, straight or curved, apex obtuse, base truncate with a marginal frill, 7–15 × 2.5–3.5 μm. Ascospores germinating after 24 h on MEA by several germ tubes (from polar ends and lateral), that grow irregular in direction; spore darkening, and distorting upon germination.
Cultural characteristics and illustrations — Wingfield et al. (1995).
Specimen examined. Chile, on leaves of Eucalyptus sp., 1994, coll. M.J. Wingfield, holotype deposited at PREM, isol. P.W. Crous, culture ex-type CPC 355 = CBS 122905.
Notes — Teratosphaeria xenocryptica was originally reported from Chile, as ‘M. cryptica’ based on its ascospore shape and unique anamorph. It was collected on hosts such as E. bicostata, E. globulus, E. maidenii and E. nitens (Wingfield et al. 1995, Crous 1998). In the present study, however, this species was shown to cluster close to, but apart from authentic strains of T. cryptica originating from Australia (Fig. 2, part 1). A re-examination of the material revealed ascospores to be smaller than normally seen in T. cryptica, and the ascospore germination patterns differ from those of the typical Type A reported from T. cryptica (Crous 1998, Crous et al. 2006f).
Zasmidium-complex (Mycosphaerella-like and Rasutoria teleomorphs; Mycosphaerellaceae; Clade 8 in Crous et al. 2009b)
Zasmidium lonicericola (Y.H. He & Z.Y. Zhang) Crous & U. Braun, comb. nov. — MycoBank MB509759
Basionym. Cladosporium lonicericola Y.H. He & Z.Y. Zhang, Mycosystema 20: 469. 2001; and in Zhang et al., Flora Fungorum Sinicorum 14: 116. 2003.
≡ Stenella lonicericola (Y.H. He & Z.Y. Zhang) K. Schub., H.D. Shin & U. Braun, Fung. Diversity 20: 204. 2005.
= Cladosporium lonicerae Sawada, Rep. Gov. Res. Inst. Formosa 86: 163. 1943, nom. inval.
Leaf spots inconspicuous, visible only as pale to dark olivaceous-brown discolouration on the abaxial leaf surface. Description on host (adapted from Schubert & Braun 2005): Colonies hypophyllous, inconspicuous, between or on leaf hairs. Primary mycelium internal, subcuticular; external mycelium creeping or climbing leaf hairs, sparingly branched, 2–4 μm wide, septate, pale to medium olivaceous or brown, walls slightly thickened, verruculose, but at the base of conidiophores smooth or almost so, often somewhat swollen and darker, medium to medium dark brown, concolourous with conidiophores. Conidiophores solitary, arising from superficial hyphae, erect, straight to flexuous, cylindrical-oblong or often filiform, unbranched, 22–350 × 3–5.5 μm, pluriseptate, occasionally slightly constricted at the septa, medium to medium dark brown, not or somewhat paler towards the apex, smooth, thick-walled; occasionally proliferating enteroblastically. Conidiogenous cells integrated, terminal, rarely intercalary, 6–34 μm long, occasionally terminally, somewhat swollen, with several, often somewhat crowded conidiogenous loci, slightly convex, 0.5–1.5 μm wide, only slightly thickened and darkened-refractive. Conidia catenate, in unbranched or branched chains, ovoid, ellipsoid, fusiform, 3–19 × 1.5–5 μm, usually aseptate, rarely with a single or up to 3 septa, septa not very conspicuous, pale brown, smooth, walls unthickened or only slightly thickened, apex rounded or attenuated towards the apex and base, with up to 4 apical hila, hila slightly convex, 0.5–1.5 μm wide, only slightly thickened, somewhat refractive or darkened-refractive. Description on WA with sterile pine needles: Mycelium pale brown, consisting of septate, branched, smooth to finely verruculose hyphae, 1.5–2.5 μm wide. Conidiophores arising singly from superficial mycelium, medium brown, finely verruculose, 1–6-septate, subcylindrical, straight to slightly curved, unbranched, or branched above, up to 120 μm long, 3–4 μm wide. Conidiogenous cells terminal, but also lateral, unbranched, subcylindrical, but apex frequently swollen, straight or once geniculate, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially, at times with a weakly developed rachis, 15–20 × 3–5 μm. Ramoconidia rarely observed, subcylindrical, medium brown, finely verruculose, 15–25 × 2 μm. Secondary ramoconidia, 10–17 × 2–3 μm. Terminal and intercalary conidia medium brown, finely verruculose, subcylindrical to ellipsoid, in branched chains, aseptate, (4–)7–9(–11) × 2–2.5(–3) μm; hila and scars somewhat thickened and refractive, 1 μm wide.
