Abstract
Despite its early discovery and high sequence homology to the other VEGF family members, the biological function of VEGF-B remained debatable for a long time, and VEGF-B has received little attention from the field thus far. Recently, we and others have found that (1) VEGF-B is a potent survival factor for different types of cells by inhibiting apoptosis via suppressing the expression of BH3-only protein and other apoptotic/cell death-related genes. (2) VEGF-B has a negligible role in inducing blood vessel growth in most organs. Instead, it is critically required for blood vessel survival. VEGF-B targeting inhibited pathological angiogenesis by abolishing blood vessel survival in different animal models. (3) Using different types of neuro-injury and neurodegenerative disease models, VEGF-B treatment protected endangered neurons from apoptosis without inducing undesired blood vessel growth or permeability. Thus, VEGF-B is the first member of the VEGF family that has a potent survival/anti-apoptotic effect, while lacking a general angiogenic activity. Our work thus advocates that the major function of VEGF-B is to act as a “survival,” rather than an “angiogenic” factor and implicates a therapeutic potential of VEGF-B in treating different types of vascular and neurodegenerative diseases.
Key words: VEGF-B, survival factor, angiogenesis, apoptosis, vascular biology
Introduction
More than a decade ago, when vascular endothelial growth factor B (VEGF-B) was first discovered,1,2 we were excited. Who would not be excited? VEGF-A was then, and still is, in the spotlight of biomedical research. Loss of even one allele of VEGF-A caused early embryonic lethality in mice.3,4 VEGF-B, as a newly discovered VEGF-A homolog at that time,1,2 might prove to be equally, if not more, important, we expected. However, the subsequent journey to reveal the biological function of VEGF-B had been largely full of disappointments. As a VEGF-A homolog, VEGF-B failed to produce most of the functionality of VEGF-A, for example, inducing new blood vessel growth and vascular permeability, etc. Further, unlike VEGF-A, loss of VEGF-B in mice did not seem to matter greatly, since VEGF-B-null mice appeared largely healthy.5–8Years of work investigating the biological function of VEGF-B had mostly led to negative findings. Is VEGF-B a redundant molecule? This was suspected. With the recent advances in VEGF-B research,9–15 perhaps now is the time to answer that question with greater confidence. And the answer is no.
The VEGF family includes five structurally related ligands that bind differentially to three receptor tyrosine kinases (VEGFR-1, 2 and 3) and the semaphorin receptors neuropilin (NP)-1 and -2. Among the five VEGF family members, VEGF-A is considered to be the prototype angiogenic factor with a potent and “universal” angiogenic effect under most physiological and pathological conditions.16–18 The placenta growth factor (PlGF) is believed to play important roles in pathological angiogenesis.19 However, when PlGF-1 is produced in the same population of cells with VEGF-A, it can act as a natural antagonist of VEGF-A.20,21 VEGF-C and VEGF-D are mainly involved in lymphangiogenesis.22,23 VEGF-B shares a high degree of sequence homology to VEGF-A and PlGF. Like VEGF-A and PlGF, VEGF-B binds to the tyrosine kinase VEGF receptor-1 (VEGFR-1) and its co-receptor neuropilin-1.1,24 Even though VEGF-B is highly expressed in most tissues and organs,1,25,26 VEGF-B under most conditions appeared to be “redundant” or “inert” without an obvious function. The in vivo role of VEGF-B therefore remained elusive for a long time. This review summarizes the recent advances on VEGF-B studies, with particular interest on its survival versus angiogenic effects.9–15
Is VEGF-B an Angiogenic Factor?
The angiogenic activity of VEGF-B has been a debatable issue for a long time. Because of its high sequence homology and similar receptor binding patterns to VEGF-A,27,28 VEGF-B was naturally considered as an angiogenic factor. However, studies along this line have only led to inconsistent results. VEGF-B was reported to be angiogenic in some studies,29–32 but lack of an angiogenic activity in others.5,7,33,34 Below is an overview of the reports with regard to the angiogenic activity of VEGF-B.
VEGF-B deficiency does not affect angiogenesis in most organs.
