Abstract
Changes in cell shape are associated with a variety of processes including cell migration, axon outgrowth, cell division and vesicle trafficking. C. elegans UNC-53 and its vertebrate homologs, the Navigators, are required for the migration of cells and the outgrowth of neuronal processes. The identification of novel molecular interactions and live imaging studies have revealed that UNC-53/NAVs are signal transducers associated with actin filaments, microtubules and intermediate filaments. In addition to modulating cytoskeletal dynamics at the leading edge of migrating or outgrowing cells, both UNC-53 and the navigators are expressed in adult cells, conspicuously those with specialized roles in endocytosis or secretion. Collectively, these results suggest that UNC-53/NAVs may be a central regulator of cytoskeletal dynamics, responsible for integrating signaling cues to multiple components of the cytoskeleton to coordinate rearrangement during cell outgrowth or trafficking.
Key words: cytoskeleton, actin filaments, microtubules, growth cone, endocytosis
The stepwise outgrowth of a growth cone is thought to be comprised of repeated cycles of: (1) protrusion, where actin rich filopodia and lamellipodia are extended from the leading edge of the cell (2) engorgement, characterized by microtubule invasion and the transport of vesicles and organelles into the periphery, and (3) consolidation, where the axon shaft is stabilized through cortical actin depolymerization and subsequent plasma membrane shrinkage around microtubule tracks concomitant with the establishment of bidirectional transport.1 The molecules and mechanisms that control the coordinated rearrangements of the actin and microtubule cytoskeleton that take place during these repeated cycles of protrusion, engorgement and consolidation during axon outgrowth are slowly coming to light.
We have been studying the role of unc-53 (uncoordinated-53), a novel component of a signal transduction pathway controlling cell motility and growth cone extension in the model nematode Caenorhabditis elegans. Hypomorphic alleles of unc-53 display guidance defects in the anterior migration of the sex myoblasts,2 and the outgrowth of mechanosensory neurons, motor neurons and the excretory canals.3 In contrast, overexpression of UNC-53 in muscle cells results in exaggerated outgrowth during embryogenesis.3 UNC-53 is a cytoplasmic protein that functions cell autonomously to control cell migration.3,4
Three mammalian UNC-53 homologs, NAV-1, NAV-2 and NAV-3 (Neuron Navigator-1,2,3) coined Navigators for their role in axon guidance have been identified,5,6 one of which, NAV-2, was shown to be retinoic acid inducible in the developing nervous system.5 Mice hypomorphic for NAV-2 have sensory deficits subsequent to morphological defects consistent with a role for NAV-2 in neurite outgrowth.7 NAV-2 is expressed in nerve tissue, kidney, placenta6 and in the heart5 while the expression of NAV-1 is restricted to the nervous system.8 NAV-2 is a true orthologue of UNC-53 as it can rescue the mechanosensory neuron outgrowth defects of unc-53 mutants when expressed in those cells.9 NAV-3 mRNA is expressed throughout the nervous system, but is especially prominent at the synapses at neuromuscular junctions.10 In neuroblastomas, expression of NAV3 is reduced while upregulated in nerve cells after brain injury, indicating that NAV3 is involved in neuron growth and regeneration as well as neural tumorigenesis.11 Deletions or re-arrangements of human NAV-3 was shown to be present in 50% of patients with early stage primary cutaneous T-cell lymphomas and 85% of patients with advanced disease, suggesting that UNC-53/NAV3 may be a tumor suppressor in lymphoid tissue.12
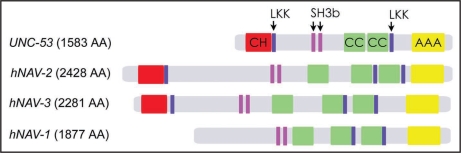
Several protein motifs are shared between UNC-53 and the Navigators including a calponin homology (CH) domain in the N-terminus, several coiled-coil regions, polyproline rich SH3 binding motifs, and an ATPases associated with diverse cellular activities domain (AAA domain) (Fig. 1).3,6 UNC-53 and the navigators are subject to multiple facets of gene regulation including multiple promoters and alternative splicing of RNA transcripts, giving rise to several protein isoforms.3,4,6 Consistent with the protein motif analysis, UNC-53 interacts genetically and physically with the SH2-SH3 adaptor protein SEM-5/GRB-2,2,3 suggesting a role in signal transduction.