Culture characteristics — Colonies on MEA spreading with smooth, lobate margins, and moderate aerial mycelium, olivaceous-grey; reverse greenish black, reaching 40 mm diam after 1 mo; on OA olivaceous-grey with moderate aerial mycelium and smooth margins, forming a diffuse red pigment in the agar; fertile.
Specimens examined. Korea, Yangpyong, on leaves of Lonicera japonica, 23 July 2004, H.-D. Shin (HAL); Hongchon, on leaves of Lonicera japonica, 30 Oct. 2004, coll. H.-D. Shin, epitype designated here CBS H-20271 (dried culture), isol. P.W. Crous, cultures ex-epitype CPC 11671 = CBS 125008, CPC 11672, 11673. – Taiwan, Taipei, on leaves of Lonicera japonica var. sempervillosa, 20 Dec. 1914, K. Sawada, BPI 427243 syntype of Cladosporium lonicerae.
Notes — The taxonomic history of Cladosporium lonicerae and C. lonicericola was discussed by Schubert & Braun (2005). On account of the scar type and the presence of verruculose, superficial hyphae, this species was reallocated to Stenella. Zhang et al. (2003) cited additional collections of this species on Abelia biflora, Leycesteria formosa, Lonicera japonica and Lonicera japonica var. sempervillosa from China.
The availability of Stenella lonicericola cultures enabled us to study the fungus in more detail. Although the overall morphology agrees with the description given by Schubert & Braun (2005), conidia could be clearly distinguished into primary and secondary ramoconidia, as well as intercalary and terminal conidia. The latter were aseptate, and much smaller, (4–)7–9(–11) × 2–2.5(–3) μm, than the range provided from host material, 3–19 × 1.5–5 μm, which is understandable, as it incorporated different conidial types. Based on its ITS DNA phylogeny (see Crous et al. 2006f bottom of fig. 3, as ‘Stenella sp. CPC 11671’) and scar type (planate instead of pileate), this species is better placed in Zasmidium.