VEGF-A or VEGF-C deficiency caused embryonic lethality in mice.3,4,35 VEGF-B deficient mice, however, are largely healthy with regard to embryonic development, lifespan, fertility, angiogenesis, etc.5–8 PlGF deficient mice display impaired pathological angiogenesis.19,36 VEGF-B deficiency, however, does not affect pathological angiogenesis in most organs studied, such as wounded skin, hypoxic lung, ischemic retina or ischemic limb.10 Even though one study reported a role of VEGF-B in pathological (inflammatory) angiogenesis using arthritis models,37 we did not see such an effect in our study thus far (unpublished observation). In contrast to VEGF-A and PlGF, VEGF-B is not required for neovessel formation in proliferative retinopathy7 or blood vessel remodeling in pulmonary hypertension.8 Taken together, genetic deletion of VEGF-B in mice showed that VEGF-B, at least under most conditions studied thus far, has a minimum role in angiogenesis in most organs tested in development, normal physiology or under pathological conditions.
VEGF-B transgenic expression in different organs induces no or minimum angiogenesis.
Transgenic expression of all the other VEGF family members, such as VEGF-A,38–40 PlGF,41 VEGF-C,42 VEGF-D43 or VEGF-E,44 induced either angiogenesis or lymphangiogenesis. VEGF-B is the only member of the VEGF family, transgenic overexpression of which in different organs did not induce angiogenesis or lymphangiogenesis.14,31 VEGF-B over-expression in cardiac myocytes under the alpha-myosin heavy chain promoter did not induce angiogenesis in the heart.14 Instead, blood vessel density was decreased in the hearts overexpressing VEGF-B.14 Furthermore, VEGF-B transgenic expression in endothelial cells under Tie2 promoter did not induce angiogenesis in different types of organs (liver, heart, kidney, etc).31 Moreover, VEGF-B transgenic expression in the skin under keratin-14 promoter led to only marginal angiogenesis.14 Thus, VEGF-B transgenic expression in different types of organs did not induce angiogenesis, or only led to minimum angiogenesis, in contrast to the other VEGF family members.
VEGF-B gene or protein delivery does not induce angiogenesis in most organs and under most conditions.
Several studies have shown that VEGF-B gene or protein transfer into different types of organs did not induce angiogenesis under most conditions. Adenoviral gene transfer of the other VEGF family members, such as VEGF-A, VEGF-C and VEGF-D, into rabbit hindlimb skeletal muscles induced strong angiogenesis, vascular permeability, or lymphangiogenesis.33 VEGF-B adenoviral gene transfer, however, did not induce angiogenesis or lymphangiogenesis in this model.33 Similarly, adenoviral gene transfer of VEGF-A and VEGF-D to adventitia induced robust adventitial angiogenesis, whereas VEGF-B adenoviral gene transfer failed to do so.34,45 Another study also showed that VEGF-B167 gene delivery to the skin or ischemic limb did not induce blood vessel growth.10 Furthermore, VEGF-B167 recombinant protein injection into mouse eyes at a dose effective for retinal neuron survival did not induce ocular angiogenesis.9 Poesen K, et al. has also shown recently that VEGF-B186 recombinant protein intracerebroventricular injection did not cause blood vessel growth or blood-brain barrier leakiness.15 Thus, reports from different groups indicate that VEGF-B gene or protein delivery did not induce angiogenesis in most organs under most conditions studied.
VEGF-B does not induce blood vessel permeability.
It is known that all the other VEGF family members, VEGF-A,46 PlGF,36 VEGF-C,47 VEGF-D33 and VEGF-E48 induce blood vessel permeability. However, numerous studies using a variety of different tools and approaches, such as VEGF-B deficient mice, VEGF-B transgenic mice, recombinant proteins, or gene transfer, have shown that VEGF-B does not affect blood vessel permeability. VEGF-B deficient mice displayed no difference in blood vessel permeability as compared with wild-type mice.5,7 Transgenic expression of VEGF-B in endothelial cells, or application of VEGF-B protein to the skin of mice did not affect blood vascular permeability using Miles assay.31 Intradermal injection of VEGF-A165, VEGF-A121 and VEGF-C in mice ears increased vascular permeability, while VEGF-B administration had no such effect using a modified Miles assay.49 In addition, VEGF-B167 recombinant protein injection into mouse brain or eye did not induce blood vessel permeability.9 It is reported that in preserved lung grafts, VEGF-A and VEGF-C, but not VEGF-B mediate increased vascular permeability.50 Indeed, when overexpressed in the lung by adenoviral gene transfer, VEGF-B had no effect on blood vessel permeability.8 Adenoviruses expressing VEGF-A and VEGF-D delivered into rabbit hindlimb skeletal muscles induced vascular permeability using the modified Miles assay, while adenoviruses expressing VEGF-B did not affect blood vessel permeability in skeletal muscles in rabbit.33 Thus, data derived from different model systems showed that VEGF-B is the only member of the VEGF family that does not induce blood vessel permeability.
