Abstract
The class Dacrymycetes is a rather small group of brown-rot wood-decaying jelly fungi characterised by forked basidia and an orange to yellow gelatinous to cartilaginous fruit body. In Japan, dacrymycetous fungi had not been investigated for a long time, justifying a taxonomic re-examination. In the present study we attempted an investigation of the dacrymycetous fungal flora of Japan, and recognised 28 taxonomic entities, including five new taxa, i.e. Dacrymyces ancyleus, D. aureosporus, D. pinacearum, D. subarcticus and Dacryopinax sphenocarpa, and nine new records. Due to the present survey, the total number of dacrymycetous species recorded from Japan increased from 28 to 42. Of the newly described species, Dacrymyces ancyleus is characterised by recurved, cylindrical basidiocarps and hyphae with clamp connections. Dacrymyces aureosporus resembles D. chrysospermus, but differs in wall thickness of its marginal hyphae. Dacrymyces pinacearum and D. subarcticus represent new coelomycetous anamorphic species. Dacryopinax sphenocarpa has sharp, spathulate basidiocarps, and hyphae with clamp connections. Descriptions, illustrations and photographs of fruit bodies are presented with some taxonomic notes. Molecular phylogenetic analyses were conducted to verify the species identification, and the remaining problems in Dacrymycetes taxonomy are discussed based on these data.
Keywords: Dacrymycetes, Japanese species, molecular phylogeny, new species, taxonomy
INTRODUCTION
Dacrymycetes is a rather small class established by Doweld (2001) in the subphylum Agaricomycotina of Basidiomycota with one order, two families, nine genera and 101 species (Kirk et al. 2001, 2008, Hibbett et al. 2007). Dacrymycetes is characterised by forked (bifurcate) basidia, except in Dacrymyces unisporus. Their fruit bodies are gelatinous or cartilaginous, yellow to orange coloured, and vary in shape from thin membranous to pulvinate, spathulate and dendroid. In their hyphae, the dolipore-type septa are surrounded by parenthesomes without perforations (Wells 1994). All species belonging to this class are wood-decaying fungi that cause brown-rot (Oberwinkler 1993, Kirk et al. 2001). Recent molecular phylogenetic studies have shown that Dacrymycetes is a member of the Agaricomycotina clade with the Agaricomycetes and Tremellomycetes, and a sister group of Agaricomycetes (James et al. 2006, Hibbett 2006, Hibbett et al. 2007).
The only order in the class, Dacrymycetales, was established by Hennings (1898; as Dacryomycetinieae) composed of the single family Dacrymycetaceae, introduced by Schröter (1889; as Dacryomycetini) with several genera, until Jülich erected an additional family Cerinomycetaceae to accommodate the genus Cerinomyces, species of which produce resupinate basidiocarps (Jülich 1981, Kirk et al. 2001). In recent years, a taxonomic review of the genera in Dacrymycetaceae was conducted by McNabb (1964, 1965a, b, c, d, e, 1966, 1973). He re-examined the validity of described genera based on the type specimens and original descriptions, and finally recognised eight genera in the Dacrymycetaceae: Calocera, Cerinomyces, Dacrymyces, Dacryopinax, Ditiola, Femsjonia, Guepiniopsis and Heterotextus. He classified these genera mainly based on the external shapes of basidiocarps and the wall thickness of marginal hyphae in the sterile parts of basidiocarps (McNabb & Talbot 1973). The ninth genus, Dacryonaema, is monotypic and rarely reported (Nannfeldt 1947). The taxonomic history of dacrymycetalean genera has been reviewed by Oberwinkler (1993). Some studies discussing the phylogenetic relationship in this class have been published (Weiss & Oberwinkler 2001, Shirouzu et al. 2007).
The fungal floristic data of a certain region adds basic information on the classification and distribution of the species occurring in this region. Some mycologists reported the taxonomic study of dacrymycetous fungi distributed in a certain country, e.g. Reid (1974; UK) and Liu & Fan (1990; China). In Japan, 28 species representing seven genera have been recorded (Table 1), but systematic investigation of Japanese dacrymycetous fungi has not been conducted for 70 years since the works of Kobayasi (1939a, b). Although he described 14 new dacrymycetous species in Japan, several have remained doubtful (McNabb 1965a, b, e, 1973), as their holotypes have not been located. Given this situation, a re-examination of dacrymycetous fungi in Japan was urgently required.
Table 1.
Species of Japanese Dacrymycetes.
| Name | Literature | McNabb’s opinion3 |
|---|---|---|
| Calocera alba Kobayasi | Kobayasi (1939b) | = C. cornea? |
| Calocera coralloides Kobayasi | Kobayasi (1939b) | = C. cornea? |
| Calocera cornea (Batsch) Fr. | Kobayasi (1939b) | distinct species |
| Calocera cornea forma glacilis Kobayasi | Kobayasi (1939b) | = C. cornea? |
| Calocera corniformis Kobayasi | Kobayasi (1939b) | = C. cornea? |
| Calocera furcata (Fr.) Fr. | Kobayasi (1939b); as Calocera flavida Lloyd | distinct species |
| Calocera viscosa (Pers.) Fr. | Kobayasi (1939b) | distinct species |
| Cerinomyces albosporus Boidin & Gilles | Maekawa (1986); as Cerinomyces aculeatus N. Maek. | – |
| Dacrymyces albidus Kobayasi | Kobayasi (1954) | distinct species? |
| Dacrymyces applanatus Kobayasi | Kobayasi (1939a) | distinct species? |
| Dacrymyces chrysocomus (Bull.) Tul. | Kobayasi (1939a) | distinct species |
| Dacrymyces chrysospermus Berk. & M.A. Curtis | Kobayasi (1939a); as Dacrymyces palmatus (Schwein.) Burt., | |
| D. roseotinctus Lloyd, D. puniceus Kobayasi | distinct species | |
| Dacrymyces kohyasanus Kobayasi | Kobayasi (1984) | – |
| Dacrymyces minor Peck | Kobayasi (1939a) | distinct species |
| Dacrymyces nikkomontanus Kobayasi | Kobayasi (1939a) | distinct species? |
| Dacrymyces pezizoides Kobayasi | Kobayasi (1939a) | = D. minutus? |
| Dacrymyces pulcher Kobayasi | Kobayasi (1939a) | distinct species? |
| Dacrymyces punctiformis Newhoff | Kobayasi (1939a) | distinct species |
| Dacrymyces san-augustinii Kobayasi | Kobayasi (1939a) | distinct species |
| Dacrymyces stillatus Nees | Kobayasi (1939a); as Dacrymyces deliquescens (Bull.) Duby | distinct species |
| Dacrymyces subalpinus Kobayasi | Kobayasi (1939a) | distinct species? |
| Dacrymyces tremellosus Kobayasi | Kobayasi (1939a) | = D. capitatus? |
| Dacryopinax imazekiana (Kobayasi) Lowy | Kobayasi (1939b); as Guepinia imazekiana Kobayasi | = D. dennisii? |
| Dacryopinax spathularia (Schwein.) G.W. Martin | Kobayasi (1939b); as Guepinia fissa Berk., G. spathularia (Schwein.) Fr. | distinct species |
| Femsjonia orientalis Kobayasi | Kobayasi (1939b) | = F. peziziformis? |
| Femsjonia peziziformis (Lèv.) P. Karst. | Kobayasi (1939b); as Femsjonia luteo-alba Fr. | distinct species |
| Guepiniopsis buccina (Pers.) L.L. Kenn. | Kobayasi (1939a); as Guepiniopsis merulinus (Pers.) Pat. | distinct species |
| Heterotextus alpinus (Tracy & Earle) Martin | Kobayasi (1939a); as Guepiniopsis alpinus (Tracy & Earle) Brasf. | distinct species |
1 McNabb (1965a, b, e, 1973).
Bold: Species collected in this study.
In the present study we attempt to survey the dacrymycetous fungi of Japan. Fungal specimens collected from 2005 to 2008 were examined. Their descriptions and illustrations are presented with some taxonomic notes in this paper. Molecular phylogenetic analyses are also conducted using Japanese and foreign strains to verify species identification. Finally, the problems remaining in Dacrymycetes taxonomy are discussed based on these data.
MATERIALS AND METHODS
Fungal collections and morphological observations
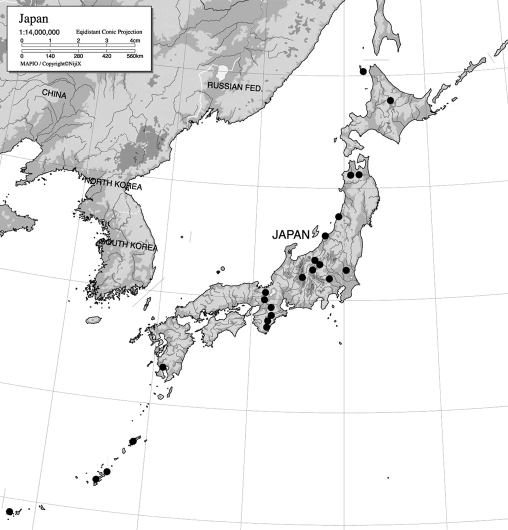
To collect basidiocarps of dacrymycetous fungi, we selected approximately 50 sites in Japan with coniferous, deciduous broad-leaved and evergreen broad-leaved forests. The 23 major sites are shown in Fig. 1. These sites were sampled from 2005 to 2008. When sampling, basidiocarps were collected together with the substrata on which they occurred. The tree species or substrates were identified when possible. Collected samples were quickly air-dried, wrapped with paper and transported to the laboratory.
Fig. 1.
Map of the Japanese Islands. Major sampling sites are shown as •.
In the laboratory, dried samples were soaked in distilled water to observe their morphological features. Basidiocarp shapes were observed with the naked eye and under a stereomicroscope. Basidiocarps were then sliced using a freezing microtome (RUB-2100, MC-802A; Yamato Kohki, Saitama, Japan) to a thickness of 10–40 μm to make preparations for microscopic examination. The microscopic features, such as basidia and basidiospores, were observed using a high-powered light microscope (150–1500×). Species identification was performed referring to the descriptions of Kobayasi (1939a), Olive (1944), McNabb (1964, 1965a, b, c, e, 1973), Oberwinkler & Tschen (1989) and Reid (1974). Identified specimens were deposited in the fungal herbarium of the National Museum of Nature and Science (TNS), Tsukuba, Ibaraki, Japan.
Fungal isolations
Pure cultures were established by means of single spore isolation. The established cultures were preserved in slants of 0.1 % cornmeal agar (0.2 % CMA; Nissui, Tokyo, Japan) + 1.25 % malt agar (2.5 % MA; Nissui, Tokyo, Japan) medium (0.2 % CMA 8.5 g, 2.5 % MA 22.5 g, yeast extract 1 g, distilled water 1 L), and deposited in the MAFF Genbank (National Institute of Agrobiological Sciences, Tsukuba, Ibaraki, Japan).
The colony growth rates and characteristics (texture, colour, etc.) were recorded based on 10 d old colonies grown in slants of 0.1 % CMA + 1.25 % MA medium at room temperature (c. 25 °C). Conidiogenesis was observed on colonies grown on 0.2 % CMA plates.
Molecular phylogenetic analyses
Fungal cultures for DNA sequencing were obtained from Japanese Dacrymycetes samples collected in this study, and from the Fungal Biodiversity Centre of the Centraalbureau voor Schimmelcultures (CBS) in Utrecht, The Netherlands. The list of samples used for phylogenetic analyses is shown in Table 2.
Table 2.
Fungal samples used in molecular phylogenetic analysis.