Figure 1.
General domain organization of neuronal navigator family proteins. NAV family proteins display a highly conserved domain organization containing multiple domains involved in signal transduction and cytoskeletal binding. Domains include a calponin homology domain (red), LKK actin-binding motifs (blue), polyproline rich SH3 binding motifs (purple), coiled coil domains (green) and a AAA ATPase associated with diverse cellular activities (yellow).
UNC-53/NAVs contains several domains observed in actin binding proteins3 and UNC-53 associates with F-actin in vitro (Stringham EG, unpublished) suggesting a role in actin cytoskeleton dynamics. We recently identified ABI-1 (abelson kinase interactor), a regulator of Arp2/3 mediated actin filament nucleation, as a molecular partner of UNC-53/NAV-2, and found that this interaction is mediated through the N-terminal calponin homology (CH) domain of UNC-53.4 ABI-1 is expressed in several of the same cells as UNC-53, and loss of abi-1 leads to migration defects similar to those found when unc-53 or members of the WAVE or Arp2/3 complex are removed.4 The UNC-53-ABI-1 interaction suggests a mechanism whereby UNC-53/NAVs may control cellular outgrowth by regulating the assembly of branched actin filaments nucleated by the Arp2/3 complex at the leading edge. Interestingly, while the CH domain is conserved in the N-terminus of UNC-53 and the vertebrate homologs NAV-2, and NAV-3, this domain is absent in mammalian NAV-1 (Fig. 1), and also in the shortest UNC-53 isoforms that are transcribed from intronic promoters.3,6
Additionally, several studies of the vertebrate UNC-53/NAVs have revealed a potential role for these proteins in microtubule organization. Murine NAV-1 associates with microtubule plus-ends on developing neuronal growth cones and its overexpression causes microtubule bundling while depletion results in a loss of directionality in migrating cells.13 These authors identified a novel type of microtubule binding domain located centrally in the protein between two coiled-coil motifs.13
Human NAV-2 has also been found to localize to microtubules as well as neurofilaments in growing neurites.9 NAV-2 did not appear to be directly involved with microtubule assembly per se as its association with microtubules lagged behind polymerization.9 Notably, these authors also identified a region required for association with the microtubule cytoskeleton that is conserved among the navigators.9
Even more recently, all three mammalian navigators have been shown to be plus end tracking proteins (+TIPs) that can promote the formation of ectopic neurite-like extensions when expressed in non-neuronal cells grown in culture.14 For at least NAV-1, the capacity to induce membrane protrusions was found to be dependent on its ATPase activity, as a mutation in the AAA cassette that abolishes nucleotide binding virtually eliminated the formation of ectopic extensions.14
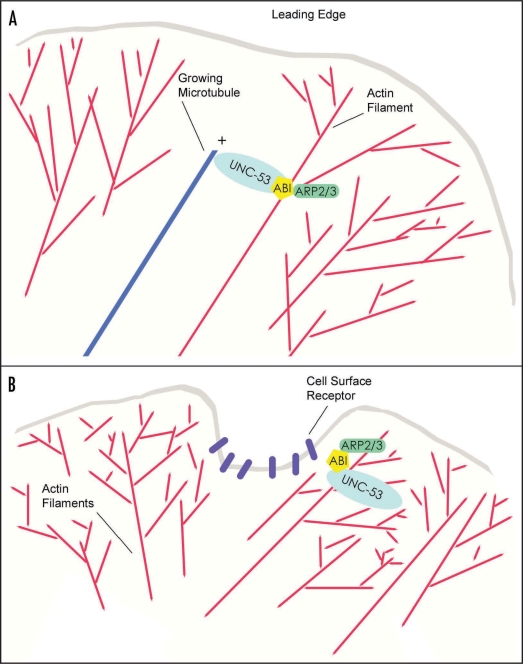
We have proposed that UNC-53 may act as a scaffold to localize regulators, such as ABI-1, to the actin cytoskeleton machinery to modulate the formation of branched actin filaments at the leading edge of motile cells. On the other hand, the vertebrate NAVs have been shown to associate with the plus ends of microtubules on outgrowing cells. As such, UNC-53/NAVs join a growing list of proteins that interact with both actin filaments and microtubules in the growth cone.1,15 This group includes MT stabilizing proteins such as MAP2c,16 the Type V and VI unconventional myosin motors,17 and the crosslinking spectraplakin ACF7. In the case of the latter, ACF7 contains an actin regulated ATPase domain that is essential for epidermal cell migration and for the targeting of microtubules along actin filaments to focal adhesions.18 Similarly, the intrinsic ATPase activity of UNC-53/NAVs may be important for controlling interactions between multiple cytoskeletal components. In summary, UNC-53/NAVs have been found associated with actin filaments, microtubules and intermediate filaments, potentially making the navigators excellent candidates as linkers of actin filaments to microtubule capture and/or stabilization during the engorgement or consolidation phases of axon outgrowth (Fig. 2).