Cardiac-specific angiogenic activity of VEGF-B.
VEGF-B is most abundantly expressed in the heart.1,25 Therefore, even though VEGF-B gene or protein delivery did not induce angiogenesis in most of the other organs, we still studied the role of VEGF-B in cardiac ischemia by occlusion of the left coronary artery in mice. We found that VEGF-B deficiency led to decreased blood vessel density in ischemic myocardium in mice, even though no vascular defect was found in other organs studied, such as wounded skin, hypoxic lung, ischemic retina or limb.10 Further, VEGF-B167 protein or gene transfer increased blood vessel density in the infarct and ischemic border zone in mouse hearts. These data thus suggest that VEGF-B has a restricted role in the revascularization of ischemic myocardium.10,11 Indeed, this observation was also recently reported by another study demonstrating that VEGF-B186 gene transfer induced myocardium-specific angiogenesis and arteriogenesis in both pigs and rabbits.13 Adenoviral VEGF-B (AdVEGF-B186) gene transfer upregulated the expression of many antiapoptotic genes in cardiomyocytes.13 Yet another study further showed that AdVEGF-B167 gene transfer into murine hearts with myocardial infarction led to increased capillary density.51 Taken together, our and others work has shown that even though VEGF-B is dispensable for blood vessel growth in development, normal physiology and pathological conditions in most organs, VEGF-B has an angiogenic activity specifically in the heart.
However, one question remains unverified. It is thus far unclear whether the heart-specific angiogenic activity of VEGF-B is a direct “growth promoting” effect on vascular cells, or an indirect effect through its survival effect on different types of cells, including nonvascular cells, such as cardiac myocytes. Since VEGF-B upregulated the expression of many antiapoptotic genes in cardiac myocytes, and therefore has a direct survival effect on them,13 it is possible that the angiogenic activity of VEGF-B in the heart could be an indirect effect subsequent to the direct survival effect of VEGF-B on cardiac myocytes, which in turn may lead to an increased angiogenesis. Further studies are needed to verify this.
VEGF-B is a Survival, Rather than an Angiogenic Factor
In our recent work,12 using different animal models including cornea pocket assay, hind limb ischemia model, hyaloid blood vessel regression assay and oxygen-induced retinal blood vessel degeneration model, we demonstrated that VEGF-B is dispensable for blood vessel growth under most conditions. However, VEGF-B is critically required for blood vessel survival.12 In vivo, VEGF-B deficiency led to poorer blood vessel survival in the cornea after growth factor withdrawal, fewer surviving hyaloid vessels in postnatal mouse eyes, and greater oxygen-induced retinal vascular degeneration in neonatal mice.12 In vitro, VEGF-B deficient vascular cells displayed increased apoptosis when the cells were challenged by serum deprivation or oxidative stress.12 Moreover, VEGF-B protein treatment rescued blood vessel cells from apoptosis in vitro and led to better blood vessel survival in vivo.12
Our data thus advocates a conceptual change of the function of VEGF-B as a “survival” rather than an “angiogenic” factor (Fig. 1). This explains and reconciles, at least to a certain extent, the controversies on the “angiogenic” nature of VEGF-B in previous studies. That is—as a survival factor, VEGF-B has a negligible role in inducing blood vessel growth under most conditions. This explains why most of the time VEGF-B did not induce blood vessels growth after protein or gene delivery.5,7,9,33,34 On the other hand, under other conditions where blood vessels are challenged and need to struggle to survive, VEGF-B is then critically required and VEGF-B treatment can indeed lead to more (survived) blood vessels.9,29,30,32 Under such conditions, VEGF-B can appear to be “angiogenic.”10,29,30,32 However, this “angiogenic” effect of VEGF-B is most likely only due to its survival effect on both vascular and non-vascular cells (Figs. 1 and 3). Ongoing work in our laboratory demonstrated that VEGF-B is expressed by different types of vascular cells and VEGF-B expression level is upregulated in pathological conditions (unpublished data).