| Name | Sample no. | Specimen no.1 | Culture no.2 | GenBank accession no.3 |
|---|---|---|---|---|
| Present study | ||||
| Calocera cornea | HNo.267 | TNS-F-15701 | MAFF240116 | AB299068 |
| HNo.358 | TNS-F-15702 | MAFF240117 | AB299076 | |
| HNo.376 | TNS-F-15703 | MAFF240118 | AB299077 | |
| HNo.452 | TNS-F-21061 | MAFF241186 | AB472722† | |
| HNo.513 | TNS-F-21065 | MAFF241188 | AB472725† | |
| Calocera viscosa | HNo.175 | TNS-F-15704 | MAFF240119 | AB299048 |
| HNo.466 | TNS-F-15705 | MAFF240120 | AB299082 | |
| Cerinomyces albosporus | HNo.191 | TNS-F-15706 | MAFF240121 | AB299050 |
| Cerinomyces canadensis | HNo.199 | TNS-F-21034 | MAFF241162 | AB472696 |
| HNo.208 | TNS-F-21035 | MAFF241163 | AB472697 | |
| HNo.216 | TNS-F-21036 | – | AB472698 | |
| HNo.219 | TNS-F-21037 | MAFF241164 | AB472699 | |
| Cerinomyces pallidus | HNo.505 | TNS-F-21064 | – | AB472724 |
| Dacrymyces adpressus | HNo.355 | TNS-F-21045 | MAFF241172 | AB472707 |
| HNo.554 | TNS-F-21069 | MAFF241191 | AB472729 | |
| Dacrymyces ancyleus | HNo.382 | TNS-F-21051 | MAFF241177 | AB472713 |
| Dacrymyces aureosporus | HNo.215 | TNS-F-15711 | MAFF240126 | AB299057 |
| HNo.354 | TNS-F-21044 | MAFF241171 | AB472706 | |
| HNo.385 | TNS-F-21053 | – | AB472715† | |
| HNo.486 | TNS-F-15714 | MAFF240129 | AB299084 | |
| HNo.665 | TNS-F-21074 | MAFF241195 | AB472734† | |
| Dacrymyces capitatus | HNo.182 | TNS-F-15707 | MAFF240122 | AB299049 |
| HNo.212 | TNS-F-15709 | MAFF240124 | AB299055 | |
| HNo.471 | TNS-F-21062 | MAFF241187 | AB472723† | |
| HNo.734 | TNS-F-21077 | MAFF241197 | AB472737† | |
| Dacrymyces capitatus (anam.) | HNo.181 | TNS-F-21033 | MAFF241161 | AB472695 |
| Dacrymyces chrysospermus | HNo.320 | TNS-F-15712 | MAFF240127 | AB299073 |
| HNo.446 | TNS-F-21060 | MAFF241185 | AB472721† | |
| HNo.468 | TNS-F-15713 | MAFF240128 | AB299083 | |
| HNo.620 | TNS-F-21072 | MAFF241194 | AB472732† | |
| Dacrymyces lacrymalis | HNo.209 | TNS-F-15716 | MAFF240131 | AB299053 |
| HNo.235 | TNS-F-15717 | MAFF240132 | AB299062 | |
| HNo.243 | TNS-F-21039 | MAFF241166 | AB472701 | |
| HNo.261 | TNS-F-15718 | MAFF240133 | AB299066 | |
| HNo.271 | TNS-F-21041 | MAFF241168 | AB472703 | |
| HNo.277 | TNS-F-21042 | MAFF241169 | AB472704 | |
| HNo.279 | TNS-F-21043 | MAFF241170 | AB472705 | |
| HNo.281 | TNS-F-15719 | MAFF240134 | AB299069 | |
| HNo.563 | TNS-F-21070 | MAFF241192 | AB472730 | |
| Dacrymyces lacrymalis (anam.) | HNo.250 | TNS-F-21040 | MAFF241167 | AB472702 |
| Dacrymyces microsporus | HNo.368 | TNS-F-21049 | MAFF241175 | AB472711 |
| HNo.371 | TNS-F-21050 | MAFF241176 | AB472712 | |
| HNo.390 | TNS-F-21054 | MAFF241180 | AB472716† | |
| Dacrymyces minor | HNo.224 | TNS-F-15720 | MAFF240135 | AB299059 |
| HNo.237 | TNS-F-15721 | MAFF240136 | AB299063 | |
| Dacrymyces minutus | HNo.282 | TNS-F-15722 | MAFF240137 | AB299070 |
| HNo.648 | TNS-F-21073 | – | AB472733† | |
| Dacrymyces novae-zelandiae | HNo.225 | TNS-F-21038 | MAFF241165 | AB472700 |
| Dacrymyces pinacearum (anam.) | HNo.418 | TNS-F-21056 | MAFF241182 | AB472718 |
| Dacrymyces punctiformis | HNo.196 | TNS-F-15723 | MAFF240138 | AB299052 |
| HNo.213 | TNS-F-15724 | MAFF240139 | AB299056 | |
| HNo.285 | TNS-F-15725 | MAFF240140 | AB299071 | |
| Dacrymyces san-augustinii | HNo.441 | TNS-F-15726 | MAFF240141 | AB299081 |
| HNo.666 | TNS-F-21075 | MAFF241196 | AB472735† | |
| Dacrymyces stillatus | HNo.233 | TNS-F-15727 | MAFF240142 | AB299061 |
| HNo.256 | TNS-F-15728 | MAFF240144 | AB299065 | |
| HNo.383 | TNS-F-21052 | MAFF241178 | AB472714 | |
| Dacrymyces stillatus (anam.) | HNo.252 | TNS-F-15729 | MAFF240143 | AB299064 |
| HNo.411 | TNS-F-21055 | MAFF241181 | AB472717 | |
| HNo.421 | TNS-F-21057 | MAFF241183 | AB472719 | |
| Dacrymyces subalpinus | HNo.228 | TNS-F-15730 | MAFF240145 | AB299060 |
| HNo.570 | TNS-F-21071 | MAFF241193 | AB472731† | |
| Dacrymyces subarcticus (anam.) | HNo.544 | TNS-F-21067 | – | AB472727† |
| HNo.722 | TNS-F-21076 | – | AB472736† | |
| Dacrymyces unisporus | HNo.332 | TNS-F-15731 | MAFF240146 | AB299074 |
| Dacrymyces variisporus | HNo.263 | TNS-F-15732 | MAFF240147 | AB299067 |
| HNo.300 | TNS-F-15733 | MAFF240148 | AB299072 | |
| HNo.352 | TNS-F-15734 | MAFF240149 | AB299075 | |
| Dacryopinax spathularia | HNo.367 | TNS-F-21048 | MAFF241174 | AB472710 |
| HNo.379 | TNS-F-15735 | MAFF240150 | AB299078 | |
| HNo.398 | TNS-F-15736 | MAFF240151 | AB299079 | |
| Dacryopinax sphenocarpa | HNo.356 | TNS-F-21046 | MAFF241173 | AB472708 |
| HNo.364 | TNS-F-21047 | – | AB472709 | |
| HNo.430 | TNS-F-21059 | MAFF241184 | AB472720 | |
| HNo.534 | TNS-F-21066 | MAFF241189 | AB472726† | |
| HNo.552 | TNS-F-21068 | MAFF241190 | AB472728 | |
| Femsjonia peziziformis | HNo.439 | TNS-F-15737 | MAFF240152 | AB299080 |
| Guepiniopsis buccina | HNo.562 | TNS-F-15738 | MAFF240153 | AB299085 |
| Centraalbureau voor Schimmelcultures (CBS) | ||||
| Dacrymycetes | ||||
| Calocera cornea | CBS124.84 | – | – | AB472738† |
| CBS125.84 | – | – | AB472739† | |
| Calocera viscosa | CBS292.82 | – | – | AB472740† |
| Dacrymyces capitatus | CBS293.82 | – | – | AB472741† |
| Dacrymyces novae-zelandiae | CBS295.82 | – | – | AB472742† |
| Dacrymyces stillatus | CBS296.82 | – | – | AB472743† |
| Dacryopinax spathularia | CBS197.63 | – | – | AB472744† |
| Guepiniopsis buccina | CBS297.82 | – | – | AB472745† |
| DNA Data Bank of Japan (DDBJ) | ||||
| Dacrymycetes | ||||
| Calocera cornea | AFTOL-ID 438 | – | – | AY701526 |
| Calocera viscosa | AFTOL-ID 1679 | – | – | DQ520102 |
| Cerinomyces crustulinus | – | – | – | AY600248 |
| Dacrymyces chrysospermus | – | – | – | AF287855 |
| Dacrymyces stillatus | – | – | – | AF291309 |
| Dacryomitra pussila | – | – | – | AJ406406 |
| Dacryopinax spathularia | AFTOL-ID 454 | – | – | AY701525 |
| Dacryoscyphus chrysochilus | – | – | – | AY604567 |
| Ditiola haasii | – | – | – | AF291314 |
| Femsjonia peziziformis | – | – | – | AF291330 |
| Guepiniopsis buccina | AFTOL-ID 888 | – | – | AY745711 |
| Agaricomycetes (out group) | ||||
| Exidia uvapassa | AFTOL-ID 461 | – | – | AY645056 |
| Pseudohydnum gelatinosum | AFTOL-ID 1875 | – | – | DQ520094 |
1 Herbarium of the National Museum of Nature and Science (TNS).
2 Culture collection of National Institute of Agrobiological Science (MAFF).
3 † Sequenced by Macrogen, Inc.
DNA was extracted from mycelia cultured on 2.5 % malt extract liquid medium following the modified CTAB method described by Matsuda & Hijii (1999). The 28S rDNA D1/D2 region was amplified with primers D1 (Peterson 2000) and NL4 (O’Donnell 1993). Polymerase chain reactions (PCR) were performed using a HotStarTaq Master Mix (Qiagen, Mississauga, Canada). Each PCR tube contained a 50 μL mixture (21 μL distilled water, 25 μL Master Mix, 3 μL template DNA, and 0.5 μL each primer; final 0.25 μM). Each DNA fragment was amplified using a PCR thermal cycler (Eppendorf Mastercycler Gradient; Eppendorf, Hamburg, Germany). The thermal cycling schedule was as follows: the first cycle consisted of 15 min at 94 °C, followed by 45 cycles of 30 s at 94 °C, 30 s at 58 °C for annealing, 1 min at 72 °C, and the final cycle of 10 min at 72 °C. The reaction mixture was then cooled at 4 °C for 5 min, and PCR products purified with a QiAquick PCR Purification Kit (Qiagen, Ontario, Canada).
Samples with a dagger (†) in Table 2 were sent to Macrogen, Inc. (Seoul, Korea) and sequenced. Sequencing reactions were performed in a MJ Research PTC-225 Eltier Thermal Cycler using an ABI PRISMR BigDyeTM Terminator Cycle Sequencing Kit with AmpliTaqR DNA polymerase (FS enzyme; PE Applied Biosystems, Foster City, CA, USA), following the protocols supplied by the manufacturer. Single-pass sequencing was performed on each template. The fluorescent-labelled fragments were purified from unincorporated terminators with an ethanol precipitation protocol. The samples were resuspended in distilled water and subjected to electrophoresis in an ABI 3730xl sequencer (PE Applied Biosystems).
Samples without a dagger (†) in Table 2 were sequenced in our laboratory. Sequencing reactions were performed using a BigDye Terminator Cycle Sequencing FS Ready Reaction Kit (PE Applied Biosystems) and an Eppendorf Mastercycler Gradient according to the manufacturer’s instructions. Sequencing reaction products were purified with a DyeEx Spin Kit (Qiagen, Mississauga, Canada), and directly sequenced using an ABI PRISM 377-18 DNA Sequencing System (PE Applied Biosystems). The sequences determined in this study were deposited in GenBank; their accession numbers are shown in Table 2. In addition to the sequences generated, 13 sequences accessed from GenBank belonging to Dacrymycetes and Agaricomycetes were included in the phylogenetic analysis (Table 2).
Preliminary multiple alignments of sequences were conducted using MAFFT v6 (Katoh et al. 2005, http://align.bmr.kyushu-u.ac.jp/mafft/software). Final alignments were manually adjusted. Alignment gaps were treated as missing data, and ambiguous positions were excluded from the analysis. Maximum-parsimony (MP) analyses were carried out using PAUP v4.0b10 (Swofford 2001). MP analyses with the heuristic search option using the tree-bisection-reconstruction (TBR) algorithm with 1 000 random sequence additions were performed to find the global optimum tree. All sites were treated as unordered and unweighted. To estimate clade support, the bootstrap (BS) procedure of Felsenstein (1985) was employed with 1 000 replicates in MP analyses and 100 replicates in the Maximum-likelihood (ML) analysis, with BS values higher than 50 % shown.
RESULTS
Taxonomy
As a result of the field work, about 600 samples were collected and 493 samples were identified to species level. There were 28 species representing 6 genera, of which 5 were new species, including 2 new anamorphic species, and 9 already-described species which were new records for Japan (Table 3). The descriptions, illustrations and photographs of these species are as follows.
Table 3.
Species identified in this study.
| Species | Literature | New record for Japan1 |
|---|---|---|
| Calocera cornea (Batsch) Fr. | McNabb (1965a) | – |
| Calocera viscosa (Pers.) Fr. | McNabb (1965a) | – |
| Cerinomyces albosporus Boidin & Gilles | Boidin & Gilles (1986) | – |
| Cerinomyces canadensis (H.S. Jacks. & G.W. Martin) G.W. Martin | McNabb (1964) | ° |
| Cerinomyces pallidus G.W. Martin | McNabb (1964) | ° |
| Dacrymyces adpressus Grognot | McNabb (1973) | ° |
| Dacrymyces ancyleus Shirouzu & Tokum. | This study | ° |
| Dacrymyces aureosporus Shirouzu & Tokum. | This study | ° |
| Dacrymyces capitatus Schwein. | McNabb (1973) | ? |
| Dacrymyces chrysospermus Berk. & M.A. Curtis | McNabb (1973) | – |
| Dacrymyces dendrocalami Oberw. | Oberwinkler & Tschen (1989) | ° |
| Dacrymyces lacrymalis (Pers.) Sommerf. | McNabb (1973) | ° |
| Dacrymyces microsporus P. Karst. | McNabb (1973) | ° |
| Dacrymyces minor Peck | McNabb (1973) | – |
| Dacrymyces minutus (L.S. Olive) McNabb | McNabb (1973) | ? |
| Dacrymyces novae-zelandiae McNabb | McNabb (1973) | ° |
| Dacrymyces pinacearum Shirouzu & Tokum. | This study | ° |
| Dacrymyces punctiformis Neuhoff | Reid (1974) | – |
| Dacrymyces san-augustinii Kobayasi | Kobayasi (1939b) | – |
| Dacrymyces stillatus Nees | McNabb (1973) | – |
| Dacrymyces subalpinus Kobayasi | Kobayasi (1939b) | – |
| Dacrymyces subarcticus Shirouzu & Tokum. | This study | ° |
| Dacrymyces unisporus (L.S. Olive) K. Wells | Olive (1944) | ° |
| Dacrymyces variisporus McNabb | McNabb (1973) | ° |
| Dacryopinax spathularia (Schwein.) G.W. Martin | McNabb (1965b) | – |
| Dacryopinax sphenocarpa Shirouzu & Tokum. | This study | ° |
| Femsjonia peziziformis (Lèv) P. Karst. | McNabb (1965e) | – |
| Guepiniopsis buccina (Pers.) L.L. Kenn. | McNabb (1965c) | – |
1 ° and – indicate new and existing Japanese records; ? unresolved.
Bold: newly described species in this study.
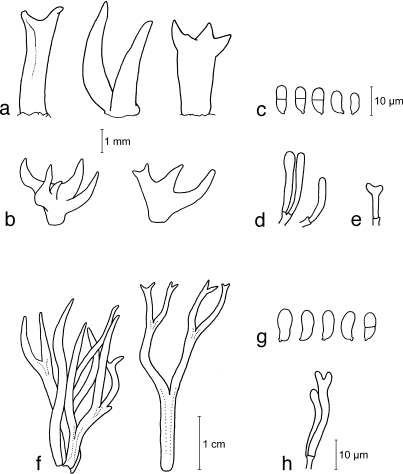
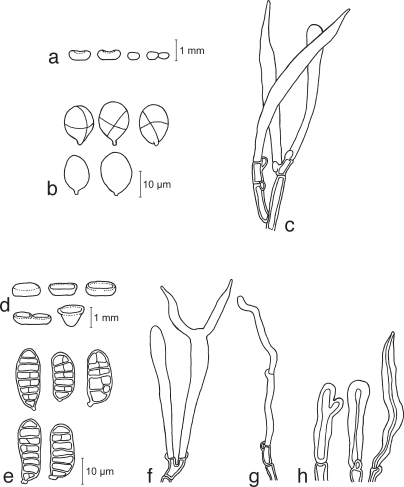
Calocera cornea (Batsch) Fr., Stirp. Agric. Femis. 5: 67. 1827 — Fig. 2a–e, 14a–d
Fig. 2.
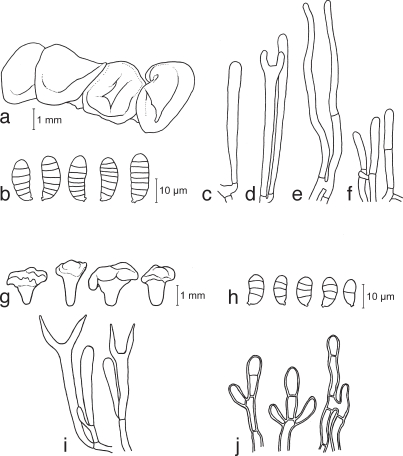
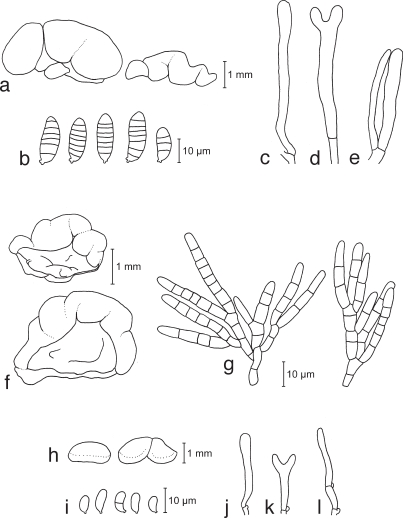
a–e. Calocera cornea TNS-F-15701 (a, c–e; HNo.267), TNS-F-21061 (b, HNo.452). a. Simple cylindrical basidiocarps; b. palmate or dendroid basidiocarps; c. basidiospores; d. probasidia; e. developing basidium. — f–h. Calocera viscosa TNS-F-15705 (HNo.466). f. Basidiocarps; g. basidiospores; h. probasidium and developing basidium.