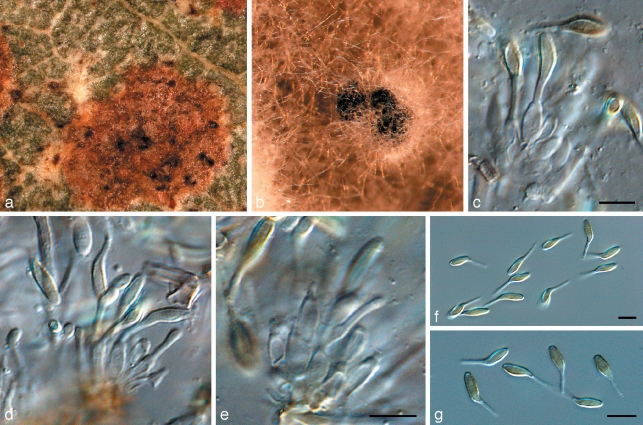
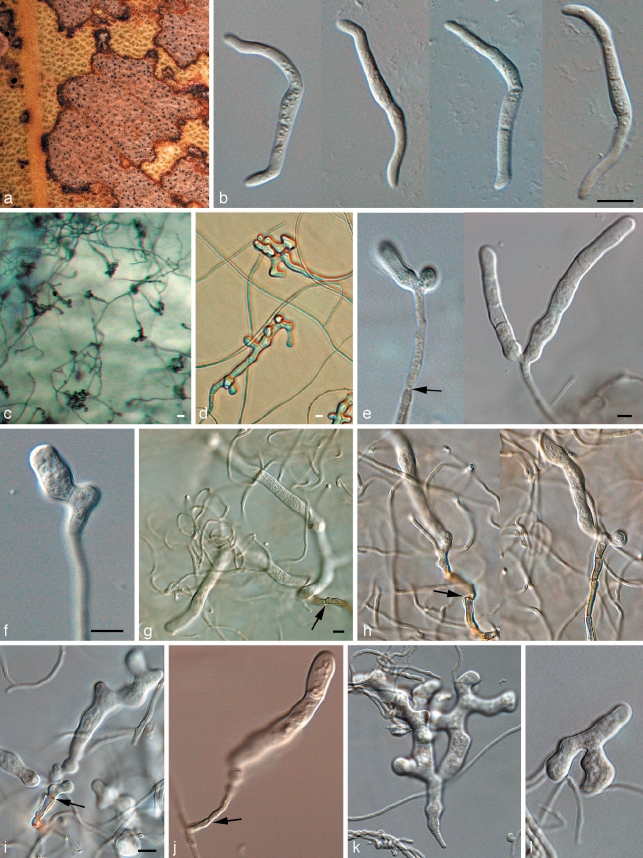
Zasmidium nocoxi Crous, sp. nov. — MycoBank MB509760; Fig. 19
Fig. 19.
Zasmidium nocoxi (CPC 14044). a. Collection site, Front Royal, Virginia; b. colony on PDA; c–f. synanamorph forming in aerial hyphae (arrow denotes point of attachment); g. conidia of synanamorph; h–k. conidia and conidiogenous cells of Zasmidium state. — Scale bars = 10 μm.
Conidiophora modice brunnea, verruculosa, 1–6-septata, subcylindrica, usque ad 100 μm longa, 2–3 μm lata. Cellulae conidiogenae terminales, non ramosae, sympodialiter proliferantes, 15–30 × 2–2.5 μm. Conidia ramicatenulata, modice brunnea, subtiliter verruculosa, anguste obclavata vel subcylindrica, (0–)3–6-septata, (20–)40–60(–80) × 2–3(–3.5) μm.
Etymology. Name refers to the DNA Barcoding meeting organised by the Consortium for the Barcode of Life (CBOL) at Front Royal, Virginia, where it was decided that Cox1 would not be the standard DNA barcode locus for fungi, and that the ITS region would rather be used.
Occurring on twig litter of unidentified host. Mycelium internal and external, medium brown, consisting of septate, branched, finely verruculose hyphae, 1.5–2.5 μm wide. Sporulating on WA with sterile pine needles. Conidiophores arising singly from superficial mycelium, medium brown, finely verruculose or verruculose, 1–6-septate, subcylindrical, straight to flexuous, predominantly unbranched, up to 100 μm long, 2–3 μm wide. Conidiogenous cells terminal, unbranched, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially, 15–30 × 2–2.5 μm. Conidia catenulate, occurring in branched chains, medium brown, finely verruculose, narrowly obclavate to subcylindrical, apex subobtuse, base long obconically subtruncate, straight to slightly curved, (0–)3–6-septate, (20–)40–60(–80) × 2–3(–3.5) μm; hila and scars thickened, darkened and refractive, 1–1.5 μm wide. Synanamorph state formed in aerial mycelium. Conidiophores subcylindrical, straight to curved with terminal conidiogenous cells that give rise to curved, irregularly branched, hyaline propagules with numerous lateral projections that are irregularly curved, thick-walled, transversely multi-septate; terminal and lateral projections with obtusely rounded ends; short projections 5–10 μm long, 3–7 μm wide.