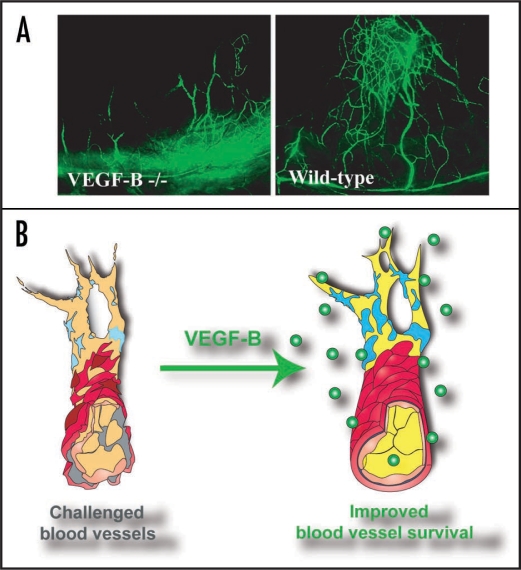
Figure 1.
VEGF-B is a vascular survival factor, rather than an angiogenic factor. (A) In a mouse cornea pocket assay, three weeks after removing the bFGF implants in the cornea, fewer bFGF-induced blood vessels (green) were left in VEGF-B deficient cornea, suggesting that VEGF-B is required for blood vessel survival. (B) Based on our and others recent work on VEGF-B, we advocate a conceptual change of the function of VEGF-B in the vascular system as a “survival,” rather than an “angiogenic” factor. As a survival factor, VEGF-B has a negligible role in inducing blood vessel growth in most organs. However, under conditions where blood vessels are stressed and need to struggle to survive (left), VEGF-B is then critically required, and VEGF-B treatment under such conditions can indeed lead to more (survived) blood vessels. Under such conditions, VEGF-B may appear to be “angiogenic,” but most likely only due to its vascular survival effect. Our ongoing work in our laboratory shows that VEGF-B is expressed by different types of vascular cells, and its expression is upregulated under stressed conditions (unpublished data).
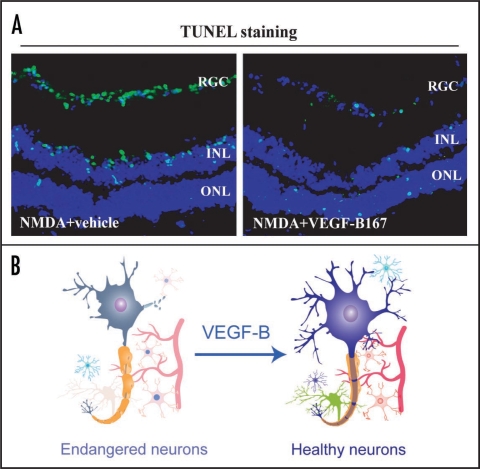
Figure 3.
VEGF-B is a survival factor for multiple types of neurons. (A) N-methyl-D-aspartic acid (NMDA) intravitreal injection led to massive apoptosis (green color) in different layers in the mouse retina as shown by the TUNEL staining (green color). VEGF-B167 protein treatment significantly reduced the number of apoptotic cells in all the three layers of the retina injured by NMDA. RGC: retinal ganglion cell, INL: inner nuclear layer, ONL: outer nuclear layer. (B) Ours and others data have shown that VEGF-B has a survival effect on different types of neurons. VEGF-B treatment inhibited apoptosis of brain cortex neurons and reduced stroke volume in a mouse stroke model. VEGF-B treatment increased survival of retinal ganglion cells in vivo. Thus, VEGF-B is a potent survival factor for different types of neurons and may have therapeutic implications in treating different types of neurodegenerative diseases.
The Survival Effect of VEGF-B is Pleiotropic
One important feature of the survival activity of VEGF-B is that it appears to be a general effect on many different types of cells, including three types of vascular cells, different types of neurons, and cardiac myocytes. VEGF-B is highly expressed in many different types of tissues.1,25,27 The receptors used by VEGF-B, VEGFR-1 and NP-1 are also expressed by various types of cells.52,53 Therefore, future work might reveal other unknown cellular targets of VEGF-B.
VEGF-B is a survival factor for different types of vascular cells.