Fig. 14.
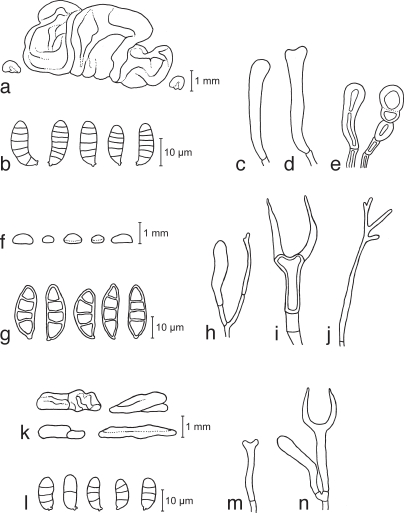
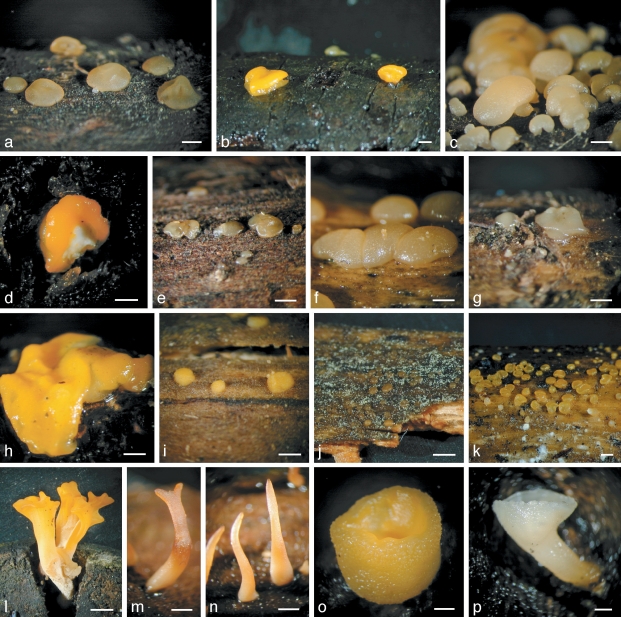
Basidiocarps. a, b. Calocera cornea TNS-F-15701 (HNo.267); c, d. Calocera cornea TNS-F-21061 (HNo.452); e. Calocera viscosa TNS-F-15705 (HNo.466); f. Cerinomyces albosporus TNS-F-15706 (HNo.191); g. Cerinomyces canadensis TNS-F-21034 (HNo.199); h. Cerinomyces pallidus TNS-F-21064 (HNo.505); i. Dacrymyces adpressus TNS-F-21045 (HNo.355); j. Dacrymyces ancyleus TNS-F-21051 (HNo.382); k. Dacrymyces aureosporus TNS-F-15714 (HNo.486); l. Dacrymyces capitatus TNS-F-15708 (HNo.192); m. Dacrymyces chrysospermus TNS-F-15712 (HNo.320); n. Dacrymyces dendrocalami TNS-F-15715 (HNo.210); o. Dacrymyces lacrymalis TNS-F-15717 (HNo.235); p. Dacrymyces microsporus TNS-F-21050 (HNo.371). — Scale bars: a–d, f–p = 1 mm; e = 10 mm.
Basionym. Clavaria cornea Batsch, Elench. Fung. 1: 139. 1783.
For other synonyms see Reid (1974).
Basidiocarps scattered, cylindrical, subulate, simple, slightly branched, palmate or dendroid, white to yellow, soft-cartilaginous, 1–5 mm high, 1–2 mm diam. Structure showing in transverse section composed of a central core of compact parallel hyphae surrounded by a zone of loosely interwoven hyphae enclosed by the hymenium. Internal hyphae branched, thin- or thick-walled, septate, sub-hyaline, 2–4.5 μm diam, without clamp connections. Hymenium amphigenous. Probasidia cylindrical to clavate, pale yellow, 10–28 × 3.5–5.5 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline, 7.5–12.5 × 3.5–6.5 μm (av. 10 × 4.5 μm; n = 20), 0–1-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 18 mm diam, velvety, pale orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5–6 × 3 μm.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of Quercus sp., 30 June 2006, T. Shirouzu, TNS-F-15702 (HNo.358); an unknown broad-leaved tree, 4 Oct. 2006, T. Shirouzu, TNS-F-21065 (HNo.513); Takaragaike, on dead branches of Quercus sp., 1 July 2006, T. Shirouzu, TNS-F-15703 (HNo.376); Nagano, Shioda, on dead branches of Pinus densiflora, 16 July 2006, T. Shirouzu, TNS-F-21061 (HNo.452); Wakayama, Mt Shirami, on dead branches of an unknown broad-leaved tree, 30 Apr. 2006, T. Shirouzu, TNS-F-15701 (HNo.267), culture MAFF240116.
Notes — This species is characterised by hyphae without clamp connections, comparatively small, simple basidiocarps and 1-septate basidiospores. We found two types of basidiocarps for this species: simple or slightly branched basidiocarps and palmate or dendroid basidiocarps. As in earlier studies (McNabb 1965a, Reid 1974, Tubaki & Hosoya 1987), this species was frequently found on woody materials of broad-leaved trees. This was previously reported in Japan (Kobayasi 1939b, Tubaki & Hosoya 1987).
Calocera viscosa (Pers.) Fr., Stirp. Agric. Femis. 5: 67. 1827 — Fig. 2f–h, 14e
Basionym. Clavaria viscosa Pers., Neues Mag. Bot. 1: 117. 1794.
For other synonyms see McNabb (1965a).
Basidiocarps scattered, dendroid composed of cylindrical dichotomous branches, yellow to orange, white near the base, soft-cartilaginous, 10–30 mm high, 1–2 mm diam. Structure showing in transverse section composed of a central core of compact parallel hyphae surrounded by a zone of loosely interwoven hyphae enclosed by the hymenium. Internal hyphae branched, thin-walled, septate, pale yellow, 2–5 μm diam, without clamp connections. Hymenium amphigenous. Probasidia cylindrical, yellow, 25–37 × 3–5 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, pale yellow, 7.5–15 × 3.5–6.5 μm (av. 10 × 4.5 μm; n = 20), 0–1-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 5 mm diam, velvety to lanose, white-yellow. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Nagano, Sugadairakougen, on litter of Abies veitchii Lindl., 10 Aug. 2005, T. Shirouzu, TNS-F-15704 (HNo.175), culture MAFF240119; Saitama, Chichibu, on dead branches of an unknown conifer, 20 July 2006, Y. Takahashi, TNS-F-15705 (HNo.466).
Notes — This species is characterised by hyphae without clamp connections and large, dichotomously branched orange-coloured basidiocarps. In contrast to C. cornea, C. viscosa has been frequently found on woody materials of coniferous trees (McNabb 1965a, Reid 1974). In Japan, this has been also reported from coniferous wood (Kobayasi 1939b).
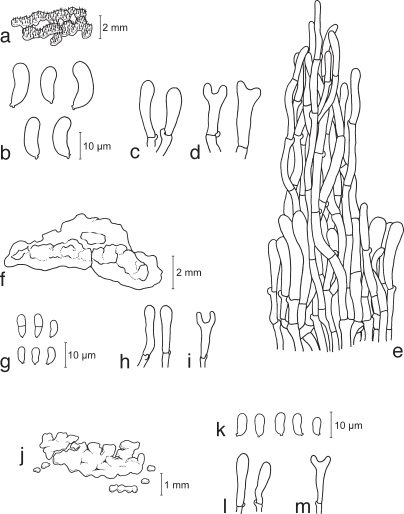
Cerinomyces albosporus Boidin & Gilles, Bull. Trimestriel Soc. Mycol. France 102: 318. 1986 — Fig. 3a–e, 14f
Fig. 3.
a–e. Cerinomyces albosporus TNS-F-15706 (HNo.191). a. Basidiocarp; b. basidiospores; c. probasidia; d. developing basidia; e. hyphal peg. — f–i. Cerinomyces canadensis TNS-F-21034 (HNo.199). f. Basidiocarp; g. basidiospores; h. probasidia; i. developing basidium. — j–m. Cerinomyces pallidus TNS-F-21064 (HNo.505). j. Basidiocarps; k. basidiospores; l. probasidia; m. developing basidium.
= Cerinomyces aculeatus N. Maek., Canad. J. Bot. 65: 583. 1987.
Basidiocarps resupinate, grey-orange, velvety, 3–10 mm long, 2–10 mm wide, 150–350 μm thick, covered with hymenium. Internal hyphae branched, thin-walled, septate, sub-hyaline, 2–3 μm diam, with clamp connections. Hyphal pegs acute to cylindrical, composed of septate, thin-walled hyphae with clamp connections, up to 180 μm above the hymenium, 30–80 μm diam. Probasidia cylindrical to clavate, pale yellow, 17.5–23.5 × 3–5 μm, with a basal clamp connection, becoming bifurcate. Basidiospores cylindrical to reniform, with an apiculum at the base, thin-walled, hyaline, 15–21 × 5–8.5 μm (av. 15.5 × 6.5 μm; n = 10), 0-septate, germination via germ tubes.
Cultural characteristics — Colonies attaining about 4 mm diam, wet, white. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 3 μm.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of Pinus densiflora, 20 Apr. 2006, T. Shirouzu, TNS-F-15706 (HNo.191), culture MAFF240121; Nagano, Sugadairakougen, on dead branches of P. densiflora, 18 Aug. 2006, T. Shirouzu, TNS-F-21063 (HNo.478).
Notes — Cerinomyces albosporus is characterised by hyphae with clamp connections, presence of hyphal pegs and cylindrical to reniform basidiospores. This species has been reported in Japan by Maekawa (1987) as C. aculeatus. All the Japanese materials have been found on wood of Pinus densiflora.
Cerinomyces canadensis (H.S. Jacks. & G.W. Martin) G.W. Martin, Mycologia 41: 85. 1949 — Fig. 3f–i, 14g
Basionym. Ceracea canadensis H.S. Jacks. & G.W. Martin, Mycologia 32: 693. 1940.
Basidiocarps resupinate, yellow, soft-waxy, 2–5 mm long, 2–3 mm wide, 100–380 μm thick, covered with hymenium. Internal hyphae branched, thin- or thick-walled, septate, sub-hyaline, 2–5 μm diam, with clamp connections. Probasidia cylindrical to clavate, pale yellow, 28.5–32.5 × 4–5 μm, with a basal clamp connection, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline, 8–13 × 3.5–5.5 μm (av. 10 × 4.5 μm; n = 15), 0–1-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 6 mm diam, velvety, white. Conidiogenesis not observed.
Specimens examined. Japan, Kyoto, Midorogaike, on dead branches of Rhododendron macrosepalum, 21 Apr. 2006, T. Shirouzu, TNS-F-21036 (HNo.216); Mt Daimonji, on dead branches of Pinus densiflora, 20 Apr. 2006, T. Shirouzu, TNS-F-21034 (HNo.199), culture MAFF241162; Mt Kiyomizu, on dead branches of Castanopsis cuspidata, 22 Aug. 2006, T. Shirouzu, TNS-F-21037 (HNo.219); Takaragaike, on dead branches of Clethra barbinervis, 21 Apr. 2006, T. Shirouzu, TNS-F-21035 (HNo.208).
Notes — Cerinomyces canadensis is characterised by yellow-coloured basidiocarps, absence of dikaryophyses and hyphal pegs, and relatively large, 0–1-septate basidiospores. This is the first report from Japan.
Cerinomyces pallidus G.W. Martin, Mycologia 41: 83. 1949 — Fig. 3j–m, 14h
Basidiocarps resupinate, grey-olive to amber, soft-waxy, 5–20 mm long, 2–5 mm wide, 120–160 μm thick, covered with hymenium. Internal hyphae branched, thin-walled, septate, sub-hyaline, 2–5 μm diam, with clamp connections. Probasidia cylindrical to clavate, pale yellow, 22–29 × 4–6 μm, with a basal clamp connection, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, pale yellow, 7–12 × 3.5–5.5 μm (av. 9 × 4.5 μm; n = 15), 0-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 8 mm diam, velvety, white. Conidiogenesis not observed.
Specimen examined. Japan, Nagano, Shioda, on dead branches of Pinus densiflora, 27 Sept. 2006, T. Shirouzu, TNS-F-21064 (HNo.505).
Notes — Cerinomyces pallidus is characterised by olive to subdued-yellow basidiocarps, absence of dikaryophyses and hyphal pegs, and relatively small, 0-septate basidiospores. Compared with the description by McNabb (1964), the size of basidiospores was slightly larger in our specimens. This is the first report from Japan.
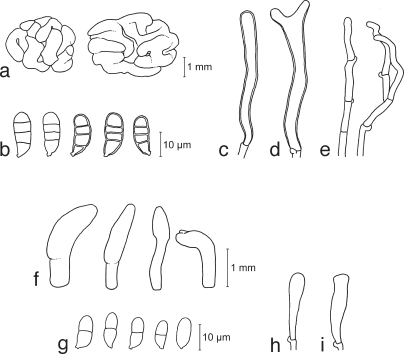
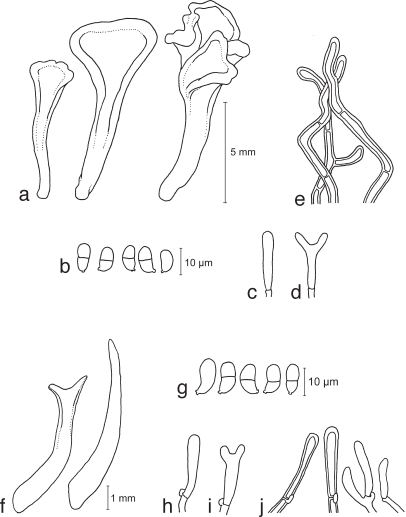
Dacrymyces adpressus Grognot, Pl. Crypt. Sâone-et-Loire: 200. 1863 — Fig. 4a–e, 14i
Fig. 4.
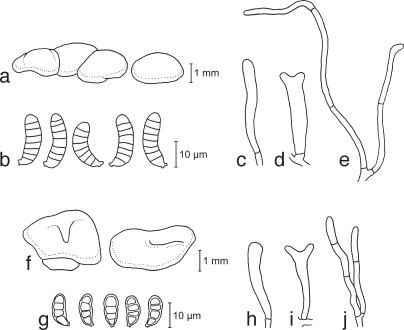
a–e. Dacrymyces adpressus TNS-F-21045 (HNo.355). a. Basidiocarps; b. basidiospores; c. probasidium; d. developing basidium; e. dikaryophyses. — f–i. Dacrymyces ancyleus TNS-F-21051 (HNo.382). f. Basidiocarps; g. basidiospores; h. probasidium; i. developing basidium.