Culture characteristics — Colonies on MEA flat, spreading with moderate aerial mycelium; surface folded, margin entire, catenulate, iron-grey; reverse greenish black; reaching 40 mm diam after 1 mo; on OA olivaceous-grey with sparse aerial mycelium and entire, catenulate margin, reaching 45 mm diam after 1 mo.
Specimen examined. USA, Virginia, Front Royal, on twig debris, 14 May 2007, P.W. Crous, CBS H-20272 holotype, cultures ex-type CPC 14044 = CBS 125009.
Notes — Zasmidium nocoxi is particularly interesting as it has a synanamorph resembling that observed in Zasmidium aerohyalinosporum, illustrating the fact that this synanamorph occurs in more than one clade. Presently no species in GenBank has a similar sequence to that of Z. nocoxi (Fig. 1), and it is consequently described as new.
Zasmidium-like (Mycosphaerellaceae; Clade 2 in Crous et al. 2009b)
Zasmidium nabiacense Crous & Carnegie, sp. nov. — MycoBank MB509761; Fig. 20
Fig. 20.
Zasmidium nabiacense (CPC 12748). a. Leaf spot; b–e. conidiogenous cells; f–i. conidia. — Scale bar = 10 μm.
Stenellae pseudoparkii similis, sed locis conidiogenis saepe solitariis, conidiis longioribus, saepe 3–5-septatis, (20–)35–45(–55) × (2–)3–3.5 μm.
Etymology. Named for Nabiac, the locality where this fungus was collected in Australia.
Leaf spots amphigenous, irregular to angular, 3–15 mm diam, medium brown with a concolorous, raised border. Mycelium internal and external, pale to medium brown, consisting of septate, branched, finely verruculose to verruculose hyphae, 2–3 μm wide. Sporulating on WA with sterile pine needles. Conidiophores arising singly from superficial mycelium, medium brown, finely verruculose or verruculose, 1–2-septate, or reduced to conidiogenous cells, subcylindrical, straight to slightly curved, unbranched, up to 70 μm long, 2.5–3.5 μm wide. Conidiogenous cells terminal, unbranched, medium brown, finely verruculose, tapering to one or two flat-tipped apical loci, proliferating sympodially, 15–30 × 2.5–3 μm. Conidia solitary, medium brown, finely verruculose, guttulate, narrowly obclavate, apex obtuse, base long obconically subtruncate, straight to slightly curved, 3–5-septate, (20–)35–45(–55) × (2–)3–3.5 μm; hila and scars thickened, darkened and refractive, 1–1.5 μm wide.
Culture characteristics — Colonies on MEA spreading with sparse aerial mycelium and smooth, lobate margins, striate, greenish black with patches of pale olivaceous-grey; reverse greenish black, reaching 30 mm diam after 1 mo; on OA flat, spreading with sparse to moderate aerial mycelium, margins entire, smooth, olivaceous-grey with sectors of pale olivaceous-grey, reaching 30 mm after 1 mo.
Specimen examined. Australia, New South Wales, Nabiac, on leaves of Eucalyptus sp. (red gum), 30 Nov. 2005, coll. A.J. Carnegie, CBS H-20273 holotype, isol. P.W. Crous, cultures ex-type CPC 12748 = CBS 125010, CPC 12749, 12750.
Notes — Morphologically and phylogenetically, Z. nabiacense is distinct from other species of ‘Stenella’ presently known from Eucalyptus (Crous 1998, Crous et. al. 2006f, 2007c). Although it resembles other taxa in the Zasmidium clade, the fungus clusters in a sister clade with taxa in the Mycosphaerella parkii complex, which also have Zasmidium-like anamorphs (Fig. 1).