We recently found that VEGF-B is a survival factor for multiple types of vascular cells, including vascular endothelial cells (EC), pericytes (PC) and smooth muscle cells (SMC) (Fig. 2).12 In vitro, using both isolated primary vascular cells and established vascular cell lines, VEGF-B167 protein treatment increased survival of not only ECs, but also that of PCs and SMCs as measured by TUNEL staining, when the cells were cultured in serum-free medium.12 On the contrary, VEGF-B shRNA treatment led to apoptosis in both ECs and PCs. Moreover, VEGF-B deficient ECs and SMCs isolated from VEGF-B deficient mice displayed increased apoptosis when cultured in serum-free medium or under H2O2-induced oxidative stress.12 Thus, both gain- and loss-of-function analysis showed that VEGF-B is required for the survival of multiple types of vascular cells (Fig. 2).
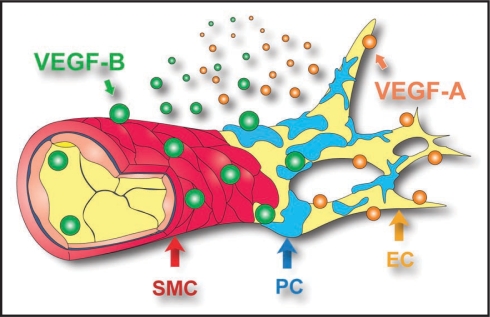
Figure 2.
Pleiotropic survival effect of VEGF-B on multiple types of vascular cells. VEGF-A (orange particles) targets mainly vascular endothelial cells (EC, yellow). VEGF-B (green particles), however, targets not only vascular ECs, but also pericytes (PC, blue) and smooth muscle cells (SMC, red). Since blood vessels covered by PCs or SMCs are more resistant to antiangiogenic reagents and difficult to prune, antiangiogenic strategies targeting not only vascular ECs, but also PCs and SMCs simultaneously are desired. Our data showed that VEGF-B may be one such multi-target molecule.
VEGF-B is a survival factor for multiple types of neurons.
We and others have shown that VEGF-B has a survival effect on different types of neurons, including brain cortex neurons,9,54 retinal neurons,9 (Fig. 3) and motor neurons in the spinal cord.15 In vitro, VEGF-B protein treatment dose-dependently increased survival of cultured primary brain cortex neurons.9,54 In vivo, VEGF-B treatment inhibited apoptosis of brain cortex neurons and reduced stroke volume using a middle cerebral artery ligation-induced brain stroke model.9 In the retina, we have shown that VEGF-B treatment protected different types of retinal neurons from apoptosis under different pathological conditions. In the optic nerve crush injury model, VEGF-B treatment increased survival of retinal ganglion cells. In the NMDA-induced retinal neuron apoptosis model, VEGF-B treatment protected retinal neurons in the ganglion cell layer, inner nuclear layer and outer nuclear layer.9 Moreover, Poesen K, et al. has recently shown that VEGF-B treatment protected cultured primary motor neurons from apoptosis.15 Indeed, VEGF-B deficient mice developed a more severe form of motor neuron degeneration when intercrossed with mutant SOD1 mice, whereas VEGF-B intracerebroventricular injection prolonged the survival of mutant SOD1 rats.15 Taken together, different in vivo models have shown that VEGF-B is a survival factor for different types of neurons (Fig. 3).
VEGF-B is a survival factor for cardiac myocytes.
Apart from its survival effect on different types of vascular cells and neurons, several recent studies have also demonstrated a survival effect of VEGF-B on cardiac myocytes. AdVEGF-B186 delivery to the heart upregulated the expression of many antiapoptotic genes in cardiomyocytes, and inhibited cardiac myocyte apoptosis, demonstrating a survival effect of VEGF-B186 on them.13 Indeed, VEGF-B overexpression in cardiac myocytes under the alpha-myosin heavy chain promoter did not induce angiogenesis in the heart, but, instead, protected cardiomyocytes from damage after ischemia insult.14 Moreover, intravenous injection of AdVEGF-B167 in mice with myocardial infarction led to increased cardiomyocyte area and improved myocardial function,51 also indicating a protective effect of VEGF-B on cardiac myocytes. Taken together, data derived from different groups showed that VEGF-B has a survival effect on cardiac myocytes.