For synonyms see McNabb (1973).
Basidiocarps scattered, pulvinate to cerebriform, sometimes applanate, sessile, orange to amber, soft-cartilaginous to firm-gelatinous, 1–3 mm high, 1–5 mm diam. Sterile parts of basidiocarps covered with simple or branched cylindrical to clavate, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, sub-hyaline, 2–6 μm diam, with clamp connections. Hymenium limited to the superior surface of the basidiocarp. Probasidia cylindrical to clavate, thick-walled, pale yellow, 45–73.5 × 5–8 μm, with a basal clamp connection, becoming bifurcate. Dikaryophyses simple or branched, septate, thin-walled, pale yellow, 40–90 × 3–4.5 μm. Basidiospores cylindrical to reniform, with an apiculum at the base, thin- or thick-walled, pale yellow, 15–24 × 6–10 μm (av. 19 × 8 μm; n = 20), 3-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 10 mm diam, velvety, white-yellow. Conidiogenesis not observed.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of an unknown broad-leaved tree, 30 June 2006, T. Shirouzu, TNS-F-21045 (HNo.355), culture MAFF241172; Wakayama, Mt Shirami, on dead branches of an unknown woody plant, 12 Oct. 2006, T. Shirouzu, TNS-F-21069 (HNo.554).
Notes — Dacrymyces adpressus is characterised by pulvinate, brown to amber-coloured basidiocarps, hyphae with clamp connections, simple dikaryophyses and relatively large, thin- or thick-walled, 3-septate basidiospores. This species sometimes produces applanate basidiocarps and it may be confused with Cerinomyces species. Dacrymyces adpressus Kobayasi was described in Japan is a later homonym, and a different species from D. adpressus Grognot (McNabb 1973), so this is the first record from Japan.
Dacrymyces ancyleus Shirouzu & Tokum., sp. nov. — MycoBank MB514036; Fig. 4f–i, 14j
Basidiocarpia sparsa, cylindrica, stipitata cum pileo subgloboso vel cylindrico ancyleo, flavida, cartilaginea vel gelatinosa, 1–2 mm alta, 0.5 mm lata. Hyphae interaneae ramosae, tenuitunicatae vel crassitunicatae, gelatinosae, hyalinae, 2–6 μm latae, cum colligationibus unciformibus. Probasidia cylindrica vel clavata, flavida, 30–45 × 4.5–10 μm, bifurcatascentia. Basidiosporae subglobosae vel reniformae, tenuitunicatae, sub-hyalinae, 10.5–19.5 × 4–9 μm, 0–1-septatae.
Etymology. Named after its recurved basidiocarps.
Basidiocarps scattered, cylindrical, stipitate, bearing a subglobose to cylindrical, recurved pileus, yellow, soft-cartilaginous to firm-gelatinous, 1–2 mm high, 0.5 mm diam. Internal hyphae branched, thin- or thick-walled, gelatinous, septate, hyaline, 2–6 μm diam, with clamp connections. Hymenium limited to surface of the pileus. Probasidia cylindrical to clavate, pale yellow, 30–45 × 4.5–10 μm, with a basal clamp connection, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline, 10.5–19.5 × 4–9 μm (av. 15.5 × 7.5 μm; n = 20), 0–1-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 4 mm diam, velvety, white. Conidiogenesis not observed.
Specimen examined. Japan, Kyoto, Takaragaike, on dead branches of Rhododendron sp., 1 July 2006, T. Shirouzu, holotype TNS-F-21051 (HNo.382), culture ex-type MAFF241177.
Notes — Dacrymyces ancyleus is characterised by basidiocarps that are recurved, cylindrical or stipitate with a subglobose pileus, hyphae with clamp connections and 0–1-septate basidiospores. This fungus is likely to be included in the genus Calocera based on the cylindrical basidiocarps; however, the internal hyphal structure of the basidiocarp could not be divided into three zones, differing from Calocera species. Furthermore, this fungus is not a member of Dacryopinax since the hymenium is not amphigenous and cortical hairs at the sterile surface could not be recognised; therefore, we regard this dacrymycetous fungus to belong to the genus Dacrymyces.
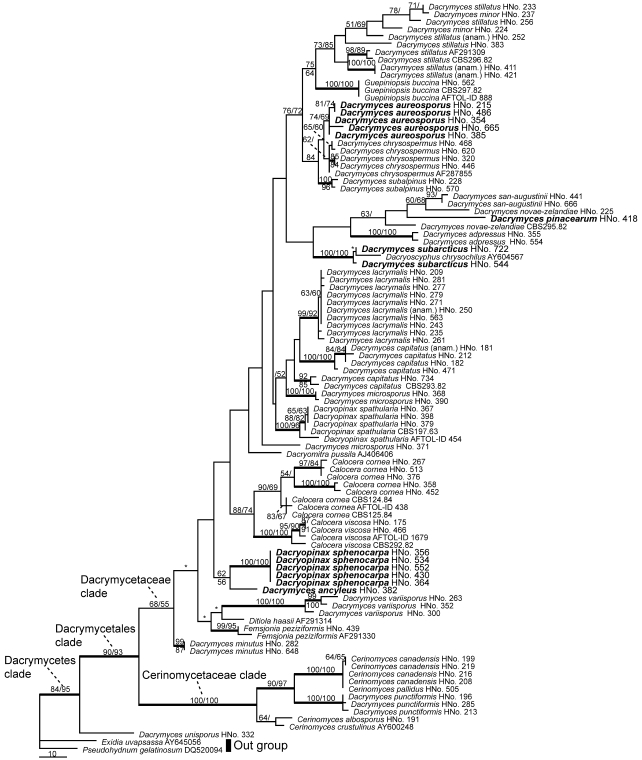
Dacrymyces microsporus is similar in basidiocarp and basidiospore shape to D. ancyleus, but the hyphae composing basidiocarps of D. microsporus do not have clamp connections. Dacrymyces flabelliformis is also similar to D. ancyleus in basidiocarp shape and by having clamp connections on hyphae (Burdsall & Laursen 2004), but D. flabelliformis is different from this fungus in basidiospore size (12.5–14 × 5–6 μm) and the septal number (3-septate); therefore, we consider this fungus to be a new species of Dacrymyces. In molecular phylogenetic analyses (Fig. 16), this species represented a monophyletic group with Dacryopinax sphenocarpa, but the phylogenetic position in the Dacrymycetes lineage was uncertain.
Fig. 16.
Most parsimonious tree of Dacrymycetes using 28S rDNA D1/D2 region sequences. One of the 24 most parsimonious trees. Length = 901, CI = 0.3563, RI = 0.8137. Branch with asterisk (*) collapse in the strict consensus tree of all most parsimonious trees. Numbers above the node or to the left of slashes (/) indicate support above 50 % in 1 000 bootstrap replicates with parsimony analyses. Numbers above the node or to the right of slashes (/) indicate support above 50 % in 100 bootstrap replicates with likelihood analyses. Bold nodes are supported more than 80 % MP and 80 % ML bootstrap values. Newly described species in this study are shown in bold. TreeBASE SN4250.
Dacrymyces aureosporus Shirouzu & Tokum., sp. nov. — MycoBank MB514037; Fig. 5a–f, 14k
Fig. 5.
a–f. Dacrymyces aureosporus TNS-F-15714 (HNo.486). a. Basidiocarps; b. basidiospores; c. probasidium; d. basidium and probasidium; e. dikaryophysis; f. marginal hyphae. — g–j. Dacrymyces capitatus TNS-F-15708 (HNo.192). g. Basidiocarps; h. basidiospores; i. probasidia and basidia; j. marginal hyphae.
Basidiocarpia sparsa vel gregaria, turbinata vel cerebriformia, sessilia vel stipitata cum pileo semigloboso rugoso vel convoluto, aurantiaca, gelatinosa, 1–3 mm alta, 1–6 mm lata. Pili corticales steriles cylindrici vel clavati, tenuitunicati. Hyphae interaneae ramosae, tenuitunicatae, gelatinosae, hyalinae, 2–3 μm latae. Probasidia cylindrica, sub-hyalina, 46.5–80 × 5.5–8 μm, bifurcatascentia. Basidiosporae cylindricae vel allantoideae, tenuitunicatae, flavidae, 17–26 × 6.5–11 μm, 7-septatae.
Etymology. Named after its yellow basidiospores.
Basidiocarps scattered or gregarious, sometimes coalesced, turbinate to cerebriform, sessile or stipitate bearing a rugose to convolute semiglobose pileus, pale orange, firm-gelatinous, 1–3 mm high, 1–6 mm diam. Sterile parts of basidiocarps covered with simple cylindrical to clavate, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the pileus. Probasidia cylindrical, sub-hyaline, 46.5–80 × 5.5–8 μm, becoming bifurcate. Basidiospores cylindrical to allantoid, with an apiculum at the base, thin-walled, yellow, 17–26 × 6.5–11 μm (av. 21.5 × 8.5 μm; n = 20), 7-septate, germination via the production of conidia and germ tubes.
Culture characteristics — Colonies attaining about 6 mm diam, velvety, pale orange to orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 4 μm.
Specimens examined. Japan, Kyoto, Midorogaike, on dead branches of Quercus serrata, 21 Apr. 2006, T. Shirouzu, TNS-F-15711 (HNo.215); on dead branches of Cryptomeria japonica, 1 July 2006, T. Shirouzu, TNS-F-21053 (HNo.385); Mt Daimonji, on dead branches of an unknown broad-leaved tree, 30 June 2006, T. Shirouzu, TNS-F-21044 (HNo.354); Nagano, Kijimadaira, on a fallen tree of Fagus crenata, 2 Sept. 2006, T. Shirouzu, holotype TNS-F-15714 (HNo.486), culture ex-type MAFF240129; Okinawa, Iriomote Island, on a fallen tree of an unknown broad-leaved tree, 9 June 2007, T. Shirouzu, TNS-F-21074 (HNo.665).
Notes — Dacrymyces aureosporus is characterised by turbinate to cerebriform basidiocarps, hyphae without clamp connections, thin-walled, marginal hypha and multi-septate basidiospores. This fungus resembles D. chrysospermus in morphological features, except for the absence of thick-walled terminal cells on the sterile surface of the basidiocarps. Dacrymyces aureosporus also produces terminal cells but its walls do not become thick. Kobayasi (1939a) reported a similar dacrymycetous fungus to D. palmatus, which is a synonym of D. chrysospermus, and the terminal cells of the fungus are also thin-walled. In molecular phylogenetic analyses, D. chrysospermus and D. aureosporus were clearly separated (Fig. 16). We considered this to be a new Dacrymyces species closely related to D. chrysospermus.
Dacrymyces capitatus Schwein., Trans. Amer. Philos. Soc., Ser. II, 4: 186. 1832 — Fig. 5g–j, 14l
For synonyms see McNabb (1973) and Reid (1974).
Basidiocarps scattered, turbinate to stoutly cylindrical, stipitate bearing a concave or rugose semiglobose pileus, yellow to orange, firm-gelatinous, sometimes white tomentose at the base, 1.5–2 mm high, 2–4 mm diam. Sterile parts of basidiocarps densely covered with simple or branched, cylindrical to clavate, septate, hyaline, thin- or thick-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–3.5 μm diam, without clamp connections. Hymenium limited to the upper surface of the pileus. Probasidia cylindrical to clavate, pale yellow, 25–42.5 × 3–6 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline to pale yellow, 10–15 × 5–7.5 μm (av. 13 × 6.5 μm; n = 20), 0–3-septate, germination via conidia and germ tubes. Anamorphic fruit bodies sometimes present with basidiocarps, pulvinate, sessile, orange, gelatinous, 1 mm high, 1–3 mm diam. Conidia holoblastic, cylindrical to subglobose, with a separation scar at the base, thin- or thick-walled, 20–30 × 7.5–10 μm, 0–1-septate.
Culture characteristics — Colonies attaining about 4 mm diam, velvety, yellow to orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of Pinus densiflora, 29 Mar. 2006, T. Shirouzu, TNS-F-21033 (HNo.181; with anamorph stage); 20 Apr. 2006, T. Shirouzu, TNS-F-15708 (HNo.192), culture MAFF240123; TNS-F-15707 (HNo.182); Takaragaike, on dead branches of Clethra barbinervi, 21 Apr. 2006, T. Shirouzu, TNS-F-15709 (HNo.212); Nagano, Sugadairakougen, on dead branches of Picea sp., 19 July 2006, T. Shirouzu, TNS-F-21062 (HNo.471); on dead branches of Pinus densiflora, 7 Aug. 2007, T. Shirouzu, TNS-F-21077 (HNo.734).
Notes — This species is characterised by turbinate or sub-stipitate basidiocarps, hyphae without clamp connections, densely arranged thin- or thick-walled marginal hyphae, and thin-walled, 3-septate basidiospores. In this study, we collected material with anamorphic fruit bodies. Oberwinkler (1993) also reported that the species produced conidium-like diaspores by hyphal phragmentation. According to the taxonomic opinion of McNabb (1973), Dacrymyces capitatus might be reported in Japan as D. tremellosus (Kobayasi 1939a).
Dacrymyces chrysospermus Berk. & M.A. Curtis, Grevillea 2: 20. 1873 — Fig. 6a–e, 14m
Fig. 6.
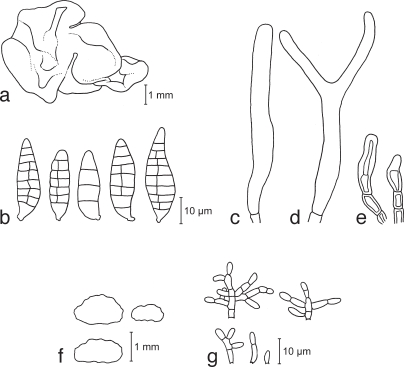
a–e. Dacrymyces chrysospermus TNS-F-15712 (HNo.320). a. Basi-diocarps; b. basidiospores; c. probasidium; d. developing basidium; e. marginal hyphae. — f–j. Dacrymyces dendrocalami TNS-F-15715 (HNo.210). f. Basidiocarps; g. basidiospores; h. probasidium and dikaryophysis; i. basidium; j. dikaryophysis. — k–n. Dacrymyces lacrymalis TNS-F-15717 (HNo.235). k. Basidiocarps; l. basidiospores; m. developing basidium; n. pro-basidium and basidium.
For synonyms see McNabb (1973) and Reid (1974).