Zasmidium aerohyalinosporum Crous & Summerell, sp. nov. — MycoBank MB509762; Fig. 21
Fig. 21.
Zasmidium aerohyalinosporum (CPC 14636). a. Leaf spot with black spermatogonia (small black dots); b. germinating ascospores on MEA; c–l. conidiogenous cells giving rise to conidia (arrow denotes point of attachment). — Scale bars = 10 μm.
Conidiophora brunnea, variabiliter longa, ad 40 μm, 3–4 μm lata, non ramosa, loco singulo, conidia hyaline, crassitunicata, subcylindrica, 3–30 transverse septata, irregulariter inflata, interdum ramosa, 4–7 μm lata, 40–150 μm longa, apice conidiorum et ramulorum obtuso.
Etymology. Name refers to the dominant asexual state in this fungus, which forms hyaline, twisted aerial conidia.
Leaf spots amphigenous, irregular grey-brown, surrounded by a dark brown to red-purple margin; spots varying from specks to spots, up to 7 mm diam; hypophyllous surface well colonised by black, submerged spermatogonia. Cultures derived from single ascospores, sporulating on WA with sterile pine needles, as well as OA (not on MEA), forming mycelium consisting of subhyaline to pale brown, smooth, branched, septate, 1–1.5 μm wide hyphae; hyphae giving rise to huge swollen propagules that occur terminal or lateral on hyphal strands. Conidiophores medium to dark brown, variable in length, up to 40 μm long and 3–4 μm wide, unbranched, smooth to verruculose, becoming constricted at septa, eventually disarticulating, with each conidiophore giving rise to a single conidium. Conidia hyaline, thick-walled, subcylindrical, with 3–30 transverse septa, cells 5–15 μm long, developing irregular swellings which can form branches, 4–7 μm wide, 40–150 μm long, apex and lateral branches with obtuse ends; body granular, basal cell tapering prominently towards the conidiophore. Typical brown, verruculose, obclavate conidia with thickened scars rarely observed.
Culture characteristics — Colonies on MEA erumpent, irregular with lobed edge, center mouse-grey, edge pale mouse-grey, reverse iron-grey, reaching 17 mm diam after 1 mo; on OA spreading with radial striations and lobed margin; surface pale olivaceous-grey with sparse, flattened aerial mycelium; reaching 27 mm diam after 1 mo.
Specimen examined. Australia, New South Wales, Road to Robin Falls, 13°31’01.3"S, 131°16’22.5"E, 126 m, on leaves of Eucalyptus tectifica, 23 Sept. 2007, coll. B.A. Summerell, CBS H-20274 holotype, isol. P.W. Crous, cultures ex-type CPC 14636 = CBS 125011, CPC 14637.
Notes — The fungus was grown in culture from discharged single ascospores that were hyaline, 10–14 × 3–4 μm. Attempts to relocate fertile ascomata were unsuccessful. Conidia of this anamorph state have been observed previously in species of Mycosphaerellaceae grown in culture, and they have hitherto generally been overlooked. However, in Z. aerohyalinosporum, these propagules were observed to clearly detach, forming loose conidia. In older cultures (especially MEA), the lateral conidial branches were observed to become constricted at the septa, and to disarticulate into single-celled, subcylindrical, hyaline conidial propagules. No such propagules have been observed in vivo. It is possible that if they form on leaves, the conidial propagules would occur as the smaller, single-celled propagules observed on MEA. Similar propagules have been observed in cultures of Zasmidium species and treated as a synanamorph. The only published record of an anamorph such as this is found in ‘Mycosphaerella’ scytalidii, which forms a Zasmidium and hyaline, aerial synanamorph in culture and is phylogenetically associated with the ‘Mycosphaerella’ endophytica complex and ‘Mycosphaerella’ stramenti (Crous et al. 2006f).