Figure 2.
Proposed models for the function of the navigators in cytoskeletal remodelling. (A) The navigators may control actin filament-microtubule capture during cell outgrowth or migration. The navigators are microtubule + end TIPs that also bind ABI, a regulator of Arp2/3 mediated actin filament assembly. Therefore, UNC-53/Navigators may link branched actin filament assembly in protrusions at the leading edge of migrating cells to the plus ends of microtubules, thereby stabilizing and/or promoting directional outgrowth. (B) Navigators are involved in endocytic pathways. As opposed to promoting filopodia formation or stabilization directly, the navigators may influence outgrowth by regulating localized actin filament mediated endocytosis of receptors and/or signals at the cell surface. Similarly, UNC-53/NAVs may coordinate actin dependent pinocytosis and synaptic vesicle trafficking in adult cells.
While UNC-53 clearly has a role in the migration of several cells and the guidance of the developing nervous system it is also required in adult cells. Immunofluorescence of adult hermaphrodites with UNC-53 antisera stains the excretory canals and several neurons suggesting that UNC-53 activity is continually required in these cells after completion of outgrowth.4 The expression of the mammalian Navigators are also expressed in a range of adult tissues including brain, heart and kidney.7,11,13 Murine NAV-3 mRNA is found concentrated at the synapses of neuromuscular junctions.10 Likewise, expression of UNC-53 is evident in adult sensory and motor neurons that undergo continuous trafficking of synaptic vesicles, and unc-53 was isolated in a screen for genes involved in synaptic connectivity.19
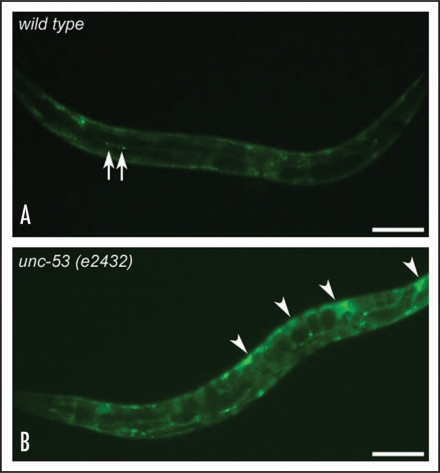
UNC-53 is also highly expressed in coelomocytes, specialised cells that endocytose pseudocoelomic fluid in C. elegans. Using an in vivo assay where GFP produced in body muscle is secreted into the pseudocoelom and then endocytosed into coelomocytes,20 we have demonstrated a coelomocyte-uptake (cup) defect in worms depleted of unc-53 (Fig. 3), or arx-2/Arp2 (data not shown). Similarly, knockdown of unc-53 causes defects in receptor mediated endocytosis, as determined by an assay where yolk protein is selectively endocytosed into oocytes of the hermaphrodite.21 These observations suggest that UNC-53/NAVs might have a general role in coordinating cytoskeletal rearrangements during vesicle trafficking (Fig. 2).
Figure 3.
Coelomocyte endocytosis is disrupted in unc-53 mutant animals. Coelomocyte uptake is measured using the transgenic reporter pmyo-3::ssgfp (arIs37).20 In this assay, ssGFP is expressed in body-wall muscle under the control of the myo-3 promoter and is secreted into the pseudocoelomic cavity where it is efficiently endocytosed and degraded by coelomocytes. Wild-type animals (A) show GFP expression in bodywall muscle and coelomocytes (only two coelomocytes shown, arrows) with little expression in the body cavity. In contrast, unc-53 (e2432) (B) animals have GFP accumulation in the pseudocoelom (arrowheads) in addition to the bodywall and coelomocytes. Scale bar is 100 µm.