Basidiocarps scattered or gregarious, sometimes coalesced, turbinate to cerebriform, sessile or stipitate bearing a rugose to convolute semiglobose pileus, orange, firm-gelatinous, 2–5 mm high, 6–13 mm diam. Sterile parts of basidiocarps covered with simple cylindrical to clavate, septate, 2–3-celled, hyaline, conspicuously thick-walled terminal cells, 25–50 × 7.5–16 μm. Internal hyphae branched, thin-walled, gelatinous, septate, pale yellow, 2–3 μm diam, without clamp connections. Hymenium limited to upper surface of the pileus. Probasidia cylindrical to clavate, yellow to orange, 30–48 × 5–8 μm, becoming bifurcate. Basidiospores cylindrical to curved-cylindrical, with an apiculum at the base, thin-walled, yellow, 12–23 × 5–10 μm (av. 18 × 7 μm; n = 20), 3–7-septate, germinated by production of conidia and germ tubes.
Culture characteristics — Colonies of the primary mycelium attaining about 10 mm diam, velvety, orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 2 μm.
Specimens examined. Japan, Kagoshima, Amami Island, on dead branches of Pinus luchuensis, 18 Jan. 2007, T. Shirouzu, TNS-F-21072 (HNo.620); Nagano, Sugadairakougen, on dead branches of Abies sp., 22 June 2006, T. Shirouzu, TNS-F-15712 (HNo.320), culture MAFF240127; a log of Larix kaempferi, 16 July 2006, T. Shirouzu, TNS-F-21060 (HNo.446); a standing log of L. kaempferi, 19 July 2006, T. Shirouzu, TNS-F-15713 (HNo.468).
Notes — This species is characterised by turbinate to cerebriform basidiocarps, hyphae without clamp connections, thick-walled terminal cells and multi-septate, cylindrical to curved, thin-walled basidiospores. It has previously been recorded in Japan as Dacrymyces palmatus, D. roseotinctus and D. puniceus (Kobayasi 1939a).
Dacrymyces dendrocalami Oberw., Trans. Mycol. Soc. Japan 30: 350. 1989 — Fig. 6f–j, 14n
Basidiocarps scattered, pustulate to pulvinate, sessile, pale white to pale orange, firm-gelatinous, 0.2–0.5 mm high, 1–3 mm diam. Sterile parts of basidiocarps covered with simple or branched, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, 2–3 μm diam, without clamp connections. Hymenium limited to the superior surface of the basidiocarp. Probasidia cylindrical to clavate, thin- or thick-walled, hyaline to pale yellow, 24–37.5 × 5–7.5 μm, becoming bifurcate. Dikaryophyses simple or branched, septate, thin-walled, sub-hyaline, 2–3 μm diam. Basidiospores naviculate, straight or curved, with an apiculum at the base, thick-walled, sub-hyaline, 16–22.5 × 6–8 μm (av. 20 × 7 μm; n = 20), 0–3-septate, germination via germ tubes.
Culture characteristics — Culture not obtained.
Specimens examined. Japan, Kyoto, Takaragaike, on dead branches of an unknown broad-leaved tree, 21 Apr. 2006, T. Shirouzu, TNS-F-15715 (HNo.210); Nara, Mt Tamaki, on dead branches of Parabenzoin trilobum, 7 July 2006, T. Shirouzu, TNS-F-21058 (HNo.426).
Notes — Dacrymyces dendrocalami is characterised by pustulate to pulvinate, sessile basidiocarps, hyphae without clamp connections, and straight- or curved-naviculate, thick-walled basidiospores. This species has been reported only once from Taiwan (Oberwinkler & Tschen 1989). This is the second record for this species, and the first from Japan.
Dacrymyces lacrymalis (Pers.) Sommerf., Suppl. Fl. Lapp. (Oslo) no. 1753: 308. 1826 — Fig. 6k–n, 14o
Basionym. Tremella lacrymalis Pers., Syn. Meth. Fung. 2: 628. 1801.
For other synonyms see McNabb (1973).
Basidiocarps scattered or gregarious, sometimes coalesced, pulvinate or irregularly discoid, applanate, gyrose or centrally depressed, sessile or sub-stipitate, yellow, firm-gelatinous, 0.5–2 mm high, 1–4 mm diam. Sterile parts of basidiocarps covered with simple or branched, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the basidiocarp. Probasidia cylindrical to clavate, sub-hyaline to pale yellow, 20.5–30 × 3.5–5 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline to pale yellow, 9.5–15 × 3.5–6 μm (av. 11.5 × 5 μm; n = 20), 0–3-septate, germinated by production of conidia and germ tubes. Anamorphic fruit bodies sometimes present with basidiocarps, pulvinate, sessile, yellow, gelatinous, 1 mm high, 1–3 mm diam. Conidia holoblastic, cylindrical to subglobose, with a separation scar at the base, thin-walled, 5–10 × 3–5 μm, 0–1-septate.
Culture characteristics — Colonies attaining about 8 mm diam, velvety, yellow. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Kyoto, Takaragaike, on dead branches of an unknown broad-leaved tree, 21 Apr. 2006, T. Shirouzu, TNS-F-15716 (HNo.209); Nagano, Sugadairakougen, on dead branches of Abies sp., 8 May 2006, T. Shirouzu, TNS-F-21041 (HNo.271); on dead branches of Alnus japonica, 14 May 2006, D. Hirose, TNS-F-15719 (HNo.281); on dead branches of Larix sp., 8 May 2006, T. Shirouzu, TNS-F-21042 (HNo.277); L. kaempferi, 28 May 2006, T. Shirouzu, TNS-F-21043 (HNo.279); Nara, Mt Tamaki, on dead branches of Fagus crenata, Nara, 27 Apr. 2006, T. Shirouzu, TNS-F-15718 (HNo.261); on dead branches of an unknown broad-leaved tree, 13 Oct. 2006, T. Shirouzu, TNS-F-21070 (HNo.563); Oodaigahara, on dead branches of F. crenata, 24 Apr. 2006, T. Shirouzu, TNS-F-15717 (HNo.235), culture MAFF240132; on dead branches of an unknown conifer, 24 Apr. 2006, T. Shirouzu, TNS-F-21040 (HNo. 250; with anamorph stage); on dead branches of F. crenata, 24 Apr. 2006, T. Shirouzu, TNS-F-21039 (HNo.243).
Notes — Dacrymyces lacrymalis is characterised by irregularly discoid, gyrose, sessile basidiocarps, hyphae without clamp connections and thin-walled, 3-septate basidiospores. Although McNabb (1973) did not report the anamorphic stage of this species, anamorphic fruit bodies bearing holoblastic conidia were observed together with basidiocarps in this study. According to the result of this study and McNabb (1973), D. lacrymalis has been frequently found on woody substrata of broad-leaved trees. This record is the first for Japan.
Dacrymyces microsporus P. Karst., Bidrag Kannedom Finlands Natur Folk 48: 459. 1889 — Fig. 7a–c, 14p
Fig. 7.
a–c. Dacrymyces microsporus TNS-F-21050 (HNo.371). a. Basidiocarps; b. basidiospores; c. probasidia. — d–g. Dacrymyces minor TNS-F-15720 (HNo.224). d. Basidiocarps; e. basidiospores; f. probasidium; g. developing basidium. — h–m. Dacrymyces minutus TNS-F-15722 (HNo.282). h. Basidiocarps; i. basidiospores; j. probasidium; k. developing basidium; l. dikaryophysis; m. marginal hyphae.
Basidiocarps scattered or gregarious, sometimes coalesced, turbinate to stoutly cylindrical, stipitate bearing a concave or rugose semiglobose pileus, pale yellow, soft-cartilaginous to firm-gelatinous, 1–1.5 mm high, 1–2 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled, marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–4 μm diam, without clamp connections. Hymenium limited to upper surface of the pileus. Probasidia cylindrical to clavate, sub-hyaline, 27.5–42.5 × 3.5–4.5 μm, becoming bifurcate. Basidiospores reniform, with an apiculum at the base, thin-walled, sub-hyaline, 10–14 × 3.5–6.5 μm (av. 11.5 × 5 μm; n = 20), 0–1-septate, germination by means of conidial production and germ tubes.
Culture characteristics — Colonies of the primary mycelium attaining about 7 mm diam, wet, yellow. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 3 μm.
Specimens examined. Japan, Kyoto, Mt Kiyomizu, on dead branches of an unknown broad-leaved tree, 2 July 2007, T. Shirouzu, TNS-F-21054 (HNo.390); Takaragaike, on a fallen tree of Quercus sp., 1 July 2006, T. Shirouzu, TNS-F-21050 (HNo.371), culture MAFF241176; Wakayama, Mt Nachi, on dead branches of an unknown woody plant, 6 July 2006, T. Shirouzu, TNS-F-21049 (HNo.368).
Notes — Dacrymyces microspores is characterised by basiodiocarps that are turbinate to stipitate, bearing a concave or rugose, semiglobose pileus, hyphae without clamp connections and thin-walled, 0–1-septate basidiospores. This is the first record from Japan.
Dacrymyces minor Peck, Ann. Rep. N. Y. State Mus. 30: 49. 1878 — Fig. 7d–g, 15a
For synonyms see McNabb (1973).
Basidiocarps scattered, pustulate to pulvinate, sessile or sub-stipitate, pale orange, firm-gelatinous, 0.5 mm high, 1–2 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled, marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to superior surface of the basidiocarp. Probasidia cylindrical to clavate, sub-hyaline, 30–47 × 4–9 μm, becoming bifurcate. Basidiospores reniform, with an apiculum at the base, thin- or thick-walled, pale yellow, 12.5–18 × 5–9.5 μm (av. 15 × 6.5 μm; n = 20), 1–3-septate, germination by means of conidial production and germ tubes.
Culture characteristics — Colonies of the primary mycelium attaining about 6 mm diam, velvety, orange to white. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 3 μm.
Specimens examined. Japan, Nara, Kasuga Shrine, on dead branches of Podocarpus nagi, 23 Apr. 2006, T. Shirouzu, TNS-F-15721 (HNo.237); Mt Kasuga, on dead branches of an unknown broad-leaved tree, 23 Apr. 2006, T. Shirouzu, TNS-F-15720 (HNo.224), culture MAFF240135.
Notes — Dacrymyces minor is characterised by relatively small, pustulate to pulvinate basidiocarps, hyphae without clamp connections, and thin- or thick-walled, 1–3-septate basidiospores. This species was first recorded from Japan by Kobayasi (1939a).
Dacrymyces minutus (L.S. Olive) McNabb, New Zealand J. Bot. 11: 497. 1973 — Fig. 7h–m, 15b
Fig. 15.
Basidiocarps. a. Dacrymyces minor TNS-F-15720 (HNo.224); b. Dacrymyces minutus TNS-F-15722 (HNo.282); c. Dacrymyces novae-zelandiae TNS-F-21038 (HNo.225); d. Dacrymyces pinacearum TNS-F-21056 (HNo.418); e. Dacrymyces punctiformis TNS-F-15723 (HNo.196); f. Dacrymyces san-augustinii TNS-F-21075 (HNo.666); g. Dacrymyces stillatus TNS-F-15727 (HNo.233); h. Dacrymyces subalpinus TNS-F-21071 (HNo.570); i. Dacrymyces subarcticus TNS-F-21067 (HNo.544); j. Dacrymyces unisporus TNS-F-15731 (HNo.332); k. Dacrymyces variisporus TNS-F-15733 (HNo.300); l. Dacryopinax spathularia TNS-F-15735 (HNo.379); m, n. Dacryopinax sphenocarpa TNS-F-21046 (HNo.356); o. Femsjonia peziziformis TNS-F-15737 (HNo439); p. Guepiniopsis buccina TNS-F-15738 (HNo.562). — Scale bars: a–p = 1 mm.
Basionym. Guepiniopsis minuta L.S. Olive, Bull. Torrey Bot. Club 81: 334. 1954.
Basidiocarps scattered, turbinate or irregularly discoid, sub-stipitate or stipitate bearing a centrally depressed semiglobose pileus, yellow-orange, firm-gelatinous, 1–3 mm high, 2–5 mm diam. Sterile parts of basidiocarps covered with simple cylindrical to clavate, hyaline, conspicuously thick-walled terminal cells, 25–32 × 6–10 μm. Internal hyphae branched, thin-walled, septate, hyaline, 2–3 μm diam, with clamp connections. Hymenium limited to the upper surface of the pileus or disc. Probasidia cylindrical to clavate, sub-hyaline to pale yellow, 30–38 × 5–7.5 μm, with a basal clamp connection, becoming bifurcate. Dikaryophyses cylindrical, simple, sub-hyaline, 45–52 × 2.5 μm, with a basal clamp connection. Basidiospores cylindrical to reniform, with an apiculum at the base, thin-walled, sub-hyaline to pale yellow, 11.5–15.5 × 4–6 μm (av. 14 × 5 μm; n = 20), 1–3-septate, germinated by production of conidia and germ tubes.
Culture characteristics — Colonies slow growing, attaining about 2 mm diam, velvety, orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Gunma, Mt Motoshirane, on dead branches of Abies veitchii, 28 May 2007, T. Shirouzu & D. Hirose, TNS-F-21073 (HNo.648); Nagano, Sugadairakougen, on dead branches of Larix leptolepis, 28 May 2006, T. Shirouzu, TNS-F-15722 (HNo.282), culture MAFF240137.
Notes — Dacrymyces minutus is characterised by turbinate to irregularly discoid basidiocarps, hyphae with clamp connections, cylindrical to clavate, thick-walled terminal cells, and thin-walled, 3-septate basidiospores. According to the results of our survey and that of McNabb (1973), D. minutus occurs frequently on woody materials of conifers. If D. pezizoides is thought of as a synonym of D. minutus as McNabb (1973) suggested, this species has already been reported from Japan by Kobayasi (1939a).
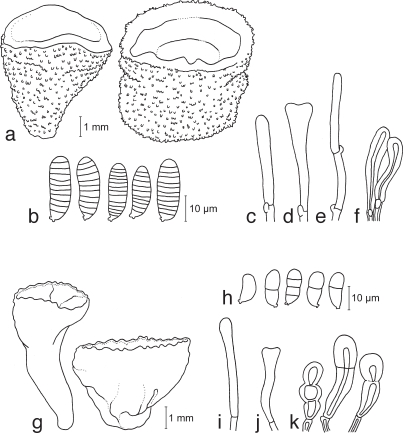
Dacrymyces novae-zelandiae McNabb, New Zealand J. Bot. 11: 493. 1973 — Fig. 8a–e, 15c
Fig. 8.
a–e. Dacrymyces novae-zelandiae TNS-F-21038 (HNo.225). a. Basi-diocarps; b. basidiospores; c. probasidium; d. developing basidium; e. dikaryophysis and probasidium. — f, g. Dacrymyces pinacearum TNS-F-21056 (HNo.418). f. Conidiocarps; g. conidia. — h–l. Dacrymyces punctiformis TNS-F-15723 (HNo.196). h. Basidiocarps; i. basidiospores; j. probasidium; k. developing basidium; l. dikaryophysis.