DISCUSSION
In this study several new species are introduced that would previously have been described in Mycosphaerella or one of its formerly accepted anamorph genera. We have rather applied the taxonomic foundation proposed by Crous et al. (2009b), in which more natural genera are defined, employing the concept of a single generic name per clade. Thus, this study employs these concepts to describe 20 novel species representing the genera Phaeophleospora, Pseudocercospora, Zasmidium (Mycosphaerellaceae), Penidiella, Phaeothecoidea, Readeriella and Teratosphaeria (Teratosphaeriaceae) as they are defined by Crous et al. (2009b).
The application of single generic names to new species in this study relates strongly to the interpretation of Article 59 of the International Code of Botanical Nomenclature (ICBN). This article sought to accommodate a dual nomenclature for the fungi and thus to present an acceptable mechanism to accommodate names of sexual and asexual states. In recent years, phylogenetic inference based on DNA sequence analyses has made it clear that a single nomenclature for fungal groups where the phylogenetic relationships are known would be preferable, although this issue has been the subject of considerable debate (Gams 1995, Seifert et al. 2000, Rossman 2006). In Seifert et al. (2000), the underlying idea was that fungal taxonomy should strive to obtain a 1 : 1 correlation of teleomorph and anamorph generic concepts. Although this goal seemed attainable for some groups, such as Calonectria : Cylindrocladium (Crous & Wingfield 1994, Crous et al. 2004c, 2006b), others like the Botryosphaeria-complex represented numerous different genera, and required a different solution (Crous et al. 2006e, Marincowitz et al. 2008, Phillips et al. 2008).
A recent emendment to the ICBN has provided a means to name asexual states of fungi in sexual genera where these relationships are known from phylogenetic inference, even though these states were not physically observed. Crous et al. (2009b) applied this approach to the Mycosphaerellaceae and Teratosphaeriaceae, and we have utilised the genera provided in this taxonomic study for the naming of several species from leaf specimens. Furthermore, we support the view of Rossman & Samuels (2005) that the oldest generic name should have priority. Although the code has made careful provision for naming anamorphs by installing Article 59, the latter need not be followed, and if relationships are known, a single (teleomorph) name can be chosen to name a novel, apparently asexual species. In the case at hand, this option appears to be the only way forward for the taxonomy of several genera within the Teratosphaeriaceae and Mycosphaerellaceae.
The fungal collections that made this study possible originated from various parts of the world, but the majority were from Australia. The fact that so many new taxa continue to arise from relatively limited collections reaffirms the view of Crous et al. (2008b, 2009a) that many more species of capnodialean fungi remain to be discovered. Most of these species could never have been recognised prior to the advent of DNA sequence comparisons for species recognition. It is commonly accepted that only a small percentage of the fungi have been collected and named (Hawksworth 1991, 2004, Crous et al. 2006d) and it seems likely that many of these will be species residing in the Mycosphaerellaceae and the Teratosphaeriaceae.
‘Mycosphaerella’ was recognised as a megadiverse group long before DNA-based phylogenetic inference was used to define species in this group (Barr 1972). If a single genus were retained for the growing numbers of species in the Mycosphaerellaceae and Teratosphaeriaceae, these fungi would be impossible to handle effectively. This would also raise serious practical problems, especially because many of these morphologically similar taxa represent important plant pathogens on a diverse range of hosts. Thus segregating this complex into different families, and families into genera that correlate with monophyletic lineages attributed to single generic names, is much more practical. Although the majority of the taxa described in this study can still be identified based on morphology, this will soon no longer be the case, as the thousands of species all have an ascospore range from approximately 8–30 × 2–6 μm. Furthermore, the majority of these species are sterile in culture, suggesting that morphology is of limited value in this group, and new technologies such as DNA barcoding (Summerbell et al. 2005, Seifert 2009), may be the only solution for ready identification in future. This conclusion also supports initiatives to recollect novel and already described species, to enable mycologists to build the DNA barcode library of ‘Mycosphaerella’, to enable users of these data to identify these pathogens when they are encountered on agricultural and forestry produce.