Expression of the UNC-53 interactor ABI-1 also persists in adulthood and several lines of evidence suggest that ABI-1 may function in vesicle trafficking and synapse function. Human ABI-1 binds Syntaxin-1 through a SNARE domain22 that is conserved in C. elegans ABI-1. Moreover, UNC-64, the C. elegans syntaxin-1 homologue, is expressed in the same major neuronal processes as ABI-1 including the dorsal and ventral cords. UNC-64/syntaxin-1 interacts with the synaptic vesicle trafficking protein SNB-1/synaptobrevin,23,24 and with synaptojanin and dynamin, both with clear roles in endocytosis.25–27 Finally, it has been shown in rat hippocampal neurons that ABI-1 is essential for dendrite and synapse formation.28 Thus, the UNC-53-ABI-1 interaction may be an important modulator of Arp2/3 mediated actin filament assembly in the trafficking of components at synapses.
In addition, it is well understood that a large amount of trafficking, both inward and outward, occurs at the growth cone. Signaling cues and their receptors are endocytosed while trafficking of lipids and membrane proteins to the leading edge is required for polarized membrane expansion. For example, in response to EGF signaling, Exo70, a component of the exocyst interacts with the Arp2/3 complex. Inhibition of Exo 70 by RNAi blocks the formation of actin rich membrane protrusions and disrupts cell motility29 while overexpression of Exo70 induces the formation of filopodia.30 By modulating Arp2/3 mediated assembly of actin filaments, UNC-53/NAVs may be involved in coordinating the trafficking of components to and from the membrane of migrating cells (Fig. 2).
Several questions remain unanswered. If UNC-53/NAVs are so important in coordinating cytoskeletal rearrangements, why is UNC-53 only required for some longitudinal migrations in the worm and for only the second half of these trajectories? One possibility is that as an outgrowing growth cone becomes more distant from the cell body, coordination of actin filament assembly with microtubule capture becomes physically more difficult, requiring a scaffolding molecule such as UNC-53/NAVs to concentrate components in the correct place. Alternatively, via their ATPase activity, the navigators may regulate the trafficking of components along microtubules and/or actin filaments in the growth cone to promote membrane protrusion. Whatever the answer, clearly, UNC-53 and the navigators define a family of proteins with a multifaceted role in cytoskeletal dynamics, and identifying the molecular interactions modulated by these proteins should provide fresh insights into cell rearrangements during trafficking, migration and outgrowth.
Acknowledgements
This work was supported by an NSERC Discovery grant and a Canada Research Chair to E.G.S.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/9451
References
- 1.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen EB, Branda CS, Stern MJ. Genetic enhancers of sem-5 define components of the gonad-independent guidance mechanism controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev Biol. 1997;182:88–100. doi: 10.1006/dbio.1996.8473. [DOI] [PubMed] [Google Scholar]
- 3.Stringham E, Pujol N, Vandekerckhove J, Bogaert T. Unc-53 controls longitudinal migration in C. elegans. Development. 2002;129:3367–3379. doi: 10.1242/dev.129.14.3367. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development. 2009;136:563–574. doi: 10.1242/dev.016816. [DOI] [PubMed] [Google Scholar]
- 5.Merrill RA, Plum LA, Kaiser ME, Clagett-Dame M. A mammalian homolog of unc-53 is regulated by all-trans retinoic acid in neuroblastoma cells and embryos. Proc Natl Acad Sci USA. 2002;99:3422–3427. doi: 10.1073/pnas.052017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes T, Barcelo A, Buesa C. Neuron navigator: A human gene family with homology to unc-53, a cell guidance gene from Caenorhabditis elegans. Genomics. 2002;80:21–30. doi: 10.1006/geno.2002.6799. [DOI] [PubMed] [Google Scholar]