VEGFR-1 and NP-1 Mediate the Survival Effect of VEGF-B
VEGF-B binds to VEGFR-1 and NP-1.1,24 It is recently reported that NP-1 is expressed in vascular endothelial cells55,56 and smooth muscle cells,57,58 and plays an important role in vascular cell survival.59 In our recent study,12 using an NP-1 neutralizing antibody that blocks VEGF-B binding to NP-1, in both in vitro and in vivo assays, the vascular survival effect of VEGF-B was abolished by the NP-1 neutralizing antibody to a certain extent, suggesting that NP-1 is required for the vascular survival effect of VEGF-B. The other receptor used by VEGF-B, VEGFR-1, is also expressed in vascular and neuronal cells and plays a role in cell survival.60,61 In our study,12 we found that in vitro, VEGFR-1 neutralizing antibody abolished the regulatory effect of VEGF-B on the expression of many pro-survival genes in immortalized retinal pericytes. In vivo, VEGFR-1 neutralizing antibody largely abolished the survival effect of VEGF-B on retinal vasculature in the oxygen-induced blood vessel regression model. Thus, VEGFR-1 also plays a role in mediating the vascular survival effect of VEGF-B. Since NP-1 and VEGFR-1 interact with each other and can form heterodimers,62 it is possible that the vascular survival effect of VEGF-B can be mediated by the NP-1-VEGFR-1 complex.
Conclusion
In summary, VEGF-B is the only member of the VEGF family that has a potent survival/anti-apoptotic effect, while lacking a general angiogenic activity (except in the heart). Thus, VEGF-B can hardly be regarded as a “vascular endothelial growth factor.” Instead, VEGF-B has a potent survival effect on a broad spectrum of different types of cells. VEGF-B promotes the survival of vascular cells, neurons and cardiac myocytes by inhibiting cell apoptosis via suppressing the expression of the BH3-only protein and other apoptotic/cell death-related genes. Even though VEGF-B has a negligible role in inducing blood vessel growth in most organs, VEGF-B targeting inhibited pathological angiogenesis by abolishing blood vessel survival in different animal models. On the other hand, in different types of neuro-injury and neurodegenerative disease models, VEGF-B treatment protected neurons from apoptosis without inducing undesired blood vessel growth or permeability. Our work thus advocates that VEGF-B is a “survival,” rather than an “angiogenic/growth” factor, and further implicates a therapeutic value of VEGF-B in treating different types of vascular and neurodegenerative diseases.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/9459
References
- 1.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimmond S, Lagercrantz J, Drinkwater C, Silins G, Townson S, Pollock P, et al. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Res. 1996;6:124–131. doi: 10.1101/gr.6.2.124. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 5.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:29–35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 7.Reichelt M, Shi S, Hayes M, Kay G, Batch J, Gole GA, Browning J. Vascular endothelial growth factor-B and retinal vascular development in the mouse. Clin Exp Ophthalmol. 2003;31:61–65. doi: 10.1046/j.1442-9071.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Louzier V, Raffestin B, Leroux A, Branellec D, Caillaud JM, Levame M, et al. Role of VEGF-B in the lung during development of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003;284:926–937. doi: 10.1152/ajplung.00247.2002. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Tjwa M, Van Hove I, Enholm B, Neven E, Paavonen K, et al. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol. 2008;28:1614–1620. doi: 10.1161/ATVBAHA.107.158725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claesson-Welsh L. VEGF-B taken to our hearts: specific effect of VEGF-B in myocardial ischemia. Arterioscler Thromb Vasc Biol. 2008;28:1575–1576. doi: 10.1161/ATVBAHA.108.170878. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahteenvuo JE, Lahteenvuo MT, Kivela A, Rosenlew C, Falkevall A, Klar J, et al. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 14.Karpanen T, Bry M, Ollila HM, Seppanen-Laakso T, Liimatta E, Leskinen H, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103:1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poesen K, Lambrechts D, Van Damme P, Dhondt J, Bender F, Frank N, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its Ligand VEGF-B in motor neuron degeneration. J Neurosci. 2008;28:10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 19.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, et al. Placenta Growth Factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 22.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 23.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Aase K, Li H, von Euler G, Eriksson U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors. 2001;19:49–59. doi: 10.3109/08977190109001075. [DOI] [PubMed] [Google Scholar]