Acknowledgments
Prof. dr U. Braun (Martin-Luther-Universität, Halle, Germany) is thanked for providing the Latin diagnoses. The authors thank the technical staff, A. van Iperen (cultures), M. Vermaas (photo plates), and M. Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance. Various colleagues collected material used in this study, for which we are grateful, namely Prof. dr A.C. Alfenas (University of Viçosa, MG, Brazil) and Dr I.W. Smith (University of Melbourne, Australia). We are also grateful to Alex Buchanan, Tasmanian Herbarium and Ian Cowie, Northern Territory Herbarium for assistance with the collection and identification of Eucalyptus spp. and to Richard Johnston and Andrew Orme, Royal Botanic Gardens and Domain Trust for the collection of Eucalyptus in New South Wales.
REFERENCES
- Andjic V, Barber PA, Carnegie AJ, Hardy GEStJ, Wingfield MJ, Burgess TI. 2007. Phylogenetic reassessment supports accommodation of Phaeophleospora and Colletogloeopsis from eucalypts in Kirramyces. Mycological Research 111: 1184 – 1198 [DOI] [PubMed] [Google Scholar]
- Aptroot A. 2006. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1 – 231 [Google Scholar]
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater M-F, Buddenhagen IW, Viljoen A, Crous PW. 2008. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19 – 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. 1972. Preliminary studies on the Dothideales in temperate North America. Contributions of the University of Michigan Herbarium 9: 523 – 638 [Google Scholar]
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 232 – 244 [DOI] [PubMed] [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JG, Hoog SG de. 2003. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress 2: 3 – 18 [Google Scholar]
- Braun U, Dick MA. 2002. Leaf spot diseases of eucalypts in New Zealand caused by Pseudocercospora species. New Zealand Journal of Forestry Science 32: 221 – 234 [Google Scholar]
- Carnegie AJ, Burgess TI, Beilharz V, Wingfield MJ. 2007. New species of Mycosphaerella from Myrtaceae in plantations and native forests in eastern Australia. Mycologia 99: 461 – 474 [DOI] [PubMed] [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, Toanan C. 2008. Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77 – 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinas MN, Crous PW, Wingfield BD, Wingfield MJ. 2006. Multi-gene phylogenies and phenotypic characters distinguish two species within the Colletogloeopsis zuluensis complex associated with Eucalyptus stem cankers. Studies in Mycology 55: 133 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. 1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 [Google Scholar]
- Crous PW, Aptroot A, Kang J-C, Braun U, Wingfield MJ. 2000. The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107 – 121 [Google Scholar]
- Crous PW, Braun U. 2003. Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1 – 571 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33 – 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Ferreira FA, Sutton BC. 1997. A comparison of the fungal genera Phaeophleospora and Kirramyces (Coelomycetes). South African Journal of Botany 63: 111 – 115 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004a. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Crous PW, Groenewald JZ, Groenewald M, Caldwell P, Braun U, Harrington TC. 2006a. Species of Cercospora associated with grey leaf spot of maize. Studies in Mycology 55: 189 – 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. 2004b. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195 – 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD. 2006b. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213 – 226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hywel-Jones NL. 2004c. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415 – 430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. 2009a. Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38 – 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Hong L, Wingfield MJ, Wingfield BD, Kang J. 1999. Uwebraunia and Dissoconium, two morphologically similar anamorph genera with distinct teleomorph affinity. Sydowia 52: 155 – 166 [Google Scholar]
- Crous PW, Kang J-C, Braun U. 2001. A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081 – 1101 [Google Scholar]