Basidiocarps scattered or gregarious, sometimes coalesced, pulvinate to convoluted, sessile or sub-stipitate, yellow, firm-gelatinous, 1–2 mm high, 1–4 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, sub-hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the basidiocarp. Probasidia cylindrical to clavate, thin-walled, pale yellow, 55–72 × 5–8.5 μm, becoming bifurcate. Dikaryophyses simple, thin-walled, pale yellow, 50–60 × 3–4 μm. Basidiospores cylindrical to reniform, with an apiculum at the base, thin-walled, yellow, 15–27 × 7–12.5 μm (av. 20.5 × 8.5 μm; n = 20), 0–7-septate, germination via the production of conidia and germ tubes.
Culture characteristics — Colonies attaining about 6 mm diam, velvety, white-orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 3 μm.
Specimen examined. Japan, Nara, Kasuga Shrine, on dead branches of Podocarpus nagi, 23 Apr. 2006, T. Shirouzu, TNS-F-21038 (HNo.225), culture MAFF241165.
Notes — Dacrymyces novae-zelandiae is characterised by pustulate to pulvinate, sessile basidiocarps, hyphae without clamp connections, simple dikaryophyses and thin-walled, multi-septate basidiospores. This is the first record from Japan.
Dacrymyces pinacearum Shirouzu & Tokum., sp. nov. — MycoBank MB514038; Fig. 8f, g, 15d
Conidiomata sparsa, pulvinata vel flabellata, sessilia, aurantiaca, fragilia, 1 mm alta, 1–2 mm lata. Hyphae interaneae ramosae, tenuitunicatae, flavidae, 2–3 μm latae. Cellulae conidiogenae micronematicae, cylindricae, flavidae. Conidia holoblastica, ramosa, dendroidea, cum ramis cylindricis 1–8-cellularibus 27–65 × 5–9 μm tenuitunicatis aurantiacis.
Etymology. Named after the family name of its host.
Conidiocarps scattered, pulvinate to flabellate, sessile, orange, sometimes white at the lower-side, fragile, 1 mm high, 1–2 mm diam. Internal hyphae branched, thin-walled, septate, pale yellow, 2–3 μm diam, without clamp connections. Hymenium limited to the orange part of the basidiocarp. Conidiogenous cells micronematous, cylindrical, pale yellow. Conidia holoblastic, branched, dendroid, composed of cylindrical, 1–8-celled branches of 27–65 × 5–9 μm, thin-walled, orange.
Culture characteristics — Colonies attaining about 8 mm diam, velvety, white to pale yellow. Conidiogenesis not observed.
Specimen examined. Japan, Nara, Oodaigahara, on a dead trunk of Abies sp., 4 July 2006, T. Shirouzu, holotype TNS-F-21056 (HNo.418), culture ex-type MAFF241182.
Notes — Dacrymyces pinacearum is characterised by pulvinate to flabellate conidiocarps and holoblastic, dendroid conidia. Coelomycetous anamorphs of Dacrymycetales are usually linked with the genus Dacrymyces and have pulvinate fruit bodies and arthric conidia as represented by D. stillatus. An unusual coelomycetous anamorph of Dacrymycetales having holoblastic, 2–4-armed stauroconidia was described by Kirschner & Yang (2005) as Dacryoscyphus chrysochilus. Our fungus resembles the anamorphic species of Kirschner & Yang, but is different from D. chrysochilus in having dendroid conidia composed of branches without accurate tips. In molecular phylogenetic analyses, Dacrymyces pinacearum was clearly separate from Dacryoscyphus chrysochilus and formed a clade with Dacrymyces san-augustinii and D. novae-zelandiae (Fig. 16); therefore, we consider that this fungus is a new anamorphic species of Dacrymyces.
Dacrymyces punctiformis Neuhoff, Schweiz. Z. Pilzk. 12: 81. 1934 — Fig. 8h–l, 15e
For synonyms see Reid (1974).
Basidiocarps scattered or gregarious, sometimes coalesced, pustulate to pulvinate, sessile, pale yellow, firm-gelatinous, 0.5–1 mm high, 1–2 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, sub-hyaline, 2–3.5 μm diam, with clamp connections. Hymenium limited to the superior surface of the basidiocarp. Probasidia cylindrical to clavate, thick-walled, pale yellow, 30–50 × 5 μm, with a basal clamp connection, becoming bifurcate. Dikaryophyses simple, septate, thin-walled, sub-hyaline, 30–50 × 2.5 μm. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline, 7–13 × 4–6 μm (av. 9 × 5 μm; n = 15), 0–1(–3)-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 4 mm diam, velvety, white. Conidiogenesis not observed.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of Pinus densiflora, 20 Apr. 2006, T. Shirouzu, TNS-F-15723 (HNo.196), culture MAFF240138; Takaragaike, on dead branches of Clethra barbinervi, 21 Apr. 2006, T. Shirouzu, TNS-F-15724 (HNo.213); Nagano, Shioda, on dead branches of Pinus densiflora, 20 May 2006, T. Shirouzu, TNS-F-15725 (HNo.285).
Notes — Dacrymyces punctiformis is characterised by pustulate to pulvinate, sessile basidiocarps, hyphae with clamp connections, simple dikaryophyses and thin-walled, 0–1(–3)-septate basidiospores. This study as well as others (Kobayasi 1939a, McNabb 1973, Reid 1974), it was mentioned that this species frequently occurred on conifers wood.
Dacrymyces san-augustinii Kobayasi, Sci. Rep. Tokyo Bunrika Daigaku, Sect. B, 4: 122. 1939 — Fig. 9a–e, 15f
Fig. 9.
a–e. Dacrymyces san-augustinii TNS-F-21075 (HNo.666). a. Basi-diocarps; b. basidiospores; c. probasidium; d. developing basidium; e. dikaryophysis. — f–j. Dacrymyces stillatus TNS-F-15727 (HNo.233). f. Basidiocarps; g. basidiospores; h. probasidium; i. developing basidium; j. dikaryophysis.
Basidiocarps scattered or gregarious, sometimes coalesced, pustulate to pulvinate, sessile, pale orange to pale amber, firm-gelatinous, 1–2 mm high, 1–3 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, sub-hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the basidiocarp. Probasidia cylindrical to clavate, thin-walled, pale yellow, 38–58 × 5.5–7 μm, becoming bifurcate. Dikaryophyses simple or branched, septate, thin- or thick-walled, pale yellow, 40–120 × 4 μm. Basidiospores curved-allantoid, with an apiculum at the base, thin-walled, sub-hyaline, 16–27.5 × 6–10 μm (av. 21.5 × 7.5 μm; n = 20), 0–7-septate, germination via conidial production and germ tubes.
Culture characteristics — Colonies attaining about 20 mm diam, velvety, white-orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Okinawa, Iriomote Island, on dead branches of an unknown broad-leaved tree, 10 June 2007, T. Shirouzu, TNS-F-21075 (HNo.666), culture MAFF241196; Wakayama, Mt Shirami, on dead branches of an unknown woody plant, 8 July 2006, T. Shirouzu, TNS-F-15726 (HNo.441).
Notes — Dacrymyces san-augustinii is characterised by pustulate to pulvinate, sessile basidiocarps, hyphae without clamp connections, branched dikaryophyses and thin-walled, multi-septate basidiospores. Dacrymyces novae-zelandiae resembles D. san-augustinii in morphological characteristics. McNabb (1973) noted that these species can be distinguished by the presence of branched dikaryophyses, which are present in D. san-augustinii and absent in D. novae-zelandiae. This species was described by Kobayasi (1939a) on a specimen collected from the trunk of a broad-leaved tree in Japan.
Dacrymyces stillatus Nees, Syst. Mycol. 2: 250. 1822 — Fig. 9f–j, 15g
For synonyms see McNabb (1973) and Reid (1974).
Basidiocarps scattered, pustulate, pulvinate to applanate, sometimes centrally depressed, sessile, pale yellow to pale amber, firm-gelatinous, 0.5–1 mm high, 1–3 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the basidiocarp. Probasidia cylindrical to clavate, sub-hyaline, 25–42.5 × 4.5–7 μm, becoming bifurcate. Basidiospores reniform, with an apiculum at the base, thick-walled, pale yellow, 12.5–17 × 5–8 μm (av. 14.5 × 6 μm; n = 20), 1–3-septate, germination via the production of conidia and germ tubes. Anamorphic fruit bodies pustulate to pulvinate, sessile, orange, firm-gelatinous, 0.5 mm high, 0.5–1.5 mm diam. Conidia arthric, cylindrical, thin-walled, pale orange, 7.5–12.5 × 2.5–3.5 μm, 0–1-septate.
Culture characteristics — Colonies attaining about 15 mm diam, velvety, pale orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Kyoto, Midorogaike, on dead branches of Quercus glauca, 1 July 2006, T. Shirouzu, TNS-F-21052 (HNo.383); Nara, Kasuga Shrine, on dead branches of Podocarpus nagi, 23 Apr. 2006, T. Shirouzu, TNS-F-15727 (HNo.233), culture MAFF240142; Mt Tamaki, on dead branches of Deutzia crenata, 27 Apr. 2006, T. Shirouzu, TNS-F-15729 (HNo.252; anamorphic stage); on dead branches of an unknown conifer, 27 Apr. 2006, T. Shirouzu, TNS-F-15728 (HNo.256); Oodaigahara, on dead branches of Fagus crenata, 4 July 2006, T. Shirouzu, TNS-F-21057 (HNo.421; anamorphic stage); on dead branches of an unknown conifer, 4 July 2006, T. Shirouzu, TNS-F-21055 (HNo.411; anamorphic stage).
Notes — Dacrymyces stillatus is characterised by pustulate to pulvinate basidiocarps, hyphae without clamp connections and thick-walled, 1–3-septate basidiospores. This species is similar to D. minor in morphology on the whole, but McNabb (1973) emphasised that these species could be distinguished by the difference in basidiocarp size. In this study, we identified materials with basidiocarps of 2 mm or more in diameter as D. stillatus, but from the results of molecular phylogenetic analyses, these two species were nested in the same clade (Fig. 16). This species was reported in Japan by Kobayasi (1939a; as D. deliquescens) and Tubaki & Hosoya (1987).
Dacrymyces subalpinus Kobayasi, Sci. Rep. Tokyo Bunrika Daigaku, Sect. B, 4: 120. 1939 — Fig. 10a–e, 15h
Fig. 10.
a–e. Dacrymyces subalpinus TNS-F-21071 (HNo.570). a. Basidiocarp; b. basidiospores; c. probasidium; d. basidium; e. marginal hyphae. — f, g. Dacrymyces subarcticus TNS-F-21067 (HNo.544). f. Conidiocarps; g. conidia.
Basidiocarps scattered or gregarious, sometimes coalesced, turbinate to cerebriform, sessile or stipitate, bearing a rugose to convoluted, semiglobose pileus, yellow to orange, firm-gelatinous, 1–2 mm high, 4–6 mm diam. Sterile parts of basidiocarps covered with simple, cylindrical to clavate, septate, 2–3-celled, hyaline, conspicuously thick-walled terminal cells, 38–50.5 × 7–12 μm. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–4 μm diam, without clamp connections. Hymenium limited to superior surface of the pileus. Probasidia cylindrical, yellow, 63.5–90 × 8–11.5 μm, becoming bifurcate. Basidiospores cylindrical to curved-naviculate, with an apiculum at the base, thin-walled, yellow, 32–54 × 9.5–17 μm (av. 41.5 × 12 μm; n = 20), 0–14-transverse septate, occasionally with 0–7-vertical septa, germinated by production of conidia and germ tubes.
Culture characteristics — Colonies attaining about 6 mm diam, velvety, pale yellow to white. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6–7 × 4 μm.
Specimens examined. Japan, Gifu, Mt Kiso-ontake, on dead branches of Tsuga sieboldii, 25 Oct. 2006, T. Osono, TNS-F-21071 (HNo.570), culture MAFF241193; Nara, Mt Kasuga, on dead branches of Abies firma, 23 Apr. 2006, T. Shirouzu, TNS-F-15730 (HNo.228).
Notes — Dacrymyces subalpinus is characterised by hyphae without clamp connections and curved-naviculate, multi-transverse and vertically septate basidiospores. Although McNabb (1973) noted that the holotype was destroyed during the Second World War, he recognised this as a distinct species based on the descriptions by Kobayasi (1939a).
Dacrymyces subarcticus Shirouzu & Tokum., sp. nov. — MycoBank MB514039; Fig. 10f, g, 15i
Conidiomata sparsa, papilliformia vel pulvinata, sessilia, flavida, fragilia, 0.5–1 mm alta, 1–2 mm lata. Hyphae interaneae ramosae, tenuitunicatae, flavidae, 2–3 μm latae. Cellulae conidiogenae micronematicae, cylindricae, flavidae. Conidia holoblastica, irregulariter ramosa, cum ramis cylindricis 1–4-cellularibus 6–22.5 × 2–4 μm tenuitunicatis flavidis.
Etymology. Named after its subarctic habitat.
Conidiocarps scattered, pustulate to pulvinate, sessile, yellow, fragile, 0.5–1 mm high, 1–2 mm diam. Internal hyphae branched, thin-walled, septate, pale yellow, 2–3 μm diam, without clamp connections. Hymenium limited to the upper surface of the basidiocarp. Conidiogenous cells micronematous, cylindrical, pale yellow. Conidia holoblastic, irregularly branched, composed of cylindrical, 1–4-celled branches of 6–22.5 × 2–4 μm, with a separation scar at the base, thin-walled, pale yellow.
Culture characteristics — Colonies attaining about 6 mm diam, velvety, white to pale yellow. Conidiogenesis not observed.
Specimens examined. Japan, Gunma, Mt Motoshirane, on a dead trunk of an unknown conifer, 23 July 2007, T. Shirouzu & D. Hirose, TNS-F-21076 (HNo.722); Nara, Oodaigahara, on dead branches of Abies sp., 9 Oct. 2006, T. Shirouzu, holotype TNS-F-21067 (HNo.544).
Notes — Dacrymyces subarcticus is characterised by pulvinate conidiocarps and holoblastic, irregularly branched conidia. This anamorphic fungus has pulvinate conidiocarps and is similar to sporodochial anamorphs, such as D. stillatus, in the shape of conidiocarps. However, D. subarcticus differs from other sporodochial anamorphs of Dacrymyces in bearing holoblastic, irregularly branched conidia. We described this fungus as a new anamorphic species of Dacrymyces. In molecular phylogenetic analyses, D. subarcticus formed a monophyletic group with Dacryoscyphus chrysochilus (Fig. 16).
Dacrymyces unisporus (L.S. Olive) K. Wells, Mycologia 86: 31. 1994 — Fig. 11a–c, 15j
Fig. 11.
a–c. Dacrymyces unisporus TNS-F-15731 (HNo.332). a. Basidiocarps; b. basidiospores; c. basidia and probasidium. — d–h. Dacrymyces variisporus TNS-F-15733 (HNo.300). d. Basidiocarps; e. basidiospores; f. probasidium and basidium; g. dikaryophysis; h. marginal hyphae.