- 26.Aase K, Lymboussaki A, Kaipainen A, Olofsson B, Alitalo K, Eriksson U. Localization of VEGF-B in the mouse embryo suggests a paracrine role of the growth factor in the developing vasculature. Dev Dyn. 1999;215:12–25. doi: 10.1002/(SICI)1097-0177(199905)215:1<12::AID-DVDY3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33:421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 28.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19:61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, et al. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44. [DOI] [PubMed] [Google Scholar]
- 30.Wright CE. Effects of vascular endothelial growth factor (VEGF)A and VEGFB gene transfer on vascular reserve in a conscious rabbit hindlimb ischaemia model. Clin Exp Pharmacol Physiol. 2002;29:1035–1039. doi: 10.1046/j.1440-1681.2002.03773.x. [DOI] [PubMed] [Google Scholar]
- 31.Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, et al. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:60–70. doi: 10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 32.Wafai R, Tudor EM, Angus JA, Wright CE. Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind limb ischemia. J Vasc Res. 2008;46:45–54. doi: 10.1159/000139132. [DOI] [PubMed] [Google Scholar]
- 33.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 34.Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, et al. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther. 2003;14:1451–1462. doi: 10.1089/104303403769211664. [DOI] [PubMed] [Google Scholar]
- 35.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 37.Mould AW, Tonks ID, Cahill MM, Pettit AR, Thomas R, Hayward NK, Kay GF. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003;48:2660–2669. doi: 10.1002/art.11232. [DOI] [PubMed] [Google Scholar]
- 38.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 39.Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998;17:303–311. doi: 10.1038/sj.onc.1201928. [DOI] [PubMed] [Google Scholar]
- 40.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 41.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, et al. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]
- 42.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 43.Karkkainen AM, Kotimaa A, Huusko J, Kholova I, Heinonen SE, Stefanska A, et al. Vascular endothelial growth factor-D transgenic mice show enhanced blood capillary density, improved postischemic muscle regeneration, and increased susceptibility to tumor formation. Blood. 2009;113:4468–4475. doi: 10.1182/blood-2008-07-171108. [DOI] [PubMed] [Google Scholar]
- 44.Kiba A, Sagara H, Hara T, Shibuya M. VEGFR-2-specific ligand VEGF-E induces non-edematous hyper-vascularization in mice. Biochem Biophys Res Commun. 2003;301:371–377. doi: 10.1016/s0006-291x(02)03033-4. [DOI] [PubMed] [Google Scholar]
- 45.Bhardwaj S, Roy H, Heikura T, Yla-Herttuala S. VEGF-A, VEGF-D and VEGF-D induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 47.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 49.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: Role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100:727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 50.Abraham D, Taghavi S, Riml P, Paulus P, Hofmann M, Baumann C, et al. VEGF-A and -C but not -B mediate increased vascular permeability in preserved lung grafts. Transplantation. 2002;73:1703–1706. doi: 10.1097/00007890-200206150-00003. [DOI] [PubMed] [Google Scholar]
- 51.Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M, et al. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 53.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab. 2004;24:1146–1152. doi: 10.1097/01.WCB.0000134477.38980.38. [DOI] [PubMed] [Google Scholar]
- 55.Kawamura H, Li X, Goishi K, van Meeteren LA, Jakobsson L, Cebe-Suarez S, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112:3638–3649. doi: 10.1182/blood-2007-12-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee S, Mehta S, Haque I, Sengupta K, Dhar K, Kambhampati S, et al. VEGF-A165 induces human aortic smooth muscle cell migration by activating neuropilin-1-VEG-FR1-PI3K axis. Biochemistry. 2008;47:3345–3351. doi: 10.1021/bi8000352. [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Parikh AA, Stoeltzing O, Fan F, McCarty MF, Wey J, et al. Upregulation of neuropilin-1 by basic fibroblast growth factor enhances vascular smooth muscle cell migration in response to VEGF. Cytokine. 2005;32:206–212. doi: 10.1016/j.cyto.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, Mukhopadhyay D. Neuropilin-1 Modulates p53/Caspases Axis to Promote Endothelial Cell Survival. PLoS ONE. 2007;2:1161. doi: 10.1371/journal.pone.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shih SC, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest. 2003;112:50–57. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci USA. 2003;100:15859–15864. doi: 10.1073/pnas.2136855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]