- Crous PW, Liebenberg MM, Braun U, Groenewald JZ. 2006c. Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163 – 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, Botha W, Slippers B, Beer WZ de, Wingfield MJ, Hawksworth DL. 2006d. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13 – 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006e. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Mohammed C, Himaman W, Groenewald JZ. 2007c. Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26: 143 – 185 [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andijc V, Barber PA, Groenewald JZ. 2009b. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99 – 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ. 2008a. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59 – 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds), 2009c. Fungal Biodiversity. CBS Laboratory Manual Series 1 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ. 1994. A monograph of Cylindrocladium, including anamorphs of Calonectria. Mycotaxon 51: 341 – 435 [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. 2006f. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99 – 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. 1991. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628 – 632 [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ. 2008b. Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan FM, Braun U, Groenewald JZ, Crous PW. 2008. Morphological plasticity in Cladosporium sphaerospermum. Persoonia 21: 9 – 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes Commonwealth Mycological Institute, Kew: [Google Scholar]
- Gams W. 1995. How natural should anamorph genera be? Canadian Journal of Botany 73: S747 – S753 [Google Scholar]
- Goodwin SB, Dunkle DL, Zismann VL. 2001. Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91: 648 – 658 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95: 641 – 655 [Google Scholar]
- Hawksworth DL. 2004. Fungal diversity and its implications for genetic resource collections. Studies in Mycology 50: 9 – 17 [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. 2006. A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147 – 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S, Groenewald JZ, Wingfield MJ, Crous PW. 2008. Species of Botryosphaeriaceae occurring on Proteaceae. Persoonia 21: 111 – 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW. 2008. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29 – 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK: [Google Scholar]
- Rossman AY. 2006. Roundtable at IMC8 debates a separate Code for naming fungi and revisits dual nomenclature. Mycological Research 110: 1255 – 1256 [Google Scholar]
- Rossman AY, Samuels GJ. 2005. Towards a single scientific name for species of fungi. Inoculum 56, 3: 3 – 6 [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1043 – 1054 [DOI] [PubMed] [Google Scholar]
- Schubert K, Braun U. 2005. Taxonomic revision of the genus Cladosporium s.l. 4. Species reallocated to Asperisporium, Dischloridium, Fusicladium, Passalora, Pseudoasperisporium and Stenella. Fungal Diversity 20: 187 – 208 [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, Hoog GS de, Crous PW. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105 – 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA. 2009. Progress towards DNA barcoding of fungi. Molecular Ecology Resources 9: (Suppl. 1)83 – 89 [DOI] [PubMed] [Google Scholar]
- Seifert KA, Gams W, Crous PW, Samuels GJ. 2000. Molecules, morphology and classification: towards monophyletic genera in the Ascomycetes. Studies in Mycology 45: 1 – 230 [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabo L. 1999. Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491 – 1499 [Google Scholar]
- Summerbell RC, Lévesque CA, Seifert KA, Bovers M, Fell JW, Diaz MR, Boekhout T, Hoog GS de, Stalpers J, Crous PW. 2005. Microcoding: the second step in DNA barcoding. Philosophical Transactions of the Royal Society B 360: (1462)1897 – 1903 doi:10.1098/rstb.2005.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, Summerbell RC, Crous PW. 2006. Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323 – 350 [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods). Version 4 Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Taylor JE, Crous PW, Wingfield MJ. 1999. The genus Batcheloromyces on Proteaceae. Mycological Research 103: 1478 – 1484 [Google Scholar]
- Wingfield MJ, Crous PW, Peredo HL. 1995. A preliminary, annotated list of foliar pathogens of Eucalyptus spp. in Chile. South African Forestry Journal 173: 53 – 57 [Google Scholar]
- Zalar P, Hoog GS de, Schroers H-J, Crous PW, Groenewald JZ, Gunde-Cimerman N. 2007. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157 – 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Liu YL, Zhang T, Li TF, Wang G, Zhang H, He YH, Peng HH. 2003. Cladosporium, Fusicladium, Pyricularia. Flora Fungorum Sinicorum 14: 1 – 297 [Google Scholar]