Basionym. Platygloea unispora L.S. Olive, J. Elisha Mitchell Sci. Soc. 60: 17. 1944.
Basidiocarps scattered, pustulate or pulvinate, sometimes centrally depressed, sessile, olive-orange to yellow-orange, firm-gelatinous, 0.5 mm high, 0.5–1 mm diam. Sterile parts of basidiocarps covered with simple or branched, cylindrical, septate, hyaline, thin-walled, marginal hyphae. Internal hyphae branched, thick-walled, septate, hyaline, 3–5 μm diam, with clamp connections. Hymenium limited to the superior surface of the basidiocarp. Basidia cylindrical to clavate, tapering toward the apex bearing a sterigma, sub-hyaline, 40–58 × 4–7 μm, with a basal clamp connection. Basidiospores globose to subglobose, with an apiculum at the base, thin-walled, sub-hyaline, 11–17.5 × 8.5–13 μm (av. 14 × 11.5 μm; n = 20), 2–4-celled, germinated by germ tubes.
Culture characteristics — Colonies slow growing, attaining about 2 mm diam, lanose, white. Conidiogenesis not observed.
Specimen examined. Japan, Nagano, Sugadairakougen, on dead branches of Pinus densiflora, 16 June 2006, T. Shirouzu, TNS-F-15731 (HNo.332), culture MAFF240146.
Notes — The most remarkable morphological feature of Dacrymyces unisporus is a simple, not bifurcate, cylindrical basidium producing a single, globose basidiospore. This species was initially described as a species of Platygloea (Olive 1944). In 1994, Wells transferred this fungus to the genus Dacrymyces based on the fact that “very often the epibasidium and sterigma arise eccentirically suggesting a derivation from the furcated, dacrymycetoid basidium” (Wells 1994). This is the first record from Japan.
Dacrymyces variisporus McNabb, New Zealand J. Bot. 11: 504. 1973 — Fig. 11d–h, 15k
Basidiocarps scattered or gregarious, sometimes coalesced, turbinate, pulvinate or discoid, centrally depressed, sessile or sub-stipitate, orange, firm-gelatinous, 0.5–1 mm high, 0.5–1.5 mm diam. Sterile parts of basidiocarps covered with cylindrical-clavate or narrowly naviculate, simple or branched, straight or flexuous, conspicuously thick-walled, hyaline terminal cells, 17.5–32 × 5–10 μm. Internal hyphae branched, thin- or thick-walled, septate, hyaline, 2–4.5 μm diam, with clamp connections. Hymenium limited to the superior surface of the basidiocarp. Probasidia cylindrical to clavate, yellow, 44.5–62.5 × 4.5–7.5 μm, with a basal clamp connection, becoming bifurcate. Dikaryophyses cylindrical, simple, septate, pale yellow, 30–50 × 3 μm. Basidiospores cylindrical to curved-cylindrical, with an apiculum at the base, thick-walled, yellow, 18–25 × 8–10.5 μm (av. 22 × 9 μm; n = 20), 1–7-transverse septate, occasionally with 1–4-vertical septa, germination via the production of conidia and germ tubes.
Culture characteristics — Colonies attaining about 4 mm diam, velvety, sulcate, yellow-orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of Pinus densiflora, 30 June 2006, T. Shirouzu, TNS-F-15734 (HNo.352); Nagano, Sugadairakougen, on dead branches of P. densiflora, 21 May 2006, T. Shirouzu, TNS-F-15733 (HNo.300), culture MAFF240148; Nara, Mt Tamaki, on dead branches of P. densiflora, 27 Apr. 2006, T. Shirouzu, TNS-F-15732 (HNo.263).
Notes — Dacrymyces variisporus is characterised by relatively small, turbinate to pulvinate basidiocarps, hyphae with clamp connections, cylindrical to clavate, thick-walled terminal cells, and thick-walled, multi-septate basidiospores. All our materials were collected from woody substrata of conifers. This is the first record from Japan.
Dacryopinax spathularia (Schwein.) G.W. Martin, Lloydia 11: 116. 1948. — Fig. 12a–e, 15l
Fig. 12.
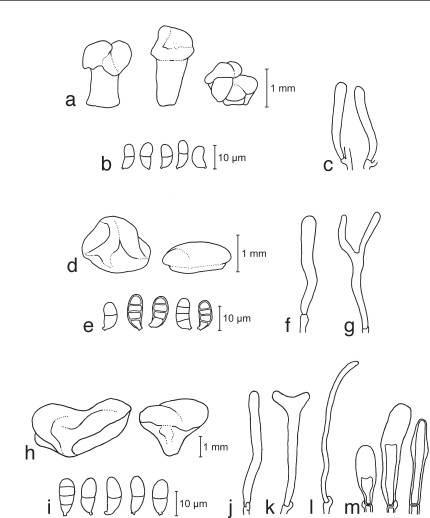
a–e. Dacryopinax spathularia TNS-F-15735 (HNo.379). a. Basidiocarps; b. basidiospores; c. probasidium; d. basidium; e. marginal hyphae. — f–j. Dacryopinax sphenocarpa TNS-F-21046 (HNo.356). f. Basidiocarps; g. basidiospores; h. probasidium; i. basidium; j. marginal hyphae.
Basionym. Merulius spathularia Schwein., Schr. Naturf. Ges. Leipzig 1: 97. 1882.
For other synonyms see McNabb (1965b).
Basidiocarps scattered or gregarious, spathulate, stipitate bearing sinuate flabellate to petaloid pileus, orange, white-yellow at the sterile surface of basidiocarps, soft-cartilaginous, 5–13 mm high, 1–2 mm diam at stipe, 3–7 mm diam at pileus. Sterile parts of basidiocarps covered with solitary or fasciculate, cylindrical, simple or branched, straight or flexuous, septate, thick-walled, pale yellow marginal hypha of 30–140 × 5–6 μm. Internal hyphae branched, thin-walled, septate, pale yellow, 2–5 μm diam, without clamp connections. Hymenium unilateral. Probasidia cylindrical to clavate, pale yellow, 23.5–34 × 3.5–7.5 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, pale yellow, 9–13.5 × 3.5–7.5 μm (av. 10.5 × 5 μm; n = 20), 0–1-septate, germinated by germ tubes.
Culture characteristics — Colonies attaining about 10 mm diam, velvety, yellow to white. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 5 × 3 μm.
Specimens examined. Japan, Kyoto, Midorogaike, on a bench made from an unknown conifer, 1 July 2006, T. Shirouzu, TNS-F-15735 (HNo.379), culture MAFF240150; Nara, Kasuga Shrine, on a handrail made from an unknown conifer, 3 July 2006, T. Shirouzu, TNS-F-15736 (HNo.398); Wakayama, Mt Nachi, on a fallen tree of an unknown palm, 6 July 2006, T. Shirouzu, TNS-F-21048 (HNo.367).
Notes — Dacryopinax spathularia is characterised by spathulate to stipitate basidiocarps with a flabellate pileus, thick-walled marginal hypha, and 0–1-septate basidiospores. This species has been reported from Japan by Kobayasi (1939b) as Guepinia spathularia.
Dacryopinax sphenocarpa Shirouzu & Tokum., sp. nov. — MycoBank MB514040; Fig. 12f–j, 15m, n
Basidiocarpia sparsa vel gregaria, spathulata vel subulata, albo-flavida vel sub-succinea, cartilaginea, 2–6 mm alta, 0.5–1 mm lata. Pili corticales steriles solitarii vel fasciculati, cylindrici vel clavati, tenuitunicati vel crassitunicati, sub-hyalini, 15–25 × 3.5–6 μm. Hyphae interaneae ramosae, tenuitunicatae, hyalinae, 2–4 μm latae, cum colligationibus unciformibus. Probasidia cylindrica vel clavata, hyalina, 15–34 × 3.5–8 μm, bifurcatascentia. Basidiosporae subglobosae vel reniformae, tenuitunicatae, hyalinae, 10–16 × 4.5–8.5 μm, 0–1-septatae.
Etymology. Named after its subulate basidiocarps.
Basidiocarps scattered or gregarious, spathulate to subulate, sometimes slightly branched at apex, yellow-white to pale amber, sometimes with simple root, soft-cartilaginous, 2–6 mm high, 0.5–1 mm diam. Sterile parts of basidiocarps covered with solitary or fasciculate, cylindrical to clavate, simple or branched, straight or flexuous, septate, thin- or thick-walled, sub-hyaline marginal hypha, 15–25 × 3.5–6 μm. Internal hyphae branched, thin-walled, septate, hyaline, 2–4 μm diam, with clamp connections. Hymenium unilateral, sometimes amphigenous. Probasidia cylindrical to clavate, hyaline, 15–34 × 3.5–8 μm, with a basal clamp connection, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, hyaline, 10–16 × 4.5–8.5 μm (av. 12.5 × 6 μm; n = 20), 0–1-septate, germination via germ tubes.
Culture characteristics — Colonies attaining about 6 mm diam, wet, yellow to orange. Conidiogenesis not observed.
Specimens examined. Japan, Kyoto, Mt Daimonji, on dead branches of an unknown conifer, 30 June 2006, T. Shirouzu, holotype TNS-F-21046 (HNo.356), culture ex-type MAFF241173; Mt Kiyomizu, on dead branches of Pinus densiflora, 7 Oct. 2006, T. Shirouzu, TNS-F-21066 (HNo.534); Nara, Mt Tamaki, on dead branches of P. densiflora, 7 July 2006, T. Shirouzu, TNS-F-21059 (HNo.430); Wakayama, Mt Nachi, on dead branches of Cryptomeria japonica, 6 July 2006, T. Shirouzu, TNS-F-21047 (HNo.364); 11 Oct. 2006, T. Shirouzu, TNS-F-21068 (HNo.552).
Notes — Dacryopinax sphenocarpa is characterised by sharp, spathulate basidiocarps, hyphae with clamp connections, solitary or fasciculate, thin- or thick-walled marginal hypha, and thin-walled, 0–1-septate basidiospores. This dacrymycetous fungus belongs to the genus Dacryopinax because of its basidiocarp morphology, having a unilateral hymenium and fasciculate, thick-walled marginal hypha. Only D. taibaishanensis is known as a species having hyphae with clamp connections in this genus (Liu & Fan 1990). Dacryopinax sphenocarpa is different from D. taibaishanensis in the shape of basidiocarps (D. taibaishanensis is discoid) and in the size (20–35 × 7–10 μm) and septal number (5–7-septate) of basidiospores. In molecular phylogenetic analyses, this species did not cluster in a monophyletic clade with another species of Dacryopinax, D. spathularia, but represented a monophyletic group with Dacrymyces ancyleus (Fig. 16).
Femsjonia peziziformis (Lév.) P. Karst., Bidrag Kannedam Finlands Natur Folk 31: 352. 1876 — Fig. 13a–f, 15o
Fig. 13.
a–f. Femsjonia peziziformis TNS-F-15737 (HNo.439). a. Basidiocarps; b. basidiospores; c. probasidium; d. developing basidium; e. dikaryophysis; f. marginal hyphae. — g–k. Guepiniopsis buccina TNS-F-15738 (HNo.562). g. Basidiocarps; h. basidiospores; i. probasidium; j. developing basidium; k. marginal hyphae.
Basionym. Exidia peziziformis Lév., Ann. Sci. Nat., Bot. 9: 127. 1848.
For other synonyms see McNabb (1965e).
Basidiocarps scattered or gregarious, turbinate to discoid, centrally depressed, sessile, yellow at hymenium, white rough tomentose at the sterile surface of basidiocarps, firm-gelatinous, 3–10 mm high, 5–10 mm diam. Sterile parts of basidiocarps densely covered with cylindrical to clavate, straight or flexuous, conspicuously thick-walled, hyaline to pale yellow terminal cells, 40–80 μm long, 4–7.5 μm diam. Internal hyphae branched, thin-walled, septate, hyaline, 2–5 μm diam, with clamp connections. Hymenium limited to the superior surface of the basidiocarp. Probasidia cylindrical to clavate, pale orange, 40–80 × 5.5–10.5 μm, with a basal clamp connection, becoming bifurcate. Dikaryophyses cylindrical, simple, septate, pale orange, 48–80 × 3–4 μm. Basidiospores cylindrical to curved-cylindrical, with an apiculum at the base, thin-walled, pale orange, 22–36 × 8.5–13 μm (av. 26.5 × 9.5 μm; n = 20), 1–13-septate, germinated by production of conidia and germ tubes.
Culture characteristics — Colonies attaining about 10 mm diam, wet, white-orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 6 × 3 μm.
Specimen examined. Japan, Nagano, Sugadairakougen, on dead branches of Pinus densiflora, 27 Sept. 2006, T. Shirouzu & T. Hosoya, TNS-F-15737 (HNo.439), culture MAFF240152.
Notes — Femsjonia peziziformis is characterised by turbinate to discoid basidiocarps that are tomentose in the sterile part, with cylindrical, thick-walled terminal cells, and multi-septate basidiospores. Reid (1974) treated this genus as a synonym of Ditiola peziziformis (as D. pezizaeformis). In this study, we classified this fungus in Femsjonia according to the opinion of McNabb (1965e). This species has been recorded from Japan by Kobayasi (1939b) as F. luteoalba, a synonym of F. peziziformis.
Guepiniopsis buccina (Pers.) L.L. Kenn., Mycologia 50: 888. 1958 — Fig. 13g–k, 15p
Basionym. Peziza buccina Pers., Syn. Meth. Fung. 2: 659. 1801.
For additional synonyms see Reid (1974).
Basidiocarps scattered, stipitate bearing a cupulate pileus, pale orange, firm-gelatinous, 4–5 mm high, 1.5–3 mm diam at the stipe, 2–3 mm high, 3–4 mm diam at the pileus. Sterile parts of basidiocarps densely covered with simple, cylindrical to clavate, septate, 2–3-celled, hyaline, conspicuously thick-walled terminal cells, 35–50 × 8–14.5 μm. Internal hyphae branched, thin-walled, gelatinous, septate, hyaline, 2–5 μm diam, without clamp connections. Hymenium limited in the cup of the pileus. Probasidia cylindrical, sub-hyaline, 31.5–55 × 3.5–8 μm, becoming bifurcate. Basidiospores subglobose to reniform, with an apiculum at the base, thin-walled, sub-hyaline, 10–16 × 5–7 μm (av. 13 × 6 μm; n = 10), 0–3-septate, germinated by germ tubes.
Culture characteristics — Colonies attaining about 5 mm diam, velvety, yellow to orange. Conidiogenous cells on vegetative hyphae, polyblastic, sympodial. Conidia subglobose, 4 × 3 μm.
Specimen examined. Japan, Nara, Mt Tamaki, on dead branches of an unknown broad-leaved tree, 13 Oct. 2006, T. Shirouzu, TNS-F-15738 (HNo.562), culture MAFF240153.