- 7.Peeters PJ, Baker A, Goris I, Daneels G, Verhasselt P, Luyten WH, et al. Sensory deficits in mice hypomorphic for a mammalian homologue of unc-53. Brain Res Dev Brain Res. 2004;150:89–101. doi: 10.1016/j.devbrainres.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Lopez MJ, Alcantara S, Mascaro C, Perez-Branguli F, Ruiz-Lozano P, Maes T, et al. Mouse neuron navigator 1, a novel microtubule-associated protein involved in neuronal migration. Mol Cell Neurosci. 2005;28:599–612. doi: 10.1016/j.mcn.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Muley PD, McNeill EM, Marzinke MA, Knobel KM, Barr MM, Clagett-Dame M. The atRA-responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation. Dev Neurobiol. 2008;68:1441–1453. doi: 10.1002/dneu.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishi M, Kummer TT, Eglen SJ, Sanes JR. LL5beta: A regulator of postsynaptic differentiation identified in a screen for synaptically enriched transcripts at the neuromuscular junction. J Cell Biol. 2005;169:355–366. doi: 10.1083/jcb.200411012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coy JF, Wiemann S, Bechmann I, Bachner D, Nitsch R, Kretz O, et al. Pore membrane and/or filament interacting like protein 1 (POMFIL1) is predominantly expressed in the nervous system and encodes different protein isoforms. Gene. 2002;290:73–94. doi: 10.1016/s0378-1119(02)00567-x. [DOI] [PubMed] [Google Scholar]
- 12.Karenko L, Hahtola S, Paivinen S, Karhu R, Syrja S, Kahkonen M, et al. Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue. Cancer Res. 2005;65:8101–8110. doi: 10.1158/0008-5472.CAN-04-0366. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Lopez MJ, Alcantara S, Mascaro C, Perez-Branguli F, Ruiz-Lozano P, Maes T, et al. Mouse neuron navigator 1, a novel microtubule-associated protein involved in neuronal migration. Mol Cell Neurosci. 2005;28:599–612. doi: 10.1016/j.mcn.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.van Haren J, Draegestein K, Keijzer N, Abrahams JP, Grosveld F, Peeters PJ, et al. Mammalian navigators are microtubule plus-end tracking proteins that can reorganize the cytoskeleton to induce neurite-like extensions. Cell Motil Cytoskeleton. 2009;66:824–838. doi: 10.1002/cm.20370. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 16.Ozer RS, Halpain S. Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol Biol Cell. 2000;11:3573–3587. doi: 10.1091/mbc.11.10.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suter DM, Espindola FS, Lin CH, Forscher P, Mooseker MS. Localization of unconventional myosins V and VI in neuronal growth cones. J Neurobiol. 2000;42:370–382. [PubMed] [Google Scholar]
- 18.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman A, Dror G, Meilijson I, Ruppin E. Gene expression of Caenorhabditis elegans neurons carries information on their synaptic connectivity. PLoS Comput Biol. 2006;2:167. doi: 10.1371/journal.pcbi.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fares H, Greenwald I. Genetic analysis of endocytosis in Caenorhabditis elegans: Coelomocyte uptake defective mutants. Genetics. 2001;159:133–145. doi: 10.1093/genetics/159.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 22.Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa H, Harada S, Sassa T, Yamamoto H, Hosono R. Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J Biol Chem. 1998;273:2192–2198. doi: 10.1074/jbc.273.4.2192. [DOI] [PubMed] [Google Scholar]
- 24.Saifee O, Wei L, Nonet ML. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell. 1998;9:1235–1252. doi: 10.1091/mbc.9.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So CW, So CK, Cheung N, Chew SL, Sham MH, Chan LC. The interaction between EEN and abi-1, two MLL fusion partners, and synaptojanin and dynamin: Implications for leukaemogenesis. Leukemia. 2000;14:594–601. doi: 10.1038/sj.leu.2401692. [DOI] [PubMed] [Google Scholar]
- 26.Schuske KR, Richmond JE, Matthies DS, Davis WS, Runz S, Rube DA, et al. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- 27.Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, et al. Abelson interacting protein 1 (abi-1) is essential for dendrite morphogenesis and synapse formation. EMBO J. 2007;26:1397–1409. doi: 10.1038/sj.emboj.7601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006;8:1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J Biol Chem. 2004;279:35958–35966. doi: 10.1074/jbc.M313778200. [DOI] [PubMed] [Google Scholar]