Notes — Guepiniopsis buccina is characterised by stipitate basidiocarps with cupulate pilei, thick-walled terminal cells, and 0–3-septate basidiospores. This species has been recorded from Japan as G. melurinus (Kobayasi 1939a).
Molecular phylogenetic analysis
The molecular phylogenetic tree using D1/D2 sequences is shown in Fig. 16. Four clades in Fig. 16 were defined as Dacrymycetes, Dacrymycetales, Cerinomycetaceae and Dacrymycetaceae, of which the Dacrymycetes, Dacrymycetales and Cerinomycetaceae clades were well supported (BS value (MP/ML) = 84/95 %, 90/93 %, 100/100 %, respectively). However, the Dacrymycetaceae clade (BS value (MP/ML) = 68/55 %) and other relatively higher-level nodes, were not well supported. At the species level, several clades such as the Guepiniopsis buccina, Calocera viscosa and Dacrymyces punctiformis clades were well supported (BS values = 100 %).
Species of the genus Dacrymyces were dispersed widely over the Dacrymycetes lineage. Dacrymyces stillatus and D. minor formed a clade with Guepiniopsis buccina (BS value (MP/ML) = 75/64 %), and Dacrymyces punctiformis formed a well-supported monophyletic group with Cerinomyces species (BS value (MP/ML) = 100/100 %).
Dacrymyces stillatus and D. minor were paraphyletic. Morphologically similar species, D. aureosporus and D. chrysospermus, represented a monophyletic group, but they were distributed in different sub-clades (BS value (MP/ML) = 74/69 %, 62/<50 %, respectively). Dacrymyces unisporus was located in the Dacrymycetes clade. Dacrymyces subarcticus formed a monophyletic group with Dacryoschyphus chrysochilus (BS value (MP/ML) = 100/100 %). Two Dacryopinax species, D. shenocarpus and D. spathularia, did not form a monophyletic group.
DISCUSSION
Japanese Dacrymycetes
In this study we recognised 28 species, including 5 new species and 9 species that were newly recorded from Japan (Table 3). Based on our survey, the total number of dacrymycetous species recorded from Japan increased from 28 to 42, although we were unable to recollect all species recorded by Kobayasi (1939a, b). This result suggests that the species diversity of dacrymycetous fungi in Japan is richer than previously thought.
The basidiocarps of Dacrymycetes could be widely collected throughout the Japanese Islands, from Hokkaido (subarctic coniferous forests) to the Yaeyama Islands (subtropical evergreen forests). However, dacrymycetous species and samples were more commonly collected from coniferous and deciduous broad-leaved forests in the subarctic and cool-temperate zone, than in the evergreen, broad-leaved forests in the warm-temperate and subtropical zone. The main diversity of Dacrymycetes may thus be from the cool-temperate to the subarctic zone.
To date, anamorphic stages with conidiocarps are known in Dacrymyces lacrymalis (present study), D. capitatus, D. minor and D. stillatus, (Oberwinkler 1993). A unique coelomycetous species, Dacryoscyphus chrysochilus (Kirschner & Yang 2005), and a hyphomycetous species, Cerinosterus luteoalbus, have also been reported (Moore 1987, Middelhoven et al. 2000). Among the newly described Dacrymyces species in this study, D. pianacearum and D. subarcticus were anamorphic species. In their fruit bodies (conidiocarps), only conidia were observed, and their holomorphs remain to be discovered. They also had various shapes of conidiocarps (flabellate and pustulate to pulvinate) and conidia (dendroid and irregularly branched). These new Japanese coelomycetous species suggest an unexpected high diversity of anamorphic stages in Dacrymycetes.
Some specimens agreed with the original description of D. subalpinus (Kobayasi 1939a). It had been thought that the holotype of this species was destroyed in the past (McNabb 1973). In this study, we reconfirmed the existence of D. subalpinus, and were able to recollected and deposit fresh specimens; however, many holotypes of other Japanese dacrymycetous species described by Kobayasi (1939a, b) have still not been found. During this study, specimens corresponding to such problematic species could not be recollected and examined (Table 1, 3), and hence their taxonomical validity remains uncertain. To confirm these doubtful species, future investigations of unlabelled dacrymycetous specimens deposited in TNS may provide useful additional information. It is suspected that these specimens may include the lost holotypes of Dacrymycetes species previously described from Japan.
Molecular phylogenetic analysis
One higher node in the molecular phylogenetic tree, the Dacrymycetaceae clade, was not well supported, and other higher phylogenetic relationships in this clade could not be resolved (Fig. 16). To define these unclear phylogenetic relationships, it may be necessary to perform additional analyses using more dacrymycetous DNA sequences. Phylogenetic analyses using multi-gene sequences, which succeeded in revealing more detailed relationships in fungal lineages (e.g. James et al. 2006), may resolve the higher phylogenetic relationships in Dacrymycetes.
A cushion-shaped Dacrymycetaceae species, Dacrymyces punctiformis, was included in the Cerinomycetaceae clade (Fig. 16). In addition, an exceptional species having non-bifurcate basidia, D. unisporus, was located basally in the Dacrymycetales clade (Fig. 16). These results suggest that a reconsideration of not only the taxonomical circumscriptions of genera, but also that of families and orders is required in this class. To solve these taxonomic problems, we may have to search for other informative criteria that correlate with the higher classification.
The molecular phylogenetic analysis of the present study in most cases supported the validity of the species identifications (Fig. 16), and also revealed the polyphyletic arrangement of Dacrymyces and Dacryopinax species throughout the Dacrymycetes lineage (Fig. 16). The classification of dacrymycetous genera is mainly based on the external morphology of the basidiocarp, and the wall thickness of marginal hyphae (McNabb & Talbot 1973), but our results underline the difficulty of using these criteria as primary characters for generic classification. Taxonomic rearrangement of dacrymycetous genera is thus necessary (Shirouzu et al. 2007). At this time, however, we have no phenotypic clues to rearrange dacrymycetous genera; therefore, it is appropriate that the newly described species such as Dacrymyces ancyleus and Dacryopinax sphenocarpa belong to genera ‘Dacrymyces’ and ‘Dacryopinax’ according to the existing taxonomic framework. Presently we are working on phylogenetic analyses that incorporate more taxa and DNA sequences, with the eventual aim to present a new interpretation to the remaining taxonomic problems.
Acknowledgments
We wish to thank Dr Takashi Osono of Kyoto University, Dr Tsuyoshi Hosoya of the Natural Museum of Nature and Science, and Dr Yukiko Takahashi of The University of Tokyo for their support during field trips. We also thank Prof. Susumu Takamatsu of Mie University, and Dr Keiichi Motohashi of Gifu University for their guidance and valuable advice on the molecular phylogenetic study, and Dr Kazuaki Tanaka of Hirosaki University for his revision of the Latin descriptions. We want to thank Prof. Pedro Crous, Prof. Dominik Begerow and two reviewers for their constructive comments on this manuscript. We acknowledge the Institute for Fermentation, Osaka (IFO) and the Fujiwara Natural History Foundation for their financial support.
REFERENCES
- Boidin J, Gilles G. 1986. Basidiomycètes Aphyllophorales de l’île de la Réunion. VI. Le genere Cerinomyces Martin. Bulletin trimestriel de la Société mycologique de France 102: 315 – 319 [Google Scholar]
- Burdsall HH, Laursen GA. 2004. Fungi of New Zealand’s subarctic islands I: Two new species of Dacrymyces (Basidiomycota). In: Miller OK, Cripps CL. (eds), Fungi in forest ecosystems: systematics, diversity and ecology: 107–111 New York Botanical Garden, USA: [Google Scholar]
- Doweld A. 2001. Prosyllabus tracheophytorum: Tentamen systematis plantarum vascularium (Tracheophyta) Geos, USSR: [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783 – 791 [DOI] [PubMed] [Google Scholar]
- Hennings P. 1898. Dacrymycetineae, Exobasidiineae, Hymenomycetineae. In: Engler A, Prantl K. (eds), Die Natürlichen Pflanzenfamilien I Teil. Abteilung 1: 96–102 Engelmann, Germany: [Google Scholar]
- Hibbett DS. 2006. A phylogenetic overview of the Agaricomycotina. Mycologia 98: 917 – 925 [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509 – 547 [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, et al. 2006. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443: 818 – 822 [DOI] [PubMed] [Google Scholar]
- Jülich W. 1981. Higher taxa of Basidiomycetes. Bibliotheca Mycologica 85: 1 – 845 [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT v5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511 – 518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. 2001. Dictionary of the Fungi 9th ed.CABI Publishing, UK: [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Dictionary of the Fungi 10th ed.CABI Europe, UK: [Google Scholar]
- Kirschner R, Yang LZ. 2005. Dacryoscyphus chrysochilus, a new staurosporous anamorph with cupulate conidiomata from China and with affinities to the Dacrymycetales (Basidiomycota). Antonie van Leewenhoek 87: 329 – 337 [DOI] [PubMed] [Google Scholar]
- Kobayasi Y. 1939a. On the Dacrymyces-group (Fungorum ordinis Tremellalium studia monographica III). Science reports of the Tokyo Bunrika Daigaku, Sect. B, 4: 105 – 128 [Google Scholar]
- Kobayasi Y. 1939b. On the genera Femsjonia, Guepinia and Calocera from Japan (Fungorum ordinis Tremellalium studia monographica IV). Science reports of the Tokyo Bunrika Daigaku, Sect. B, 4: 215 – 227 [Google Scholar]
- Kobayasi Y. 1954. Monographic studies of Japanese Tremellaceous fungi VI. Nagaoa 4: 36 – 47 [Google Scholar]
- Kobayasi Y. 1984. Miscellaneous notes of fungi (5). The Journal of Japanese Botany 59: 187 – 189 [Google Scholar]
- Liu B, Fan L. 1990. New species and new variety of Dacrymycetaceae in China. Acta Mycologica Sinica 9: 12 – 19 [Google Scholar]
- Maekawa N. 1987. A new species of the genus Cerinomyces. Canadian Journal of Botany 65: 538 – 588 [Google Scholar]
- Matsuda Y, Hijii N. 1999. Characterization and identification of Strobilomyces confusus ectomycorrhizas on momi fir by RFLP analysis of the PCR-amplified ITS region of the rDNA. Journal of Forest Research 4: 145 – 150 [Google Scholar]
- McNabb RFR. 1964. Taxonomic studies in the Dacrymycetaceae I. Cerinomyces Martin. New Zealand Journal of Botany 2: 415 – 424 [Google Scholar]
- McNabb RFR. 1965a. Taxonomic studies in the Dacrymycetaceae II. Calocera (Fries) Fries. New Zealand Journal of Botany 3: 31 – 58 [Google Scholar]
- McNabb RFR. 1965b. Taxonomic studies in the Dacrymycetaceae III. Dacryopinax Martin. New Zealand Journal of Botany 3: 59 – 72 [Google Scholar]
- McNabb RFR. 1965c. Taxonomic studies in the Dacrymycetaceae IV. Guepiniopsis Patouillard. New Zealand Journal of Botany 3: 159 – 169 [Google Scholar]
- McNabb RFR. 1965d. Taxonomic studies in the Dacrymycetaceae V. Heterotextus Lloyd. New Zealand Journal of Botany 3: 215 – 222 [Google Scholar]
- McNabb RFR. 1965e. Taxonomic studies in the Dacrymycetaceae VI. Femsjonia Fries. New Zealand Journal of Botany 3: 223 – 228 [Google Scholar]
- McNabb RFR. 1966. Taxonomic studies in the Dacrymycetaceae VII. Ditiola Fries. New Zealand Journal of Botany 4: 546 – 558 [Google Scholar]
- McNabb RFR. 1973. Taxonomic studies in the Dacrymycetaceae VIII. Dacrymyces Nees ex Fries. New Zealand Journal of Botany 11: 461 – 524 [Google Scholar]
- McNabb RFR, Talbot PHB. 1973. Holobasidiomycetidae: Exobasidiales, Brachybasidiales, Dacrymycetales. In: Ainsworth GC, Sparrow FK, Sussman AS. The fungi Vol IV B: 317 – 325 Academic Press, USA: [Google Scholar]
- Middelhoven WJ, Gueho E, Hoog GS de. 2000. Phylogenetic position and physiology of Cerinosterus cyanescens. Antonie van Leeuwenhoek 77: 313 – 320 [DOI] [PubMed] [Google Scholar]
- Moore RT. 1987. Micromorphology of yeasts and yeast-like fungi and its taxonomic implications. Studies in Mycology 30: 203 – 226 [Google Scholar]
- Nannfeldt JA. 1947. Sphaenonaema rufum Fr., a misunderstood member of the Dacrymycetaceae. Svensk Botanisk Tidskrift 11: 301 – 338 [Google Scholar]
- O’Donnell K. 1993. Fusarium and its near relatives. In: Reynolds DR, Taylor JW. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics: 225–233 CAB International, UK: [Google Scholar]
- Oberwinkler F. 1993. Genera in a monophyletic group: the Dacrymycetales. Mycologia Helvetica 6: 35 – 72 [Google Scholar]
- Oberwinkler F, Tschen J. 1989. A new Dacrymyces species from Taiwan. Transactions of the Mycological Society of Japan 30: 349 – 356 [Google Scholar]
- Olive LS. 1944. New or rare heterobasidiomycetes from North Carolina-I. The Journal of the Elisha Mitchell Scientific Society 60: 17 – 26 [Google Scholar]
- Peterson SW. 2000. Phylogenetic analysis of Penicillium species based on ITS and lsu-rDNA nucleotide sequences. In: Samson RA, Pitt JI. (eds), Integration of modern taxonomic methods for Penicillium and Aspergillus classification: 163–178 Harwood, The Netherlands: [Google Scholar]
- Reid DA. 1974. A monograph of the British Dacrymycetales. Transactions of the British Mycological Society 62: 433 – 494 [Google Scholar]
- Schröter J. 1889. Die Pilze Schlesiens Vol. 3 J.U. Kern’s Verlag, Germany: [Google Scholar]
- Shirouzu T, Hirose D, Tokumasu S. 2007. Sequence analyses of 28S rRNA gene D1/D2 region suggests Dacrymyces (Heterobasidiomycetes, Dacrymycetales) is polyphyletic. Mycoscience 48: 388 – 394 [Google Scholar]
- Swofford DL. 2001. PAUP Phylogenetic analysis using parsimony (and other methods), v4.0b10 Sinauer Associates, UK: [Google Scholar]
- Tubaki K, Hosoya T. 1987. Cultural studies of Dacrymycetales. Tsukuba Environmental Studies 10: 53 – 59 [In Japanese] [Google Scholar]
- Weiss M, Oberwinkler F. 2001. Phylogenetic relationships in Auriculariales and related groups – hypotheses derived from nuclear ribosomal DNA sequences. Mycological Research 105: 403 – 415 [Google Scholar]
- Wells K. 1994. Jelly fungi, then and now! Mycologia 86: 18 – 48 [Google Scholar]